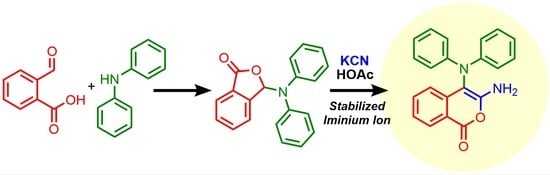
3-Amino-4-(diphenylamino)-1H-2-benzopyran-1-one
Abstract
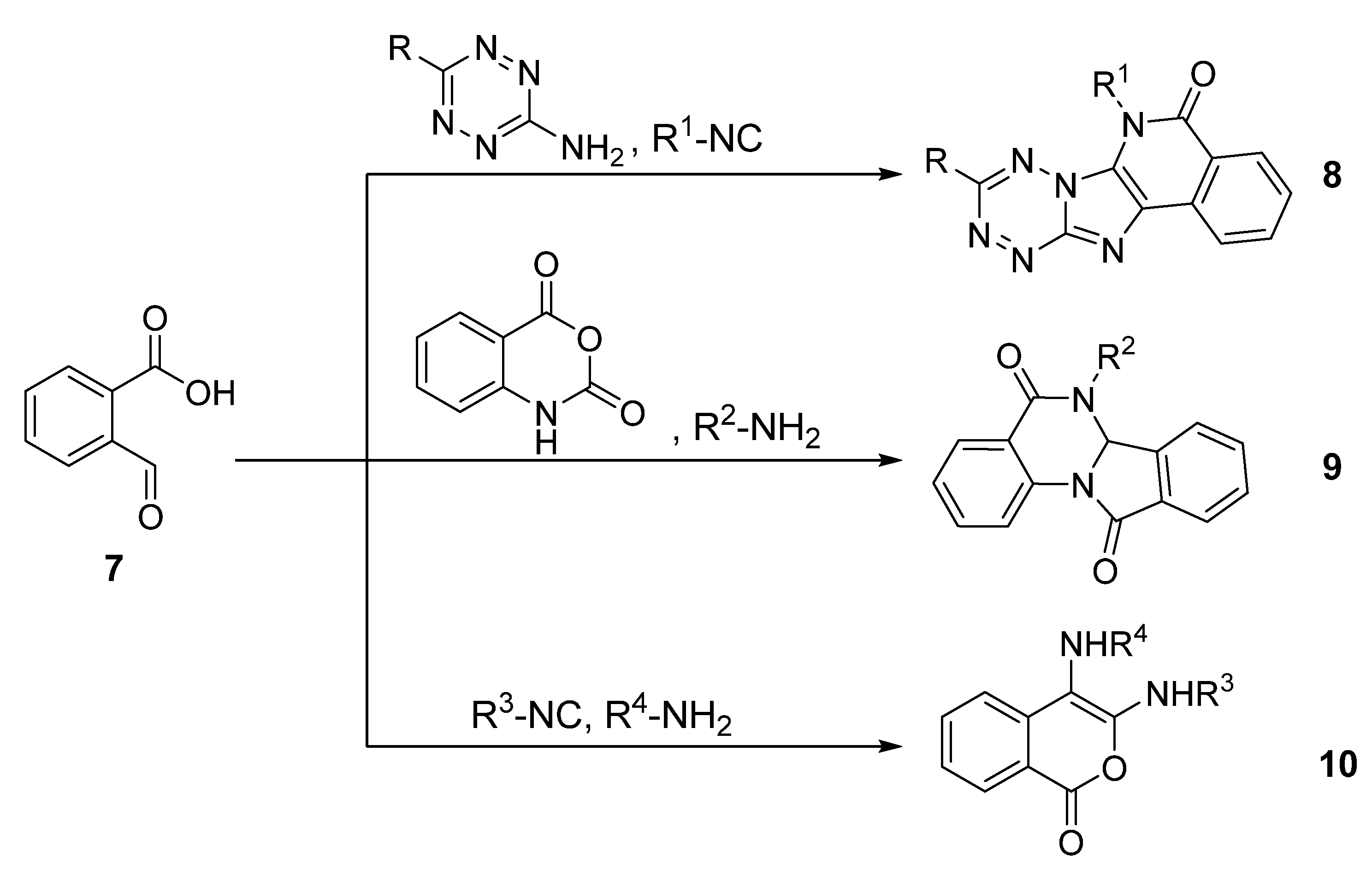
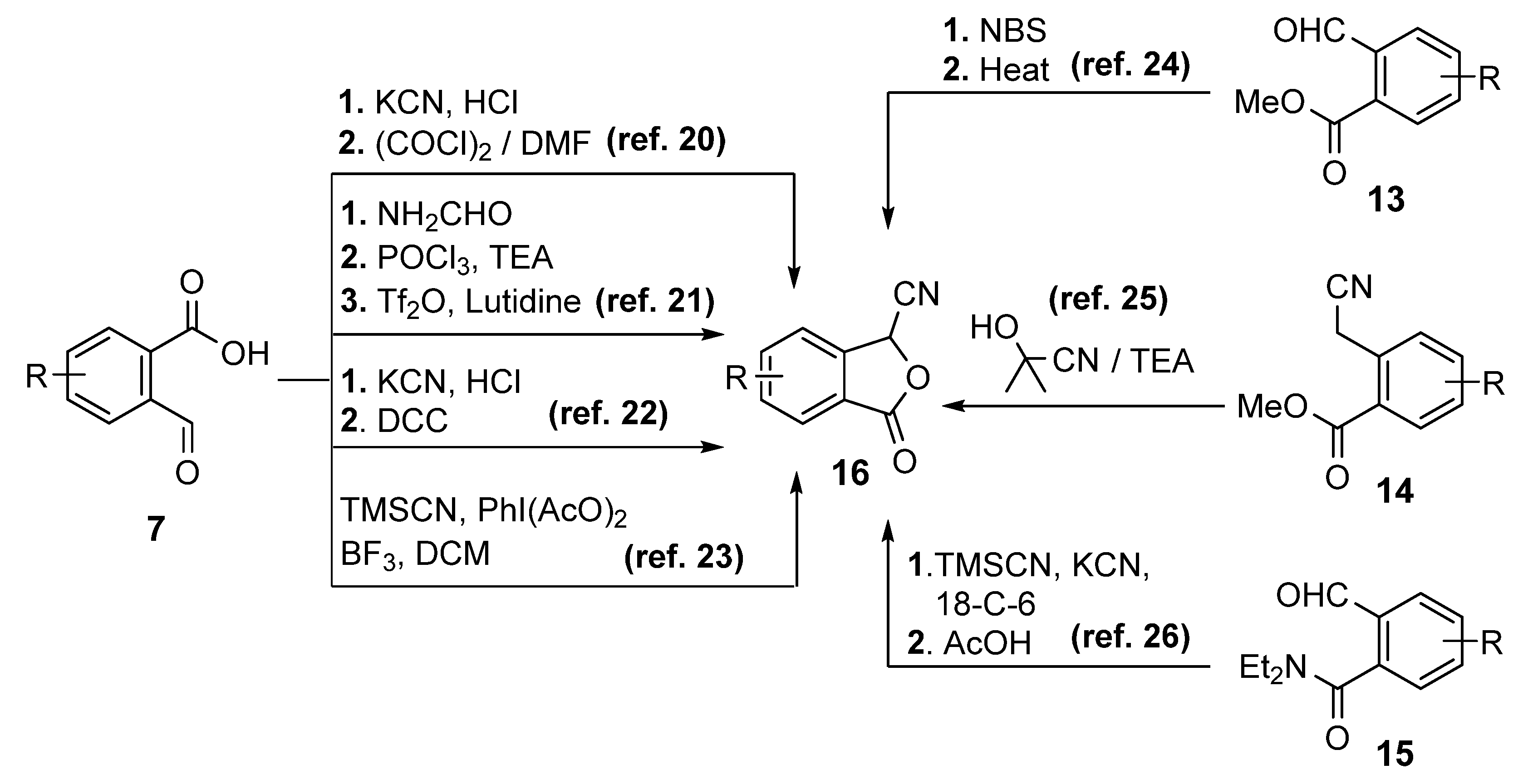
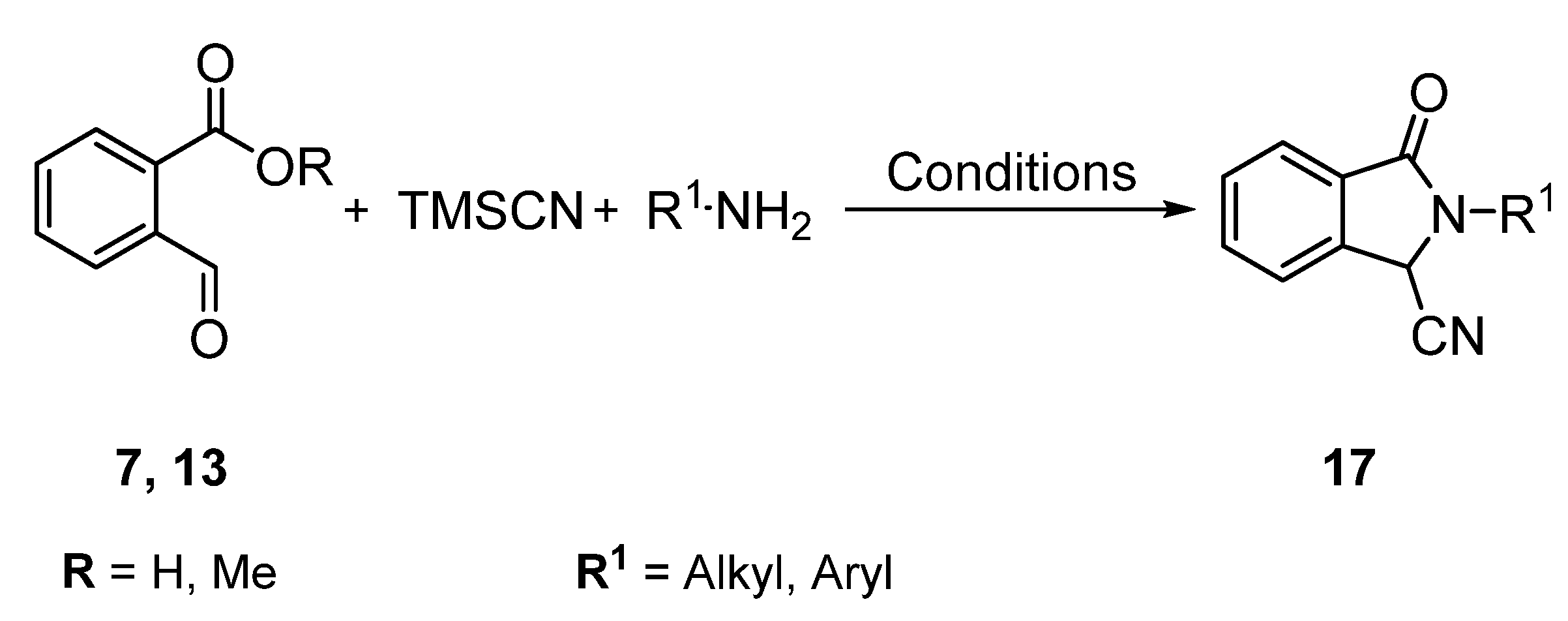
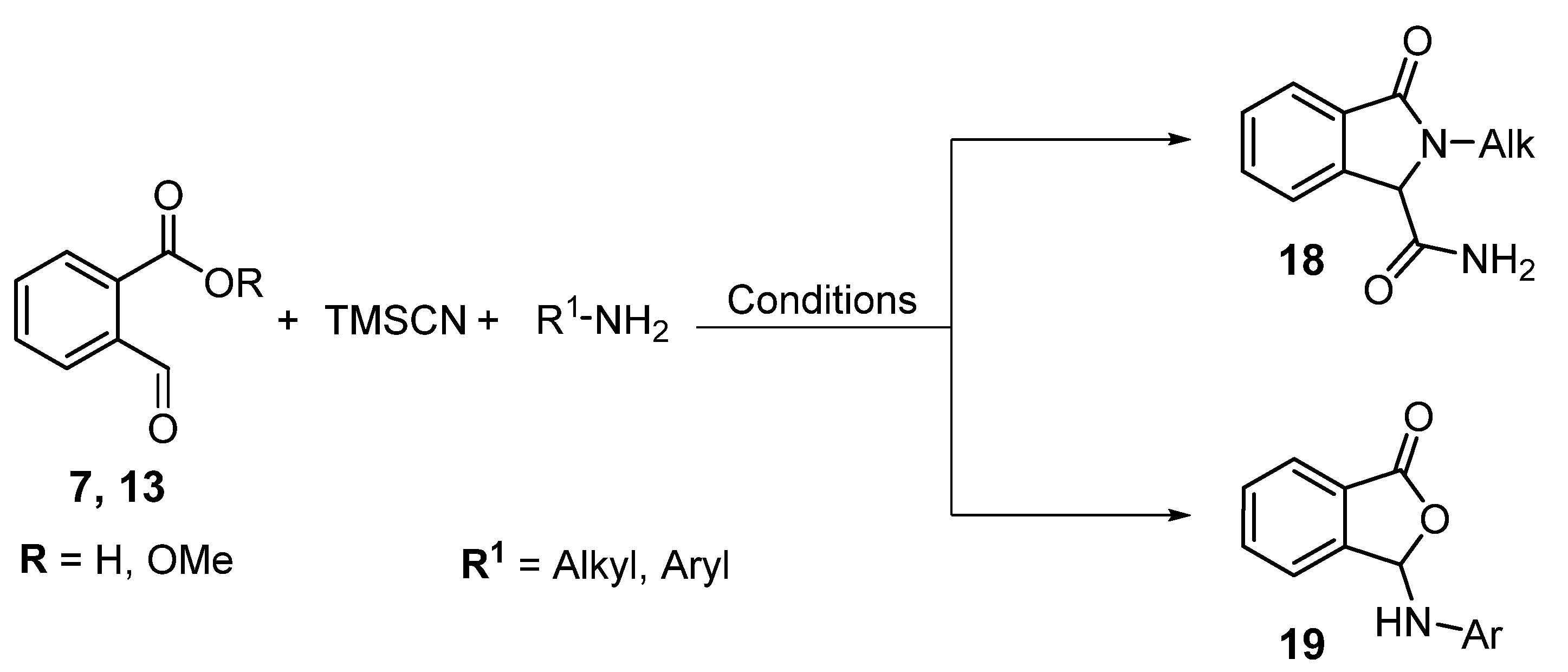
1. Introduction
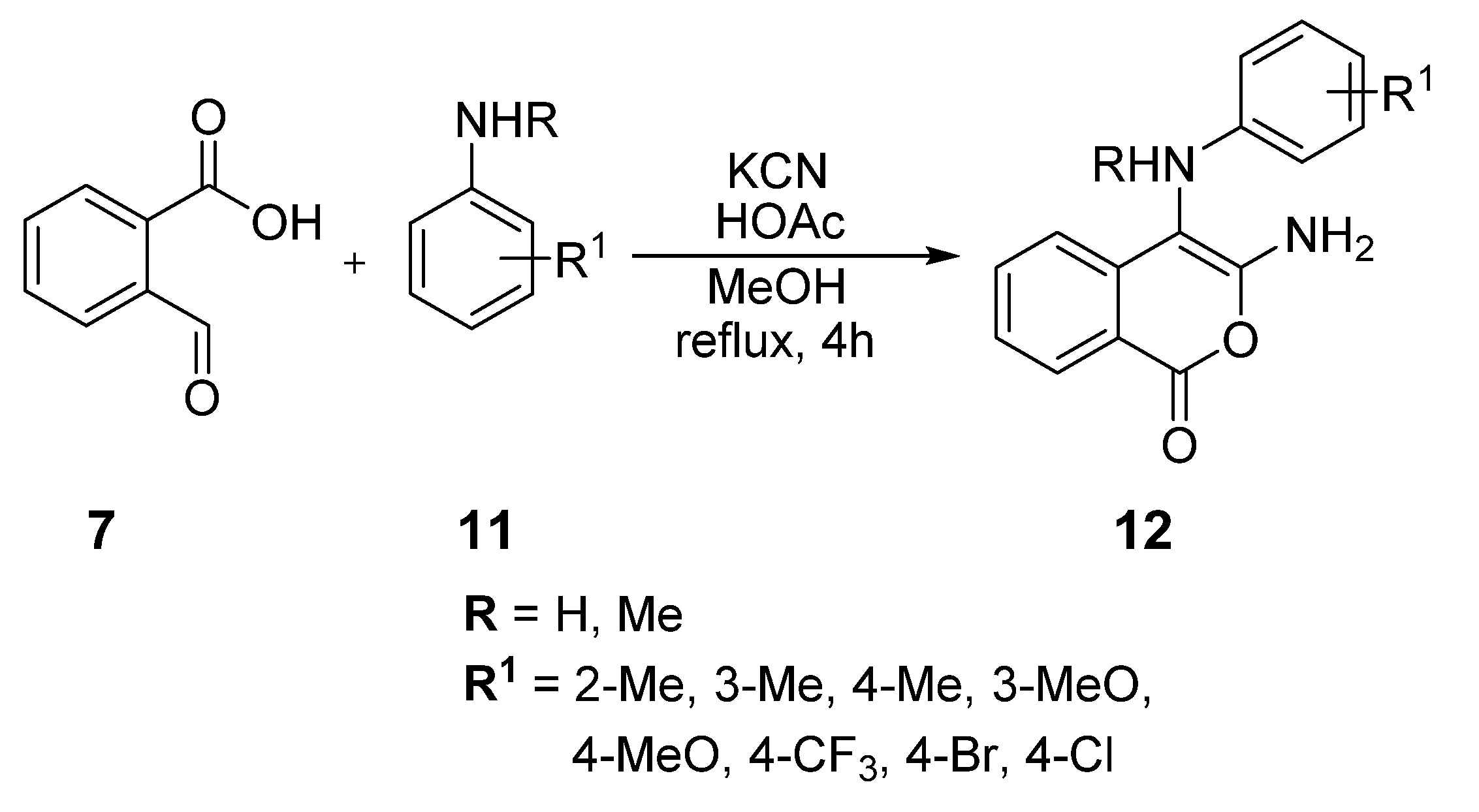
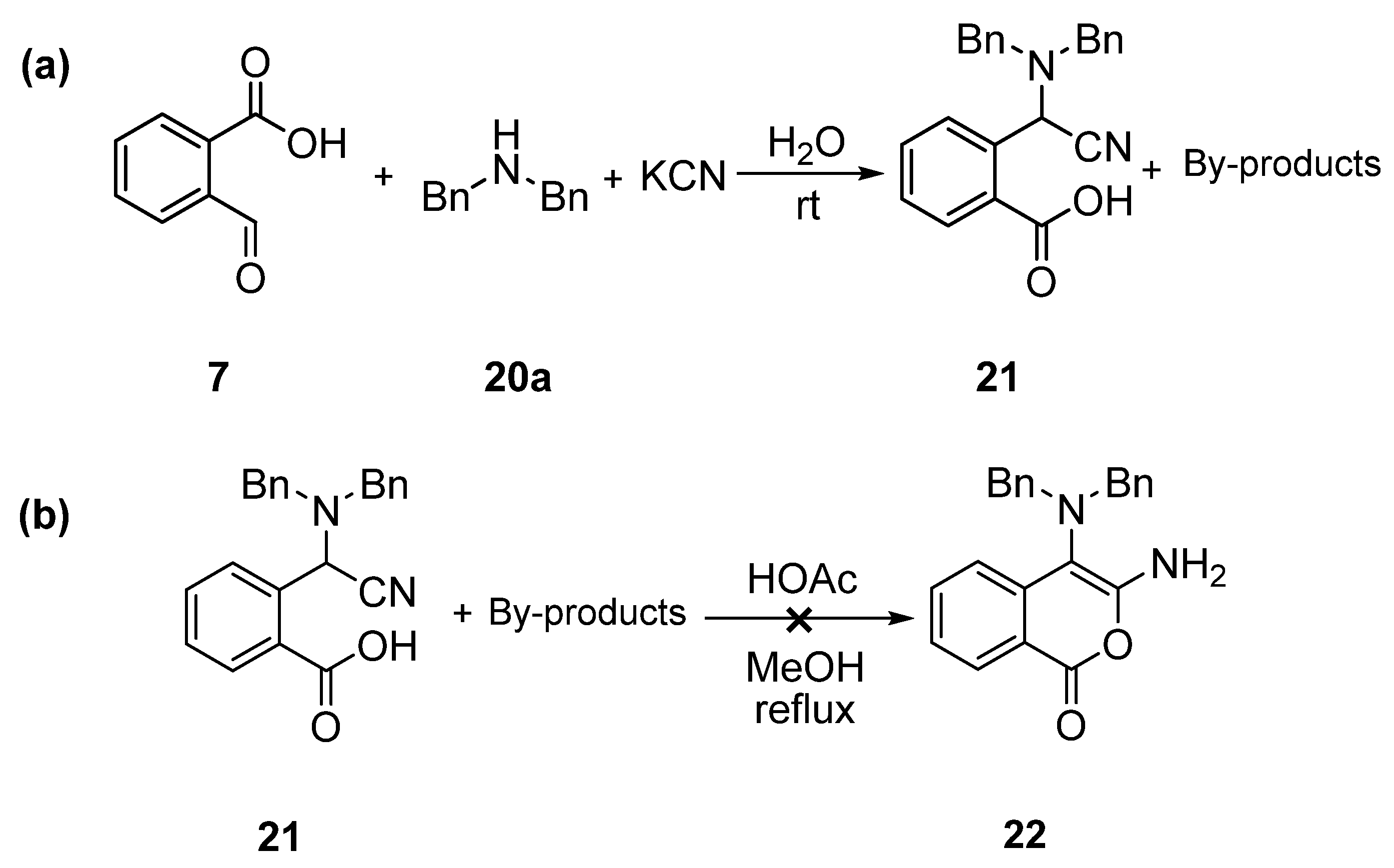
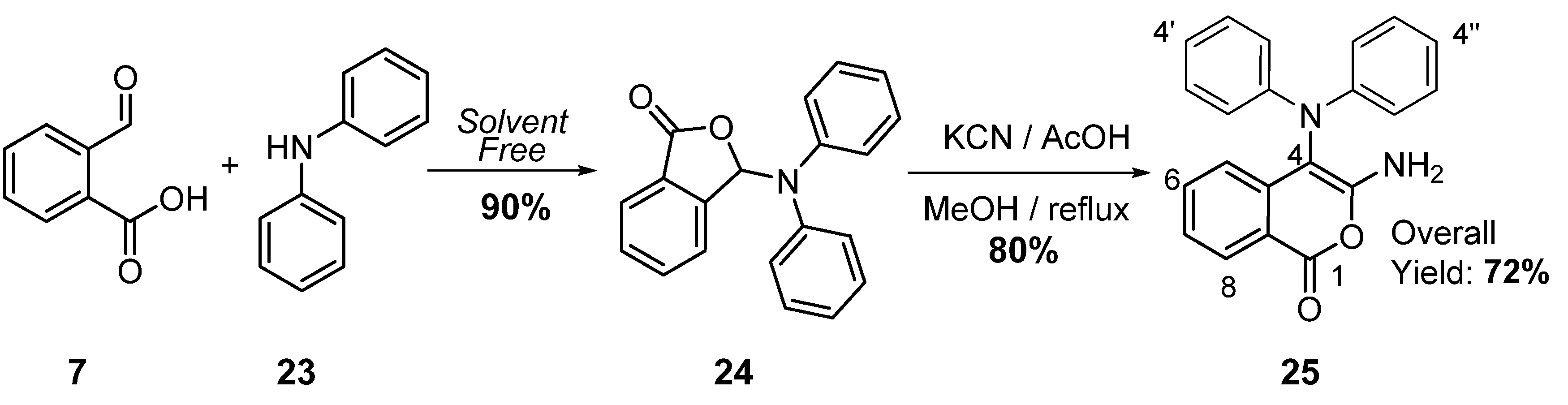
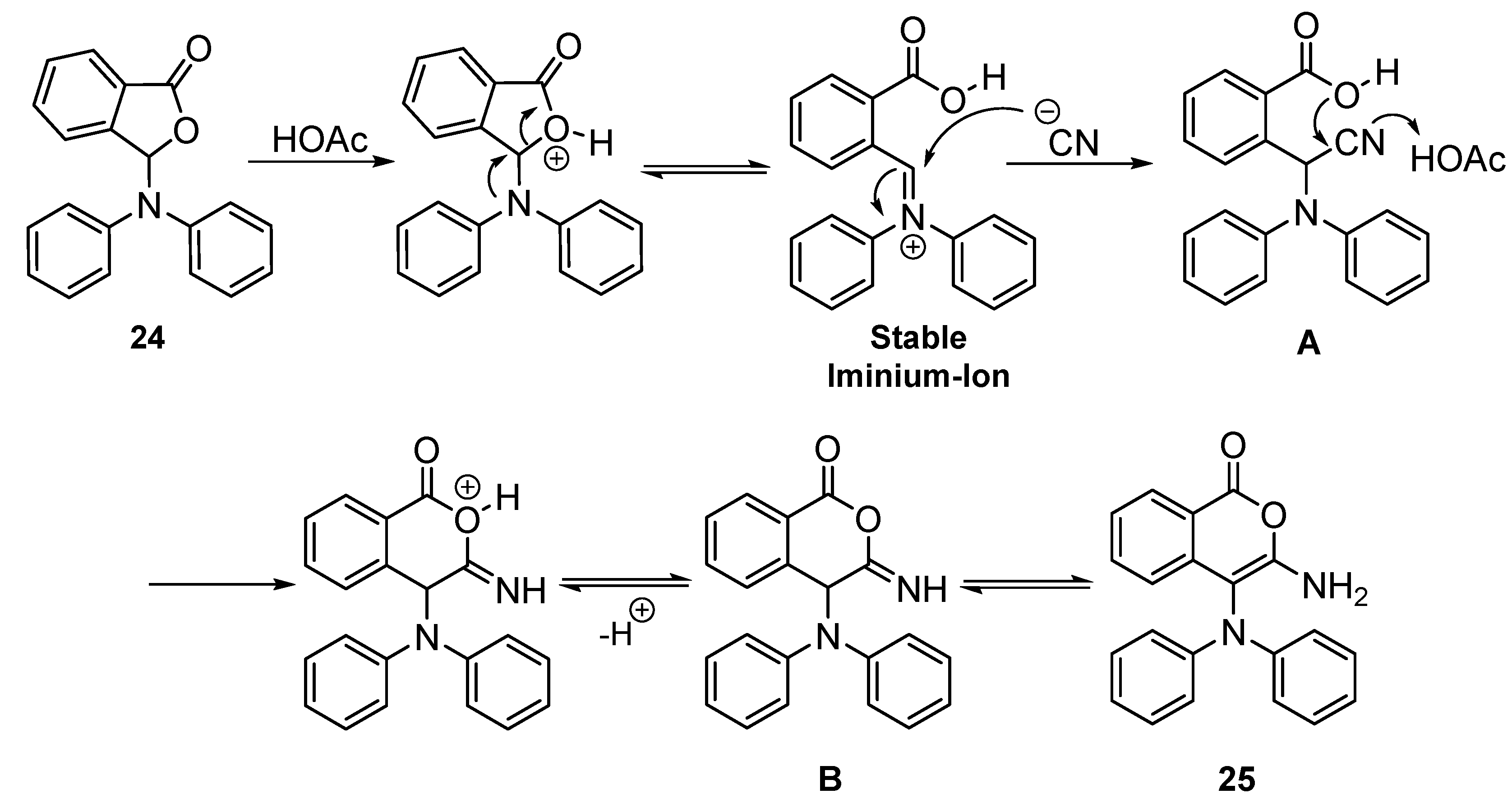
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Preparation of 3-Amino-4-(diphenylamino)-1H-isochromen-1-one (25)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, W.-N.; Cai, J.; Wang, B.; Chen, L.-Y.; Pan, C.-X.; Chen, S.-J.; Huang, G.-L.; Zheng, C.-J. A New Bioactive Isocoumarin from the Mangrove-Derived Fungus Penicillium sp. TGM112. J. Asian Nat. Prod. Res. 2021, 24, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Zinad, D.S.; Shaaban, K.A.; Abdalla, A.; Islam, T.; Schüffler, A.; Laatsch, H. Bioactive Isocoumarins from a Terrestrial Streptomyces sp. ANK302. Nat. Prod. Commun. 2011, 6, 45–48. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, C.-J.; Huang, G.-L.; Mei, R.-Q.; Wang, B.; Luo, Y.-P.; Zheng, C.; Niu, Z.-G.; Chen, G.-Y. Bioactive Meroterpenoids and Isocoumarins from the Mangrove-Derived Fungus Penicillium sp. TGM112. J. Nat. Prod. 2019, 82, 1155–1164. [Google Scholar] [CrossRef]
- Boonlarppradab, C.; Suriyachadkun, C.; Suphothina, S.; Tobwor, P. Bireticulol, a Bioactive Isocoumarin Dimer from Streptomyces sp. BCC24731. J. Antibiot. 2011, 64, 267–270. [Google Scholar] [CrossRef]
- Gao, W.; Wang, X.; Chen, F.; Li, C.; Cao, F.; Luo, D. Setosphlides A–D, New Isocoumarin Derivatives from the Entomogenous Fungus Setosphaeria rostrate LGWB-10. Nat. Prod. Bioprospect. 2021, 11, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Fu, Y.-H.; Zhou, Q.; Bai, M.; Chen, G.-Y.; Zhao, S.-Y.; Han, C.-R.; Song, X.-P. Bioactive Phenolic and Isocoumarin Glycosides from the Stems of Homalium paniculiflorum. Molecules 2018, 23, 472. [Google Scholar] [CrossRef] [PubMed]
- Meepagala, K.M.; Briscoe, W.E.; Techen, N.; Johnson, R.D.; Clausen, B.M.; Duke, S.O. Isolation of a Phytotoxic Isocoumarin from Diaporthe eres-Infected Hedera helix (English Ivy) and Synthesis of Its Phytotoxic Analogs. Pest. Manag. Sci. 2018, 74, 37–45. [Google Scholar] [CrossRef]
- Saeed, A.; Haroon, M.; Muhammad, F.; Larik, F.A.; Hesham, E.-S.; Channar, P.A. Advances in Transition Metal Catalyzed Synthesis of 3-substituted Isocoumarins. J. Organomet. Chem. 2017, 834, 88–103. [Google Scholar] [CrossRef]
- Hara, Y.; Onodera, S.; Kochi, T.; Kakiuchi, F. Catalytic Formation of α-Aryl Ketones by C-H Functionalization with Cyclic Alkenyl Carbonates and One-Pot Synthesis of Isocoumarins. Org. Lett. 2015, 17, 4850–4853. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, L.; Tong, Q.; Wu, W.; Jiang, H. Oxypalladation Initiating the Oxid ative Heck Reaction with Alkenyl Alcohols: Synthesis of Isocoumarin–Alkanones. Eur. J. Org. Chem. 2016, 2016, 663–667. [Google Scholar] [CrossRef]
- Saikia, P.; Gogoi, S. Isocoumarins: General Aspects and Recent Advances in their Synthesis. Adv. Synth. Catal. 2018, 360, 2063–2075. [Google Scholar] [CrossRef]
- Ashraf, Z. Metal-catalyzed synthesis of isocoumarin derivatives (microreview). Chem. Heterocycl. Compd. 2016, 52, 149–151. [Google Scholar] [CrossRef]
- Pellegatti, L.; Vedrenne, E.; Hiebel, M.A.; Buron, F.; Massip, S.; Leger, J.M.; Jarry, C.; Routier, S. Synthesis of [1,2,4,5]Tetrazino[6′,1′:2,3]Imidazo[4,5-c] Isoquinolin-5-Ones by Microwave-Assisted Three-Component Reaction. Tetrahedron Lett. 2011, 52, 5224–5228. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Palnati, G.R.; Dodda, R.P.; Avula, S.R.; Swami, P. Studies on Novel Synthetic Methodologies, Part XII: An Efficient One-Pot Access to 6,6a-Dihydroisoindolo[2,1-a]quinazoline-5,11-diones and 5-Phenylisoindolo[2,1-a]Quinazolin-11(6aH)-ones. Synlett 2013, 24, 105–113. [Google Scholar] [CrossRef][Green Version]
- Faggi, C.; García-Valverde, M.; Marcaccini, S.; Menchi, G. Isolation of Ugi Four-Component Condensation Primary Adducts: A Straightforward Route to Isocoumarins. Org. Lett. 2010, 12, 788–791. [Google Scholar] [CrossRef]
- Opatz, T.; Ferenc, D. Facile Preparation of 3-Amino-4-(arylamino)-1H-isochromen-1-ones by a New Multicomponent Reaction. Eur. J. Org. Chem. 2005, 2005, 817–821. [Google Scholar] [CrossRef]
- Rodríguez, R.; Vicentes, D.E.; Cobo, J.; Nogueras, M. Synthesis of new 1,2-diaryl[2]benzopyrano[3,4-d]imidazol-5(1H)-one derivatives mediated by ceric ammonium nitrate. Tetrahedron Lett. 2017, 58, 1487–1489. [Google Scholar] [CrossRef]
- Zhang, Y.; Ao, Y.; Huang, Z.; Wang, D.; Wang, M.; Zhu, J. Chiral Phosphoric Acid Catalyzed Asymmetric Ugi Reaction by Dynamic Kinetic Resolution of the Primary Multicomponent Adduct. Angew. Chem. Int. Ed. 2016, 55, 5282–5285. [Google Scholar] [CrossRef]
- Ramazani, A.; Mahyari, A. Three-Component Reaction of Isocyanides and 2-Formylbenzoic Acid with Dibenzylamine Catalyzed by Silica Nanoparticles under Solvent-Free Conditions. Helv. Chim. Acta 2010, 93, 2203–2209. [Google Scholar] [CrossRef]
- Freskos, J.N.; Morrow, G.W.; Swenton, J.S. Synthesis of functionalized hydroxyphthalides and their conversion to 3-cyano-1(3H)-isobenzofuranones. The Diels-Alder reaction of methyl 4,4-diethoxybutynoate and cyclohexadienes, J. Org. Chem. 1985, 50, 805–810. [Google Scholar] [CrossRef]
- Mal, D.; Ghosh, K.; Chakraborty, S. Synthesis, Rearrangement, and Hauser Annulation of 3-Isocyanophthalides. Synthesis 2015, 47, 2473–2484. [Google Scholar] [CrossRef]
- Russell, R.A.; Pilley, B.A.; Warrener, R.N. A High-Yielding Synthesis of 3-Cyanophthalides. Synth. Commun. 1986, 16, 425–430. [Google Scholar] [CrossRef]
- Ghosh, B.; Chakraborty, S.; Mal, D. TMSCN-PhI(OAc)2 Promoted Synthesis of 3-Cyanophthalides from Phthalaldehydic Acids. Chem. Select. 2016, 1, 3097–3099. [Google Scholar] [CrossRef]
- Kraus, G.; Cho, H.; Crowley, S.; Roth, B.; Sugimoto, H.; Prugh, S. Phthalide annulation: The synthesis of kalafungin, pachybasin and chrysophanol. J. Org. Chem. 1983, 48, 3439–3444. [Google Scholar] [CrossRef]
- Sakulsombat, M.; Angelin, M.; Ramström, O. Tandem reversible addition-intramolecular lactonization for the synthesis of 3-functionalized phthalides. Tetrahedron Lett. 2010, 51, 75–78. [Google Scholar] [CrossRef]
- Okasaki, K.; Nomura, K.; Yoshii, E. An Efficient Synthesis of 3-Cyano-1(3H)-Isobehzofuranones. Synth. Commun. 1987, 17, 1021–1027. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Suneja, A.; Bisai, V.; Singh, V.K. Approach to Isoindolinones, Isoquinolinones, and THIQs via Lewis Acid-Catalyzed Domino Strecker-Lactamization/Alkylations. Org. Lett. 2016, 18, 634–637. [Google Scholar] [CrossRef]
- Chen, T.; Cai, C. Sc(OTf)3-catalyzed three-component cascade reaction: One-pot synthesis of substituted 3-oxoisoindoline-1-carbonitrile derivatives. Catal. Comm. 2016, 74, 119–121. [Google Scholar] [CrossRef]
- Nammalwar, B.; Muddala, N.P.; Murie, M.; Bunce, R. Efficient syntheses of substituted (±)-3-oxoisoindoline-1-carbonitriles and carboxamides using OSU-6. Green Chem. 2015, 17, 2495–2503. [Google Scholar] [CrossRef]
- Ling, H.; Zha, Z.; Min, L.; Li, H. A facile and efficient method for the synthesis of N-substituted 3-oxoisoindoline-1-carbonitrile derivatives catalyzed by sulfamic acid. Arkivoc 2013, 2013, 189–198. [Google Scholar] [CrossRef]
- Soleimani, E.; Khodaei, M.M.; Mehrdadi-nejad, P.; Ghanbari, M. The synthesis of dialkylaminonitrile derivatives of 2-formylbenzoic acid by the Strecker reaction in an aqueous medium. J. Chem. Res. 2016, 40, 371–374. [Google Scholar] [CrossRef]
- Moreno-Fuquen, R.; Castillo, J.C.; Abonia, R.; Ellena, J.; Tenorio, J.C. 3-(Diphenylamino)Isobenzofuran-1(3H)-One. Acta Cryst. 2014, E70, o490. [Google Scholar] [CrossRef] [PubMed]
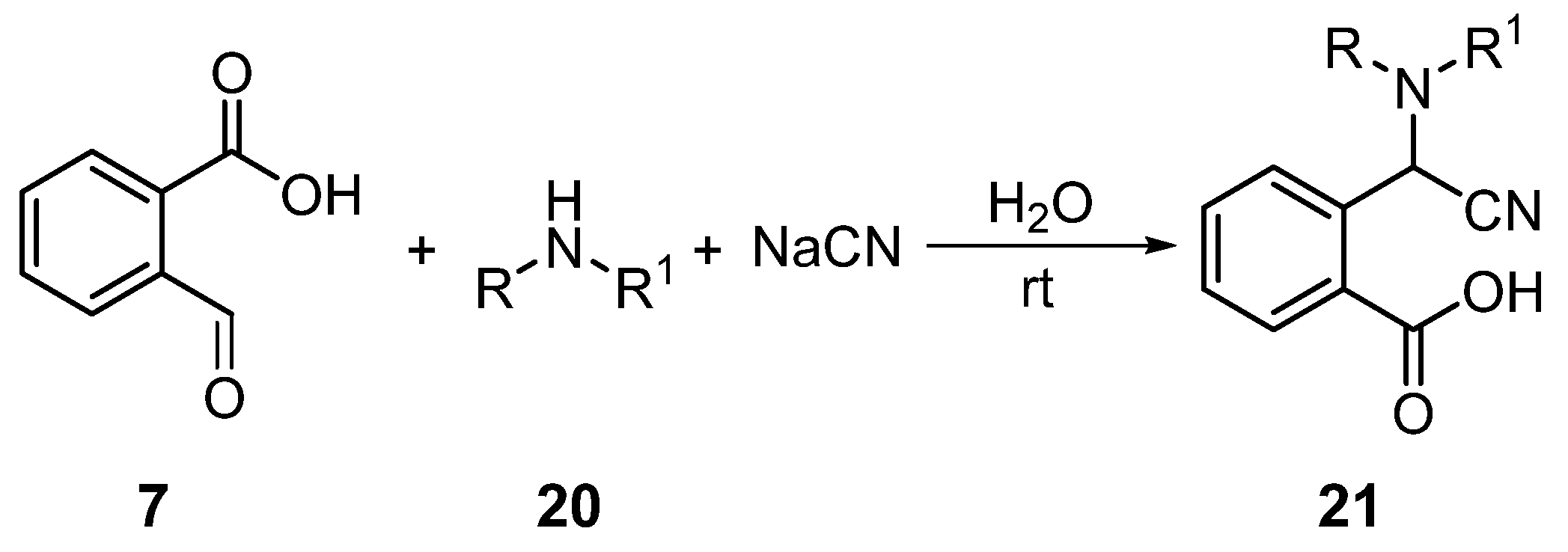

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroga-Suavita, F.; Rodríguez, R.; León, O. 3-Amino-4-(diphenylamino)-1H-2-benzopyran-1-one. Molbank 2022, 2022, M1408. https://doi.org/10.3390/M1408
Quiroga-Suavita F, Rodríguez R, León O. 3-Amino-4-(diphenylamino)-1H-2-benzopyran-1-one. Molbank. 2022; 2022(3):M1408. https://doi.org/10.3390/M1408
Chicago/Turabian StyleQuiroga-Suavita, Felipe, Ricaurte Rodríguez, and Omar León. 2022. "3-Amino-4-(diphenylamino)-1H-2-benzopyran-1-one" Molbank 2022, no. 3: M1408. https://doi.org/10.3390/M1408
APA StyleQuiroga-Suavita, F., Rodríguez, R., & León, O. (2022). 3-Amino-4-(diphenylamino)-1H-2-benzopyran-1-one. Molbank, 2022(3), M1408. https://doi.org/10.3390/M1408