An Efficient Synthesis of Novel 4-Aryl-2-thioxo-3,4-dihydro-1H-pyrimido[1,2-a][1,3,5]triazin-6(2H)-ones and Their Antibacterial Activity
Abstract
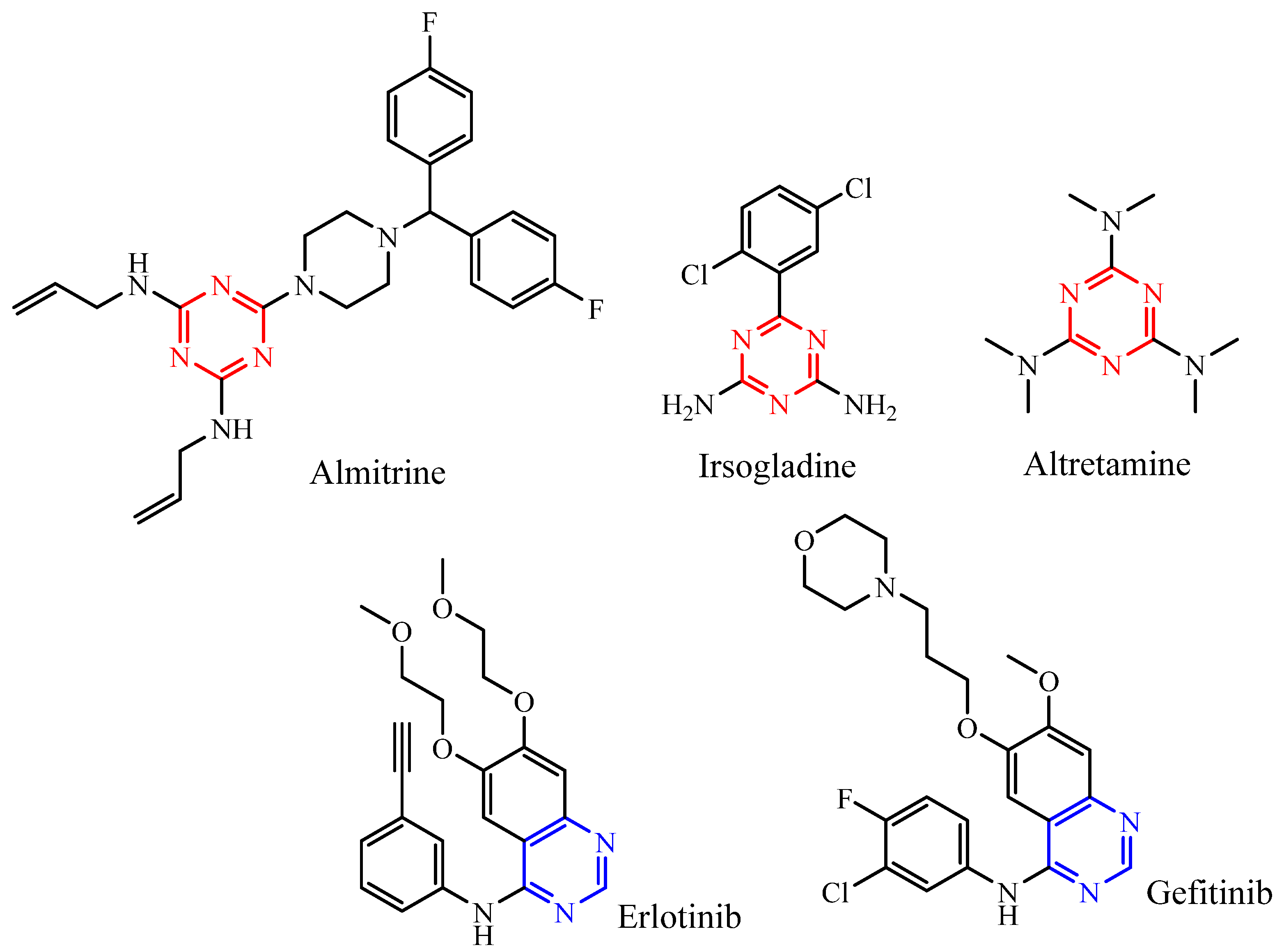
:1. Introduction
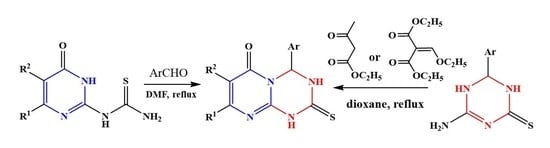
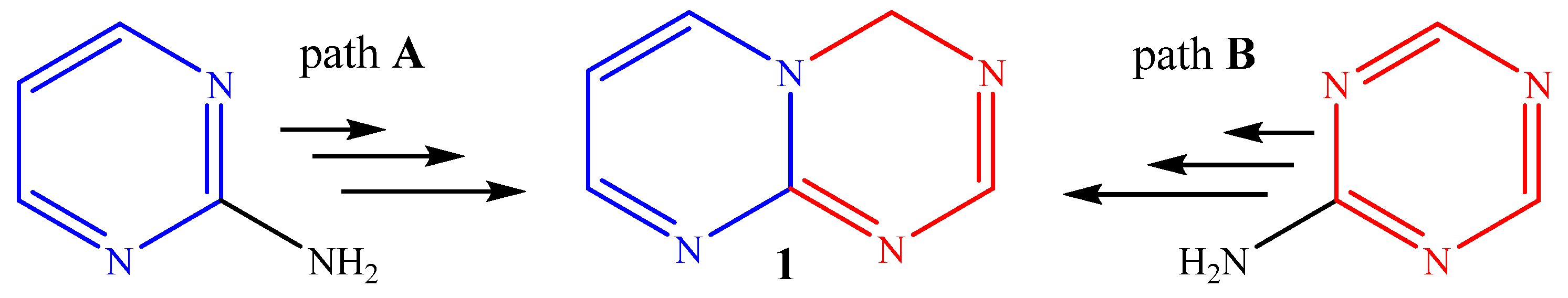
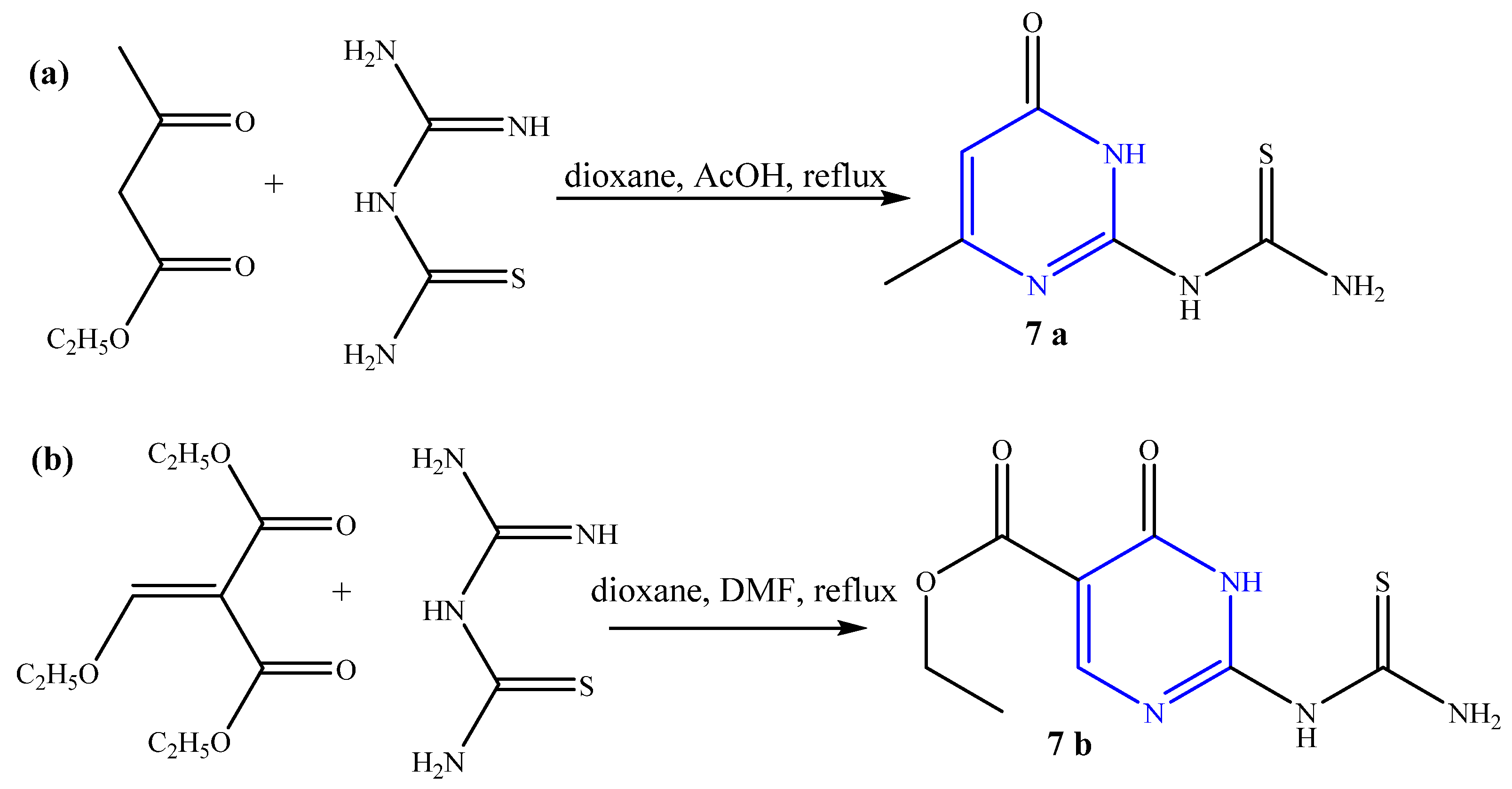
2. Results and Discussion
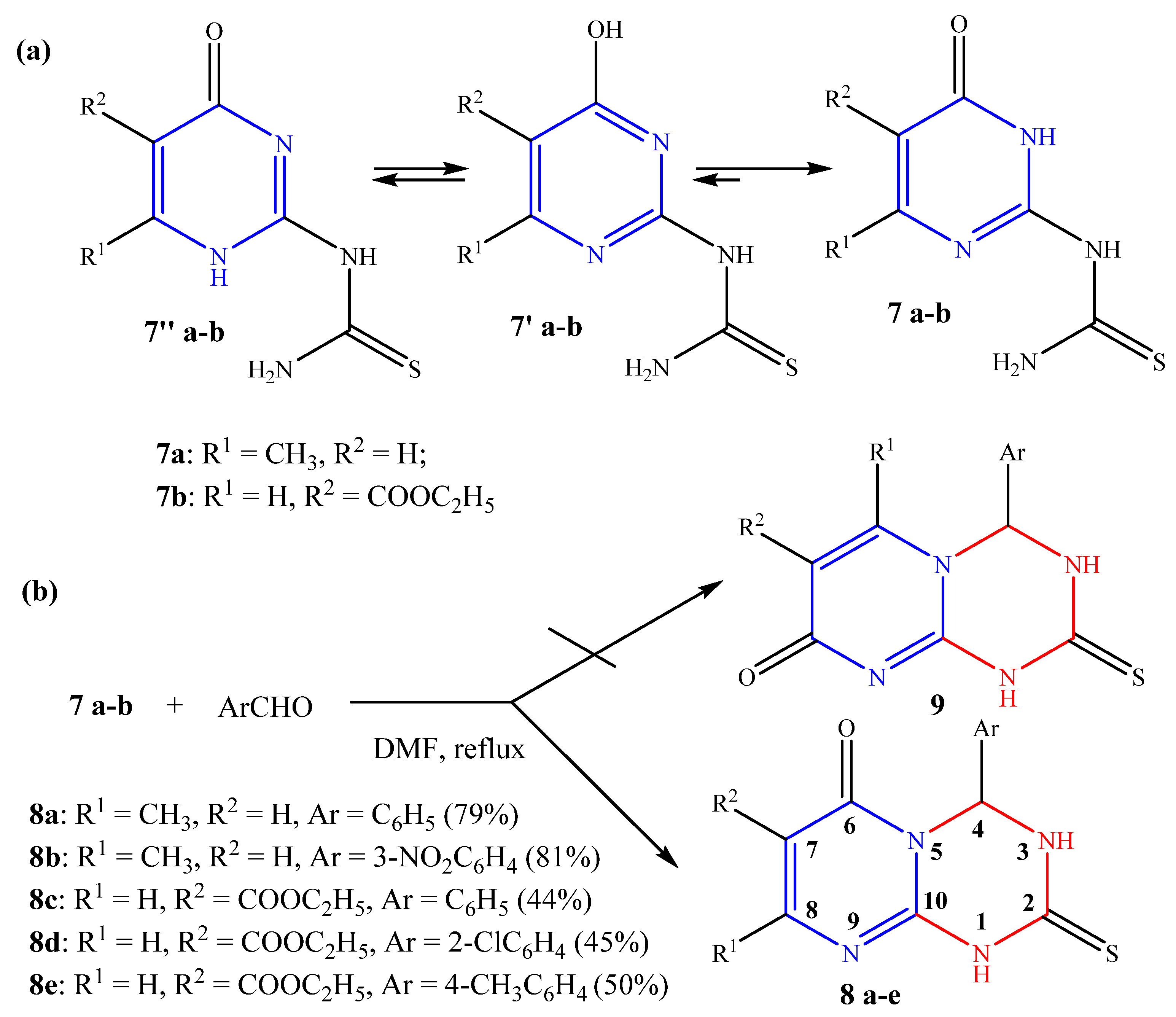
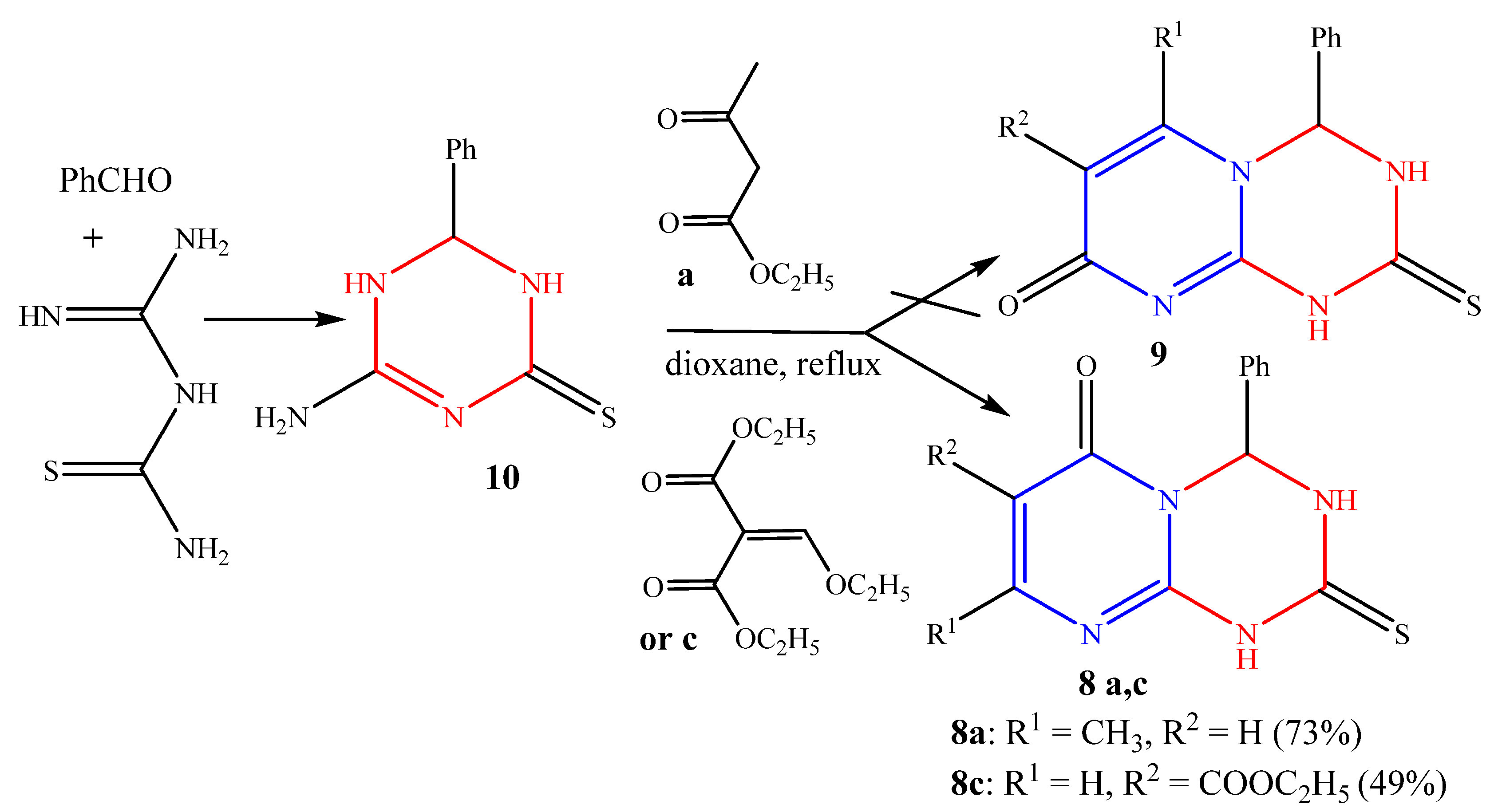
2.1. Chemistry
2.2. Antibacterial Activity of the 2-Thioxo-1,2,3,4-tetrahydro-6H-pyrimido[1,2-a][1,3,5]triazin-6-one 8a–e
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, G.; Chen, Z.; Tang, X.; Ma, J.-A. Triazines: Syntheses and Inverse Electron-demand Diels−Alder Reactions. Chem. Rev. 2021, 121, 14555–14593. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mandal, M.K.; Masih, A.; Saha, A.; Ghosh, S.K.; Bhat, H.R.; Singh, U.P. 1,3,5-Triazine: A versatile pharmacophore with diverse biological activities. Arch. Pharm. 2021, 354, 2000363. [Google Scholar] [CrossRef]
- Abbas, N.; Matada, G.S.P.; Dhiwar, P.S.; Patel, S.; Devasahayam, G. Fused and Substituted Pyrimidine Derivatives as Profound Anti-Cancer Agents. Anticancer Agents Med. Chem. 2021, 21, 861–893. [Google Scholar] [CrossRef] [PubMed]
- Stolpovskaya, N.V.; Kruzhilin, A.A.; Zorina, A.V.; Shikhaliev, K.S.; Ledeneva, I.V.; Kosheleva, E.A.; Vandyshev, D.Y. Synthesis of Substituted Aminopyrimidines as Novel Promising Tyrosine Kinase Inhibitors. Russ. J. Org. Chem. 2019, 55, 1322–1328. [Google Scholar] [CrossRef]
- Desai, N.; Trivedi, A.; Pandit, U.; Dodiya, A.; Kameswara Rao, V.; Desai, P. Hybrid bioactive heterocycles as potential antimicrobial agents: A review. Mini Rev. Med. Chem. 2016, 16, 1500–1526. [Google Scholar] [CrossRef] [PubMed]
- Diab, H.M.; Salem, M.E.; Abdelhamid, I.A.; Elwahy, A.H. Synthesis of novel star-shaped molecules based on a 1,3,5-triazine core linked to different heterocyclic systems as novel hybrid molecules. RSC Adv. 2020, 10, 44066–44078. [Google Scholar] [CrossRef]
- Al-Issa, S.A. Synthesis and anticancer activity of some fused pyrimidines and related heterocycles. Saudi Pharm. J. 2013, 21, 305–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemdan, M.M.; Abd El-Mawgoude, H.K. Synthesis and Antimicrobial Evaluation of Thieno[2,3-d]pyrimidine, Thieno[2′,3′:4,5]pyrimido[1,2-a][1,3,5]triazine, Thieno[2,3-d]-1,3-thiazine and 1,2,4-Triazole Systems. Chem. Pharm. Bull. 2015, 63, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Waly, M.A.; Abou Dobara, M.I. Syntheses and characterization of the pyrimido[1,2-][1,3,5]triazinthione as a new ring system and antibacterial agent. ChemInform. 2010, 41, 1601–1607. [Google Scholar] [CrossRef]
- Toyama, M.; Sakakibara, N.; Takeda, M.; Okamoto, M.; Watashi, K.; Wakita, T.; Baba, M. Pyrimidotriazine derivatives as selective inhibitors of HBV capsid assembly. Virus Res. 2019, 271, 197677. [Google Scholar] [CrossRef]
- Dolzhenko, A. Synthetic routes towards pyrimido[1,2-a][1,3,5]triazines (Review). Heterocycles 2011, 83, 1489–1525. [Google Scholar] [CrossRef] [Green Version]
- Lucry, L.; Enoma, F.; Estour, F.; Ménager, S.; Lafont, O.; Oulyadi, H. Synthesis and biological testing of 3-phenyloctahydro-pyrimido[1,2-a]-s-triazine derivatives. J. Heterocycl. Chem. 2002, 39, 663–670. [Google Scholar] [CrossRef]
- Takahashi, H.; Hashimoto, Y. Formaldehyde-mediated modification of natural deoxyguanosine with amines: One-pot cyclization as a molecular model for genotoxicity. Bioorg. Med. Chem. Lett. 2001, 11, 729–731. [Google Scholar] [CrossRef]
- Kumar, S.; Oakes, F.T.; Wilson, S.R.; Leonard, N.J. Synthesis and structure of a fluorescent, tricyclic analogue of 2’-deoxy-adenosine and of a prodrug by N-annelation of 2′-deoxyguanosine and 9-[(2-hydroxyethoxy) methyl] guanine (acyclovir), respectively. Heterocycles 1988, 27, 2891–2901. [Google Scholar] [CrossRef]
- Kamal, A.; Sattur, P.B. A one-pot synthesis of ring-fused 1, 3, 5-triazine-2, 4 (3H)-diones: Reactions with chlorocarbonyl isocyanate. Synthesis 1985, 1985, 892–893. [Google Scholar] [CrossRef]
- Mahmoud, M.R.; Abd-El-Halim, M.S.; Ebrahim, A.E.F.; Radwan, A.M. Fused Heterocyclic Ring Systems from Amino- and Thioxopyrimidine Derivatives. ChemInform. 1996, 27, 915. [Google Scholar] [CrossRef]
- Bossio, R.; Marcaccini, S.; Parrini, V.; Pepino, R. Synthesis of some 2-(heteroarylamino)benzimidazoles and their cyclization with phosgene. J. Heterocycl. Chem. 1985, 22, 1147–1148. [Google Scholar] [CrossRef]
- Dolzhenko, A.V.; Foo, M.C.; Tan, B.J.; Dolzhenko, A.; Chiu, G.N.C.; Chui, W.K. Synthesis and heterocyclizations of 3,4-dihydroquinazolin-2-yl guanidine in the search of new anticancer agents. Heterocycles 2009, 78, 1761–1775. [Google Scholar] [CrossRef]
- Dolzhenko, A.; Foo, M.; Tan, B.; Dolzhenko, A.; Chiu, G.; Chui, W. 4-Amino-2, 8-dimethyl-6H-pyrimido[1,2-a][1,3,5]triazin-6-one. Acta Crystallogr. Sect. E 2010, 66, 2050. [Google Scholar] [CrossRef] [Green Version]
- Sachdeva, N.; Dolzhenko, A.V.; Chui, W.K. Regioselective synthesis of pyrimido[1,2-a][1,3,5]triazin-6-ones via reaction of 1-(6-oxo-1,6-dihydropyrimidin-2-yl)guanidines with triethylorthoacetate: Observation of an unexpected rearrangement. Org. Biomol. Chem. 2012, 10, 4586–4596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolzhenko, A.V.; Sachdeva, N.; Tan, G.K.; Koh, L.L.; Chui, W.K. 2-Amino-4-(4-bromophenyl)-8-trifluoromethyl-3,4-dihydropyrimido[1,2-a][1,3,5]triazin-6(5H)-one. Acta Crystallogr. Sect. E 2009, 65, 684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolzhenko, A.V.; Chui, W.K. Simple synthesis of 2-amino-4-(het) aryl-4, 6-dihydro-1(3)(11)H-[1,3,5]triazino[2,1-b]quinazolin-6-ones. J. Heterocycl. Chem. 2008, 45, 173–176. [Google Scholar] [CrossRef]
- Ziegler, E.; Noelken, E. Synthesen von Heterocyclen, 34. Mitt.: Über kondensierte N-Heterocyclen. Monatsh. Chem. Verw. Tl. 1961, 92, 1184–1190. [Google Scholar] [CrossRef]
- Murty, M.S.R.; Ramalingam, T.; Sattur, P.B. Synthesis of novel 3-carboethoxy-6-methyl-4-oxo-4H-pyrimido[1′,2′:5,6]-[1,3,5]triazino[1,2-a]benzimidazoles. J. Heterocycl. Chem. 1990, 27, 949–950. [Google Scholar] [CrossRef]
- Dolzhenko, A.V.; Chui, W.K. Synthesis of ethyl 6-aryl-4-oxo-4, 6-dihydro-1(12)(13)H-pyrimido-[2′,1′:4,5][1,3,5]triazino[1,2-a]benzimidazole-3-carboxylates. J. Heterocycl. Chem. 2006, 43, 1513–1521. [Google Scholar] [CrossRef]
- Oziminski, W.P.; Wiśniewski, I. Quantum-chemical study on the relative stability of sildenafil tautomers. J. Struct. Chem. 2021, 32, 1733–1743. [Google Scholar] [CrossRef]
- Shinji, S.; Yuuji, K. 4-Amino-1,3-thiazine or Oxazine Derivative. U.S. Patent US2012258961A1, 11 October 2012. [Google Scholar]
- Majouga, A.G.; Beloglazkina, E.K.; Yudin, I.V.; Rakhimov, R.D.; Khlobystov, A.N.; Zyk, N.V. First example of the ring-opening transformation of thiazolidines to iminothiols on gold surface. Mendeleev Commun. 2009, 2, 92–93. [Google Scholar] [CrossRef]
- Prati, F.; De Simone, A.; Armirotti, A.; Summa, M.; Pizzirani, D.; Scarpelli, R.; Bolognesi, M.L. 3,4-Dihydro-1,3,5-triazin-2(1H)-ones as the first dual BACE-1/GSK-3β fragment hits against Alzheimer’s disease. ACS Chem. Neurosci. 2015, 6, 1665–1682. [Google Scholar] [CrossRef]
- Furukawa, M.; Okawara, T.; Noguchi, Y.; Miyazaki, R. Reaction of Biguanides and Related Compounds. XIV. Cyclization of Amidinothioureas with Some Carbonyl Compounds. Chem. Pharm. Bull. 1978, 26, 314–317. [Google Scholar] [CrossRef] [Green Version]
- Liu, D. Rapid biochemical test for measuring chemical toxicity. Bull. Environ. Contam. Toxicol. 1981, 26, 145–149. [Google Scholar] [CrossRef]
| Compound | Minimum Inhibitory Concentration (MIC), µg/mL | |
|---|---|---|
| E. coli | S. aureus | |
| 8a | 256 | 256 |
| 8b | 256 | 256 |
| 8c | 256 | 256 |
| 8d | 256 | 256 |
| 8e | 256 | 256 |
| Chloramphenicol | 128 | 256 |
| Sulfathiazole | 128 | 256 |
| Metronidazole | 128 | 256 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Quy, D.; Van Hung, N.; Stolpovskaya, N.; Kruzhilin, A.; Olshannikova, S.S.; Holyavka, M.; Sulimov, V.; Shikhaliev, K. An Efficient Synthesis of Novel 4-Aryl-2-thioxo-3,4-dihydro-1H-pyrimido[1,2-a][1,3,5]triazin-6(2H)-ones and Their Antibacterial Activity. Molbank 2022, 2022, M1417. https://doi.org/10.3390/M1417
Van Quy D, Van Hung N, Stolpovskaya N, Kruzhilin A, Olshannikova SS, Holyavka M, Sulimov V, Shikhaliev K. An Efficient Synthesis of Novel 4-Aryl-2-thioxo-3,4-dihydro-1H-pyrimido[1,2-a][1,3,5]triazin-6(2H)-ones and Their Antibacterial Activity. Molbank. 2022; 2022(3):M1417. https://doi.org/10.3390/M1417
Chicago/Turabian StyleVan Quy, Do, Nguyen Van Hung, Nadezhda Stolpovskaya, Aleksey Kruzhilin, Svetlana S. Olshannikova, Marina Holyavka, Vladimir Sulimov, and Khidmet Shikhaliev. 2022. "An Efficient Synthesis of Novel 4-Aryl-2-thioxo-3,4-dihydro-1H-pyrimido[1,2-a][1,3,5]triazin-6(2H)-ones and Their Antibacterial Activity" Molbank 2022, no. 3: M1417. https://doi.org/10.3390/M1417
APA StyleVan Quy, D., Van Hung, N., Stolpovskaya, N., Kruzhilin, A., Olshannikova, S. S., Holyavka, M., Sulimov, V., & Shikhaliev, K. (2022). An Efficient Synthesis of Novel 4-Aryl-2-thioxo-3,4-dihydro-1H-pyrimido[1,2-a][1,3,5]triazin-6(2H)-ones and Their Antibacterial Activity. Molbank, 2022(3), M1417. https://doi.org/10.3390/M1417