Abstract
Reaction of diphenylmethanol (4) with n-butyllithium and subsequent treatment with selenium resulted in 12H-dibenzo[d,g][1,2,3]triselenocin-12-ol (5) comprising a novel heterocyclic ring system. The title compound 5 was analyzed by 1H-NMR, 13C-NMR and HPLC. Additionally, the structure of 5 was confirmed by single crystal X-ray diffraction.
1. Introduction
Examples of cyclic di- and triselenides are underrepresented in the literature. E.g., dibenzotriselenonine 1 has been synthesized by reaction of selenium with (Z)-2,3-bis(2-lithiophenyl)-2-butene (Figure 1) [1]. Dibenzodiselenocines 2 and 3 have been investigated as chiral selenium-π-acid catalysts for the asymmetric, oxidative functionalization of alkenes [2]. During our studies to synthesize novel selenium-containing heterocycles, we aimed to insert selenium into ortho-lithiated diphenylmethanol by an oxidative ring closing reaction.
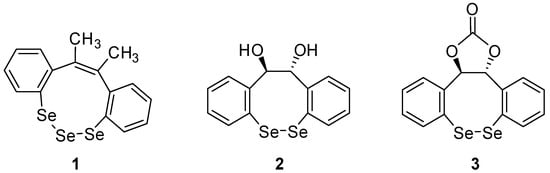
Figure 1.
Examples of cyclic di- and triselenides [1,2].
2. Results and Discussion
2.1. Chemistry
The title compound 12H-dibenzo[d,g][1,2,3]triselenocin-12-ol (5) was synthesized by n-butyllithium-promoted directed ortho-lithiation of diphenylmethanol (4) under argon atmosphere and treatment with elemental selenium according to a modified literature protocol (Scheme 1) [2]. Compound 5 was obtained in low yield (18%) and its chemical structure was determined by 1H-nuclear magnetic resonance spectroscopy (1H-NMR) (Figure S1, Supplementary Materials), 13C-NMR (Figure S2, Supplementary Materials) and single crystal X-ray diffraction (Figure 2). The purity of compound 5 was analyzed by high performance liquid chromatography (HPLC) (Figure S3, Supplementary Materials).
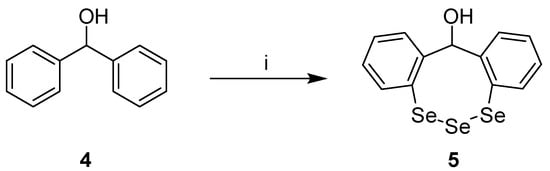
Scheme 1.
Synthesis of 12H-dibenzo[d,g][1,2,3]triselenocin-12-ol (5). (i) (a) n-Butyllithium, diethyl ether, n-hexane, 16 h, 50 °C; (b) selenium, THF, 1.5 h, 50 °C; (c) ice water, 1 h, room temperature.
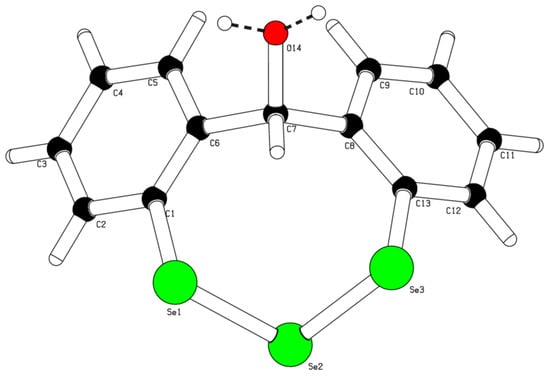
Figure 2.
Crystal structure of the title compound 5. Hydrogen atoms at O14 are half occupied.
2.2. X-ray Structure
The crystal packing of 5 features numerous hydrogen bonds, generating a three-dimensional network (Figure 3). The hydrogen atoms on the hydroxyl groups are 50% disordered, thus intermolecular hydrogen bonds are observed both in between hydroxyl groups and between hydroxyl groups and selenium atoms. The two aromatic rings form a dihedral angle of 65.3(2)°.
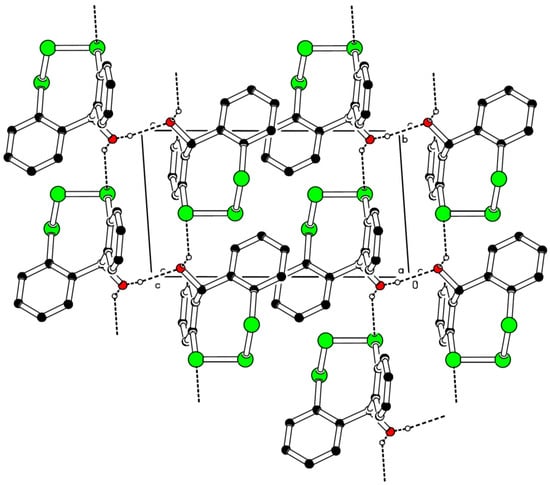
Figure 3.
Partial packing diagram of the title compound 5. Intermolecular hydrogen bonds are indicated as dashed lines.
3. Materials and Methods
3.1. General
All reagents and solvents were of commercial quality and utilized without further purification. The purity of the title compound 5 was determined by reverse phase HPLC. An Agilent 1100 Series HPLC system (Agilent Technologies, Santa Clara, CA, USA) was used, equipped with an ultraviolet diode array detector (detection at 254 nm). The chromatographic separation was performed on an XBridgeTM C18 column (150 × 4.6 mm, 5 µm) from Waters (Milford, MA, USA). The injection volume was 5 μL and the flow 1.5 mL/min using the following gradient: 0.01 M KH2PO4, pH 2.3 (mobile phase A), MeOH (mobile phase B), 40% B to 85% B in 8 min; 85% B for 5 min; 85% B to 40% B in 2 min; stop time 16 min. Column chromatography was performed on Geduran Si60 40–63 µm silica from Merck (Darmstadt, Germany) or commercial 50 µm silica columns from Interchim (Montluçon, France) using an Interchim PuriFlash XS520Plus automated flash chromatography system. NMR spectra were measured on an Avance 300 MHz NMR spectrometer from Bruker (Billerica, MA, USA). Chemical shifts are reported in parts per million (ppm) relative to tetramethylsilane. All spectra were calibrated against the (residual proton) peak of the deuterated solvent used. X-ray diffraction data were collected on a STOE IPDS 2T diffractometer (STOE & Cie, Darmstadt, Germany) using monochromated Mo Kα radiation (0.71073 Å).
3.2. Chemistry
Synthesis of 12H-Dibenzo[d,g][1,2,3]triselenocin-12-ol (5)
In a 100 mL three-neck round bottom flask diphenylmethanol (4) (0.5 g, 2.71 mmol, 1 eq.) was dissolved in dry n-hexane (12.5 mL) and dry diethyl ether (9 mL) under an argon atmosphere. n-Butyllithium (8.5 mL of a 1.6 M solution in n-hexane, 13.6 mmol, 5 eq.) was added dropwise and the mixture was stirred for 16 h at 50 °C. During the reaction, a white solid precipitated and the color of the suspension changed to purple. After cooling to room temperature, selenium (1.0 g, 13.6 mmol, 5 eq.) and dry THF (9 mL) were added, and the suspension was stirred for 1.5 h under an argon atmosphere at 50 °C. The color changed from purple to yellow. After cooling to room temperature, the mixture was poured into ice water and stirred for 1 h under aerobic conditions. The mixture was extracted with dichloromethane (DCM) (three times) and the combined organic phases were washed with water (three times), dried over MgSO4 and evaporated. The residue was purified twice by flash column chromatography (SiO2, petroleum ether/ethyl acetate gradient elution from 0% to 25%, and petroleum ether/DCM gradient elution from 50% to 90%) to yield 210 mg (18%) of the title compound as a yellowish solid. 1H-NMR (300 MHz, DMSO-d6) δ 8.24–8.13 (dd, J = 8.0 Hz, 1.3 Hz, 2H), 7.70–7.62 (dd, J = 7.7 Hz, 1.3 Hz, 2H), 7.55–7.45 (td, J = 7.9 Hz, 1.3 Hz, 2H), 7.24–7.15 (td, J = 7.5 Hz, 1.5 Hz, 2H), 6.55 (s, 1H), 6.35 (s, 1H); 13C-NMR (75 MHz, DMSO-d6) δ 152.0, 135.2, 130.2, 129.7, 129.4, 127.4, 72.3; HPLC: tR = 9.27 min (purity 95.7%).
Crystal data for C13H10OSe3 (M = 419.09 g·mol−1): triclinic space group P-1(2), a = 7.4876(6) Å, b = 7.4939(5) Å, c = 12.4930(9) Å, V = 623.66(9) Å3, Z = 2, T = 120(2) K, µ(MoKα) = 8.825 mm−1, Dcalc = 2.232 Mg m−3, 12,850 reflections measured (2.96° ≤ Θ ≤ 28.40°), 2960 unique (Rint = 0.0206, R1 = 0.0360) which were used in all calculations. CCDC 2184462 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 15 July 2022).
4. Conclusions
To the best of our knowledge, we report for the first time an example of a compound having a heterocyclic triselenocine core. The synthesis of 12H-dibenzo[d,g][1,2,3]triselenocin-12-ol (5) was performed by reaction of diphenylmethanol (4) with n-butyllithium and subsequent treatment with elemental selenium. The analytical characterization of the novel compound 5 comprised 1H-NMR, 13C-NMR, HPLC, and single crystal X-ray diffraction.
Supplementary Materials
The following supporting information are available online. Figure S1: 1H-NMR of 5; Figure S2: 13C-NMR of 5; Figure S3: HPLC chromatogram of 5.
Author Contributions
M.B., S.A., D.S. and P.K. conceived and designed the experiments; M.B. performed synthesis; M.B., S.A., D.S. and P.K. analyzed the data; M.B., S.A. and P.K. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yamazaki, S.; Yoshimura, T.; Yamabe, S.; Arai, T.; Tamura, H. Synthesis and unusual selenium extrusion reaction of a cyclic triselenide. J. Org. Chem. 1990, 55, 263–269. [Google Scholar] [CrossRef]
- Krätzschmar, F.; Ortgies, S.; Willing, R.Y.N.; Breder, A. Rational design of chiral selenium-π-acid catalysts. Catalysts 2019, 9, 153. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).