Urban Re-Greening: A Case Study in Multi-Trophic Biodiversity and Ecosystem Functioning in a Post-Industrial Landscape
Abstract
1. Introduction
2. The Case Study: Liberty State Park in Jersey City, NJ, USA
2.1. Site Description
2.2. Urban Ecosystem Function Classification
3. Functional Diversity within Our Urban Case Study
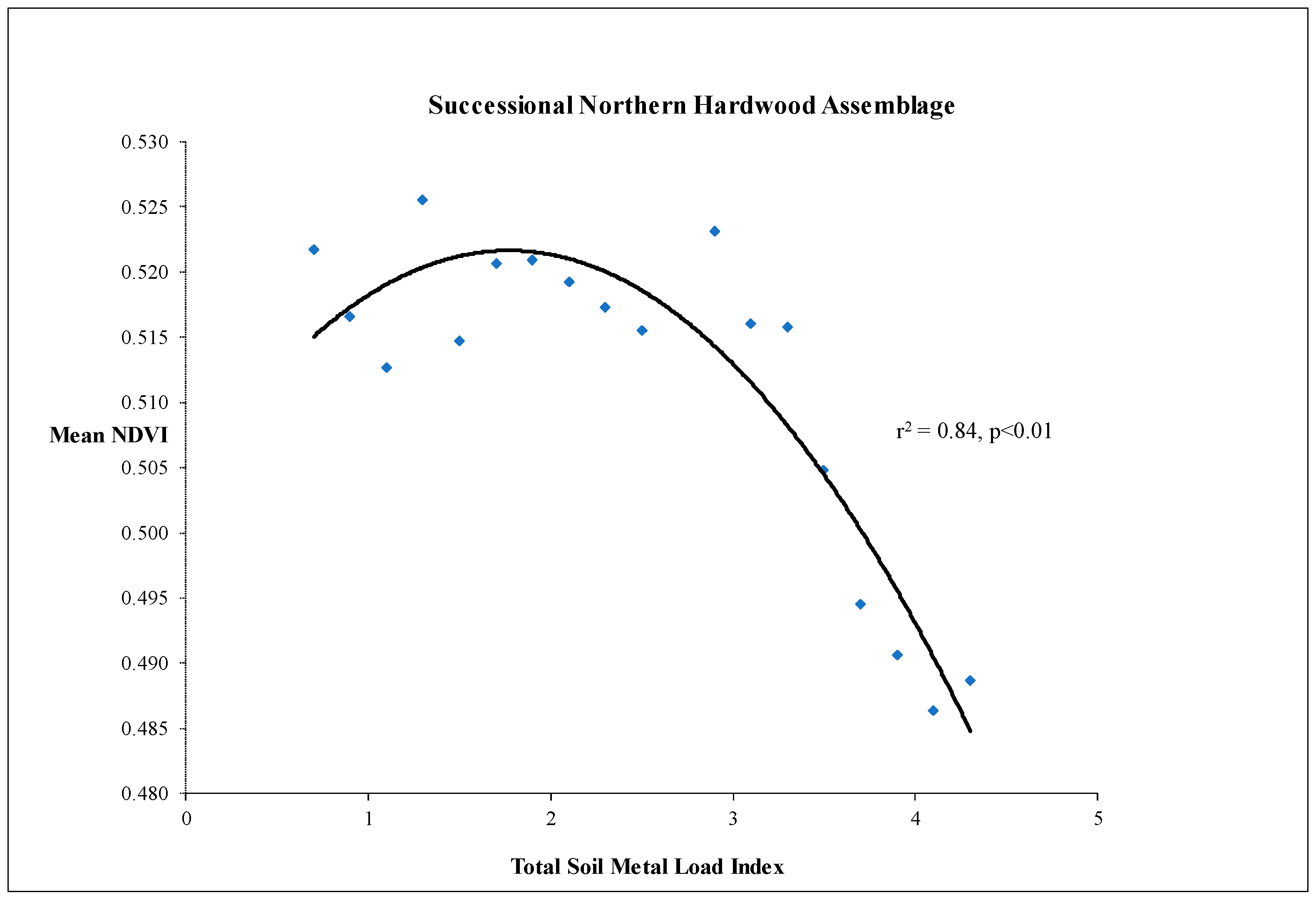
3.1. Photosynthesis and Carbon Allocation as Production Functions
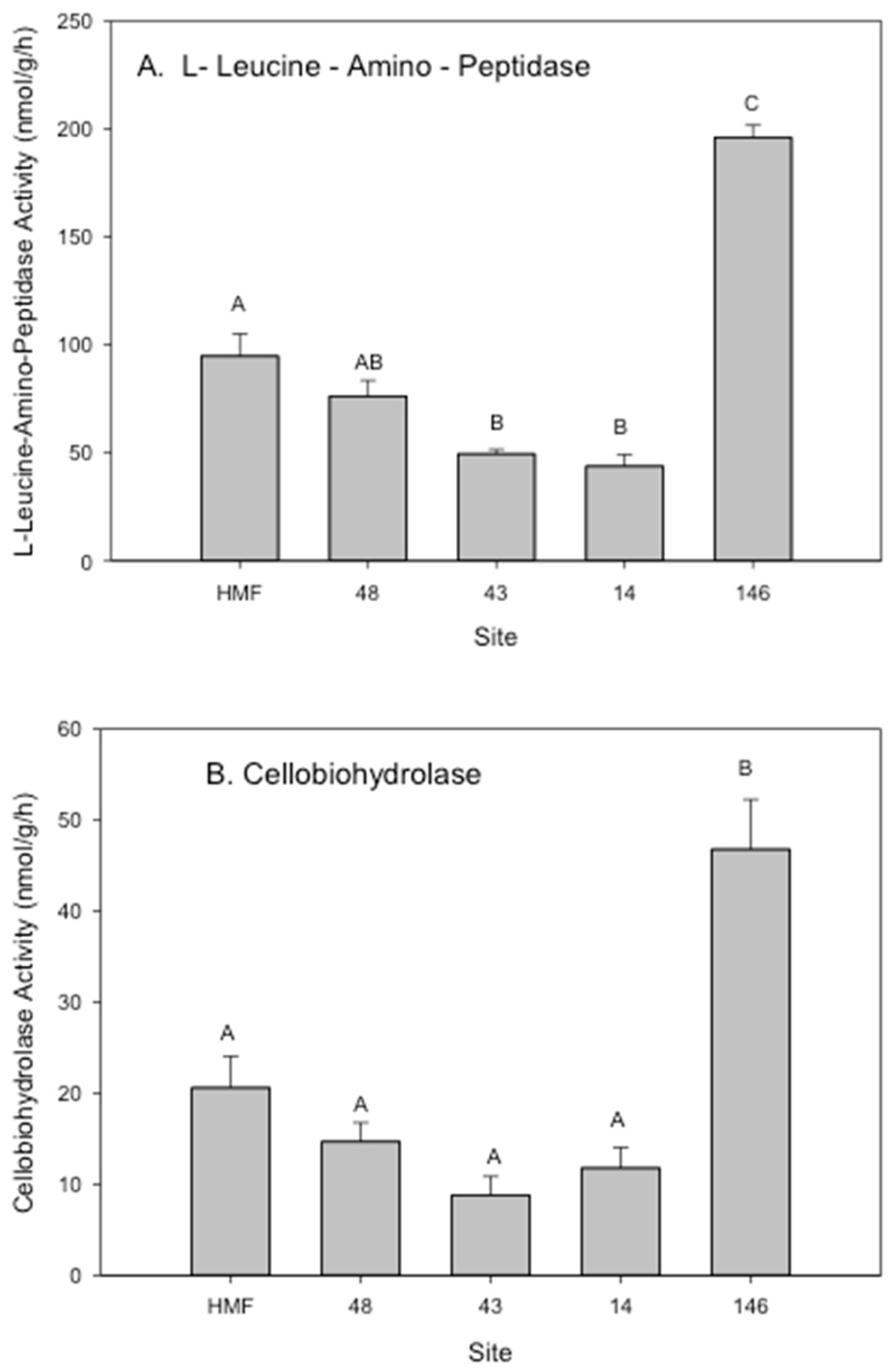
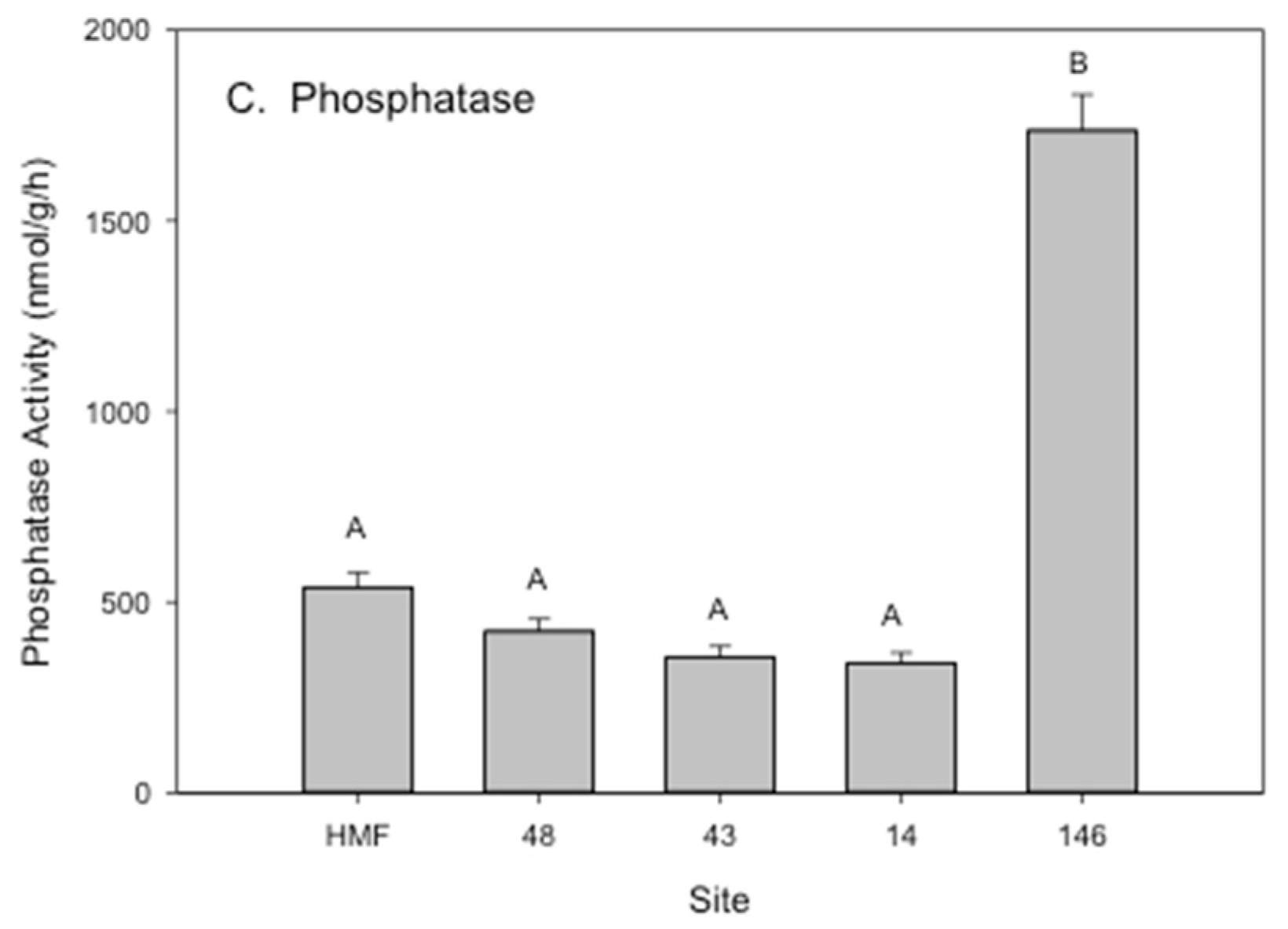
3.2. Rhizosphere Microbial Metal Transfer and Amelioration, Regulatory Functions
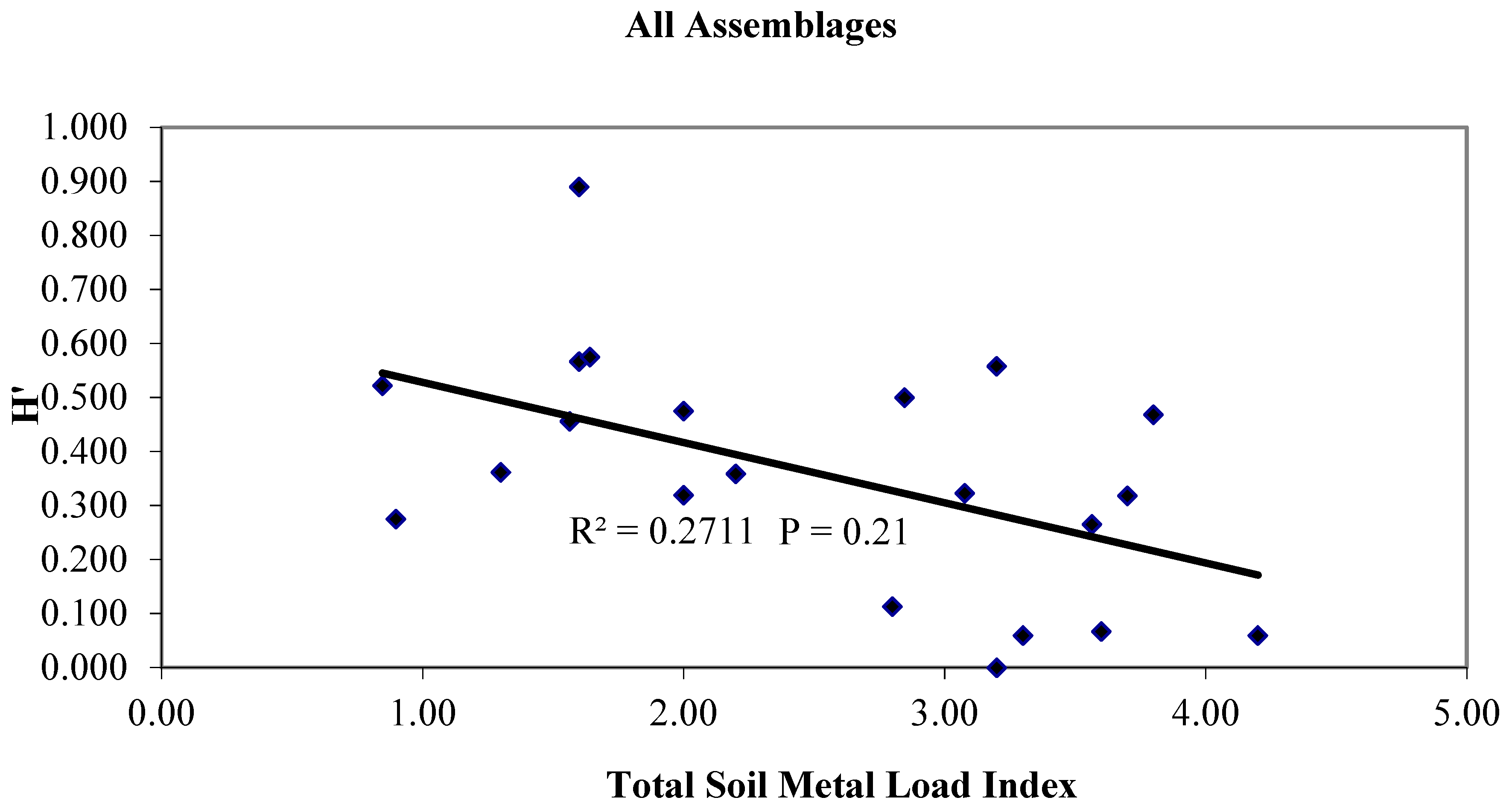
3.3. Metal Translocation and the Plant Community, a Regulatory Function
3.4. Trophic Transfer to Secondary and Tertiary Consumers, a Regulatory Function
3.5. Functional Costs of Metal Transfer and Tolerance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Site | Species | As | Cr | Cu | Pb | Zn | Va | Na | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| l | r | st | s | l | r | st | s | l | r | st | s | l | r | st | s | l | r | st | s | l | r | st | s | l | r | st | s | ||
| TP-1 | Rhus sp. | 0.00 | 0.75 | >MD | 12.5 | 0.37 | 4.92 | 0.00 | 41.3 | 5.14 | 126 | 1.3 | 124 | >MD | 121 | 1 | 453 | 54.7 | 694 | 14.8 | 309 | >MD | 6.12 | >MD | 19.6 | 0.06 | 3.95 | 0.33 | 0.80 |
| TP-3 | Rhus sp. | 0.00 | 0.92 | >MD | 38.9 | 0.58 | 10.1 | 0.00 | 70.1 | 5.58 | 35.7 | 1.6 | 379 | >MD | 27 | 1 | 341 | 29.1 | 147 | 11.3 | 168 | >MD | 11.6 | >MD | 44.2 | 0.08 | 3.02 | 0.37 | 1.04 |
| TP-8a | Artemisia vulgaris | 0.00 | 2.29 | >MD | 11.9 | 0.40 | 6.63 | 0.07 | 38.4 | 20.0 | 85.4 | 7.4 | 168 | >MD | 91 | 1 | 349 | 122 | 339 | 154 | 207 | >MD | 11.3 | >MD | 29.0 | 0.07 | 6.07 | 0.43 | 3.05 |
| TP-8b | Solidago virgaurea | 0.00 | >MD | >MD | >MD | 0.47 | >MD | 0.12 | >MD | 9.30 | >MD | 10.1 | >MD | >MD | >MD | 1 | >MD | 69.5 | >MD | 396 | >MD | >MD | >MD | >MD | >MD | 0.04 | >MD | 1.58 | >MD |
| TP-10 | Populus tremuloides | 0.00 | 20.20 | >MD | 193 | 0.33 | 9.16 | 0.00 | 61.6 | 8.99 | 78.4 | 2.5 | 257 | >MD | 93 | 4 | 500 | 1309 | 270 | 81.8 | 131 | >MD | 11.7 | >MD | 27.0 | 0.06 | 5.63 | 0.14 | 1.16 |
| TP-14 | Betula populifolia | 0.00 | 0.90 | >MD | 87.4 | 0.25 | 9.34 | 0.00 | 37.8 | 4.32 | 33.0 | 1.3 | 103 | >MD | 94 | 4 | 383 | 256 | 320 | 67 | 49.9 | >MD | 3.31 | >MD | 20.7 | 0.12 | 1.70 | 0.13 | 2.55 |
| TP-16a | Onoclea sensibilis | 0.00 | 2.91 | >MD | 35.1 | 0.72 | 13.4 | 0.51 | 53.4 | 4.62 | 48.0 | 4.2 | 67.0 | >MD | 47 | 0 | 168 | 2.69 | 37.5 | 25.5 | 38.7 | >MD | 21.8 | >MD | 72.3 | 0.14 | 4.99 | 1.05 | 1.50 |
| TP-16b | Phragmites australis | 0.00 | >MD | >MD | >MD | 0.27 | >MD | 0.00 | >MD | 5.41 | >MD | 4.2 | >MD | >MD | >MD | 1 | >MD | 2.09 | >MD | 30.2 | >MD | >MD | >MD | >MD | >MD | 0.09 | >MD | 3.55 | >MD |
| TP-18 | Betula populifolia | 0.00 | 1.77 | >MD | 33.3 | 0.06 | 10.7 | 0.00 | 27.6 | 6.93 | 120 | 1.3 | 238 | >MD | 74 | 4 | 393 | 1284 | 458 | 241 | 491 | >MD | 10.6 | >MD | 118 | 0.06 | 13.40 | 0.20 | 1.38 |
| TP-21 | Artemisia vulgaris | 0.00 | 1.68 | >MD | 37.4 | 0.38 | 5.93 | 0.37 | 38.2 | 19.0 | 81.9 | 8.2 | 345 | >MD | 67 | 1 | 563 | 95.4 | 377 | 172 | 1058 | >MD | 8.94 | >MD | 58.0 | 0.04 | 3.07 | 0.60 | 2.49 |
| TP-24 | Populus sp. | 0.00 | 0.95 | >MD | 23.6 | 0.20 | 3.18 | 0.00 | 44.0 | 8.62 | 43.6 | 3.3 | 249 | >MD | 92 | 1 | 486 | 1693 | 487 | 127 | 718 | >MD | 6.73 | >MD | 24.9 | 0.09 | 2.26 | 0.10 | 1.24 |
| TP-25 | Populus sp. | 0.04 | 3.25 | >MD | 270 | 0.16 | 2.76 | 0.00 | 40.4 | 8.05 | 113 | 3.0 | 1527 | >MD | 199 | 9 | 4640 | 1657 | 604 | 152 | 1586 | >MD | 8.87 | >MD | 54.0 | 0.06 | 5.78 | 0.25 | 6.72 |
| TP-28 | Betula populifolia | 0.01 | 1.64 | >MD | 22.5 | 0.03 | 13.7 | 0.00 | 68.5 | 5.45 | 20.1 | 1.3 | 44.3 | >MD | 56 | 4 | 173 | 109 | 145 | 64.2 | 72.5 | >MD | 16.5 | >MD | 37.8 | 0.10 | 1.85 | 0.24 | 5.26 |
| TP-40 | Artemisia vulgaris | 0.00 | 1.08 | >MD | 13.2 | 0.09 | 6.38 | 0.00 | 20.3 | 23.3 | 46.3 | 10.7 | 153 | >MD | 47 | 1 | 303 | 54.8 | 59.8 | 50.7 | 159 | >MD | 8.60 | >MD | 193 | 0.05 | 12.18 | 0.77 | 1.91 |
| TP-40 B | Rhus sp. | 0.00 | 1.21 | >MD | 24.8 | 0.04 | 6.66 | 0.12 | 46.6 | 2.98 | 53.5 | 2.7 | 212 | >MD | 54 | 0 | 421 | 1.06 | 49.2 | 4.19 | 232 | >MD | 11.8 | >MD | 66.9 | 0.09 | 2.50 | 1.33 | 2.34 |
| TP40C | Artemisia vulgaris | 0.00 | 0.55 | >MD | 14.2 | 0.62 | 2.06 | 0.15 | 20.2 | 21.4 | 57.0 | 6.6 | 224 | >MD | 22 | 1 | 421 | 107 | 374 | 118 | 603 | >MD | 2.57 | >MD | 39.0 | 0.05 | 2.87 | 0.77 | 3.83 |
| TP-41 | Betula populifolia | 0.08 | 0.91 | >MD | 13.3 | 0.13 | 1.83 | 0.00 | 9.7 | 6.11 | 28.6 | 1.6 | 229 | >MD | 60 | 3 | 449 | 1617 | 303 | 243 | 304 | >MD | 5.78 | >MD | 40.6 | 0.08 | 2.48 | 0.26 | 0.79 |
| TP-43 | Betula populifolia | 0.02 | 1.56 | >MD | 21.0 | 0.37 | 3.86 | 1.32 | 10.2 | 5.29 | 40.6 | 1.6 | 68 | >MD | 112 | 26 | 86 | 1051 | 81.3 | 70.0 | 198 | >MD | 7.92 | >MD | 118 | 0.11 | 1.71 | 0.21 | 0.67 |
| TP-48 | Betula populifolia | 0.00 | 1.26 | >MD | 10.7 | 0.48 | 9.44 | 0.12 | 16.7 | 5.14 | 38.7 | 1.3 | 166 | 10 | 125 | 10 | 333 | 1034 | 169 | 69.6 | 64 | >MD | 3.98 | >MD | 79.8 | 0.17 | 3.62 | 0.38 | 1.80 |
| TP7/8 | Rhus sp. | 0.00 | 1.24 | >MD | 57.1 | 0.31 | 2.42 | 0.00 | 50.6 | 4.44 | 17.3 | 2.9 | 199 | >MD | 28 | 3 | 409 | 2.51 | 165 | 23.5 | 218 | >MD | 3.25 | >MD | 9.31 | 0.05 | 2.74 | 0.39 | 2.32 |
| TP-14/16 | Betula populifolia | 0.05 | 3.34 | >MD | 42.8 | 0.57 | 52.8 | 0.00 | 209 | 5.57 | 51.4 | 2.2 | 76.4 | >MD | 332 | 16 | 196 | 824 | 153 | 118 | 24.9 | >MD | 28.0 | >MD | 7.37 | 0.16 | 2.18 | 0.37 | 1.42 |
| TP-21/40 | Rhus sp. | 0.00 | 0.52 | >MD | 15.7 | 0.28 | 2.67 | 0.07 | 32.1 | 3.13 | 40.6 | 1.8 | 129 | >MD | 43 | 1 | 552 | 1.81 | 239 | 21.5 | 157 | >MD | 3.94 | >MD | 0.00 | 0.04 | 2.77 | 0.83 | 1.56 |
| TP-28/17 | Polygonum cuspidatum | 0.00 | 2.56 | >MD | 12.5 | 0.51 | 12.7 | 0.06 | 43.4 | 4.29 | 40.3 | 6.5 | 48.3 | >MD | 43 | 1 | 156 | 98 | 131 | 59.1 | 96 | >MD | 22.5 | >MD | 18.1 | 0.13 | 4.19 | 1.24 | 3.00 |
| TP-43/14 | Betula populifolia | 0.00 | 7.16 | >MD | 17.6 | 0.19 | 2.83 | 0.64 | 11.4 | 6.15 | 36.0 | 3.2 | 69.9 | 8 | 181 | 22 | 117 | 1062 | 135 | 112 | 49.0 | >MD | 10.6 | >MD | 8.28 | 0.09 | 2.39 | 0.26 | 2.07 |
References
- Wilcox, B.A.; Murphy, D.D. Conservation strategy: The effects of fragmentation on extinction. Am. Nat. 1985, 125, 879–887. [Google Scholar] [CrossRef]
- Powell, G.V.N.; Powell, A.H.X. Reproduction by Great white herons Ardea herodias in Florida bay as an indicator of habitat quality. Biol. Conserv. 1986, 36, 101–113. [Google Scholar] [CrossRef]
- Canterbury, G.E.; Martin, T.E.; Petit, D.R.; Petit, L.J.; Bradford, D.F. Bird communities and habitat as ecological indicators of forest condition in regional monitoring. Conserv. Biol. 2000, 14, 544–558. [Google Scholar] [CrossRef]
- Luck, G.W. A review of the relationships between human population density and biodiversity. Biol. Rev. 2007, 82, 607–645. [Google Scholar] [CrossRef] [PubMed]
- Cincotta, R.P.; Wisnewski, J.; Engelman, R. Human population in the biodiversity hotspots. Nature 2000, 404, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Pickett, C.M.L. Beyond urban legends: An emerging framework of urban ecology, as illustrated by the Baltimore Ecosystem Study. Bioscience 2008, 58, 139–150. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed]
- McPhearson, T.; Pickett, S.T.; Grimm, N.B.; Niemelä, J.; Alberti, M.; Elmqvist, T.; Weber, C.; Haase, D.; Breuste, J. Advancing urban ecology toward a science of cities. Bioscience 2016, 66, 198–212. [Google Scholar] [CrossRef]
- Ossola, A.; Irlich, U.M.; Niemelä, J. Bringing Urban Biodiversity Research into Practice. Rout. Assoc. GSE Res. 2018, 1, 1–17. [Google Scholar]
- Aronson, M.F.J.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.A.; Williams, N.S.G.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B. 2014, 281, 2013–3330. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.M.; Hackett, B.; Guénard, B.; Youngsteadt, E.K.; Dunn, R.R. Fine-scale heterogeneity across Manhattan’s urban habitat mosaic is associated with variation in ant composition and richness. Insect Conserv. Divers. 2015, 8, 216–228. [Google Scholar] [CrossRef]
- Savard, J.P.L.; Clergeau, P.; Mennechez, G. Biodiversity Concepts and Urban Ecosystems. Landsc. Urban Plan. 2000, 48, 131–142. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Kolasa, J.; Armesto, J.J.; Collins, S.L. The Ecological Concept of Disturbance and Its Expression at Various Hierarchical Levels. Oikos 1989, 54, 129–136. [Google Scholar] [CrossRef]
- McDonnell, M.J.; Pickett, S.T.A. Humans as Components of Ecosystems: The Ecology of Subtle Human Effects and Populated Areas; Springer: New York, NY, USA, 1993. [Google Scholar]
- Grimm, N.B.; Grove, J.M.; Pickett, S.T.A. Integrated Approaches to Long Term Studies of Urban Ecological Systems. Bioscience 2000, 50, 571–584. [Google Scholar] [CrossRef]
- Adler, F.R.; Tanner, C.J. Urban Ecosystems, Ecological Principals for the Built Environment; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Hobbs, R.J.; Harris, J.A. Repairing the Earth’s Ecosystem in the New Millennium. Restor. Ecol. 2001, 9, 239–246. [Google Scholar] [CrossRef]
- Klötzli, F.; Grootjans, A.P. Restoration of Natural and Semi-Natural Wetland Systems in Central Europe: Progress and Predictability of Developments. Restor. Ecol. 2001, 9, 209–219. [Google Scholar] [CrossRef]
- Prach, P.; Hobbs, R.J. Spontaneous Succession verses Technical Reclamation in the Restoration of Disturbed Sites. Restor. Ecol. 2008, 16, 363–366. [Google Scholar] [CrossRef]
- Gallagher, F.J.; Pechmann, I.; Holzapfel, C.; Grabosky, J. Altered Vegetative Assemblage Trajectories within an Urban Brownfield. Environ. Pollut. 2011, 159, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.; Chapman, L.; Wrangham, R.W.; Isabirye-Basuta, G.; Ben-David, K. Spatial and temporal variability in the structure of a tropical forest. Afr. J. Ecol. 1997, 35, 287–302. [Google Scholar] [CrossRef]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The Influence of Functional Diversity and Composition on Ecosystem Processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Antonio, D.; Loope, C.M.; Lloyd, L.; Westbrooks, R. Biological Invasions as Global Environmental Change. Am. Sci. 1996, 84, 468. [Google Scholar]
- Faeth, S.H.; Saari, S.; Bang, C. Urban Biodiversity: Patterns, Processes and Implications for Conservation; John Wiley and Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Keddy, P.A. Assembly and response rules: Two goals for predicting community ecology. J. Veg. Sci. 1993, 3, 157–164. [Google Scholar] [CrossRef]
- Hillebrand, H.; Matthiessen, B. Biodiversity in a complex world: Consolidation and progress in functional biodiversity research. Ecol. Lett. 2009, 12, 1405–1419. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D. A trait-based approach for modelling microbial litter decomposition. Ecol. Lett. 2012, 15, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Texas Instruments (TI). Liberty State Park Ecological Study; Prepared for the Port Authority of New York and New Jersey; Texas Instruments: Dallas, TX, USA, 1976. [Google Scholar]
- Gallagher, F.J.; Pechmann, I.; Bogden, J.; Grabosky, J.; Weis, P. Soil Metal Concentrations and Plant Productivity in an Urban Brownfield. Environ. Pollut. 2008, 153, 351–361. [Google Scholar] [CrossRef] [PubMed]
- National Cooperative Soil Survey NCS. Available online: https://soilseries.sc.egov.usda.gov/OSD_Docs/L/LADYLIBERTY.html (accessed on 11 February 2018).
- Gallagher, F.J.; Pechmann, I.; Bogden, J.; Grabosky, J.; Weis, P. Soil Metal concentrations and productivity of Betula populifolia (gray birch) as measured by field spectrometry and incremental annual growth in an abandoned urban Brownfield in New Jersey. Environ. Pollut. 2008, 156, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, A.; Reinfelder, J.R.; Gallagher, F.J.; Grabosky, J.C. Long-Term Stability of Trace Element Concentrations in a Spontaneously Vegetated Urban Brownfield with Anthropogenic Soils. Soil Sci. 2017, 182, 69–81. [Google Scholar] [CrossRef]
- Bartha, S.; Meiners, S.J.; Pickett, S.T.; Cadenasso, M.L. Plant colonization windows in a mesic old field succession. Appl. Veg. Sci. 2003, 6, 205–212. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Leff, J.W.; Barberán, A.; Bates, S.T.; Betley, J.; Crowther, T.W.; Kelly, E.F.; Oldfield, E.E.; Shaw, E.A.; Steenbock, C. Biogeographic patterns in below-ground diversity in New York City’s Central Park are similar to those observed globally. Proc. R. Soc. B. 2014, 281, 20141988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Holzapfel, C.; Yuan, X. Scale-dependent Ecosystem Service. Ecos. Serv. Agr. Urban Landsc. 2013, 105–121. [Google Scholar] [CrossRef]
- Lurie, M.N.; Wacker, P.O. Mapping New Jersey: An Evolving Landscape; Rutgers University Press: New Brunswick, NJ, USA, 2009. [Google Scholar]
- Bolund, P.; Hunhammar, S. Ecosystem services in urban areas. Ecol. Econ. 2009, 29, 293–301. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Kroeger, T.; Wagner, J.E. Urban forests and pollution mitigation: Analyzing ecosystem services and disservices. Environ. Polut. 2011, 8–9, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Pataki, D.E.; Carreiro, M.M.; Cherrier, J.; Grulke, N.E.; Jennings, V.; Pincetl, S.; Pouyat, R.V.; Whitlow, T.H.; Zipperer, W.C. Coupling biogeochemical cycles in urban environments: ecosystem services, green solutions, and misconceptions. Front. Ecol. Environ. 2011, 9, 27–36. [Google Scholar] [CrossRef]
- Jim, C.Y.; Wendy, Y.; Chen, W.Y. Ecosystem services and valuation of urban for-ests in China. Cities 2009, 26, 187–219. [Google Scholar] [CrossRef]
- Sander, H.; Polasky, S.; Haight, R.G. The value of urban tree cover: A hedonic property price model in Ramsey and Dakota Counties, Minnesota, USA. Ecol. Econ. 2010, 69, 1646–1656. [Google Scholar] [CrossRef]
- Andersson, E.; Barthel, S.; Ahrné, K. Measuring social-ecological dynamics behind the generation of ecosystem services. Ecol. Appl. 2007, 17, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, G.; Marcotullio, P.; Bai, X.; Balk, D.; Braga, T.; Douglas, I.; Elmqvist, T.; Rees, W.; Satterthwaite, D.; Songsore, J.; et al. Urban Systems. In Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Current State and Trends; Island Press: Washington, DC, USA, 2005; pp. 795–825. [Google Scholar]
- De Groot, R.S.; van der Perk, J.; Chiesura, A.; Marguliew, S. Ecological functions and socio-economic values of critical natural capital as a measure for ecological integrity and environmental health. In Implementing Ecological Integrity: Restoring Regional and Global Environmental and Human Health; NATO-Science Series, IV Earth and Environmental Sciences; Kluwer Academic Publishers: London, UK, 2000. [Google Scholar]
- Dahle, G.A.; Gallagher, F.J.; Gershenson, D.; Schäfer, K.V.R.; Grabosky, J.C. Allometric and Mass Relationships of Betula populifolia in a Naturally Assembled Urban Woodlot. Urban Ecosyst. 2014, 17, 1147–1160. [Google Scholar] [CrossRef]
- Salisbury, A.; Gallagher, F.J.; Caplan, J.; Grabosky, J.C. Maintenance of photosynthetic capacity despite high load of trace metals in contaminated anthropogenic soils. Sci. Total Environ. 2018, 625, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Krumins, J.A.; Goodey, N.M.; Gallagher, F.J. Plant-Soil Interactions in Metal Contaminated Soils. Soil Biol. Biochem. 2015, 80, 224–231. [Google Scholar] [CrossRef]
- Evans, J.M.; Parker, A.; Gallagher, F.J.; Krumins, J.A. Plant Productivity, Ectomycorrhiza and Metal Contamination in Urban Brownfield Soils. Soil Sci. 2015, 180, 198–206. [Google Scholar] [CrossRef]
- Hagmann, D.; Goodey, N.M.; Matheau, C.; Evans, J.M.; Aronson, M.F.J.; Gallagher, F.J.; Krumins, J.A. Effect of metal contamination on microbial enzymatic activity in soil. Soil Biol. Biochem. 2015, 91, 291–297. [Google Scholar] [CrossRef]
- Qian, Y.; Feng, F.H.; Gallagher, F.J.; Zhu, Q.; Wu, M.; Liu, C.J.; Jones, K.; Tappero, R. Synchrotron Study of Metal Localization in Typha latifolia L. Root Sections. J. Synchrotron Radiat. 2015, 22, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.H.; Qian, Y.; Tappero, M.R.; Gallagher, F.J.; Wu, M.; Zhang, W.; Yu, L.; Zhu, Q.; Zhang, K.; Liu, C.J. Lead accumulation and association with Fe on Typha latifolia root from an urban brownfield site. Env. Sci. Pollut. Res. 2015, 20, 3743–3750. [Google Scholar] [CrossRef] [PubMed]
- Litwhiler, M. Attraction and Risk in Urban Bird Habitats. Ph.D. Thesis, New Jersey Institute of Technology, Newark, NJ, USA, 2017. [Google Scholar]
- Das, P.; Samantaray, S.; Rout, G.R. Studies on cadmium toxicity in plants: A review. Environ. Pollut. 1997, 98, 29–36. [Google Scholar] [CrossRef]
- Li, H.; Smith, S.E.; Holloway, R.E.; Zhu, Y.; Smith, F.A. Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus-fixing soil even in the absence of positive growth responses. New Phytol. 2006, 172, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. Int. J. Biochem. Cell Biol. 2006, 41, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Hofer, C.; Gallagher, F.J.; Holzapfel, C. Metal accumulation and growth performance of nestlings of passerine bird species at an urban brownfield site. Envrion. Pollut. 2010, 158, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Fasola, M.; Muhammad, A.S.A.; Malik, N.; Bostan, H.; Bokhari, M.A.; Kamran, M.N.; Shafqat, A.; Alamdar, K.M.; Ali, N.; et al. Avian feathers as a non-destructive bio-monitoring tool of trace metals signatures: A case study from severely contaminated areas. Chemosphere 2015, 119, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Dauwe, T.L.; Bervoets, R.; Blust, R.; Pinxten, M.; Eens, M. Can excrement and feathers of nestling songbirds be used as biomonitors for heavy metal pollution? Arch. Environ. Contam. Toxicol. 2000, 39, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Beasley, D.A.E.; Bonisoli-Alquati, A.; Mousseau, T.A. The use of fluctuating asymmetry as a measure of environmentally induced developmental instability: A meta-analysis. Ecol. Indic. 2013, 30, 218–226. [Google Scholar] [CrossRef]
- Wadhwa, S.; Gallagher, F.J.; Rodriguez-Saône, C.; Holzapfel, C. Analysis of fluctuating asymmetry in isopod and hardwood tree populations in brown fields. J. Environ. Indic. 2017, 76, 42–51. [Google Scholar] [CrossRef]
- Radwanski, D.; Gallagher, F.J.; Vanderklein, D.W.; Schafer, K.V.R. Physiology and carbon allocation of two co-occurring poplar species (Populus 1 deltoides and 2 Populus tremuloides) in an urban brownfield. Environ. Pollut. 2017, 23, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Schill, R.; Öhler, H.R. Energy reserves and metal-storage granules in the hepatopancreas of Oniscus asellus and Porcellio scaber (Isopoda) from a metal gradient at Avonmouth, UK. Ecotoxicology 2004, 13, 787–796. [Google Scholar] [CrossRef] [PubMed]

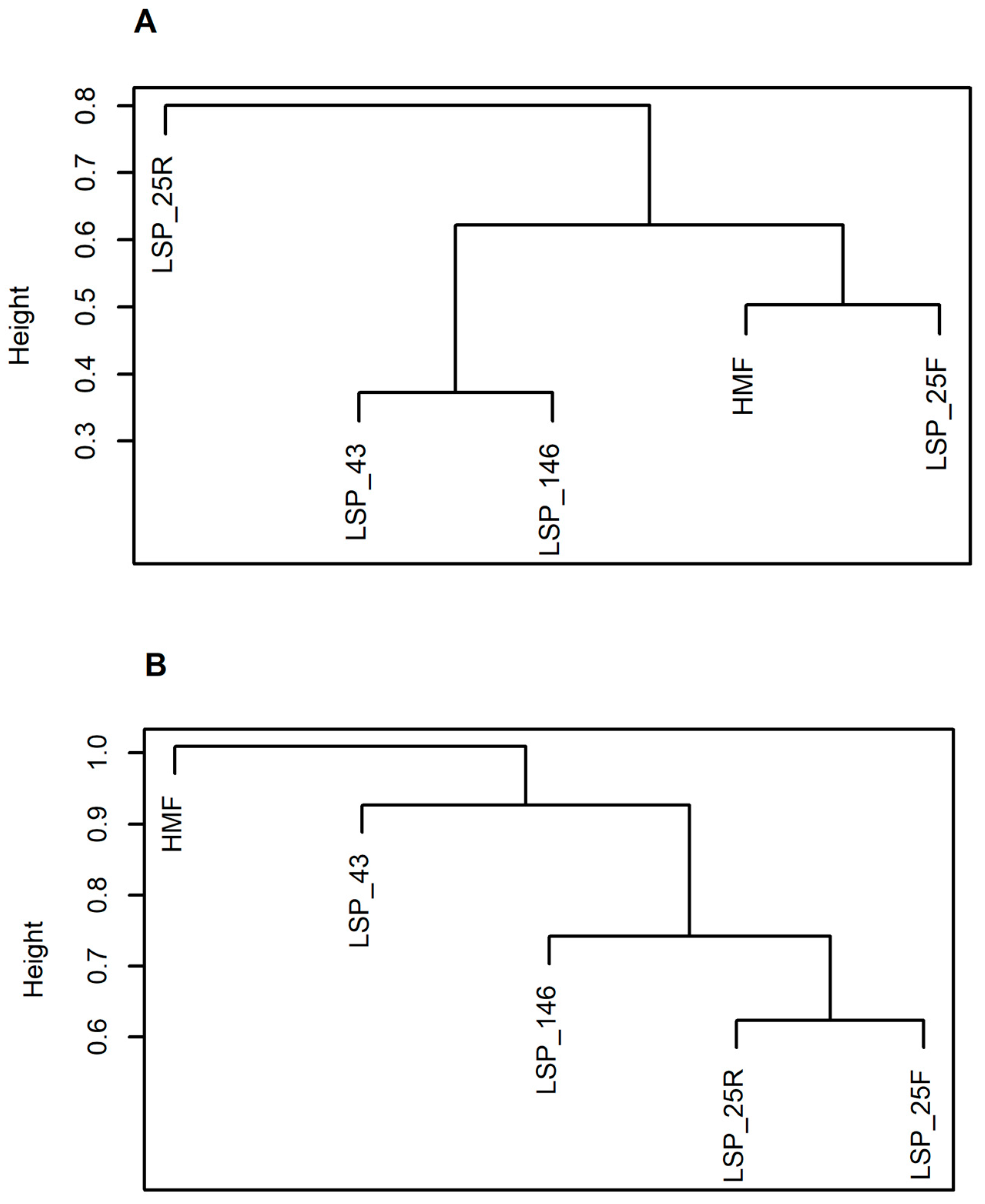
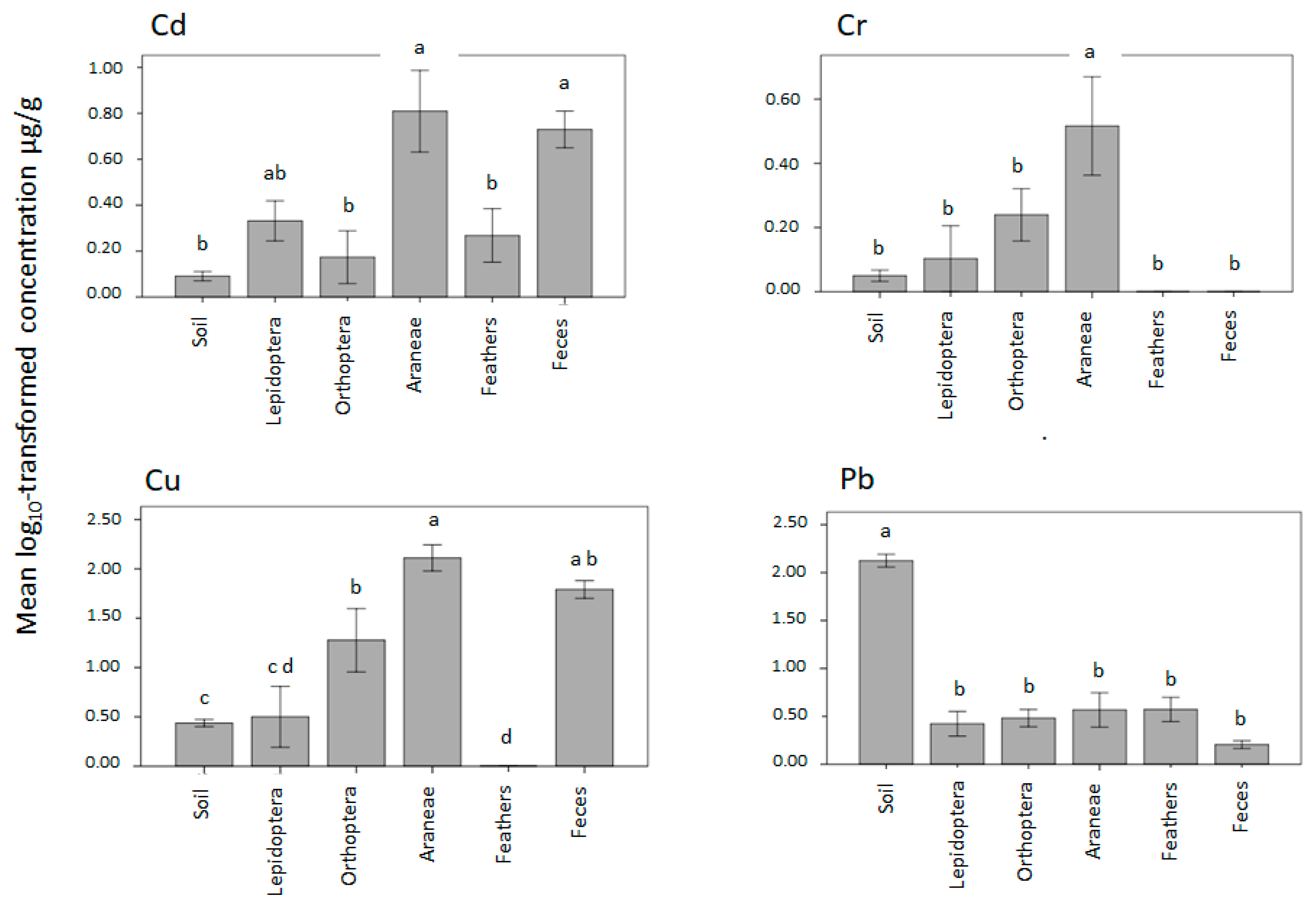
| Liberty SP | Urban Matrix in NJ | Region: NJ, Urban Developed | Region: NJ, Urban Undeveloped | |||
|---|---|---|---|---|---|---|
| Site Size | 0.1 ha | 200 ha | 0.1 ha | 200 ha | ||
| Vascular Plants | 20–65 | 185 | 6–12 | 45 | 210 | 560 |
| Birds | 8–17 | 87 | 4–7 | 21 | 45 | 210 |
| Mammals | 2–8 | 11 | 2–4 | 6 | 17 | 45 |
| Odonata | 5–7 | 12 | 1–3 | 6 | 14 | 35 |
| Lepidoptera | 12–14 | 25 | 4–5 | 11 | 24 | 50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallagher, F.; Goodey, N.M.; Hagmann, D.; Singh, J.P.; Holzapfel, C.; Litwhiler, M.; Krumins, J.A. Urban Re-Greening: A Case Study in Multi-Trophic Biodiversity and Ecosystem Functioning in a Post-Industrial Landscape. Diversity 2018, 10, 119. https://doi.org/10.3390/d10040119
Gallagher F, Goodey NM, Hagmann D, Singh JP, Holzapfel C, Litwhiler M, Krumins JA. Urban Re-Greening: A Case Study in Multi-Trophic Biodiversity and Ecosystem Functioning in a Post-Industrial Landscape. Diversity. 2018; 10(4):119. https://doi.org/10.3390/d10040119
Chicago/Turabian StyleGallagher, Frank, Nina M. Goodey, Diane Hagmann, Jay Prakash Singh, Claus Holzapfel, Megan Litwhiler, and Jennifer Adams Krumins. 2018. "Urban Re-Greening: A Case Study in Multi-Trophic Biodiversity and Ecosystem Functioning in a Post-Industrial Landscape" Diversity 10, no. 4: 119. https://doi.org/10.3390/d10040119
APA StyleGallagher, F., Goodey, N. M., Hagmann, D., Singh, J. P., Holzapfel, C., Litwhiler, M., & Krumins, J. A. (2018). Urban Re-Greening: A Case Study in Multi-Trophic Biodiversity and Ecosystem Functioning in a Post-Industrial Landscape. Diversity, 10(4), 119. https://doi.org/10.3390/d10040119




