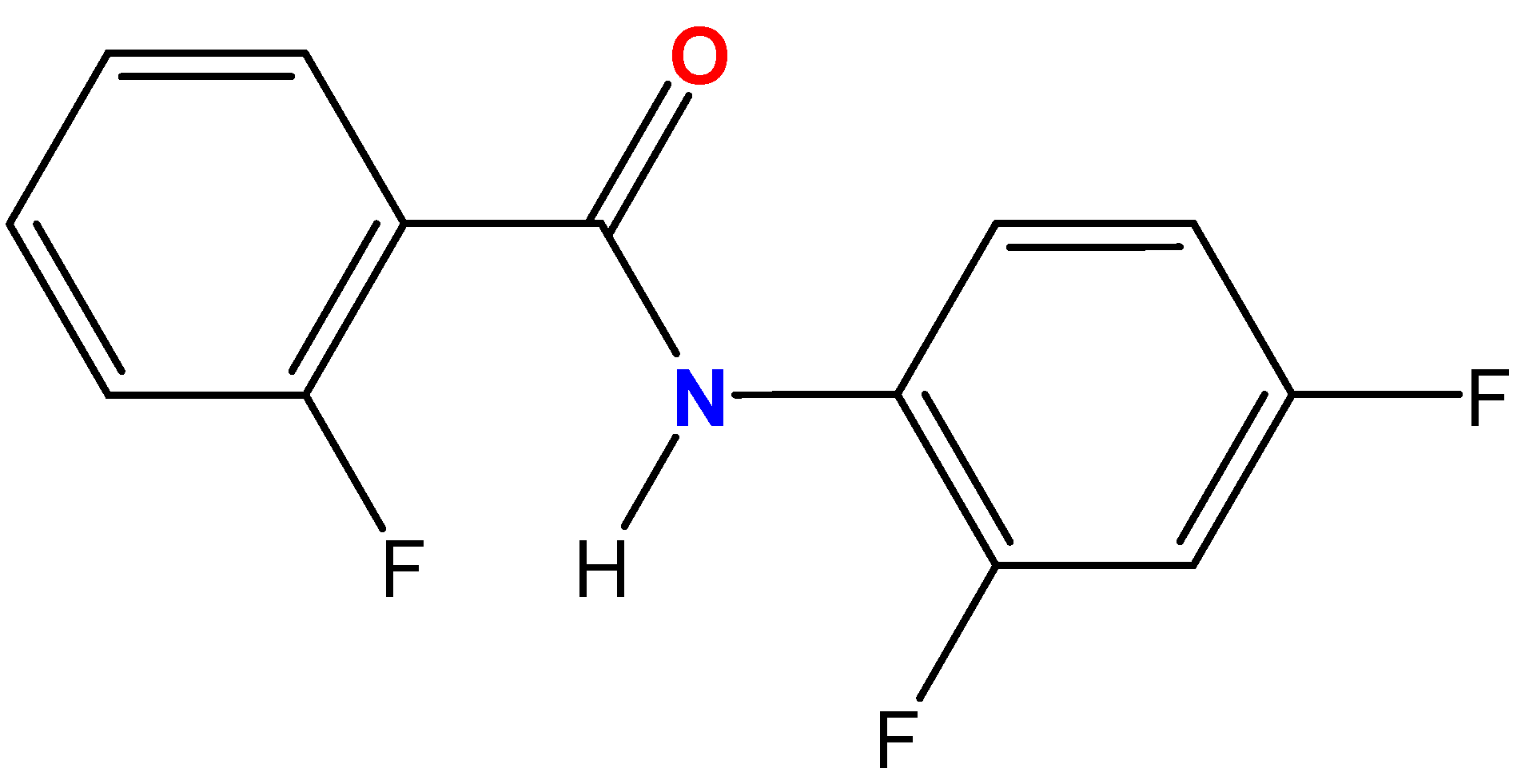
N-(2,4-Difluorophenyl)-2-fluorobenzamide
Abstract
1. Introduction
2. Experimental
2.1. Materials and Characterisation
2.2. Reaction Procedure and Characterisation: Experimental and Spectroscopic Data
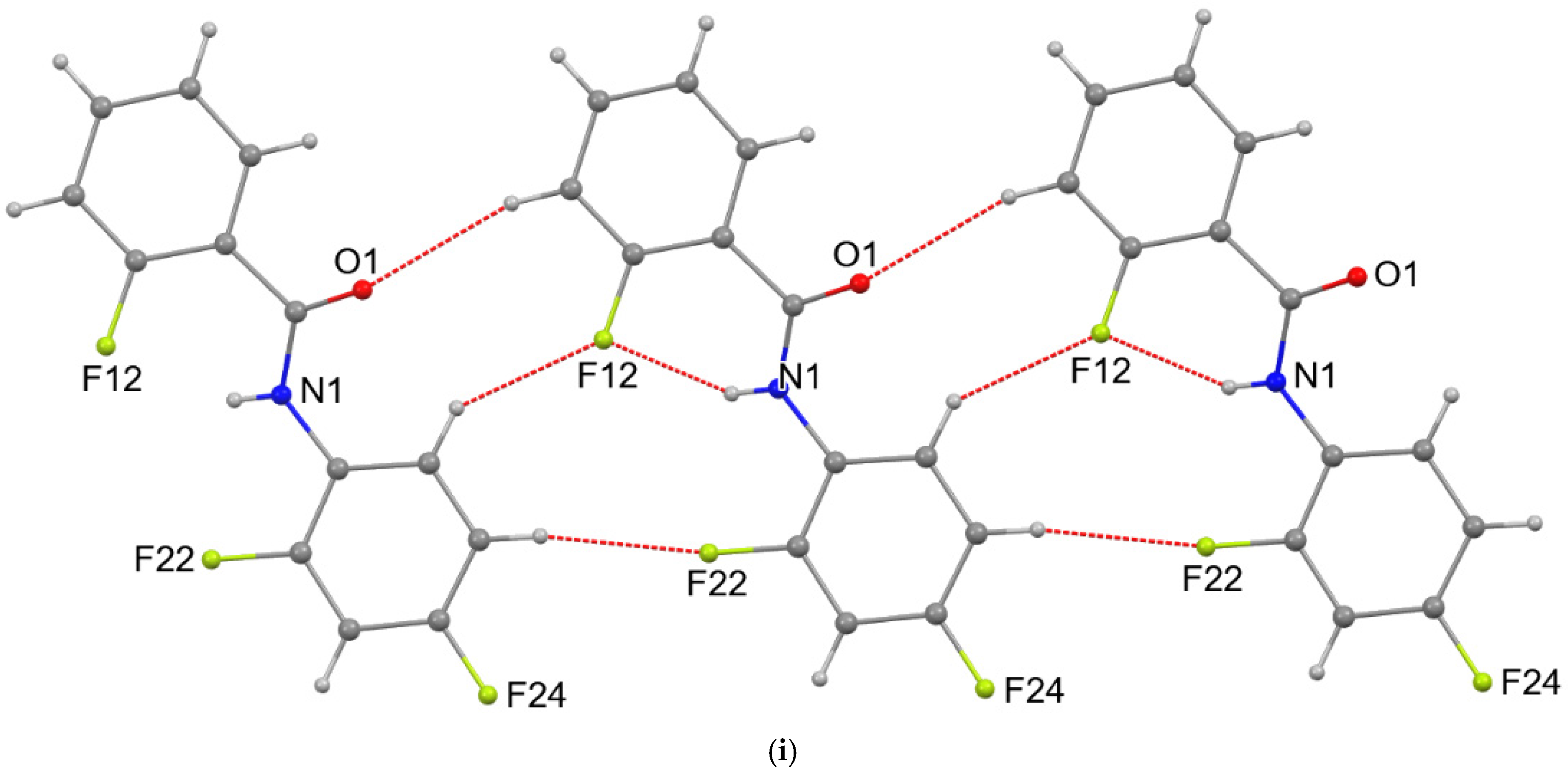
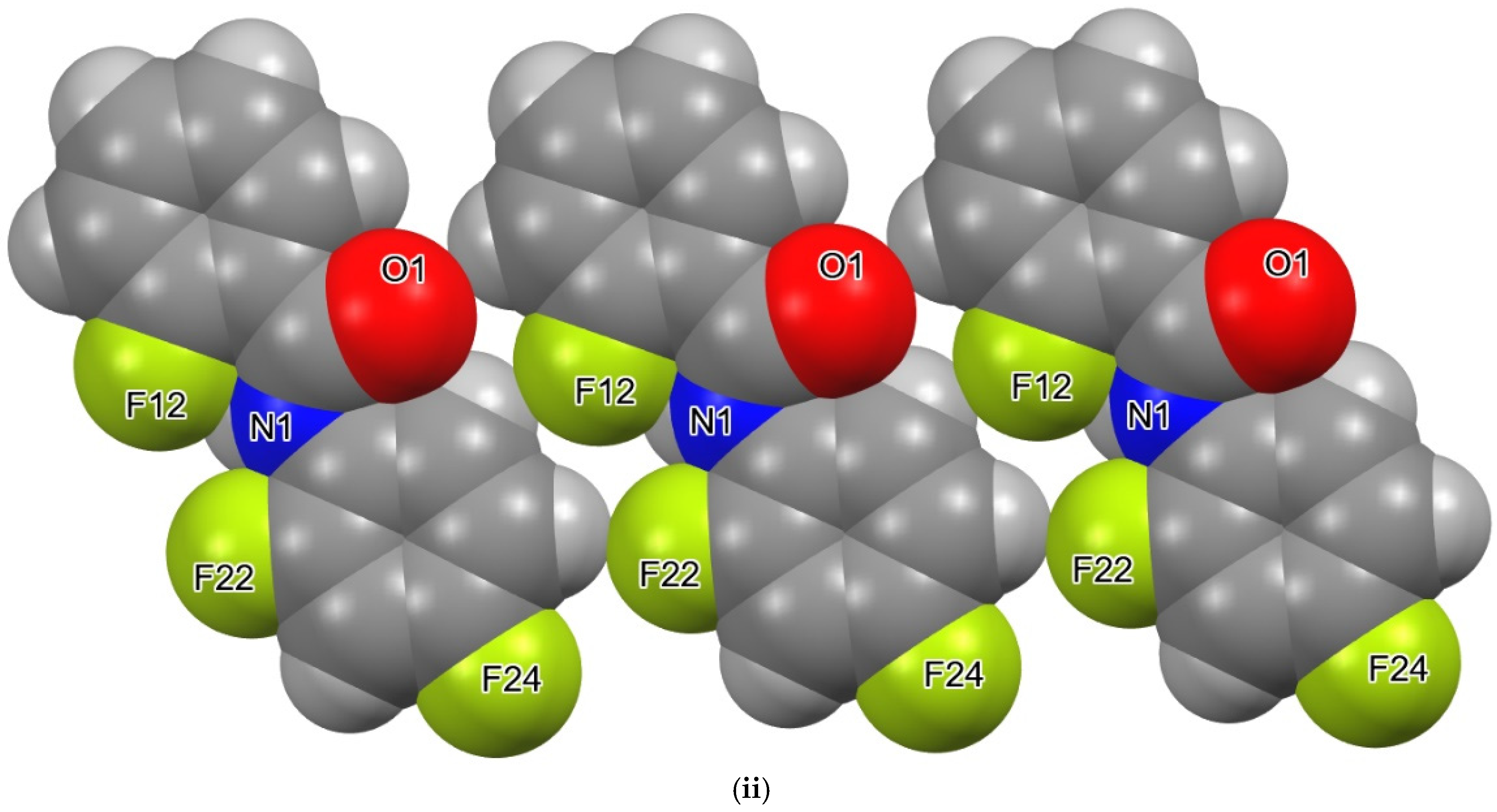
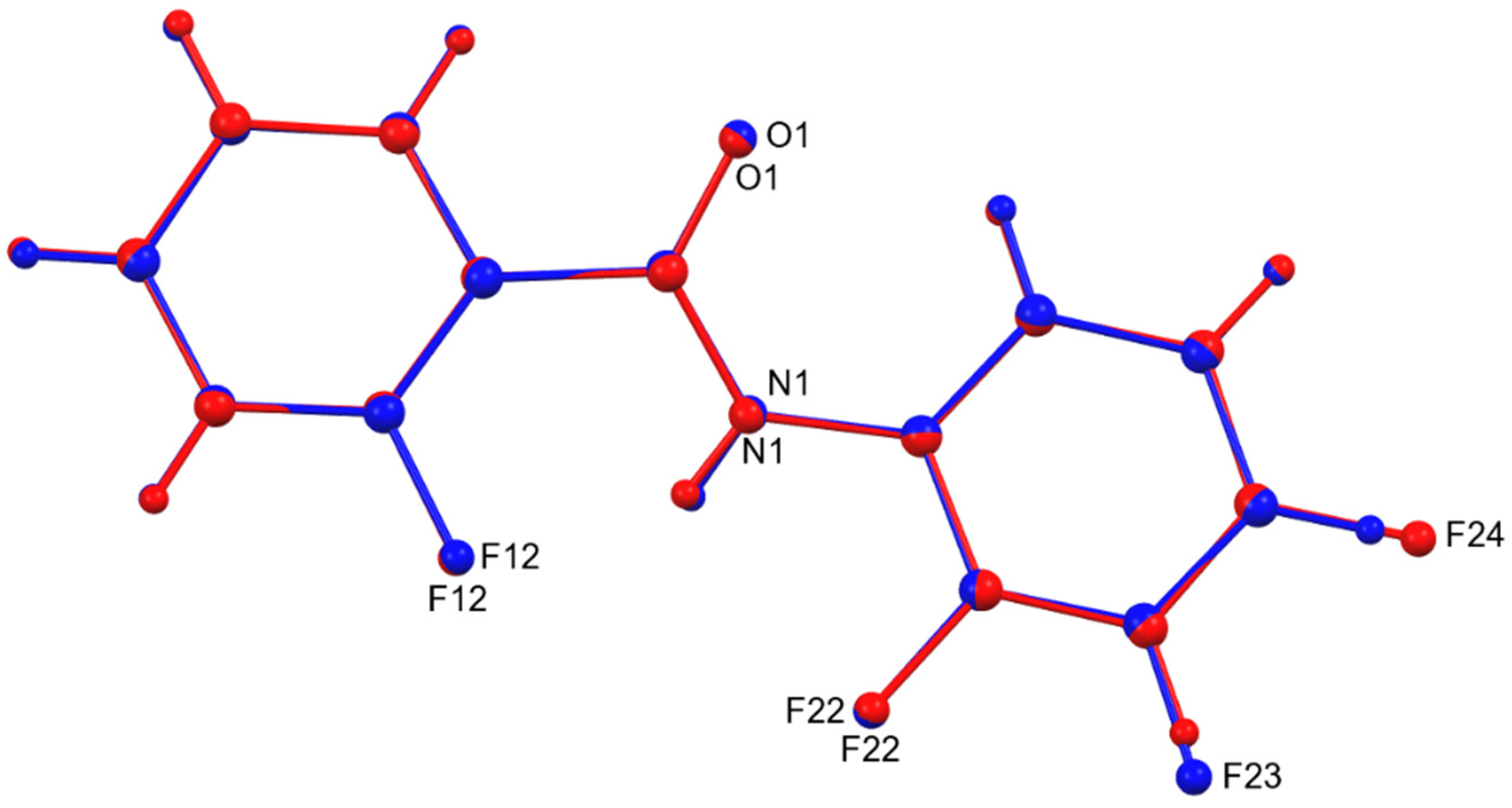
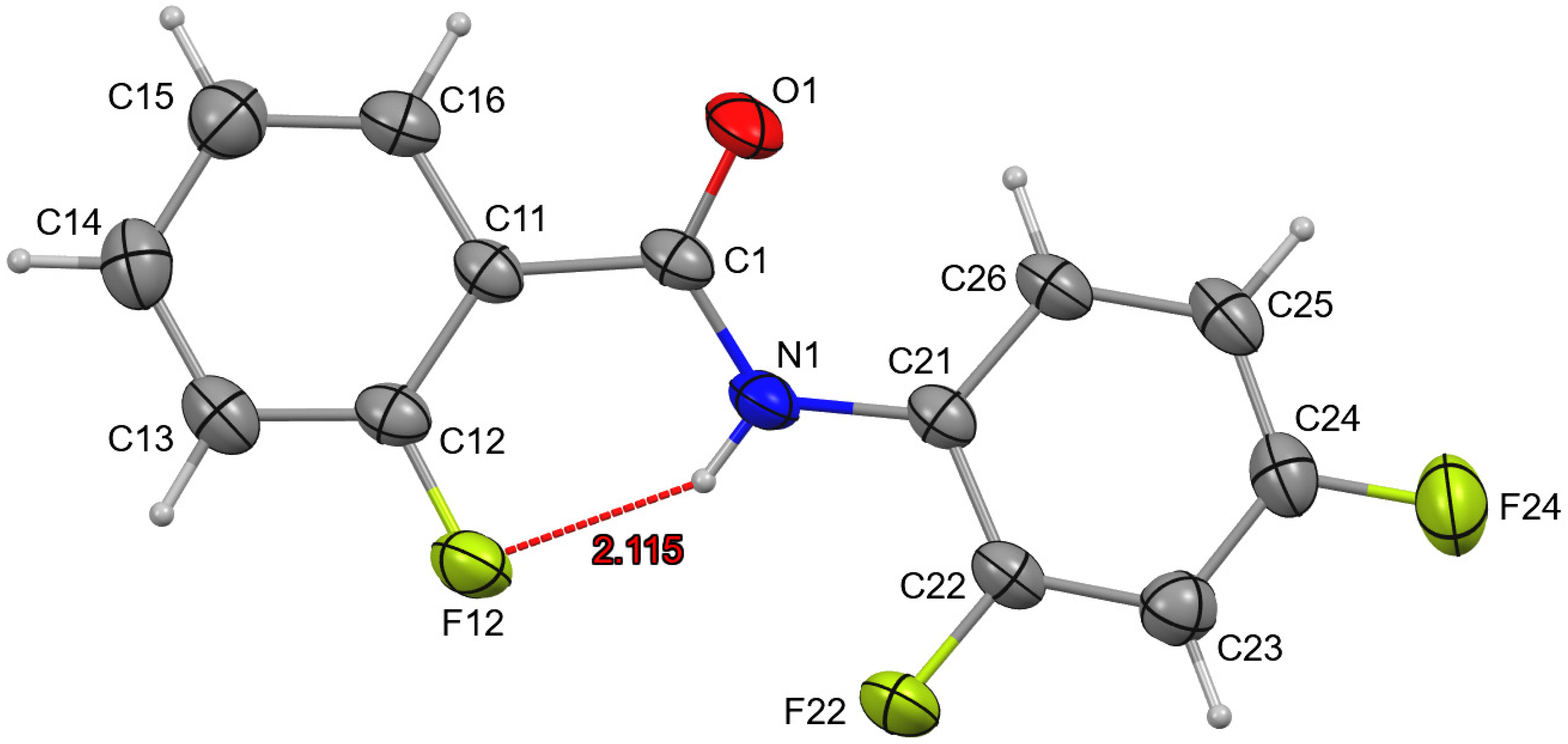
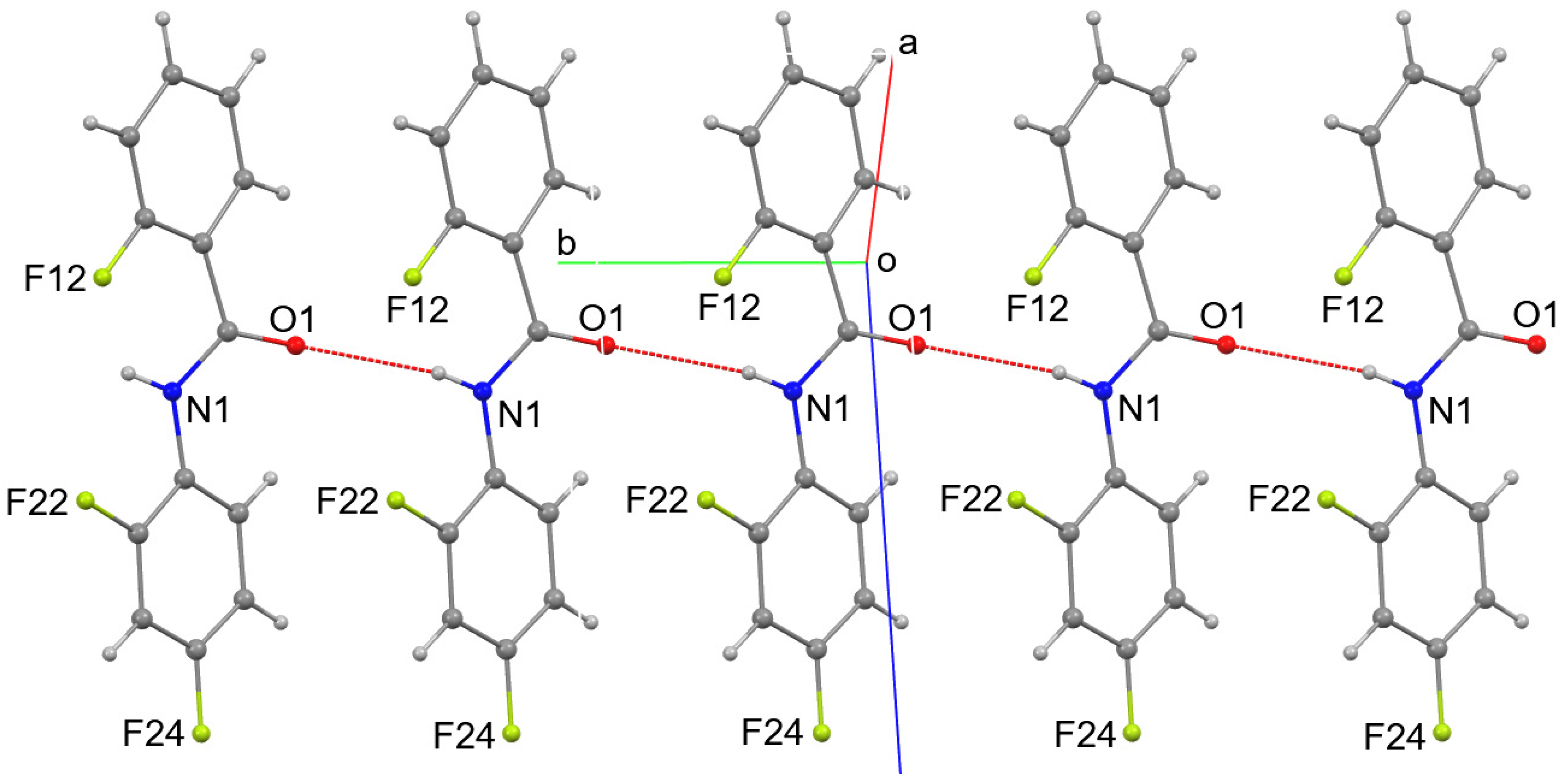
3. Results and Discussion
4. Overall Structural Results and Related Literature
5. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hagmann, W.K. The Many Roles of Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in Pharmaceuticals: Looking beyond Intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Chopra, D.; Row, T.N. Guru Row. Evaluation of the interchangeability of C–H and C–F groups: Insights from crystal packing in a series of isomeric fluorinated benzanilides. CrystEngComm 2008, 10, 54–67. [Google Scholar] [CrossRef]
- Mondal, P.K.; Chopra, D. Crystal structure landscape of conformationally flexible organo-fluorine compounds. CrystEngComm 2016, 18, 48–53. [Google Scholar] [CrossRef]
- Mondal, P.K.; Yadav, H.R.; Choudhury, A.R.; Chopra, D. Quantitative characterization of new supramolecular synthons involving fluorine atoms in the crystal structures of di- and tetrafluorinated benzamides. Acta Crystallogr. 2017, B73, 805–819. [Google Scholar] [CrossRef]
- Donnelly, K.; Lough, A.J.; Gallagher, J.F. Assembling an isomer grid: The isomorphous 4-, 3- and 2-fluoro-N’-(4-pyridyl)benzamides. Acta Crystallogr. 2008, C64, o335–o340. [Google Scholar] [CrossRef]
- Mocilac, P.; Donnelly, K.; Gallagher, J.F. Structural systematics and conformational analyses of a 3 × 3 isomer grid of fluoro-N-(pyridyl)benzamides: Physicochemical correlations, polymorphism and isomorphous relationships. Acta Crystallogr. 2012, B68, 189–203. [Google Scholar] [CrossRef]
- Osman, I.A.; Mocilac, P.; Gallagher, J.F. Short C–H⋯F interactions involving the 2,5-difluorobenzene group: Understanding the role of fluorine in aggregation and complex C–F/C–H disorder in a 2 × 6 isomer grid. CrystEngComm 2016, 18, 5764–5776. [Google Scholar]
- Hehir, N.; Gallagher, J.F. N-(2,3-difluorophenyl)-2-fluorobenzamide. Molbank 2023, 2023, M1717. [Google Scholar] [CrossRef]
- Agilent. CrysAlis PRO; Agilent Technologies Ltd.: Oxford, UK, 2014. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J Appl Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Howe, P.T.A. Recent developments in the use of fluorine NMR in synthesis and characterization. Prog. Nucl. Magn. Reson. Spectrosc. 2020, 118–119, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dolbier, W.R. Guide to Fluorine NMR for Organic Chemists; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, W.; Zhou, W.Q.; Liu, H.H.; Wei, S.H.; Fan, J.F. A new crystal structure and fluorescence property of N-2-fluorobenzoyl-N’-4-tolylthiourea. J. Mol Struct. 2011, 1004, 74–81. [Google Scholar] [CrossRef]
- Kazim, T.; Siegler, M.A.; Leckta, T. Close Amide NH⋯F Hydrogen Bonding Interactions in 1,8-Disubstituted Naphthalenes. J. Org. Chem. 2020, 85, 6195–6200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Wu, J.; Li, C.; Zhu, J.; Hou, J.L.; Li, C.H.; Jiang, X.K.; Li, Z.T. F⋯H-N and MeO⋯H-N Hydrogen-Bonding in the Solid States of Aromatic Amides and Hydrazides: A Comparison Study. Cryst. Growth Des. 2007, 7, 1490–1496. [Google Scholar] [CrossRef]
- Garia, A.; Kumar, S.; Jain, N. Selectfluor™-Mediated Tandem Fluorination and 1,3-Carbonyl Migration in ortho-Carbonyl Anilines: Charge-Transfer-Enabled ortho-Selectivity. Asian J. Org. Chem. 2022, 11, e202200164. [Google Scholar] [CrossRef]
- Flörke, U.; Saeed, A. 2-bromo-N-(2,4-dichlorophenyl)benzamide. CSD Commun. 2016, CCDC, 961227. [Google Scholar]
- Zhang, J.; Li, Y.; Wang, B.; Jia, A.-Q.; Zhang, Q.-F. Synthesis of Dichlorobenzamide Derivatives: Crystal Structures of 3,5-Dichloro-N-(2-chlorophenyl)benzamide and 3,5-Dichloro-N-(4-chlorophenyl)benzamide J. Chem. Crystallogr. 2021, 51, 108–115. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, C.; Rao, X.P.; Diao, L.-H. Crystal structure of 2-chloro-5-fluoro-N-(4-fluorophenyl)benzamide C13H8ClF2NO. Z. Kristallogr. New Cryst. Struct. 2011, 226, 145–146. [Google Scholar] [CrossRef]
- Gowda, B.T.; Foro, S.; Sowmya, B.P.; Fuess, H. 2-Chloro-N-(2,3-dichlorophenyl)benzamide. Acta Crystallogr. 2008, E64, o1342. [Google Scholar] [CrossRef] [PubMed]
- Gowda, B.T.; Foro, S.; Sowmya, B.P.; Fuess, H. 2-Chloro-N-(3,5-dichlorophenyl)benzamide. Acta Crystallogr. 2008, E64, o1294. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Wei, H.-X.; Wang, J.-Q.; Guo, C. 2,6-Dichloro-N-(4-chlorophenyl)benzamide. Acta Crystallogr. 2012, E68, o843. [Google Scholar] [CrossRef]
- Gowda, B.T.; Tokarcik, M.; Kozisek, J.; Sowmya, B.P.; Fuess, H. 2-Chloro-N-(2,6-dichlorophenyl)benzamide. Acta Crystallogr. 2008, E64, o1493. [Google Scholar] [CrossRef]
- Tokarcik, M.; Gowda, B.T.; Kozisek, J.; Sowmya, B.P.; Fuess, H. 4-Chloro-N-(2,6-dichlorophenyl)benzamide. Acta Crystallogr. 2009, E65, o1637. [Google Scholar]
- Price, S. Predicting crystal structures of organic compounds. Chem. Soc. Rev. 2014, 43, 2098–2111. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hehir, N.; Gallagher, J.F. N-(2,4-Difluorophenyl)-2-fluorobenzamide. Molbank 2024, 2024, M1771. https://doi.org/10.3390/M1771
Hehir N, Gallagher JF. N-(2,4-Difluorophenyl)-2-fluorobenzamide. Molbank. 2024; 2024(1):M1771. https://doi.org/10.3390/M1771
Chicago/Turabian StyleHehir, Niall, and John F. Gallagher. 2024. "N-(2,4-Difluorophenyl)-2-fluorobenzamide" Molbank 2024, no. 1: M1771. https://doi.org/10.3390/M1771
APA StyleHehir, N., & Gallagher, J. F. (2024). N-(2,4-Difluorophenyl)-2-fluorobenzamide. Molbank, 2024(1), M1771. https://doi.org/10.3390/M1771





