Synthesis and Anti-Inflammatory Activity of (Z)-4-(2-(3-Oxopiperazin-2-ylidene)acetyl)benzoic Acid
Abstract
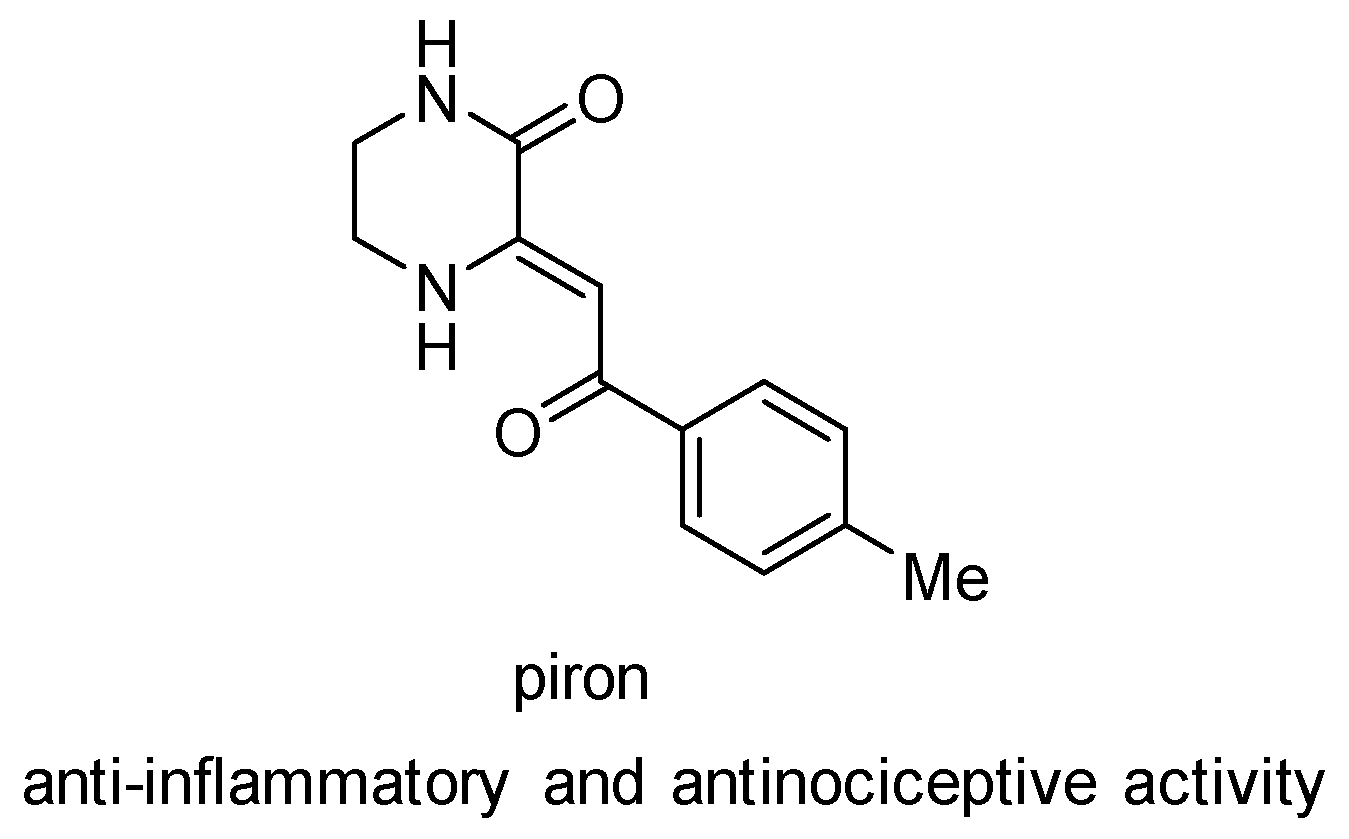
1. Introduction
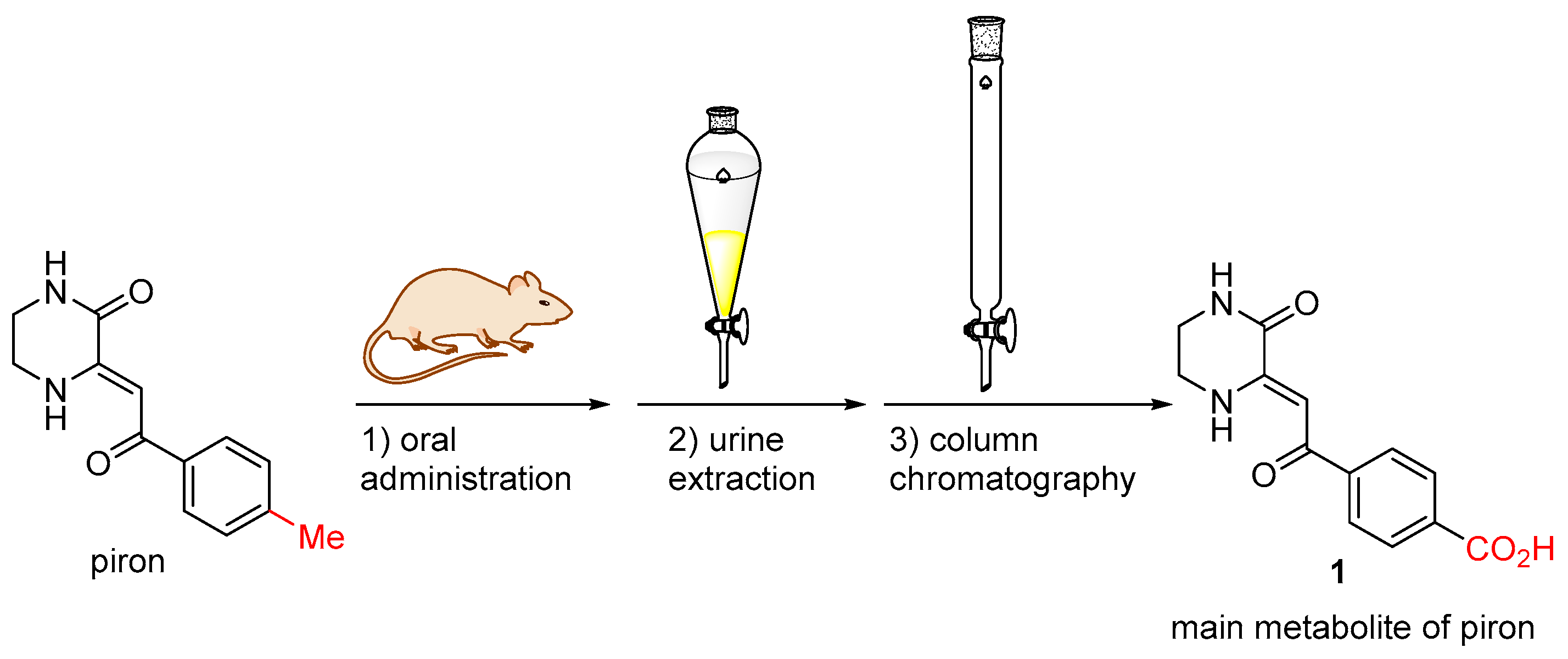
2. Results and Discussion
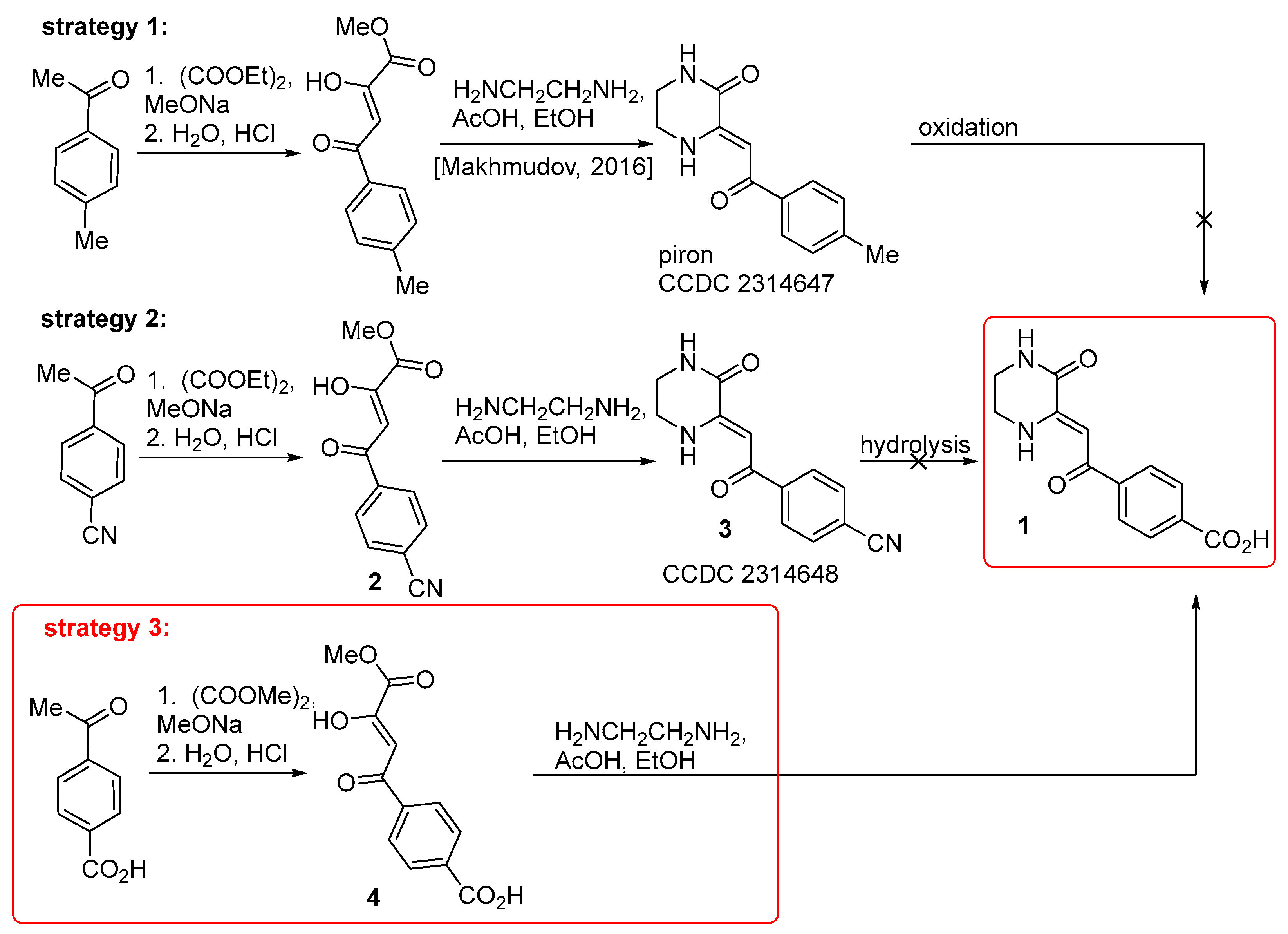
2.1. Chemistry
2.2. X-ray Analysis
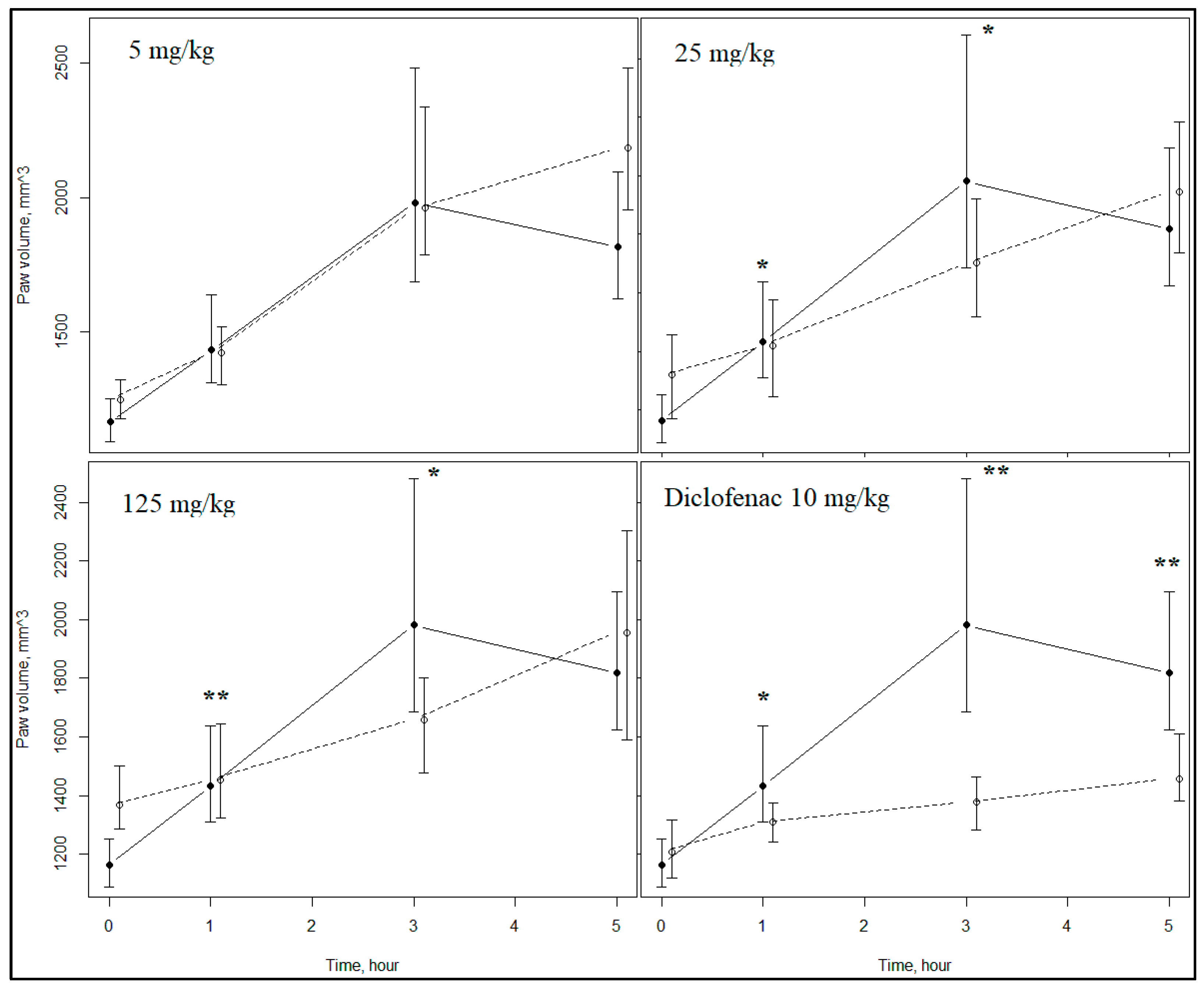
2.3. Biological Assay
3. Materials and Methods
3.1. General Information
3.2. Methyl (Z)-4-(4-cyanophenyl)-2-hydroxy-4-oxobut-2-enoate 2
3.3. (Z)-4-(2-(3-Oxopiperazin-2-ylidene)acetyl)benzonitrile 3
3.4. (Z)-4-(3-Hydroxy-4-methoxy-4-oxobut-2-enoyl)benzoic Acid 4
3.5. (Z)-4-(2-(3-Oxopiperazin-2-ylidene)acetyl)benzoic Acid 1
3.6. Biological Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Chapurlat, R.; Al-Daghri, N.; Herrero-Beaumont, G.; Bruyère, O.; Rannou, F.; Roth, R.; Uebelhart, D.; Reginster, J.Y. Safety of oral non-selective non-steroidal anti-inflammatory drugs in osteoarthritis: What does the literature say? Drugs Aging 2019, 36, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Makhmudov, R.R.; Kotegov, V.P.; Andrejchikov, J.S.; Pidemskij, E.L. Anti-inflammatory and/or antinociceptive (Z)-3-(2-oxo-2-(4-tolyl) ethylidene)piperazine-2-on, method for production thereof, pharmaceutical compositions. Patent RU2602500, 20 November 2016. [Google Scholar]
- Mashevskaya, I.V.; Kotegov, V.P.; Makhmudov, R.R.; Suldin, A.S.; Puchnina, S.V. Composition and method for producing a solid dosage form containing (Z)-3-(2-oxo-2-(4-tolyl)ethylidene)piperazin-2-one. Patent RU2657526, 14 June 2018. [Google Scholar]
- Kotegov, V.P.; Razumova, M.Y.; Andreev, A.I.; Apushkin, D.Y. Study of the specific activity of piron on a model of carrageenan inflammation. In Proceedings of the “Creation of Competitive Medicines—A Priority Direction in the Development of Pharmaceutical Sciences”, Perm, Russia, 13 December 2018. [Google Scholar]
- CrysAlisPro, Version 1.171.37.33 (Release 27-03-2014 CrysAlis171.NET), Agilent Technologies, Oxford Diffraction: Wroclaw, Poland, 2014. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 10 December 2023).
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.R.; Li, C.; Guo, X.; Myrvang, H.K.; Fischer, P.M.; Dekker, L.V. Design, synthesis, and structure− activity relationship exploration of 1-substituted 4-aroyl-3-hydroxy-5-phenyl-1 H-pyrrol-2 (5 H)-one analogues as inhibitors of the annexin A2− S100A10 protein interaction. J. Med. Chem. 2011, 54, 2080–2094. [Google Scholar] [CrossRef] [PubMed]
- Anglemyer, A.; Horvath, H.T.; Bero, L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst. Rev. 2014, MR000034. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.H.; Khaziakhmetova, V.N.; Zigashina, L.E. Rat paw oedema modeling and NSAIDs: Timing of effects. Int. J. Risk Saf. Med. 2015, 27, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Nazreen, S.; Alam, M.M.; Gupta, A.; Hamid, H.; Alam, M.S. Anti-inflammatory and anti-nociceptive activities of ethanolic extract and its various fractions from Adiantum capillus veneris Linn. J. Ethnopharmacol. 2011, 138, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Digital Anhydrous Plethysmometer. Available online: http://www.openscience.ru/index.php?page=physio&item=001 (accessed on 15 December 2023).
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Glanz, S. Primer of Biostatistics, 4th ed.; McGraw-Hill Inc.: New York, NY, USA, 1997. [Google Scholar]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Reading, MA, USA, 1977. [Google Scholar]
- ISO 3534-1:2006; Statistics. Part 1: General Statistical Terms and Terms Used in Probability. ISO (International Organization for Standardization): Geneva, Switzerland, 2006.

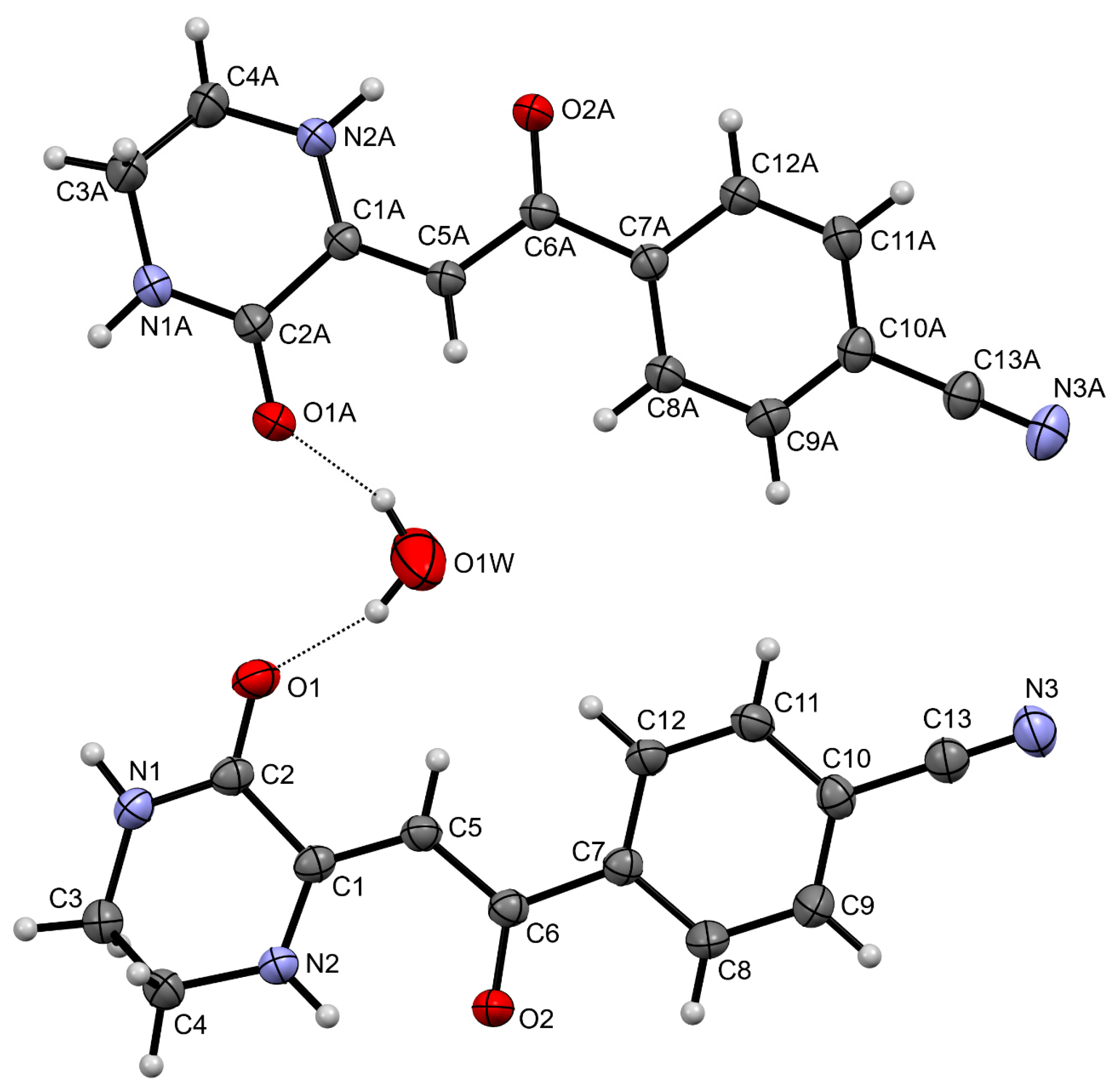

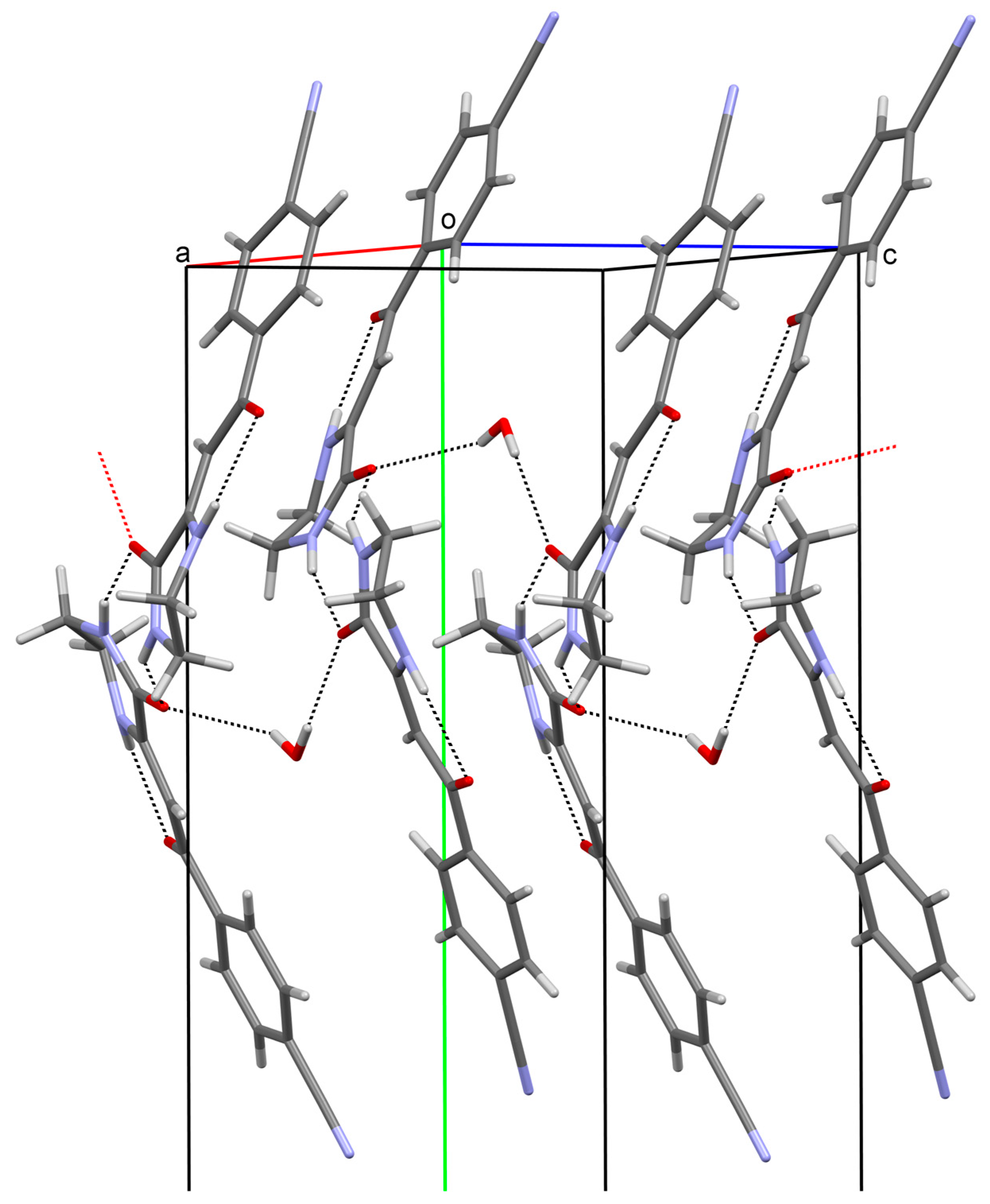
| Compound | D–H···A | D–H, Å | H···A, Å | D···A, Å | Angle D–H···A, ° |
|---|---|---|---|---|---|
| Piron | N1—H1···O1 * | 0.877(19) | 1.99(2) | 2.8649(16) | 173.0(16) |
| Piron | N2—H2···O2 | 0.90(2) | 1.96(2) | 2.6529(16) | 132.8(17) |
| Compound 3 | N1A—H1A···O1 ** | 0.91(2) | 2.03(2) | 2.915(2) | 163(2) |
| Compound 3 | N2A—H2A···O2A | 0.92(3) | 2.02(2) | 2.697(2) | 129(2) |
| Compound 3 | O1W—H1WA···O1 | 0.85 | 2.09 | 2.866(3) | 152 |
| Compound 3 | O1W—H1WB···O1A | 0.85 | 2.25 | 2.873(3) | 131 |
| Compound 3 | N1—H1···O1A *** | 0.89(3) | 2.08(3) | 2.884(2) | 150(2) |
| Compound 3 | N2—H2···O2 | 0.88(2) | 2.05(2) | 2.707(2) | 130(2) |
| Group | Edema Inhibition, % | ||
|---|---|---|---|
| 1 h | 3 h | 5 h | |
| Compound 1 5 mg/kg | 19.5 | 14.2 | −61.6 |
| Compound 1 25 mg/kg | 63.1 1 | 61.5 1 | 16.2 |
| Compound 1 125 mg/kg | 48.9 2 | 58.3 1 | 20.0 |
| Diclofenac 10 mg/kg | 77.2 1 | 73.1 2 | 59.2 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitriev, M.V.; Khramtsova, E.E.; Apuskin, D.Y.; Andreev, A.I.; Kovalenko, I.I.; Mashevskaya, I.V.; Maslivets, A.N. Synthesis and Anti-Inflammatory Activity of (Z)-4-(2-(3-Oxopiperazin-2-ylidene)acetyl)benzoic Acid. Molbank 2024, 2024, M1772. https://doi.org/10.3390/M1772
Dmitriev MV, Khramtsova EE, Apuskin DY, Andreev AI, Kovalenko II, Mashevskaya IV, Maslivets AN. Synthesis and Anti-Inflammatory Activity of (Z)-4-(2-(3-Oxopiperazin-2-ylidene)acetyl)benzoic Acid. Molbank. 2024; 2024(1):M1772. https://doi.org/10.3390/M1772
Chicago/Turabian StyleDmitriev, Maksim V., Ekaterina E. Khramtsova, Danila Y. Apuskin, Alexander I. Andreev, Ilya I. Kovalenko, Irina V. Mashevskaya, and Andrey N. Maslivets. 2024. "Synthesis and Anti-Inflammatory Activity of (Z)-4-(2-(3-Oxopiperazin-2-ylidene)acetyl)benzoic Acid" Molbank 2024, no. 1: M1772. https://doi.org/10.3390/M1772
APA StyleDmitriev, M. V., Khramtsova, E. E., Apuskin, D. Y., Andreev, A. I., Kovalenko, I. I., Mashevskaya, I. V., & Maslivets, A. N. (2024). Synthesis and Anti-Inflammatory Activity of (Z)-4-(2-(3-Oxopiperazin-2-ylidene)acetyl)benzoic Acid. Molbank, 2024(1), M1772. https://doi.org/10.3390/M1772








