Phylogeny of the Genus Dichotrachelus (Coleoptera: Curculionidae: Cyclominae)
Abstract
1. Introduction
2. Material and Methods
2.1. Samples Origin
2.2. Outgroup Selection
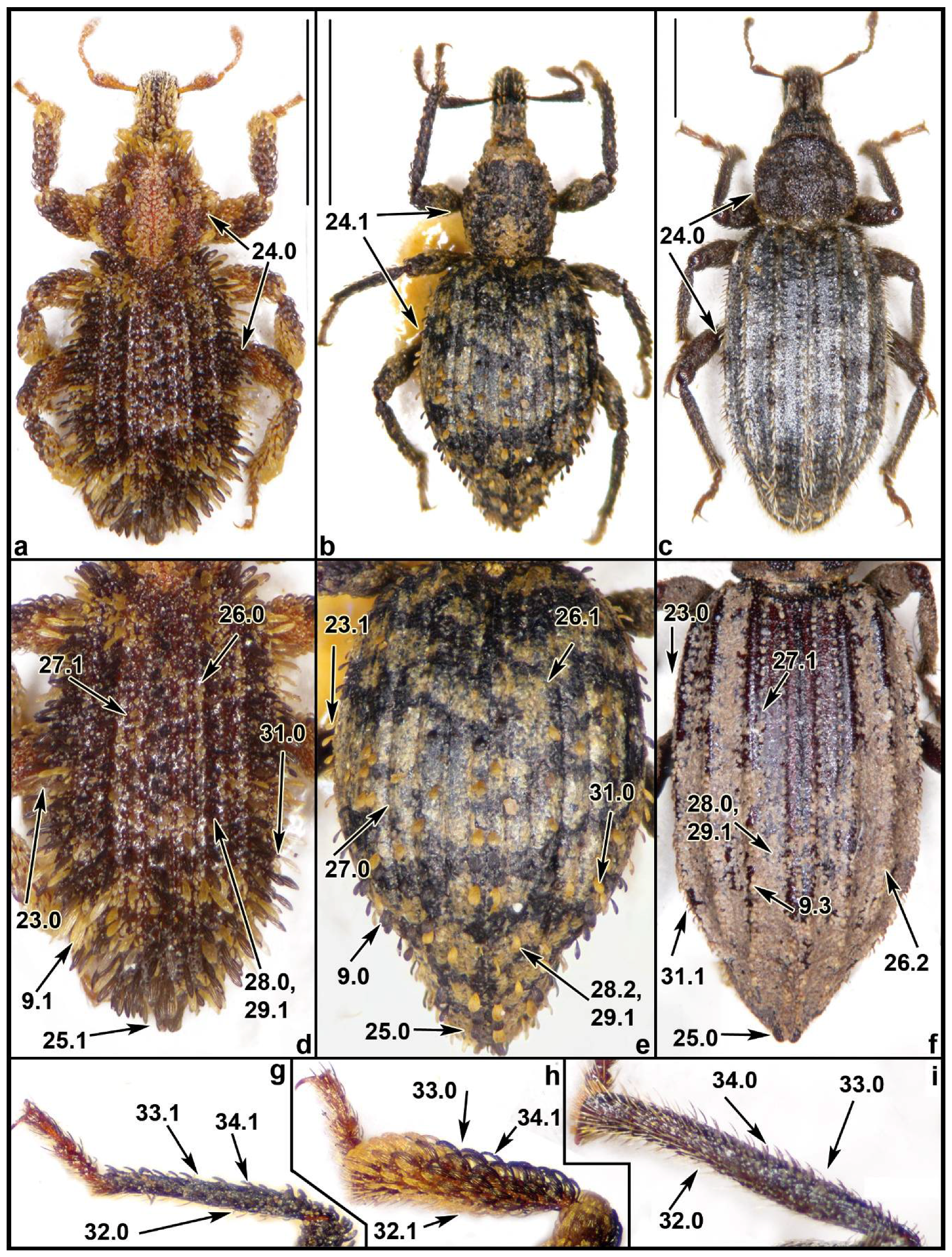
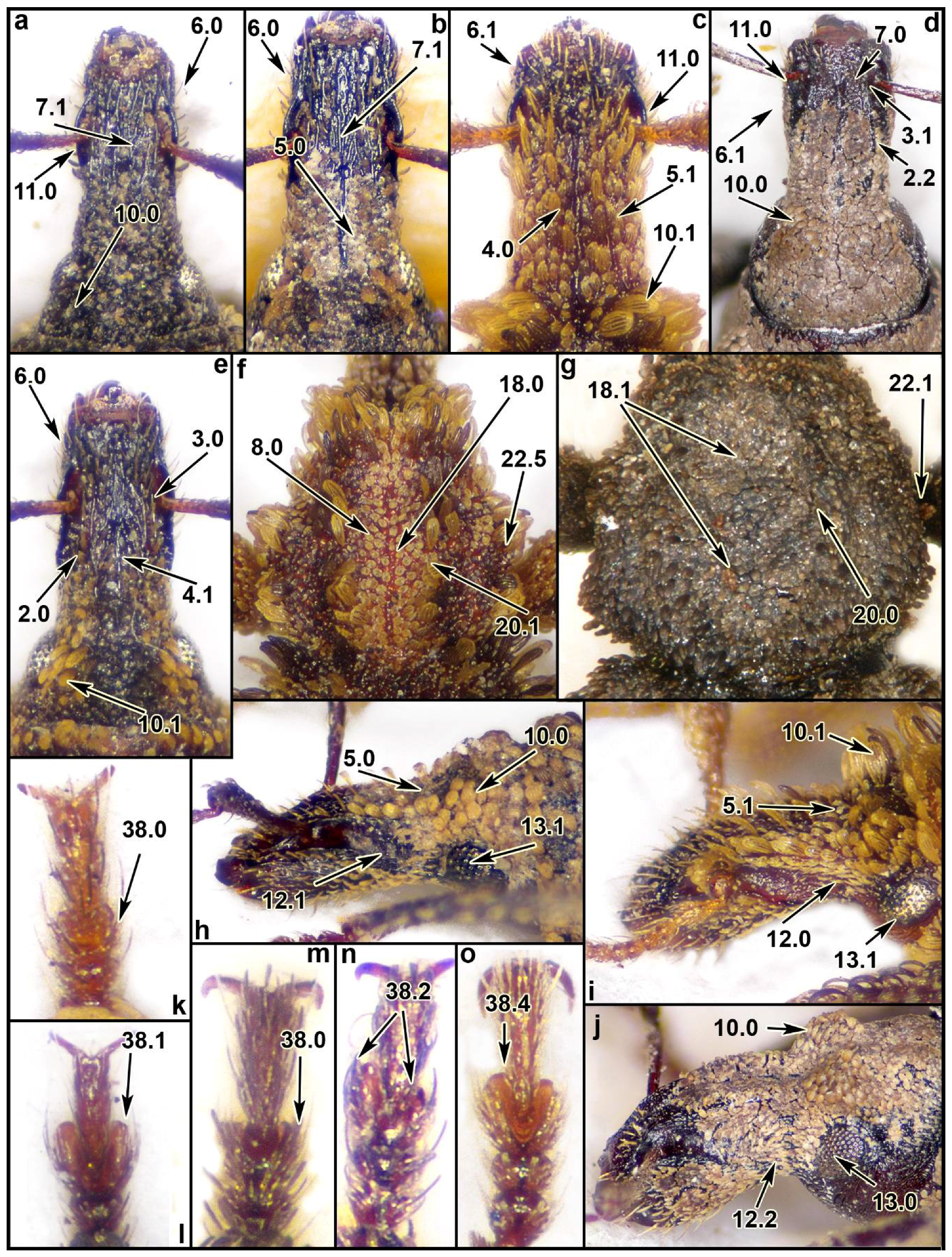
2.3. Morphological Data
2.4. Molecular Data
2.5. Phylogenetic Analysis: Morphology
2.6. Phylogenetic Analyses: mt-Cox1
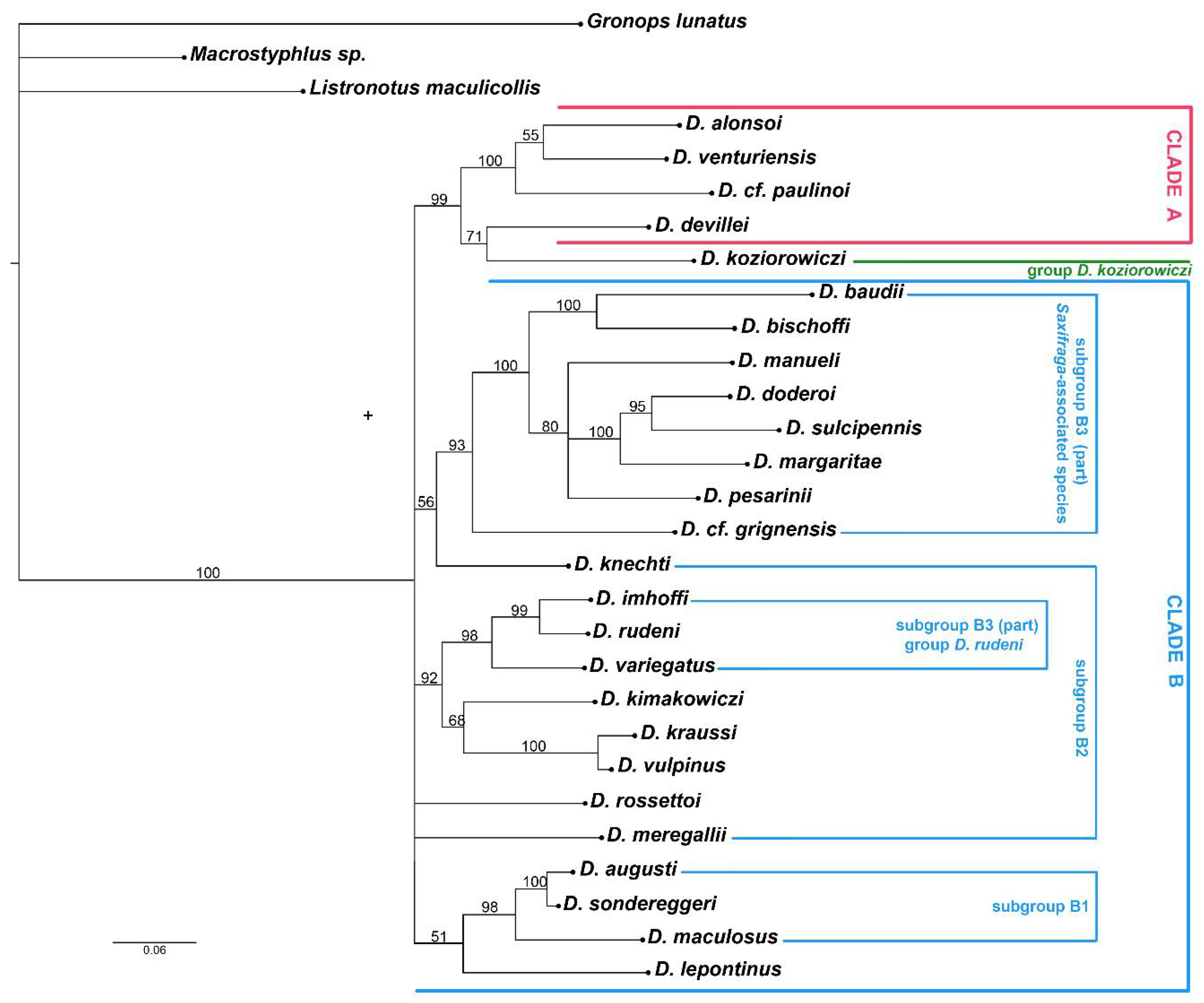
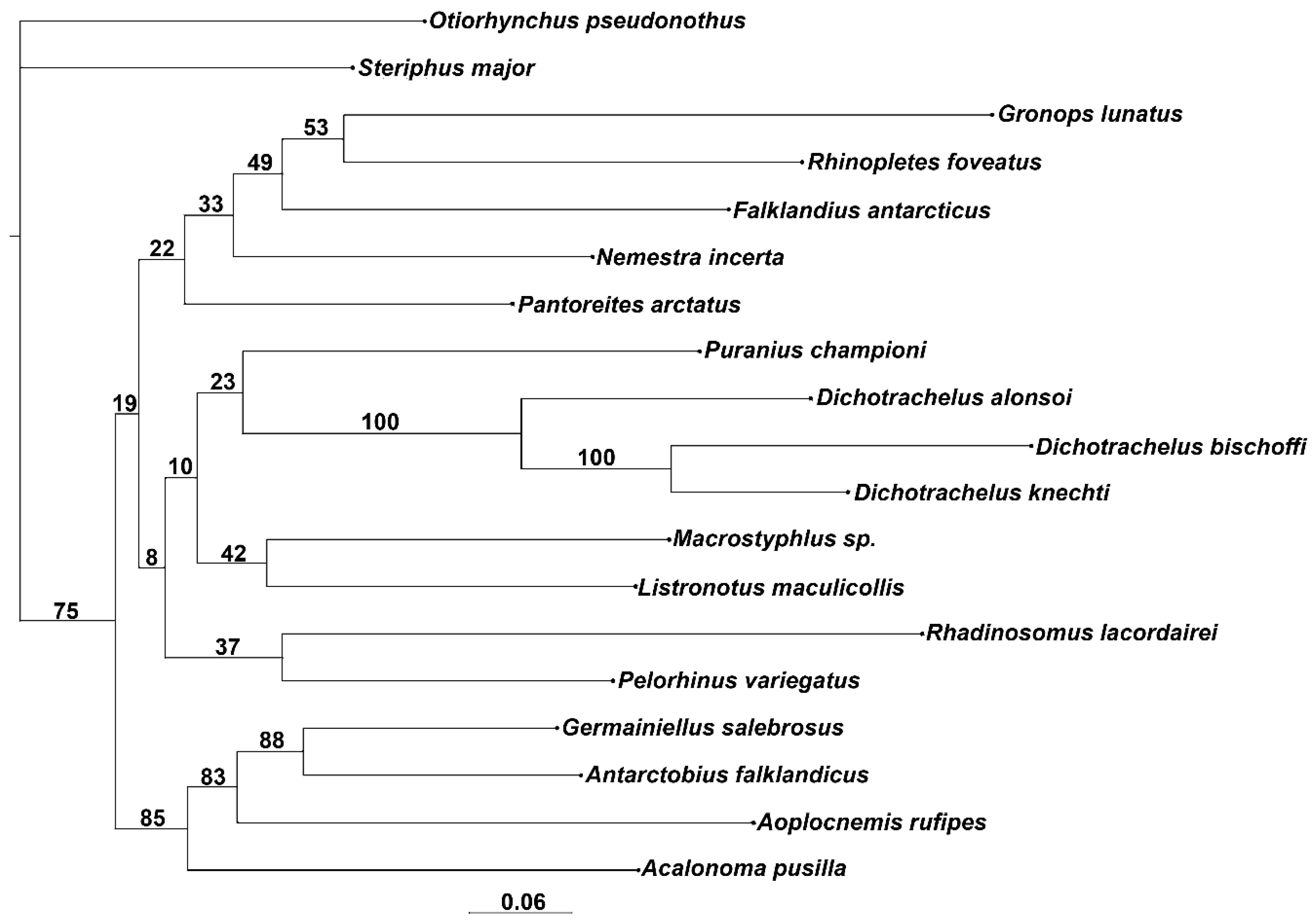
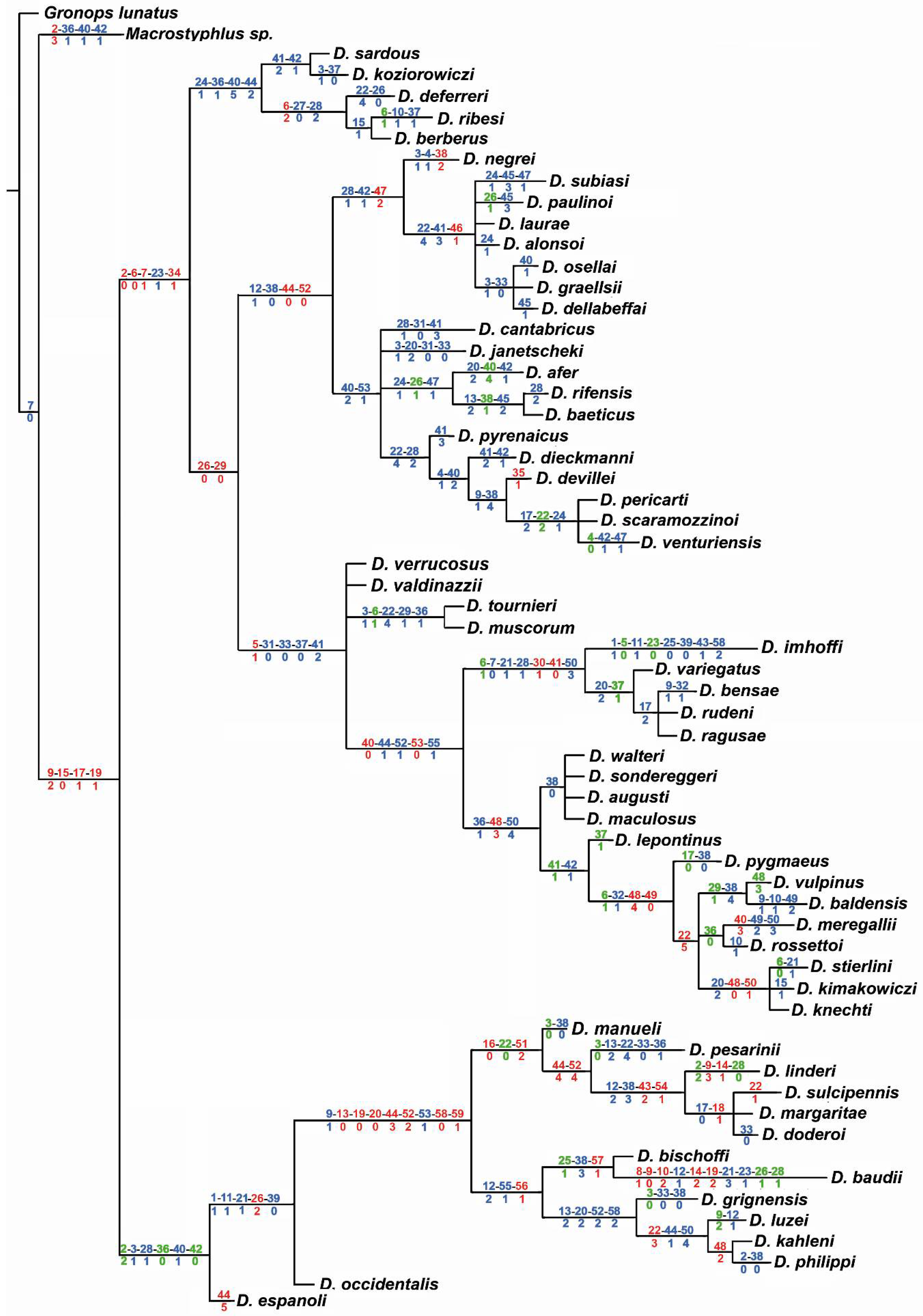
3. Results
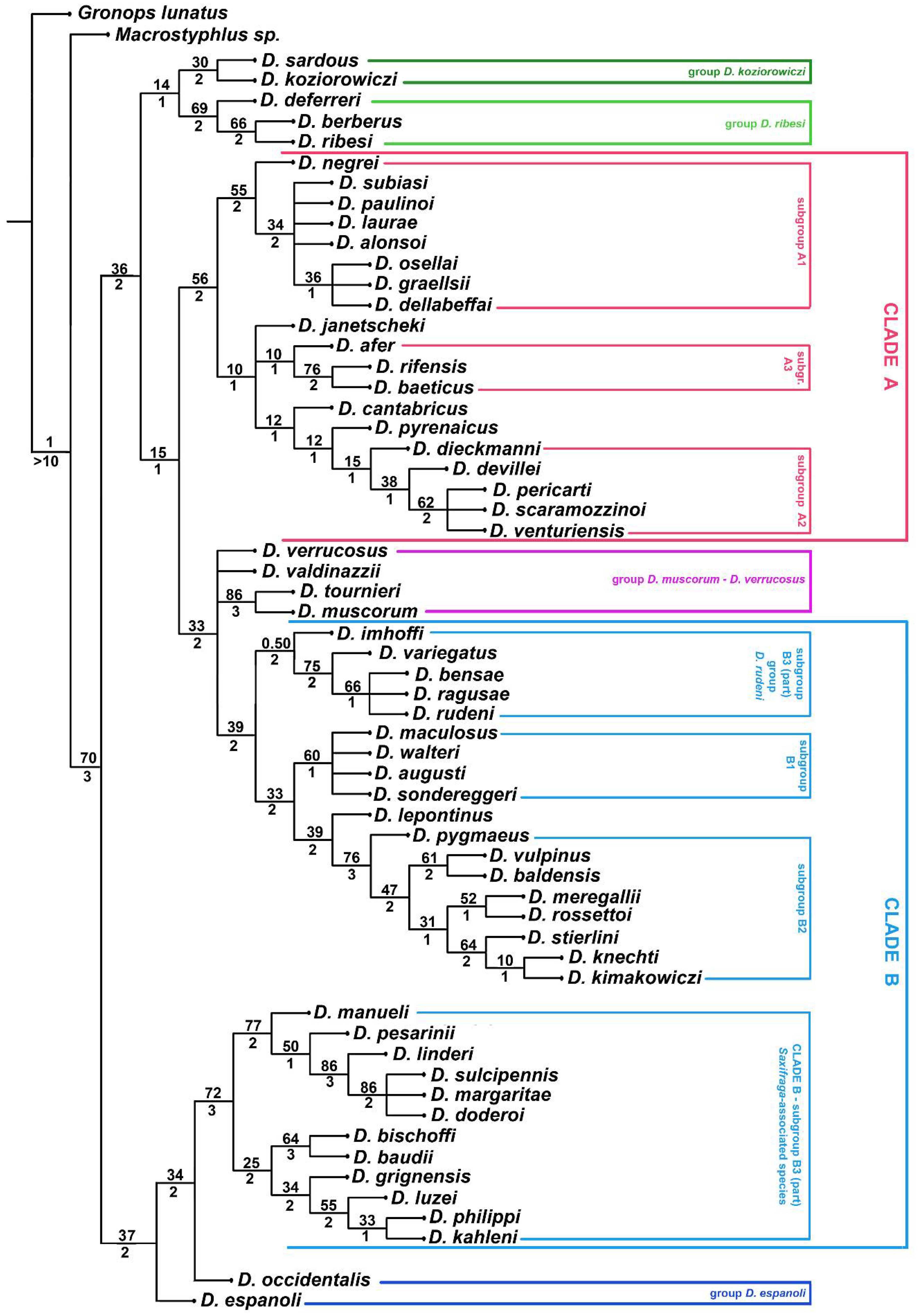
3.1. Morphological Analysis
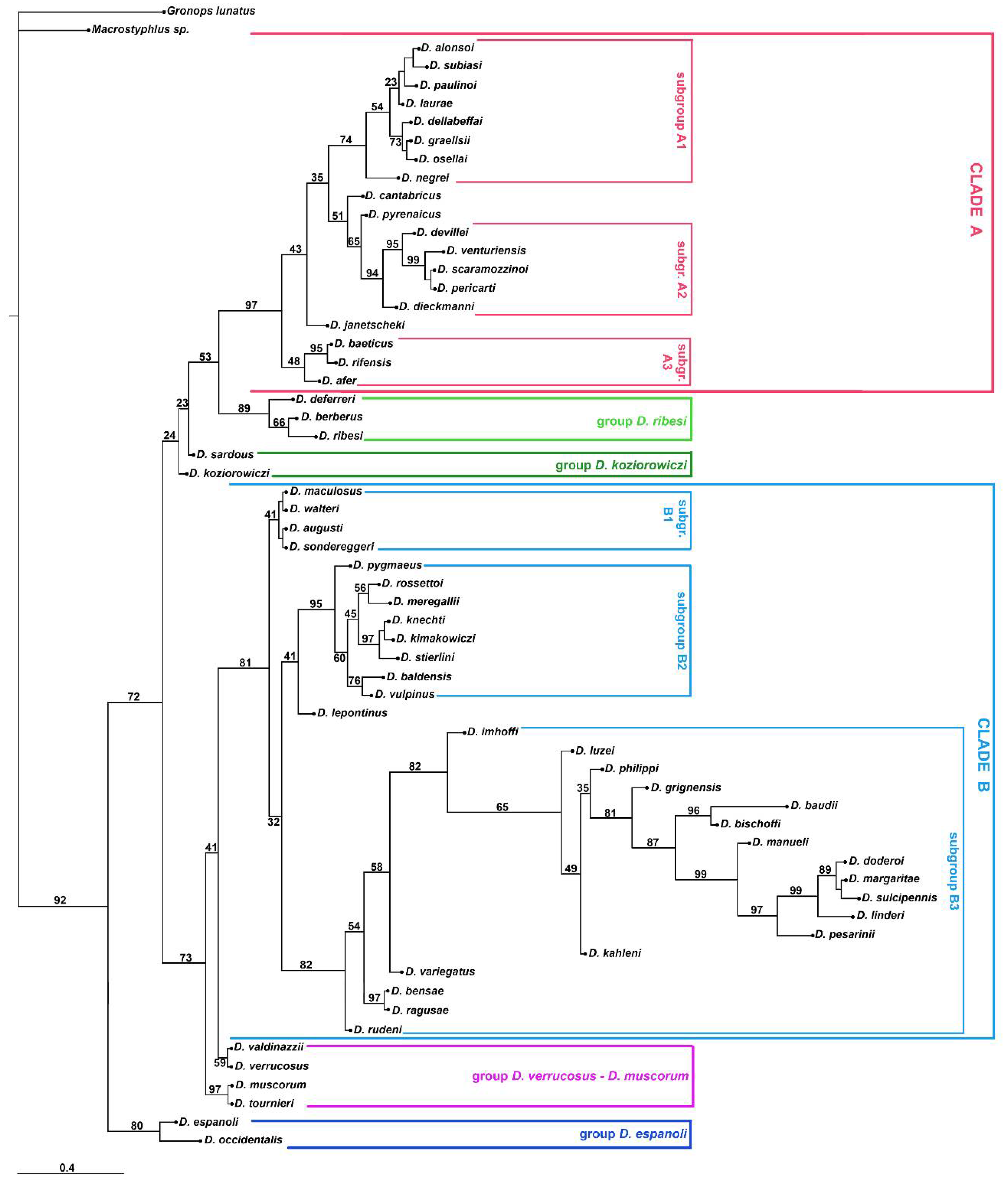
3.2. Molecular Analyses
3.3. Pairwise Distance
4. Discussion
4.1. Relationships of Dichotrachelus within Cyclominae
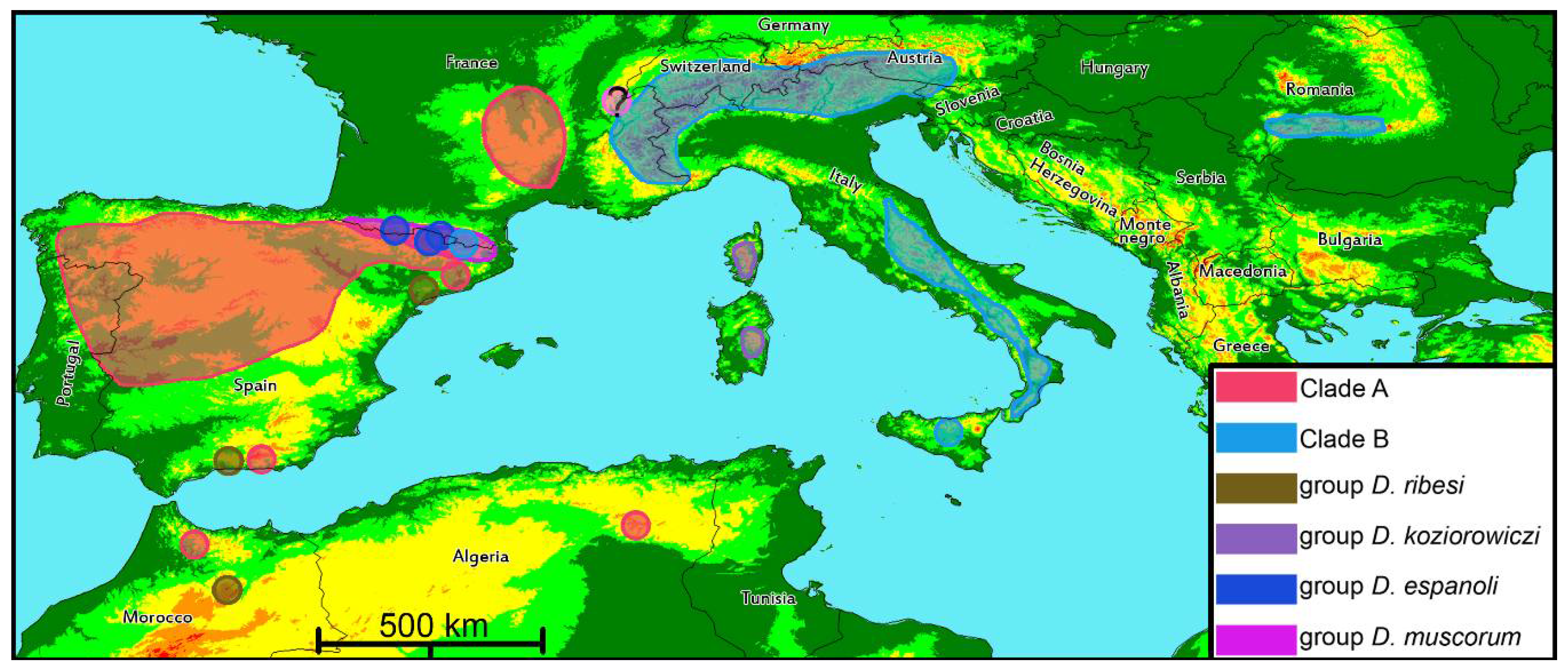
4.2. Phylogenetic Lineages in Dichotrachelus
4.3. Species-Groups
- D. graellsii, D. dellabeffai and D. osellai.
- D. alonsoi, D. subiasi, D. paulinoi and D. laurae.
- D. negrei.
- D. pericarti, D. scaramozzinoi, D. venturiensis, D. devillei and possibly D. dieckmanni.
- D. pyrenaicus.
- D. cantabricus.
- D. stierlini, D. knechti and D. kimakowiczi.
- D. baldensis, D. vulpinus and D. pygmaeus.
- D. pesarinii.
- D. linderi.
- D. sulcipennis, D. margaritae and D. doderoi.
4.4. Inter vs. Intraspecific Distance
4.5. Analysis of the Morphological Characters
5. Conclusions and Future Developments
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alonso-Zarazaga, M.A.; Barrios, H.; Borovec, R.; Bouchard, P.; Caldara, R.; Colonnelli, E.; Gültekin, L.; Hlaváč, P.; Korotyaev, B.; Lyal, C.H.C.; et al. Cooperative Catalogue of Palaearctic Coleoptera Curculionoidea. Monografías Electrónicas SEA 2017, 8, 1–729. [Google Scholar]
- Oberprieler, R.G. A reclassification of the weevil subfamily Cyclominae (Coleoptera: Curculionidae). Zootaxa 2010, 2515, 1–35. [Google Scholar]
- Stierlin, G. Revision der Dichotrachelus-Arten. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 1878, 5, 392–425. [Google Scholar]
- Stierlin, G. Fauna Coleopterorum Helvetica. Teil II; Bolli und Boecherer: Schaffhausen, Switzerland, 1898; p. 662. [Google Scholar]
- Franz, H. Die südwesteuropäischen Arten der Gattung Dichotrachelus (Col. Curc.). Koleopterol. Rundsch. 1954, 23, 93–96. [Google Scholar]
- González, M. Los Dichotrachelus ibéricos (Col. Curculionidae). Publicaciones del Instituto de Biología Aplicada 1964, 37, 5–16. [Google Scholar]
- Meregalli, M. Revisione delle specie iberiche del genere Dichotrachelus Stierlin, 1853 (Coleoptera, Curculionidae). MRSN 1987, 5, 335–418. [Google Scholar]
- Hustache, A. Curculionides gallo-rhénans (suite). Ann. Soc. Entomol. Fr. 1929, 98, 1–96. [Google Scholar]
- Hoffmann, A. Coléoptères Curculionides. Deuxième Partie. Faune Fr. 1954, 59, 488–1208. [Google Scholar]
- Barajon, M. Le specie Italiane del Gen. Dichotrachelus Stierl. (Col. Curculionidae). Estratto dalle Memorie del Museo Civico di Storia Naturale 1946, 85, 112–129. [Google Scholar]
- Barajon, M. Le specie Italiane del Gen. Dichotrachelus Stierl. II. Estratto dalle Memorie del Museo Civico di Storia Naturale Milano 1947, 86, 31–32. [Google Scholar]
- Osella, G. Revisione delle specie Italiane del Genere Dichotrachelus Stierlin (Coleoptera, Curculionidae). Estratto dalle Memorie del Museo Civico di Storia Naturale 1967, 15, 349–445. [Google Scholar]
- Osella, G. Revisione del genere Dichotrachelus Stierlin. Estratto dalle Memorie del Museo Civico di Storia Naturale 1970, 18, 449–569. [Google Scholar]
- Germann, C. About the enigmatic Dichotrachelus valesiacus Stierlin, 1878 (Coleoptera, Curculionidae, Cyclominae). Zookeys 2009, 5, 81–86. [Google Scholar] [CrossRef]
- Germann, C. Review of the Dichotrachelus alpestris Stierlin, 1878 species group with evidence for a species complex of D. augusti F. Solari, 1946, and D. sondereggeri sp. nov. from Switzerland (Coleoptera, Curculionidae). Contrib. Nat. Hist. 2011, 17, 1–21. [Google Scholar]
- Germann, C.; Baur, H. Notes on the taxonomy and biology of Dichotrachelus imhoffi Stierlin, 1857 (Coleoptera, Curculionidae) with the observation of a length dimorphism of the aedeagus. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 2010, 83, 249–260. [Google Scholar]
- Germann, C. Die Rüsselkäfer der Schweiz–Checkliste (Coleoptera, Curculionoidea) mit Verbreitungsangaben nach biogeografischen Regionen. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 2010, 83, 41–118. [Google Scholar]
- Germann, C. Supplement zur Checkliste der Rüsselkäfer der Schweiz (Coleoptera, Curculionoidea). Mitteilungen der Schweizerischen Entomologischen Gesellschaft 2011, 84, 155–169. [Google Scholar]
- Meregalli, M.; Menardo, F.; Klass, K.-D.; Cervella, P. Phylogeny of the Saxifraga-associated species of Dichotrachelus (Insecta: Coleoptera: Curculionidae), with remarks on their radiation in the Alps. Arthropod Syst. Phylogeny 2013, 71, 43–68. [Google Scholar]
- Meregalli, M.; Monguzzi, R.; Klass, K.-D.; Cervella, P.; Kahlen, M. Dichotrachelus pesarinii sp.n., a missing link between the species from the central and the western southern Alps (Coleoptera: Curculionidae: Cyclominae). Arthropod Syst. Phylogeny 2015, 73, 323–332. [Google Scholar]
- Germann, C.; Wyler, S.; Bernasconi, M. DNA barcoding of selected alpine beetles with focus on Curculionoidea (Coleoptera). Rev. Suisse Zool. 2017, 124, 15–38. [Google Scholar]
- Morrone, J.J. Annotated checklist of the tribe Listroderini (Coleoptera: Curculionidae: Cyclominae). Zootaxa 2011, 3119, 1–68. [Google Scholar]
- Marvaldi, A.E.; Sequeira, A.S.; O’Brien, C.W.; Farrell, B.D. Molecular and morphological phylogenetics of weevils (Coleoptera, Curculionoidea): Do niche shifts accompany diversification? Syst. Biol. 2002, 51, 761–785. [Google Scholar] [CrossRef] [PubMed]
- Riedel, A.; Daawia, D.; Blake, M. Deep COX1 divergence and hyperdiversity of Trigonopterus weevils in a new Guinea mountain range (Coleoptera Curculionidae). Zool. Scr. 2009, 39, 63–74. [Google Scholar] [CrossRef]
- Hughes, J.; Vogler, A.P. The phylogeny of acorn weevils (genus Curculio) from mitochondrial and nuclear DNA sequences: The problem of incomplete data. Mol. Phylogenet. Evol. 2004, 32, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Van der Mark, P.; Huelsenbeck, J.P. Bayesian phylogenetic analysis using MrBayes. In The Phylogenetic Handbook: A Practical Approach to Phylogenetic Analysis and Hypothesis Testing; Lemey, P., Salemi, M., Vandamme, A.M., Eds.; Cambridge University Press: Cambridge, UK, 2009; p. 723. [Google Scholar]
- Goloboff, P.A.; Farris, J.S.; Nixon, K.C. TNT, a free program for phylogenetic analysis. Cladistics 2008, 24, 774–786. [Google Scholar] [CrossRef]
- Silvestro, D.; Michalak, I. RAxML GUI: A graphical front-end for RAxML. Org. Divers. Evol. 2011, 12, 335–337. [Google Scholar] [CrossRef]
- Goldman, N.; Yang, Z. A codon-based model of nucleotide substitution for protein-coding DNA sequences. Mol. Biol. Evol. 1994, 11, 725–736. [Google Scholar] [PubMed]
- Baele, G.; Lemey, P. Bayesian evolutionary model testing in the phylogenomics era: Matching model complexity with computational efficiency. Bioinformatics 2013, 29, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Larget, B.; Alfaro, M.E. Bayesian phylogenetic model selection using reversible jump Markov chain Monte Carlo. Mol. Biol. Evol. 2004, 21, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.M.; Hillis, D.M. Bayesian Analysis Using a Simple Likelihood Model Outperforms Parsimony for Estimation of Phylogeny from Discrete Morphological Data. PLoS ONE 2014, 9, e109210. [Google Scholar] [CrossRef] [PubMed]
- Gillett, C.P.D.T.; Lyal, C.H.C.; Vogler, A.P.; Emerson, B.C. Statistical Evaluation of Monophyly in the ‘Broad-Nosed Weevils’ through Molecular Phylogenetic Analysis Combining Mitochondrial Genome and Single-Locus Sequences (Curculionidae: Entiminae, Cyclominae, and Hyperinae). Diversity 2018, 10, 21. [Google Scholar] [CrossRef]
- Alonso-Zarazaga, M.A.; Lyal, C.H.C. A World Catalogue of Families and Genera of Curculionoidea (Insecta: Coleoptera). (Excepting Scolytidae and Platypodidae); Entomopraxis, S.C.P., Ed.; Entomopraxis: Barcelona, Spain, 1999; p. 315. [Google Scholar]
- Bik, H.M.; Lambshead, P.J.; Thomas, W.K.; Lunt, D.H. Moving towards a complete molecular framework of the Nematoda: A focus on the Enoplida and early-branching clades. BMC Evol. Biol. 2010, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Li, H.; Song, F.; Cai, W. Molecular phylogeny of Polyneoptera (Insecta) inferred from expanded mitogenomic data. Sci. Rep. 2016, 6, 36175. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lv, S. Substitution saturation analysis of mitochondrial cytochrome C oxidase subunit 1 (Cox1) gene of Angiostrongylus cantonensis. Chin. J. Parasitol. Parasit. Dis. 2014, 32, 205–209. [Google Scholar]
- Stüben, P.E.; André Schütte, A.; Bayer, C.; Astrin, J.J. The Molecular Weevil Identification Project (Coleoptera: Curculionoidea), Part II-Towards an Integrative Taxonomy. Snudebiller 2015, 16, 1–316. [Google Scholar]
- Gillett, C.P.D.T.; Crampton-Platt, A.; Timmermans, M.J.T.N.; Jordal, B.H.; Emerson, B.C.; Vogler, A.P. Bulk de novo mitogenome assembly from pooled total DNA elucidates the phylogeny of Weevils (Coleoptera: Curculionoidea). Mol. Biol. Evol. 2014, 31, 2223–2237. [Google Scholar] [CrossRef] [PubMed]
- Morrone, J.J. Cladistics of the New World genera of Listroderina (Coleoptera: Curculionidae: Rhytirrhinini). Cladistics 1997, 13, 247–266. [Google Scholar] [CrossRef]
- Morrone, J.J. The subtribes and genera of the tribe Listroderini (Coleoptera, Curculionidae, Cyclominae): Phylogenetic analysis with systematic and biogeographical accounts. Zookeys 2013, 273, 15–71. [Google Scholar] [CrossRef] [PubMed]
- Kuschel, G. Insects of Campbell Island Coleoptera: Curculionidae of the Subantarctic islands of New Zealand. Pac. Insects Monogr. 1964, 1, 416–493. [Google Scholar]
- Kuschel, G. Entomology of the Aucklands and other islands south of New Zealand: Coleoptera: Curculionidae. Pac. Insects Monogr. 1971, 27, 225–259. [Google Scholar]
- Chown, S.L.; Scholtz, C.H. Cryptogam herbivory in Curculionidae from the sub-Antarctic Prince Edward Islands. Coleopt. Bull. 1989, 43, 165–169. [Google Scholar]
- McKenna, D.D.; Sequeira, A.S.; Marvaldi, A.E.; Farrell, B.D. Temporal lags and overlap in the diversification of weevils and flowering plants. Proc. Natl. Acad. Sci. USA 2009, 106, 7083–7088. [Google Scholar] [CrossRef] [PubMed]
- Frisch, W.; Meschede, M. Plattentektonik 2; Wissenschaftliche Buchgesellschaft: Darmstadt, Germany, 2007; p. 196. [Google Scholar]
- Péricart, J. Notes sur divers Curculionidae français avec la description d’une espèce et d’une sous-espèce nouvelles (Coleoptera). Nouv. Rev. Entomol. 1974, 4, 55–70. [Google Scholar]
- Meregalli, M. Osservazioni preliminari sulla biologia dei Dichotrachelus (Coleoptera, Curculionidae). In Proceedings of the XII Congresso Nazionale Italiano di Entomologia, Roma, Italy, 5–9 November 1980; pp. 125–133. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meregalli, M.; Germann, C.; Bernasconi, M.V.; Cervella, P. Phylogeny of the Genus Dichotrachelus (Coleoptera: Curculionidae: Cyclominae). Diversity 2018, 10, 66. https://doi.org/10.3390/d10030066
Meregalli M, Germann C, Bernasconi MV, Cervella P. Phylogeny of the Genus Dichotrachelus (Coleoptera: Curculionidae: Cyclominae). Diversity. 2018; 10(3):66. https://doi.org/10.3390/d10030066
Chicago/Turabian StyleMeregalli, Massimo, Christoph Germann, Marco V. Bernasconi, and Piero Cervella. 2018. "Phylogeny of the Genus Dichotrachelus (Coleoptera: Curculionidae: Cyclominae)" Diversity 10, no. 3: 66. https://doi.org/10.3390/d10030066
APA StyleMeregalli, M., Germann, C., Bernasconi, M. V., & Cervella, P. (2018). Phylogeny of the Genus Dichotrachelus (Coleoptera: Curculionidae: Cyclominae). Diversity, 10(3), 66. https://doi.org/10.3390/d10030066





