Abstract
The use of human mediated translocations has been an increasing component of many species recovery initiatives, including for numerous imperiled Lepidopteran species. Despite the identified need for this ex situ strategy, few such programs are conducted in a scientifically repeatable way, are executed with a structured decision-making process, are well documented throughout, or are documented only in gray literature. The International Union for Conservation of Nature’s Guidelines for Reintroductions and Other Conservation Translocations are an important tool for conservation practitioners to help implement comprehensive translocation planning. These generalized guidelines are intended to be applicable to all taxa. Though there is a growing body of literature and supplementary guidelines for many vertebrate classes, other proposed standards fail to capture the specific biology of many invertebrate groups, like Lepidoptera. Here, we present a targeted list of detailed recommendations that are appropriate for Lepidopteran translocation programs to expand on the broad and tested guidelines developed by the IUCN. We assert that the increased standardization and repeatability among Lepidopteran translocations will improve the conservation outcomes.
1. Introduction
Butterfly populations continue to decline worldwide [1]. The drivers of loss are complex and often attributable to multiple, interacting factors, several of which may be incompletely understood [2,3,4,5,6,7]. In the face of this uncertainty, effective at-risk species recovery efforts require a comprehensive toolkit of diverse options. Adaptive and well-documented techniques are powerful tools that are used for stabilizing, reestablishing, or increasing in-situ populations that have suffered significant declines [8,9,10]. Increasingly, conservation translocation, defined by the International Union for Conservation of Nature (IUCN) [11] as “the deliberate movement of organisms from one site for release in another”, has become a key component of strategies aimed at helping prevent extinction and promote recovery of compromised species, particularly those that have some official listed status by governments (i.e., as threatened or endangered under the United States (U.S.) Endangered Species Act) or international bodies (i.e., International Union for Conservation of Nature’s Red List).
There are currently thirty butterfly taxa listed under the Endangered Species Act as either Threatened or Endangered in the United States and its territories [12]. Of these, twenty-one have approved draft or final recovery plans. A total of seventeen recovery plans specifically mention organism translocation (or similar terminology, such as reintroduction, augmentation, introduction, or transplant) as part of the recommended actions that may be essential to recovery. In Canada, over 30% of the butterfly species listed on Schedule I of the Species at Risk Act (SARA) with recovery strategies identify reintroduction or research on reintroduction techniques as a priority [13]. Similarly, for the 25 British butterflies for which there are Species Action Plans, over 60% have strategic reintroduction or an assessment of the feasibility of strategic reintroduction as a low, medium, or high priority action recommendation [14].
Despite the identified need for conservation translocation activities for the recovery of many Lepidopteran species, few such programs are conducted in a scientifically repeatable way, are executed with a structured decision-making process, are well documented throughout, or are documented only in gray literature. This lack of standardization and communication of translocation results (whether successful or unsuccessful) creates a void for other programs to learn from one another and to benefit from the continued development and refinement of best practices. As organism translocations can be expensive and their success in general has been mixed [15,16], especially for Lepidopteran programs [17], such information would not only help advance the field, but also likely improve overall recovery targets. Structured-decision making processes that were developed by the IUCN [18] weigh the potential costs and benefits for all possible ex situ actions (including no action) in a transparent and informed manner. The additional IUCN Guidelines for Reintroductions and Other Conservation Translocations [11] outline the broad principles to help design and implement conservation actions that are intended to be applicable for all taxa. Our goal is to expand on these sound overarching guidelines by providing a more targeted list of detailed recommendations that are appropriate for Lepidoptera regarding conducting, documenting, and monitoring translocations. They are based in part on outcomes from a four-year series of U.S.-based workshops funded by the Institute of Museum and Library Services that focused on ex situ at-risk butterfly conservation and recovery programs [19], as well as extensive exchanges with conservation practitioners, wildlife agency personnel, and stakeholders. By doing so, we further reinforce the assertion by Sutherland et al. [20] that such standards help “ensure that, as a community, we collect a more complete and useful set of data on reintroductions to enable assessment of the timing and causes of both successes and failures, and that this information is easily accessible for future reference in other comparable reintroduction programs”. To avoid confusion, we use the typology outlined by IUCN [11], in which the term conservation translocation is overarching and encompasses reintroduction, reinforcement (i.e., augmentation), and introduction.
The following provides an overview of suggested recommendations for at-risk butterfly programs involving conservation translocation as an active or identified recovery strategy, and more broadly for all such programs involving Lepidoptera. While these represent basic standards that programs should strive to achieve over time, we realize that there may be many limitations, including budget, personnel, facilities, expertise, permitting, coordination, etc. that can directly hinder their adoption and implementation. We also recognize that there will be distinct cost/benefit trade-offs that are associated with their use and that the applicability of individual standards may vary depending on the specific recovery strategy, conservation targets, and the threats being addressed. At the very minimum, we urge all programs to develop clear a priori goals, increase detailed documentation for all releases, and broadly share results with the conservation community.
2. Proposed Actions
2.1. Document All Release Events
Similar to Sutherland et al. [20], we suggest a basic list of standardized criteria that should be collected for all release events and across all translocation projects involving Lepidoptera (Table 1).

Table 1.
List of recommended actions and types of information to be recorded.
2.2. Need for Better Communication and Coordination between Ex Situ and In Situ Program Components
Conservation and recovery efforts regularly involve a diversity of stakeholders and partners [21,22]. Although recovery teams can help to facilitate regular communication and the exchange of ideas, organizational hierarchies and boundaries, divergent communication processes, and the division of tasks and objectives often result in communication silos [23]. This gap is often most severe between various ex situ and in situ program components. As a result, transparency and coordination can suffer. This, in turn, can hinder the overall efforts and results and further program compartmentalization. Regular and consistent communication between key recovery partners, including conservation practitioners (i.e., land managers, personnel in charge of captive breeding and husbandry, etc.), scientists/researchers, and agency staff is needed to stimulate innovation, facilitate achieving the desired results, and to advance best practices.
2.3. Test and Develop More Robust Best Practices through Experimentation
The joint policy of the U.S. Fish and Wildlife Service and the National Marine Fisheries Service regarding the controlled propagation of species that are listed under the Endangered Species Act identifies the use of organisms for recovery-oriented scientific research that is expected to result in a net benefit to the listed taxon as an approved scope [24]. The continued development of best practices in reintroduction biology should be a priority. Beyond more broadly reporting program outcomes in descriptive accounts, disentangling why some efforts fail and others succeed is essential to advancing the field [20]. This can be done by more frequently designing and implementing translocation efforts in an experimental manner to test questions that are identified a priori [25]. Armstrong and Seddon [26] provide an excellent overview of key broad questions that reintroduction biologists should address in order to produce a more strategic approach in the field. Butterflies are particularly ideal for experimentation when compared to other taxa due to the potentially large numbers of organisms that can be produced in captivity. Their close host plant associations, often limited vagility, variable voltinism patterns, short adult longevity, and general ease of adult monitoring can facilitate experimental replication and data collection, as well as maximize return on effort.
2.4. Clearly Define Outcomes of Ex Situ Recovery Strategy A Priori
All restoration and recovery programs should have pre-defined goals prior to implementation. This includes all ex situ elements of the overall strategy, not just translocation. Such elements represent synergistic components and they should not be considered to be independent of one another. For example, the identified source populations, founders utilized, population genetic structure, and even colony captive management may significantly influence restoration success. Recovery programs should strive to clearly define what threats are being addressed, the strategies proposed to adequately address those threats, and the desired recovery outcomes so that success can be effectively assessed.
2.5. Promote Protocol Flexibility to Stimulate Innovation
Ex situ efforts are inherently pioneering. The vast majority of taxa of conservation concern have been poorly studied prior to substantial wild population declines. This is certainly true of insects and other invertebrates. The resulting biological and ecological data gaps pertaining to basic natural history, habitat requirements, behavior, and population biology can be substantial [27]. The corresponding charge to develop appropriate captive breeding, husbandry, and translocation protocols with limited data has often proven equally challenging. To help overcome these issues, many at-risk butterfly conservation and recovery programs regularly utilize or adapt protocols from other programs or from isolated published accounts, especially those that are focused on closely related taxa. While clearly an excellent starting point, the extensive interspecific diversity of butterfly behaviors, life history traits, and ecological requirements combined with often marked differences in program facilities, budgets, practitioner expertise, and release sites, seldom translates seamlessly. This can even be true across programs focused on the same taxon. Thus, strict adherence to a single protocol, even if proven successful in one region or facility, may curtail success in another. It is in our opinion essential to strongly support the flexibility to regularly refine existing methodologies or to generate new, workable, and innovative protocols, as needed to generate success. Detailed documentation of protocol changes or new methodologies is critical for this process. Conservation practitioners should identify levels of acceptable risk for their specific program prior to the execution of any action.
2.6. Define Objectives of Post-Release Monitoring
Monitoring of organisms following all release events is essential. This recovery program component should follow a detailed plan that clearly defines the scope, scale, and objectives of monitoring, as well as indicators of success. This is critical for assessing organism performance, evaluating program impact, and applying appropriate adaptive management measures if necessary. It is additionally valuable to ensure that the sufficient budgetary and labor resources are appropriately allocated to accomplish the predetermined objectives. For all translocation efforts, monitoring should ideally be conducted to yield reliable estimates of population density or size and to adequately assess changes in the population over time (i.e., estimates that appropriately estimate trends in population trajectory) [28]. Survey methodologies should also consider minimizing habitat impacts and accounting for organism detectability and variable phenology. Where possible, long-term, standardized monitoring should be conducted.
Beyond estimating population size, recovery programs should consider monitoring organism use of and movement across the broader landscape. This would involve collecting a variety of ecological and spatial data to evaluate the reproductive success of released individuals, organism presence, and the use of resources across the recipient site, dispersal to adjacent habitat patches or sites, successful colonization of adjacent habitat patches or sites, and the use of available corridors or stepping stones. Additional monitoring objectives might include evaluating the immediate fate and behavior of released individuals. This would require a more intensive monitoring effort in the days and weeks immediately following release, and the exact protocols would vary depending on organism life stage at release. Such data would be especially useful for documenting mortality and comparing different release strategies. Species threat assessments often seek knowledge of dispersal potential and dispersal propensity to model the probability of persistence of populations and subpopulations. Dispersal can most precisely be estimated when a species is reintroduced to a novel site and/or to a site where it had been extirpated. Assuming that the species was indeed absent (a presumption that necessitates significant monitoring prior to any release), recording the movement patterns of released individuals, particularly in the first year of such an operation before any in situ reproduction would occur, can provide some of the most detailed information of potential dispersal capacity. Dispersal capacity is a function of both the landscape of available acceptable habitat and of the propensity of individuals to move and to explore their environment. Dispersal capacity is both species-specific and condition-specific.
Lastly, recovery programs should consider some level of regular genetic monitoring of released individuals or reinforced populations. This can be particularly important if there is a limited number of initial founders, release sites are relatively small in size or spatially isolated, released organisms have limited dispersal ability, population trajectories decline severely, and/or to evaluate genetic rescue of reinforcement.
Where the use of genetic monitoring techniques is financially infeasible or logistically impractical, practitioners should take minimal steps to secure a genetically diverse set of founders with a sample size that is large enough to maximize persistence probability. For example, reintroduction planning of the large blue butterfly, Phengaris (=Maculinea) arion, in the United Kingdom called for the collection of more than 250 larvae from 11 demographically distinct and spatially separated populations in an attempt to capture maximum levels of genetic diversity. Although no genetic testing was done at the time of founder collection from the source populations, subsequent analysis 19 generations later of the reintroduced population indicated that the predetermined procedure was effective for maintaining genetic diversity during conservation translocations as well as maintaining population persistence [29].
2.7. Optimize the Contributions of Citizen Scientists
Labor costs are arguably one of the largest budgetary components of any conservation and recovery program. Although citizen scientists are ideal for projects that are spanning large spatiotemporal scales [30,31], strategically deploying a smaller number of volunteers that are well-trained to collect data and have familiarity with sensitive or protected lands can represent highly skilled and motivated contributors to a wide range of conservation efforts, including those that are involving listed taxa [32,33,34,35,36,37]. Volunteers with strong botanical or entomological interests and backgrounds or wildlife observational skills as an example can be particularly valuable for organism monitoring or habitat assessment program components.
Comprehensive training and supervision of volunteers is vital to ensure effective adherence to identified methodologies, compliance with all required regulations and permitted activities, and the generation of useable, high quality data. Similarly, care should be taken to minimize disturbance (e.g., trampling, creation of trails, etc.) to sensitive habitats and avoid “harassment” of listed species (such as defined by the U.S. Endangered Species Act of 1973).
2.8. More Robust Habitat Monitoring at Recipient Sites
In order to help maximize the potential for translocation efforts to succeed, careful planning needs to take place both prior to target organism release as well as after. A comprehensive assessment of the suitability of the habitat is an essential component, and it should ultimately aim to focus on the ability of the habitat to support the taxon for the long-term [38,39]. For butterflies, the distribution, both spatial extent and density, of known larval host plants is a primary element of suitable habitat that often delineates the organism’s distribution and drives its population abundance. Similarly, nectar source availability, including diversity, density, spatial extent, and phenology can also be critically important [6,40,41]. In addition, landscape features and the overall landscape structure should be carefully considered and documented prior to all translocation activities. Changes in elevation, water bodies, firebreaks or easements, tree lines, and even anthropogenic structures, such as roads, can influence butterfly behavior, including dispersal. Landscape structure may benefit some species’ initial establishment period by confining them in high quality habitat and discouraging dispersal into low quality environs or resulting in Allee effects at release sites [42].
Many additional habitat attributes may be critical for one life stage or another, including the presence of symbionts (e.g., ants associated with lycaenid larvae), various substrates for thermoregulation, hibernation, or mate-locating, and specific microclimatic or management conditions. Thus, when possible, a functional resource-based framework should be adopted to define the essential habitat and resource components for the target organism [43,44], followed by a scientifically sound protocol for assessing and monitoring those components over time.
Lastly, little attention is often given to evaluating the unexpected or unintended consequences of species interactions, such as interspecific competition. While numerous vertebrate examples exist [45,46], niche occupancy by another butterfly or phytophagous insect could result in resource competition sufficient enough to hinder reintroduction success. Such risk assessments are critical, especially for strategies that are involving assisted migration under climate change. Subsequent demographic monitoring should further be used to more effectively assess the resulting density-dependent processes.
2.9. Increase Use of Genetic and Health Screening
The importance of incorporating genetic data into conservation and recovery programs is well recognized. Such information offers numerous practical applications that can be used to help inform a range of decisions from listing and distribution modeling to management and recovery [47]. The rapid advancement of modern genetic and genomic techniques including next-generation sequencing (NGS) offers new, unparalleled research opportunities, while presenting many low-cost options. Similar advancements in non-destructive and non-invasive sampling methodologies have lessened stakeholder concerns about organism handling and injury and significantly broadened application.
Genetic testing can also be used to help screen for pathogens in Lepidoptera. Such evaluations should be considered when conservation programs involve any ex situ recovery and management components. Considerable attention has focused on the intracellular bacterium Wolbachia due to its high infection incidence in Lepidoptera, including many taxa of conservation concern, and ability to induce several deleterious phenotypic effects on the host population [48,49,50,51]. Concerns surrounding additional inherited bacteria that are known to demonstrate reproductive parasitism and associated infection-related effects, such as male-killing, include Arsenophonus, Cardinium, and Spiroplasma [52,53]. This type of pathogen screening can be especially useful for helping inform conservation decision-making. By contrast, testing for acute infections, such as baculoviruses, which inflict the immature stages of insects that can be particularly devastating for captive colonies of Lepidoptera serve only to confirm disease presence [54]. While useful for potentially directing changes to husbandry protocols to minimize initial infection and spread, effective treatment of afflicted organisms is unlikely.
Lastly, the relatively new discipline of landscape genomics offers the opportunity to explore the adaptive evolution of species in response to spatial environmental heterogeneity. It has direct conservation implications by helping to predict how populations will respond to climate change and to identify adaptive genetic variation [55,56,57,58]. The resulting impact of these genetic and genomic tools for ex situ efforts can be substantial. Beyond the broader examples described above, they could be used to help manage inbreeding depression in captive populations, detect adaptation to captivity, pinpoint founders that could increase the evolutionary potential of captive populations, and identify individuals for translocation efforts that may provide genetic rescue or which are most appropriate for assisted migration [59].
Despite their growing utility and potential, undertaking conservation genetic research still requires significant planning and resources. Such studies should always be conducted as part of a larger comprehensive conservation and recovery plan and strive to yield results that have practical application.
2.10. Employ Ecological Modeling to Help Inform Practice Where Possible and Appropriate
Ecological models are becoming fundamental tools to aid in conservation decision-making. Population viability analyses (PVAs) are commonly applied to study the combined impacts of all relevant variables to predict future population dynamics or risk of extinction (or quasi extinction) and help to prioritize management options for species conservation [60,61]. Such spatially explicit models (SEMs), however, are inherently data-intensive. Their parameterization requires input of very high quality demographic, habitat, and other behavioral or ecological data that can be quite limited or often entirely lacking for many imperiled species. As a result, the reliability and predictive power of such quantitative model-based approaches has often been debated [62,63,64,65]. Similar to the discussion above regarding genetic and health screening, we emphasize the tremendous utility of PVAs, but also recognize that they are but one of a series of tools that are available to conservation practitioners, and that such complex models alone do not provide “an adequate or practical overarching framework to overcome current shortcomings in recovery planning” [66].
2.11. Increase Information and Data Sharing
The IUCN/SSC Guidelines for Reintroductions and Other Conservation Translocations recognize the importance of data for planning, implementing, and disseminating the results of translocation events [18]. Regular reporting and data sharing is essential for improving best practices, avoiding redundancies and misinformation, and increasing conservation impacts. Effective dissemination of program methodologies and results is best accomplished through peer-reviewed scientific journals or at minimum in gray literature. There are the strengths and limitations of both publication types that should be carefully considered. Peer-reviewed publications have higher scholarly standards, require a strict format that often limits more detailed content, may have a prolonged publication schedule, and often have restricted access or other fee-based requirements. Gray literature offers increased formatting and content flexibility, more rapid production, and ease of sharing. In either case, conservation practitioners should develop a detailed publication plan as part of the overall translocation and recovery program. It should comprehensively outline the target audiences, content to be included, schedule of completion, collaborators, need for any additional information or expertise, and dissemination methods.
The resulting publication of program findings is still limiting. The development of a single source standardized data repository similar to the Avian Reintroduction and Translocation Database (ARTD) is sorely needed to help support the rapidly growing field of insect conservation. The ARTD includes data for over 200 taxa and some 2300 release events around the globe and represents a unique conservation resource to “help guide the design of future reintroductions, characterize past reintroduction practices and compare and contrast procedures across programs” [67]. Similarly, there have been increasing calls from the conservation community to more effectively integrate in-situ and ex-situ management processes [68]. Such a centralized and standardized Lepidoptera recovery database could help to accomplish this broad goal, stimulate new collaborations, and promote recovery-based research.
2.12. Make Use of Available Conservation Project Design, Management, and Monitoring Tools
There is a growing list of innovative tools available to the conservation community [68,69,70]. The Open Standards for the Practice of Conservation (OS) (http://cmp-openstandards.org/) and its software platform Miradi has become one of the most prominent and widely used by conservation practitioners for program design, implementation, and monitoring [71,72]. Developed by the Conservation Measures Partnership (CMP), a joint venture of some 28 leading global conservation organizations and collaborators, the OS framework incorporates the principles and best practices in adaptive management to help better assess the effectiveness of actions and to improve the overall practice of conservation [73]. Its most recently developed cloud-based system, Miradi Share, enables organizations and individuals to easily share information and facilitate cross-project learning [74]. Miradi enables the development of graphical conceptual project models and associated results chains (Figure 1) that help practitioners and associated stakeholders better conceptualize, prioritize, and implement management actions for specific conservation targets.
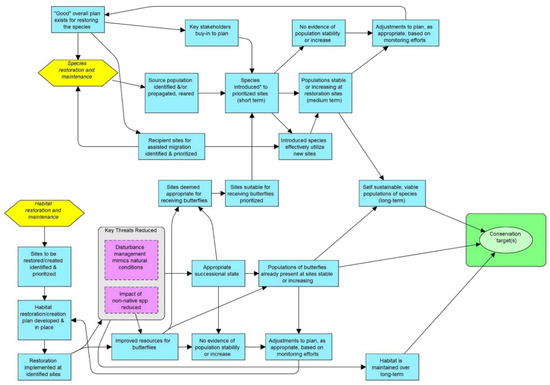
Figure 1.
Example results chain developed through Miradi. The yellow hexagons represent a subset of the specific recovery strategies, blue boxes represent intermediate actions, pink boxes represent threat reduction result of intermediate actions, and green oval represents identified conservation target (e.g., organism, species assemblage, habitat, community, system). This particular example is for an at-risk butterfly recovery project in southern California, USA where the focal species require some level of regular habitat management.
3. Concluding Remarks
The threats and challenges to butterfly, and more broadly, insect conservation and recovery continue to grow, as does the number of targeted species-specific recovery programs involving the integration of ex situ and in situ components. Such efforts demand significant commitments of labor and funds, and the time that is needed to achieve identified recovery targets may exceed what is feasible for both the program and the continued persistence of the organism. To help to maximize recovery outcomes, conservation practice needs to make better use of systematic planning and increased evidence-based assessments [75]. The field of insect conservation is still young in many ways. We have the luxury of learning from the long list of vertebrate-based examples, their successes, and maybe more importantly, their lessons learned.
Author Contributions
Conceptualization, J.C.D., E.R. and C.N.; Writing-Review & Editing, J.C.D., E.R. and C.N.
Funding
This work received no external funding.
Acknowledgments
We would like to thank Geena Hill and Chase Kimmel for their kind assistance with the manuscript preparation and initial content reviews.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thomas, J.A.; Telfer, M.G.; Roy, D.B.; Preston, C.D.; Greenwood, J.J.; Asher, J.; Fox, R.; Clarke, R.T.; Lawton, J.H. Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science 2004, 303, 1879–1881. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Schmitt, T.; Weitzel, M.; Seitz, A. The severe decline of butterflies on western German calcareous grasslands during the last 30 years: A conservation problem. Biol. Conserv. 2005, 128, 542–552. [Google Scholar] [CrossRef]
- Thomas, C.D.; Franco, A.M.A.; Hill, J.K. Range retractions and extinction in the face of climate warming. Trends Ecol. Evol. 2006, 21, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Wallisdevries, M.F.; Van Swaay, C.A.M.; Plate, C.L. Changes in nectar supply: A possible cause of widespread butterfly decline. Curr. Zool. 2012, 58, 384–391. [Google Scholar] [CrossRef]
- Van Dyck, H.; Van Strein, A.J.; Maes, D.; Van Swaay, C.A. Declines in common, widespread butterflies in a landscape under intense human use. Conserv. Biol. 2009, 23, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Breed, G.A.; Stitcher, S.; Crone, E.E. Climate-driven changes in northeastern US butterfly communities. Nat. Clim. Chang. 2012, 3, 142–145. [Google Scholar] [CrossRef]
- Gilburn, A.S.; Bunnefeld, N.; Wilson, J.M.; Botham, M.S.; Brereton, T.M.; Fox, R.; Goulson, D. Are neonicotinoid insecticides driving declines of widespread butterflies? PeerJ 2015, 3, e1402. [Google Scholar] [CrossRef] [PubMed]
- Wilhere, G.F. Adaptive Management in Habitat Conservation Plans. Conserv. Biol. 2002, 16, 20–29. [Google Scholar] [CrossRef]
- Gregory, R.; Long, G. Using Structured Decision Making to Help Implement a Precautionary Approach to Endangered Species Management. Risk Anal. 2009, 29, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Runge, M.C. An Introduction to Adaptive Management for Threatened and Endangered Species. J. Fish Wildl. Manag. 2011, 2, 220–233. [Google Scholar] [CrossRef]
- IUCN/SSC. Guidelines for Reintroductions and Other Conservation Translocations; Version 1.0; IUCN Species Survival Commission: Gland, Switzerland, 2013; ISBN 978-2-8317-1609-1. [Google Scholar]
- USFWS Environmental Conservation Online System. Listed Animals. U.S. Fish and Wildlife Service. Available online: https://ecos.fws.gov/ecp/ (accessed on 12 June 2018).
- Government of Canada. Species at Risk Registry. Available online: https://www.registrelep-sararegistry.gc.ca/species/schedules_e.cfm?id=1 (accessed on 15 June 2018).
- Butterfly Conservation. Available online: https://butterfly-conservation.org/3544/Species-ActionPlans.html (accessed on 1 June 2018).
- Seddon, P.J.; Armstrong, D.P.; Maloney, R.F. Developing the Science of Reintroduction Biology. Conserv. Biol. 2007, 21, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Soorae, P.S. (Ed.) Global Re-Introduction Perspectives: Re-Introduction Case-Studies from Around the Globe; IUCN/SSC Reintroduction Specialist Group: Abu Dhabi, UAE, 2008; ISBN 978-2-8317-1113-3. [Google Scholar]
- Schultz, C.B.; Russel, C.; Wynn, L. Restoration, Reintroduction, and captive Propagation for at-risk Butterflies: A review of British and American Conservation Efforts. Isr. J. Ecol. Evol. 2008, 54, 41–61. [Google Scholar] [CrossRef]
- IUCN/SSC. Guidelines on the Use of Ex Situ Management for Species Conservation; Version 2.0; IUCN Species Survival Commission: Gland, Switzerland, 2014; Available online: https://www.iucn.org/about/work/programmes/species/publications/iucn_guidelines_and__policy__statements (accessed on 15 June 2018).
- Daniels, J.C. (Ed.) Butterfly Conservation in North America: Efforts to Help Save our Charismatic Microfauna; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2015; ISBN 978-94-017-9851-8. [Google Scholar]
- Sutherland, W.J.; Armstrong, D.; Butchart, S.H.M.; Earnhardt, J.M.; Ewen, J.; Jamieson, I.; Jones, C.G.; Lee, R.; Newbery, P.; Nichols, J.D.; et al. Standards for documenting and monitoring bird reintroduction projects. Conserv. Lett. 2010, 3, 229–235. [Google Scholar] [CrossRef]
- Daniels, J.C. Cooperative conservation efforts to help recover an endangered south Florida butterfly. Insect Conserv. Divers. 2009, 2, 62–64. [Google Scholar] [CrossRef]
- Knight, A.T.; Cowling, R.M.; Campbell, B.M. An Operational Model for Implementing Conservation Action. Conserv. Biol. 2006, 20, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Crouse, D.T.; Mehrhoff, L.A.; Parkin, M.J.; Elam, D.R.; Chen, L.Y. Endangered species recovery and SCB Study: A U.S. Fish and Wildlife Service Perspective. Ecol. Appl. 2002, 12, 719–723. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service; National Marine Fisheries Service. Policy regarding controlled propagation of species listed under the Endangered Species Act. Fed. Regist. 2000, 65, 56916–56922. [Google Scholar]
- Moseby, K.E.; Hill, B.M.; Lavery, T.H. Tailoring Release Protocols to Individual Species and Sites: One Size Does Not Fit All. PLoS ONE 2014, 9, e99753. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.P.; Seddon, P.J. Directions in reintroduction biology. Trends Ecol. Evol. 2008, 23, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Bossart, J.L.; Carlton, C.E. Insect Conservation in America: Status and Perspectives. Am. Entomol. 2002, 48, 82–92. [Google Scholar] [CrossRef]
- Stephens, P.A.; Pettorelli, N.; Barlow, J.; Wittingham, M.J.; Cadotte, M.C. Management by proxy? The use of indices in applied ecology. J. Appl. Ecol. 2015, 52, 1–6. [Google Scholar] [CrossRef]
- Andersen, A.; Simcox, D.J.; Thomas, J.A.; Nash, D.R. Assessing reintroduction schemes by comparing genetic diversity of reintroduced and source populations: A case study of the globally threatened large blue butterfly (Maculinea arion). Biol. Conserv. 2014, 175, 34–41. [Google Scholar] [CrossRef]
- Bonney, R.; Cooper, C.B.; Dickinson, J.; Kelling, S.; Phillips, T.; Rosenberg, K.V.; Shirk, J. Citizen Science: A Developing Tool for Expanding Science Knowledge and Scientific Literacy. BioScience 2009, 59, 977–984. [Google Scholar] [CrossRef]
- Devictor, V.; Whittaker, R.J.; Beltrame, C. Beyond scarcity: Citizen science programmes as useful tools for conservation biogeography. Divers. Distrib. 2010, 16, 354–362. [Google Scholar] [CrossRef]
- Jue, D.K.; Daniels, J.C. A successful model for citizen scientist involvement in building a statewide at-risk butterfly database. J. Insect Conserv. 2014, 19, 421–431. [Google Scholar] [CrossRef]
- Chandler, M.; See, L.; Copas, K.; Bonde, A.M.Z.; López, B.C.; Danielsen, F.; Legind, J.K.; Masinde, S.; Miller-Rushing, A.J.; Newman, G.; et al. Contribution of citizen science towards international biodiversity monitoring. Biol. Conserv. 2016, 213, 280–294. [Google Scholar] [CrossRef]
- Hess, R.J.; Hess, A.N. Conserving Karner blue butterflies in Wisconsin: A development of management techniques. Am. Entomol. 2005, 61, 96–113. [Google Scholar] [CrossRef]
- Hudgins, J.A.; Hudgins, E.J.; Ali, K.; Mancini, A. Citizen science surveys elucidate key foraging and nesting habitat for two endangered marine turtle species within the Republic of Maldives. Herpetol. Notes 2017, 10, 463–471. [Google Scholar]
- Kobori, H.; Dickinson, J.L.; Washitani, I.; Sakurai, R.; Amano, T.; Komatsu, N.; Kitamura, W.; Takagawa, S.; Koyama, K.; Ogawara, T.; et al. Citizen science: A new approach to advance ecology, education, and conservation. Ecol. Res. 2016, 31, 1–19. [Google Scholar] [CrossRef]
- Tye, C.A.; McCleery, R.A.; Fletcher, R.J., Jr.; Greene, D.U.; Butryn, R.S. Evaluating citizen vs. professional data for modelling distributions of a rare squirrel. J. Appl. Ecol. 2017, 54, 628–637. [Google Scholar] [CrossRef]
- Cheyne, S.M. Wildlife reintroduction: Considerations of habitat quality at the release site. BMC Ecol. 2006, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Ewen, J.G.; Armstrong, D.P. Strategic monitoring of reintroductions in ecological restoration programmes. Ecoscience 2007, 14, 401–409. [Google Scholar] [CrossRef]
- Schultz, C.B.; Dlugosh, K.M. Nectar and hostplant scarcity limit populations of an endangered Oregon butterfly. Oecologia 1999, 119, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, J.; Wesselingh, R.A.; Van Dyck, H. Floral resource limitation severely reduces butterfly survival, condition and flight activity in simplified agricultural landscapes. Oecologia 2016, 180, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Kuussaari, M.; Heikkinen, R.K.; Heliölä, J.; Luoto, M.; Mayer, M.; Rytteri, S.; von Bagh, P. Successful translocation of the threatened Clouded Apollo butterfly (Parnassius mnemosyne) and metapopulation establishment in southern Finland. Biol. Conserv. 2015, 190, 51–59. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Shreeve, T.G.; Van Dyck, H. Towards a functional resource-based concept for habitat: A butterfly biology viewpoint. Oikos 2003, 102, 417–426. [Google Scholar]
- Dennis, R.L.H.; Shreeve, T.G.; Van Dyck, H. Habitats and Resources: The need for a resource-based definition to conserve butterflies. Biodivers. Conserv. 2006, 15, 1943–1966. [Google Scholar] [CrossRef]
- Lovari, S.; Ferretti, F.; Corazza, M.; Milder, I.; Troiani, N.; Ferrari, C.; Saddi, A. Unexpected consequences of reintroductions: Competition between increasing red deer and threatened Apennine chamois. Anim. Conserv. 2014, 17, 359–370. [Google Scholar] [CrossRef]
- Hayward, M.W.; Hayward, G.J. Activity patterns of reintroduced lion Panthera leo and spotted hyaena Crocuta crocuta in the Addo Elephant National Park, South Africa. Afr. J. Ecol. 2007, 45, 135–141. [Google Scholar] [CrossRef]
- Frankham, R. Where are we in conservation genetics and where do we need to go? Conserv. Genet. 2010, 11, 661–663. [Google Scholar] [CrossRef]
- Saarinen, E. Butterfly Conservation Genetics. In Butterfly Conservation in North America; Daniels, J., Ed.; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2015; pp. 75–101. ISBN 978-94-017-9851-8. [Google Scholar]
- Dyson, E.A.; Kamath, M.K.; Hurst, G.D.D. Wolbachia infection associated with all-female broods in Hypolimnas bolina (Lepidoptera: Nymphalidae): Evidence for horizontal transmission of a butterfly male killer. Heredity 2002, 88, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Hamm, C.A.; Handley, C.A.; Pike, A.; Forister, M.L.; Fordyce, A.J.; Nice, C.C. Wolbachia infection and Lepidoptera of conservation concern. J. Insect Sci. 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Araujo-Jnr, E.V.; Welch, J.J.; Kawahara, A.Y. Wolbachia in butterflies and moths: Geographic structure in infection frequency. Front. Zool. 2015, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Bouchon, D.; Bouton, S.; Bellamy, L.; Zhou, L.; Engelstädter, J.; Hurst, G.D. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Duplouy, A.; Hornett, E. Uncovering the hidden players in Lepidoptera biology: The heritable microbial endosymbionts. PeerJ 2018, 6, e4629. [Google Scholar] [CrossRef] [PubMed]
- Clem, R.J.; Passarelli, A.L. Baculoviruses: Sophisticated Pathogens of Insects. PLoS Pathog. 2013, 9, e1003729. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.H.; Murphy, W.J.; Ostrander, E.A.; Wayne, R.K. Genomics and conservation genetics. Trends Ecol. Evol. 2006, 21, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Joost, S.; Bonin, A.; Bruford, M.W.; Despres, L.; Conord, C.; Erhardt, G. A spatial analysis method (SAM) to detect candidate loci for selection: Towards a landscape genomics approach to adaptation. Mol. Ecol. 2007, 16, 3955–3969. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, E.V.; Hellmann, J.J. Genetic differentiation across a latitudinal gradient in two co-occurring butterfly species: Revealing population differences in a context of climate change. Mol. Ecol. 2007, 17, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Moa, R.; Yang, J.; Miao, C.; Li, Z.; Qiu, Y. Ten Years of Landscape Genomics: Challenges and Opportunities. Front. Plant Sci. 2010, 8, 2136. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, F.W.; Hohenlohe, P.A.; Luikart, G. Genomics and the future of conservation genetics. Nat. Rev. Genet. 2010, 11, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.B.; Hammond, P.C. Using population viability analysis to develop recovery criteria for endangered insects: Case study of the Fender’s blue butterfly. Conserv. Biol. 2003, 17, 1372–1385. [Google Scholar] [CrossRef]
- McIntire, E.J.B.; Schultz, C.B.; Crone, E.E. Designing a network for butterfly habitat restoration: Where individuals, populations, and landscapes interact. J. Appl. Ecol. 2007, 44, 725–736. [Google Scholar] [CrossRef]
- Radchuk, C.; Johst, K.; Groeneveld, J.; Turlure, C.; Grimm, V.; Schtickzelle, N. Appropriate resolution in time and model structure for population viability analysis: Insights from a butterfly metapopulation. Biol. Conserv. 2014, 169, 345–354. [Google Scholar] [CrossRef]
- Coulson, T.; Mace, G.M.; Hudson, E.; Possingham, H. The use and abuse of population viability analysis. Trends Ecol. Evol. 2001, 16, 219–221. [Google Scholar] [CrossRef]
- Brook, B.W.; Burgman, M.A.; Akçakaya, H.R.; O’Grady, J.J.; Frankham, R. Critiques of PVA ask the wrong questions: Throwing the heuristic baby out with the numerical bath water. Conserv. Biol. 2002, 16, 262–263. [Google Scholar] [CrossRef]
- Reed, M.J.; Mills, L.S.; Dunning, J.B., Jr.; Menges, E.S.; McKelvey, K.S.; Frye, R.; Beissinger, S.R.; Anstett, M.; Miller, P. Emerging Issues in Population Viability Analysis. Conserv. Biol. 2002, 16, 7–19. [Google Scholar] [CrossRef]
- Wolf, S.; Hartl, B.; Carroll, C.; Neel, M.C.; Greenwald, D.N. Beyond PVA: Why Recovery under the Endangered Species Act Is More than Population Viability. BioScience 2015, 65, 200–207. [Google Scholar] [CrossRef]
- Lincoln Park Zoo. Avian Reintroduction and Translocation Database. Available online: http://www.lpzoo.org/ARTD (accessed on 20 June 2018).
- Schwartz, K.R.; Parsons, E.C.M.; Rockwood, L.; Wood, T.C. Integrating In-Situ and Ex-Situ Data Management Processes for Biodiversity Conservation. Front. Ecol. Evol. 2017, 5, 120. [Google Scholar] [CrossRef]
- Sarkar, S.; Pressey, R.L.; Faith, D.P.; Margules, C.R.; Fuller, T.; Stoms, D.M.; Moffett, A.; Wilson, K.A.; Williams, K.J.; Williams, P.H.; et al. Biodiversity Conservation Planning Tools: Present Status and Challenges for the Future. Annu. Rev. Environ. Resour. 2006, 31, 123–159. [Google Scholar] [CrossRef]
- Moilanen, E. Generalized Complementarity and Mapping of the Concepts of Systematic Conservation Planning. Conserv. Biol. 2008, 22, 1655–1658. [Google Scholar] [CrossRef] [PubMed]
- Dietz, L.A.; Brown, M.; Swaminathan, V. Increasing the impact of conservation projects. Am. J. Primatol. 2010, 72, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Deiner, K.; Forrester, T.; Grof-Tisza, P.; Muir, M.J.; Santos, M.J.; Souza, L.E.; Wilkerson, M.L.; Zylberberg, M. Perspectives on the Open Standards for the Practice of Conservation. Biol. Conserv. 2012, 155, 169–177. [Google Scholar] [CrossRef]
- Conservation Measures Partnership. Available online: http://www.conservationmeasures.org/ (accessed on 30 May 2018).
- Miradi Share. Available online: https://www.miradishare.org/ (accessed on 2 June 2018).
- Sutherland, W.J.; Pullin, A.S.; Dolman, P.M.; Knight, T.M. The need for evidence-based conservation. Trends Ecol. Evol. 2004, 19, 305–308. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).