Winter Roost Tree Selection and Phenology of the Long-Eared Owl (Asio otus) in Crimea
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
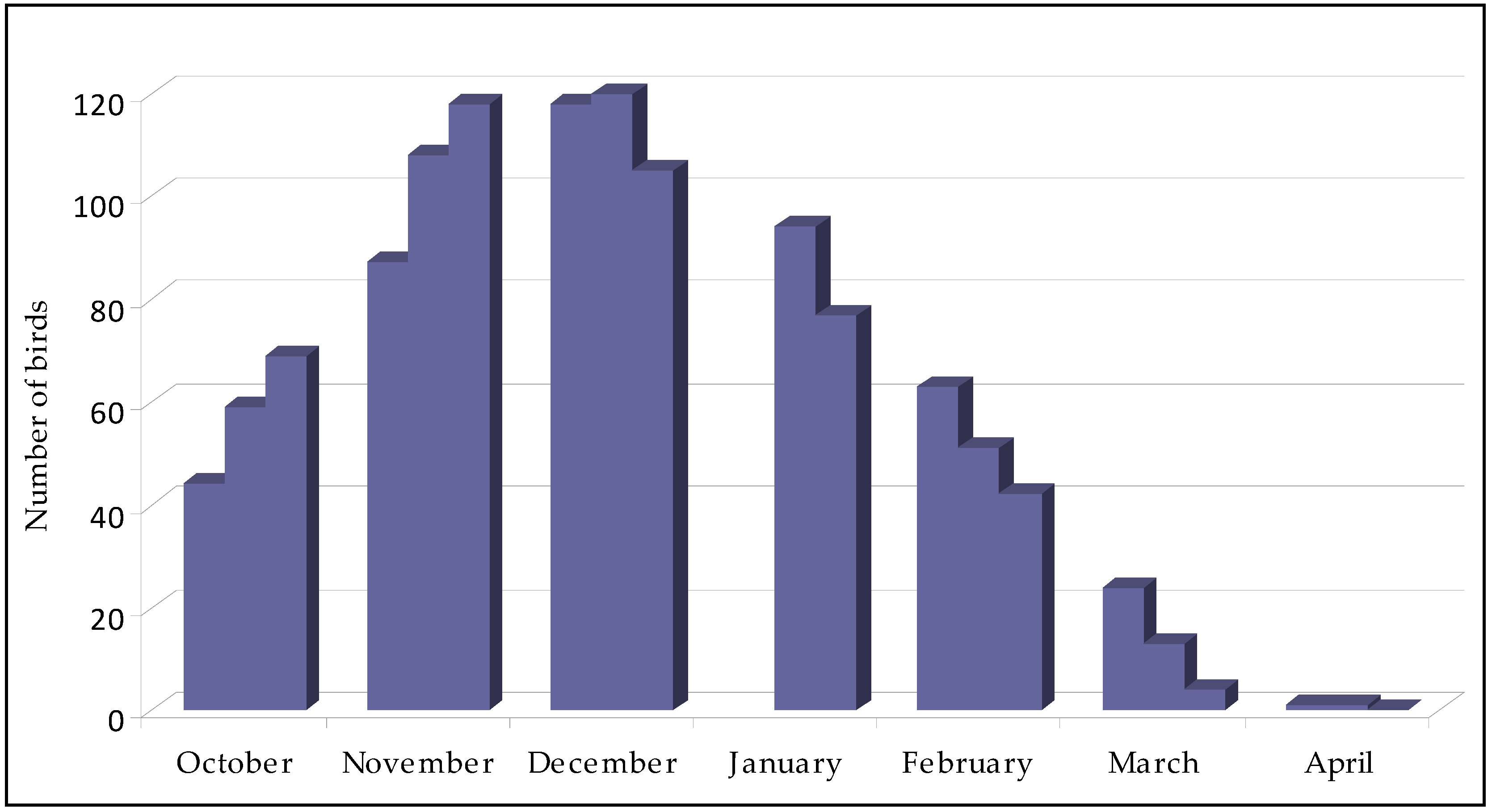
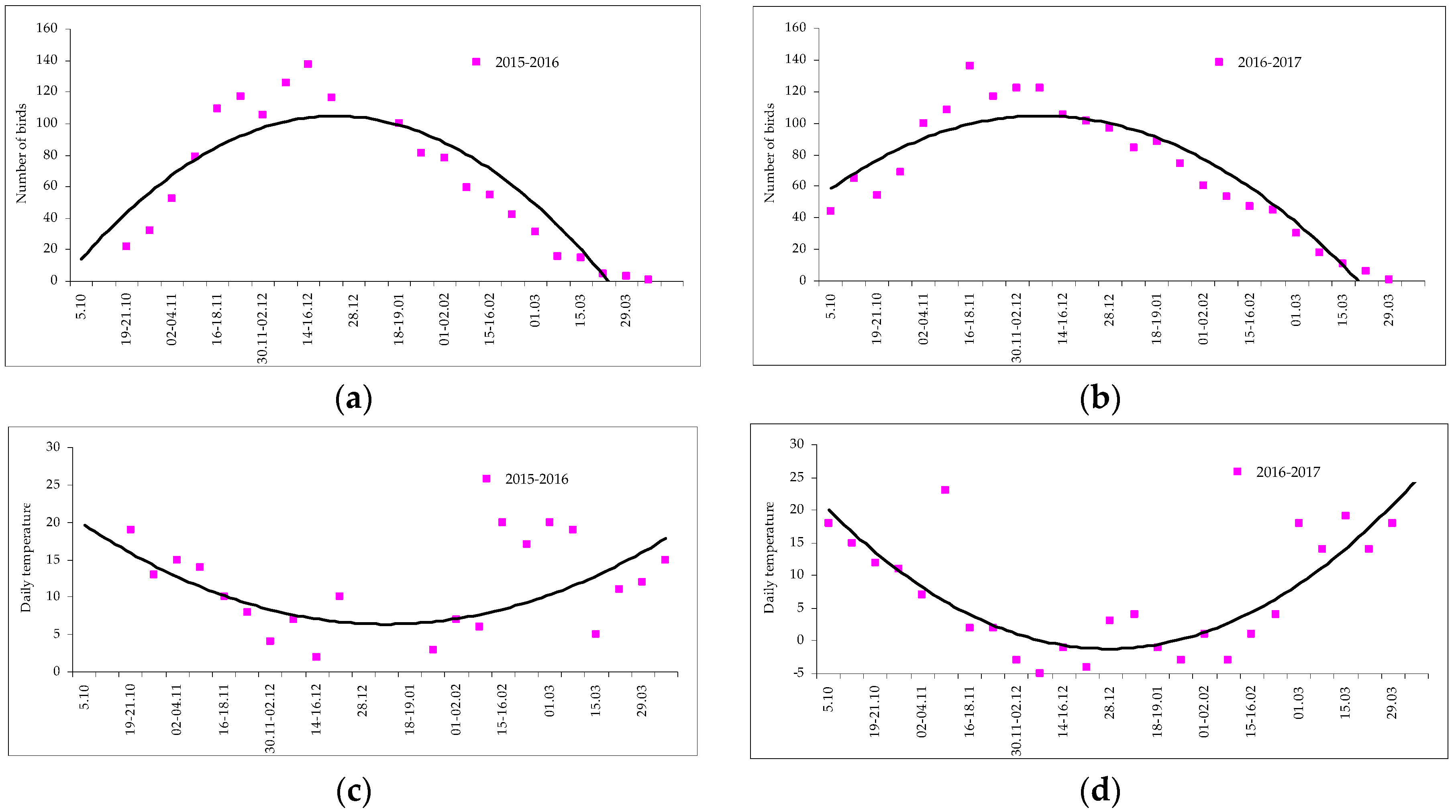
- In Crimea, the maximum number of roosting long-eared owls was recorded from the end of November to the middle of December, and the minimum in October and April. The number of owls is changed smoothly in the winter roost, and there was no sharp increase due to migratory birds.
- The birds exhibited a strong preference for roosting in conifers. The distribution of owls on some trees differed significantly under different weather conditions.
- The temperature decrease curve caused a practically synchronous change in the dynamics of the increase of the number of birds, but abrupt short-term anomalies did not have an effect.
Author Contributions
Funding
Conflicts of Interest
References
- Cramp, S.; Simmons, K. (Eds.) The Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1985; Volume 4, p. 970. ISBN 0198575076. [Google Scholar]
- Marti, C. A review of prey selection by the Long-Eared Owl. Condor 1976, 78, 331–336. [Google Scholar] [CrossRef]
- Makarova, T.V.; Sharikov, A.V. Winter roost place selection of Long-eared Owl in European Russia. J. Raptor Res. 2015, 49, 333–336. [Google Scholar] [CrossRef]
- Smith, D.G. Winter roost site fidelity by Long-eared Owls in central Pennsylvania. Am. Birds 1981, 35, 33–339. [Google Scholar]
- Bosakowsky, T. Roost selection and behavior of the Long-eared Owl (Asio otus) wintering in New-Jersey. Raptor Res. 1984, 18, 137–142. [Google Scholar]
- Alivizatos, H.; Goither, V. Winter diet of the Barn Owl (Tyto alba) and Long-eared Owl (Asio otus) in north-eastern Greece: A comparison. J. Raptor Res. 1999, 33, 160–163. [Google Scholar]
- Tovpinets, N.N.; Evstav’ev, I.L. Small mammals in the winter diet of Long-eared Owl (Asio otus) from the Crimea: Ecological and epizootological aspects. Berkut 2013, 22, 113–121. [Google Scholar]
- Glass, M.; Nielsen, H. The evening departure of the Long-eared Owl (Asio otus) from the winter roost. Dansk. Ornithol. Foren. Tids 1967, 61, 100–106. [Google Scholar]
- Pirovano, A.; Rubolini, D.; Michelis, S. Winter roost occupancy and behavior at evening departure of urban Long-eared Owls. Ital. J. Zool. 2000, 67, 63–66. [Google Scholar] [CrossRef]
- Sharikov, A.V.; Makarova, T.V.; Ganova, E.V. Long-term dynamics of Long-eared Owls Asio otus at a northern winter roost in European Russia. Ardea 2013, 101, 171–176. [Google Scholar] [CrossRef]
- Mestecăneanu, A.; Gava, R. The evaluation of the strength of Long-eared Owl (Asio otus) that wintered in the Argeş country (2014–2015). Preliminary study. Curr. Trends Nat. Sci. 2015, 4, 56–65. [Google Scholar]
- Poluda, A.M. On migratory movements of owls in Ukraine. In Birds of Prey in the Dynamic Environment of the Third Millennium: Status and Prospects, Proceeding of the 6th International Conference on Birds of Prey and Owls of North Eurasia, Kryvyi Rih, Ukraine, 27–30 September 2012; Belik, V.P., Bragin, A.E., Eds.; Kryvyj Rih Press: Kryvyj Rih, Ukraine, 2012; pp. 500–507. ISBN 978-966-2775-03-7. [Google Scholar]
- Kostin, Y.V. Birds of Crimea; Nauka: Moscow, Russia, 1983; pp. 1–239. [Google Scholar]
- Hendrichsen, D.K.; Christiansen, P.; Nielsen, E.K.; Dabelsteen, T.; Sunde, P. Exposure affects the risk of an owl being mobbed—Experimental evidence. J. Avian Biol. 2006, 37, 13–18. [Google Scholar] [CrossRef]
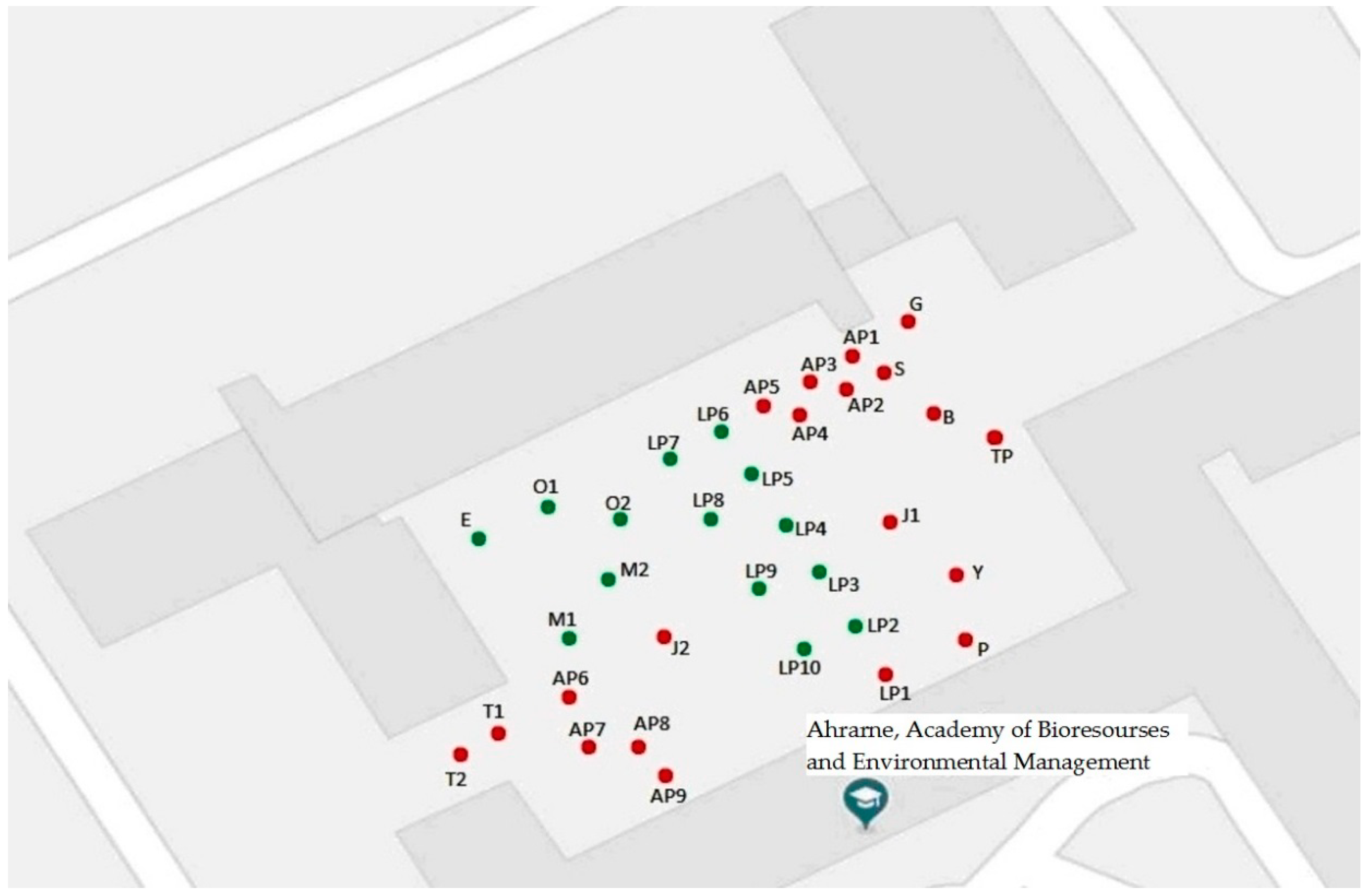
| Tree Species | Label on the Figure 1 | % Owls Sitting on a Tree under Different Weather Conditions from the Total Number of Owls in This Weather | A One-Way ANOVA | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Sunny | Cloudy | Overcast | Rain, Fog | Snow | ||||
| Platanus sp. | LP1 | 7.4 | 8.1 | 3.7 | 4.5 | 0.1 | F = 4.69 | p < 0.001 |
| Taxus baccata | Y | 1.2 | 2.5 | 3.2 | 2.3 | 2.7 | F = 10.69 | p < 0.001 |
| Juniperus sp. | J1 | 5.6 | 5.1 | 8.4 | 6.3 | 6.5 | F = 13.57 | p < 0.001 |
| Juniperus sp. | J2 | 0.6 | 2.8 | 0.4 | 2.0 | 2.2 | F = 3,04 | p < 0.05 |
| Pinus brutia | TP | 20.3 | 23.1 | 13.4 | 13.1 | 11.4 | F = 11.48 | p < 0.001 |
| Pinus nigra | AP1 | 29.7 | 24.8 | 37.0 | 32.2 | 35.0 | F = 39.69 | p < 0.001 |
| Pinus nigra | AP3 | 7.5 | 5.6 | 10.1 | 11.4 | 12.9 | F = 23.03 | p < 0.001 |
| Pinus nigra | AP4 | 5.5 | 7.6 | 7.3 | 9.6 | 10.2 | F = 30.18 | p < 0.001 |
| Pinus nigra | AP5 | 1.1 | 1.4 | 2.2 | 1.7 | 3.3 | F = 11.52 | p < 0.001 |
| Pinus nigra | AP6 | 0.1 | 0.1 | 0 | 0.3 | 0.5 | F = 4.87 | p < 0.001 |
| Pinus nigra | AP7 | 5.0 | 3.6 | 7.3 | 7.9 | 6.4 | F = 15.48 | p < 0.001 |
| Pinus nigra | AP8 | 2.7 | 1.6 | 2.9 | 4.4 | 3.9 | F = 16.57 | p < 0.001 |
| Pinus nigra | AP9 | 0.4 | 0.4 | 0 | 0.2 | 2.7 | F = 4.00 | p < 0.001 |
| Picea sp. | S | 0.1 | 1.1 | 0.4 | 0.3 | 0 | F = 3.12 | p < 0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucherenko, V.; Kalinovsky, P. Winter Roost Tree Selection and Phenology of the Long-Eared Owl (Asio otus) in Crimea. Diversity 2018, 10, 105. https://doi.org/10.3390/d10040105
Kucherenko V, Kalinovsky P. Winter Roost Tree Selection and Phenology of the Long-Eared Owl (Asio otus) in Crimea. Diversity. 2018; 10(4):105. https://doi.org/10.3390/d10040105
Chicago/Turabian StyleKucherenko, Volodymyr, and Pavel Kalinovsky. 2018. "Winter Roost Tree Selection and Phenology of the Long-Eared Owl (Asio otus) in Crimea" Diversity 10, no. 4: 105. https://doi.org/10.3390/d10040105
APA StyleKucherenko, V., & Kalinovsky, P. (2018). Winter Roost Tree Selection and Phenology of the Long-Eared Owl (Asio otus) in Crimea. Diversity, 10(4), 105. https://doi.org/10.3390/d10040105





