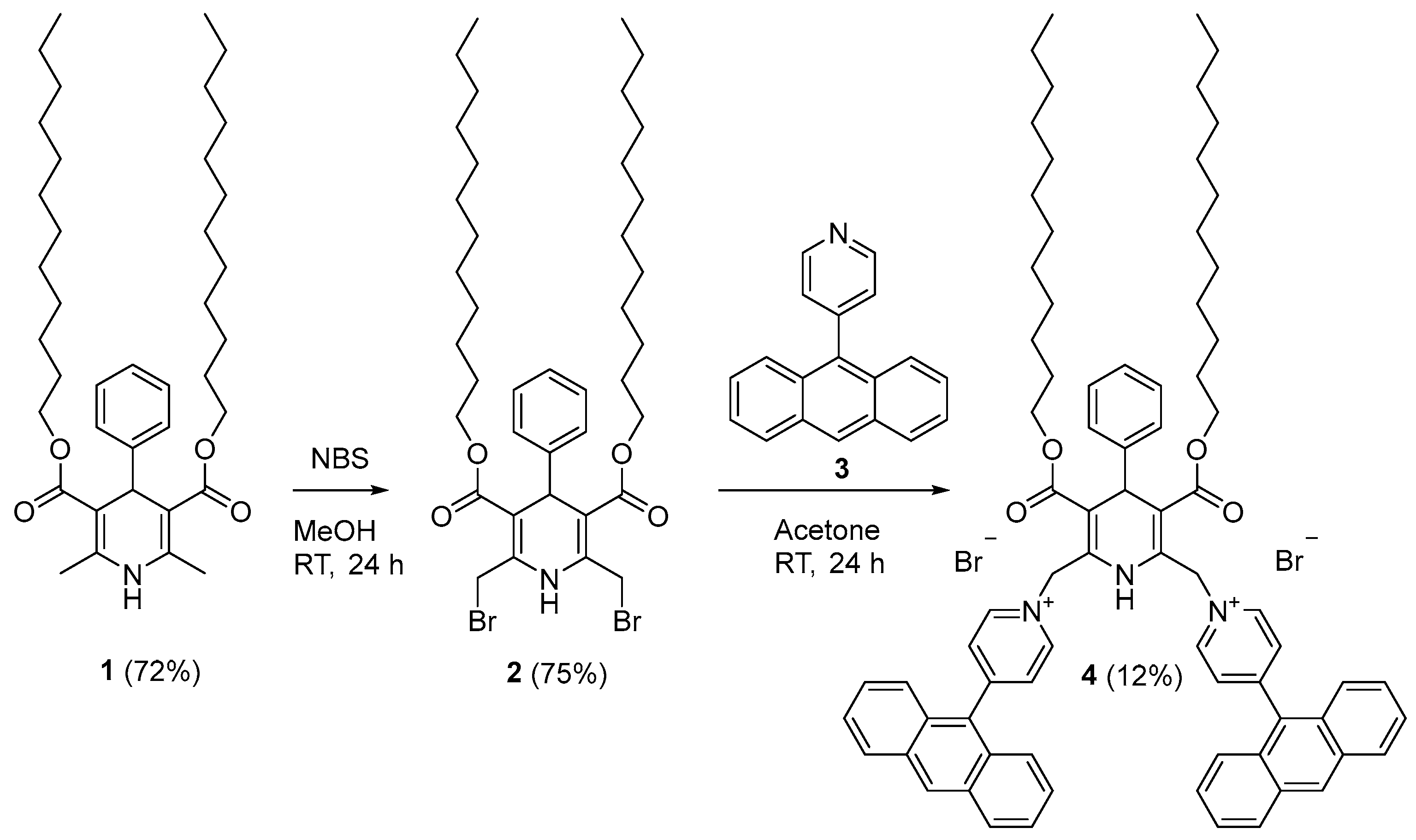
1,1′-{[3,5-Bis((dodecyloxycarbonyl)-4-phenyl-1,4-dihydropyridine-2,6-diyl]bis(methylene)}bis[4-(anthracen-9-yl)pyridin-1-ium] Dibromide
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of 1,1′-{[3,5-Bis((dodecyloxycarbonyl)-4-phenyl-1,4-dihydropyridine-2,6-diyl]bis-(methylene)}bis[4-(anthracen-9-yl)pyridin-1-ium] Dibromide (4)
3.3. Self-Assembling Properties of Compound 4 Using Dynamic Light Scattering Measurements
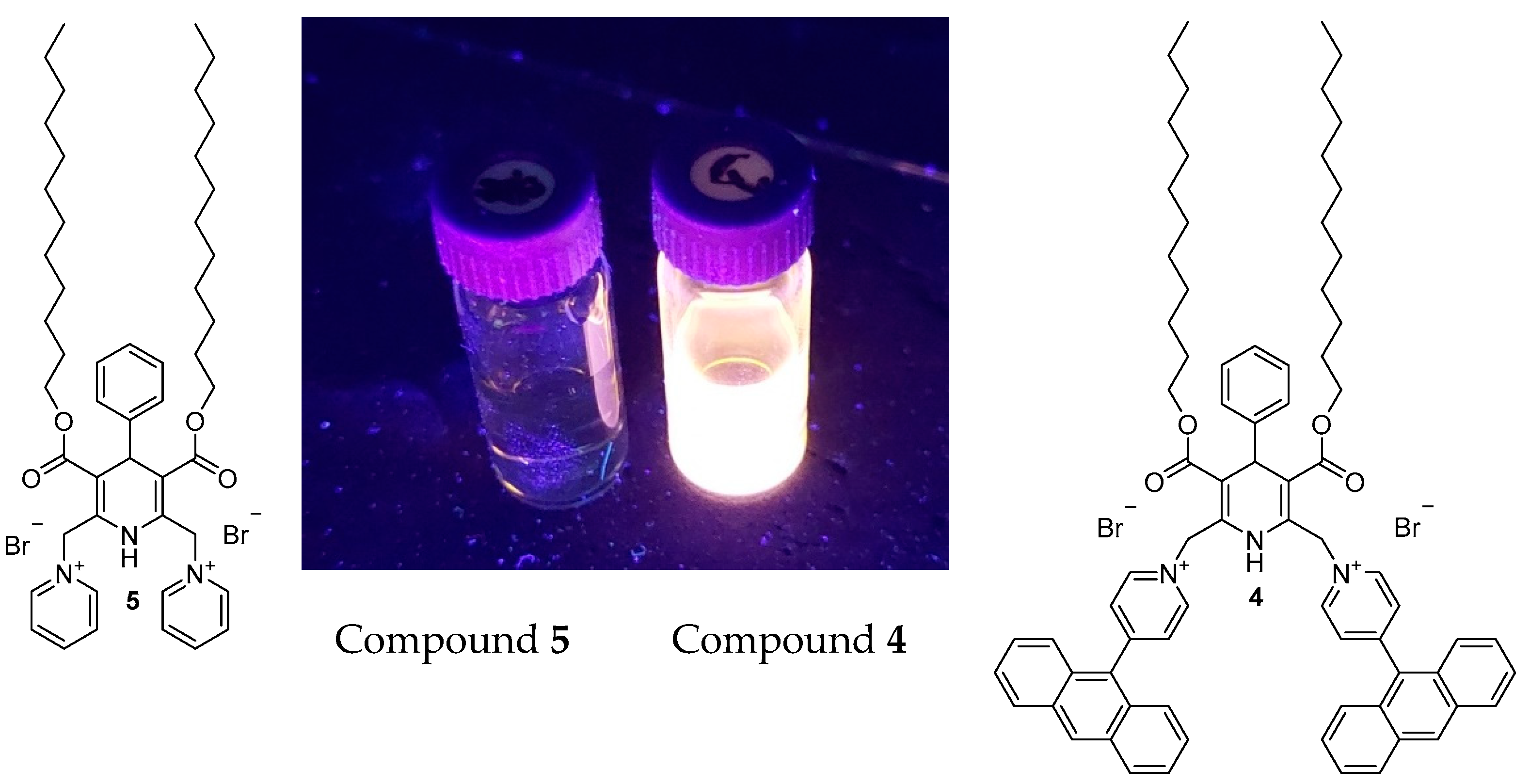
3.4. Photoluminescence Measurements
3.5. Characterization of Monolayers Formed by 1,4-DHP Amphiphiles or Surface Pressure–Area (p–A) Isotherms
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sguizzato, M.; Esposito, E.; Cortesi, R. Lipid-based nanosystems as a tool to overcome skin barrier. Int. J. Mol. Sci. 2021, 22, 8319. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Mirgorodskaya, A.B.; Koroleva, M.Y.; Kushnazarova, R.A.; Mishchenko, E.V.; Petrov, K.A.; Lenina, O.A.; Vyshtakalyuk, A.B.; Voloshina, A.D.; Zakharova, L.Y. Microemulsions and nanoemulsions modified with cationic surfactants for improving the solubility and therapeutic efficacy of loaded drug indomethacin. Nanotechnology 2022, 33, 155103. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Tang, W.L.; Tang, W.H.; Li, S.D. Cancer theranostic applications of lipid-based nanoparticles. Drug Discov. Today 2018, 23, 1159–1166. [Google Scholar] [CrossRef]
- Charron, D.M.; Chen, J.; Zheng, G. Theranostic lipid nanoparticles for cancer medicine. Cancer Treat. Res. 2015, 166, 103–127. [Google Scholar] [CrossRef]
- Zingale, E.; Romeo, A.; Rizzo, S.; Cimino, C.; Bonaccorso, A.; Carbone, C.; Musumeci, T.; Pignatello, R. Fluorescent Nanosystems for Drug Tracking and Theranostics: Recent Applications in the Ocular Field. Pharmaceutics 2022, 14, 955. [Google Scholar] [CrossRef]
- Baviera, G.S.; Donate, P.M. Recent advances in the syntheses of anthracene derivatives. Beilstein J. Org. Chem. 2021, 17, 2028–2050. [Google Scholar] [CrossRef] [PubMed]
- Duraimurugan, K.; Harikrishnan, M.; Madhavan, J.; Siva, A.; Lee, S.J.; Theerthagiri, J.; Choi, M.Y. Anthracene-based fluorescent probe: Synthesis, characterization, aggregation-induced emission, mechanochromism, and sensing of nitroaromatics in aqueous media. Environ. Res. 2021, 194, 110741. [Google Scholar] [CrossRef]
- Li, R.; Xiao, S.; Li, Y.; Lin, Q.; Zhang, R.; Zhao, J.; Yang, C.; Zou, K.; Li, D.; Yi, T. Polymorphism-dependent and piezochromic luminescence based on molecular packing of a conjugated molecule. Chem. Sci. 2014, 5, 3922–3928. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, H.; Xu, X.; Lu, Y.; Xue, S.; Zhang, H.; Yang, W. 9,10-Bis((Z)-2-phenyl-2-(pyridin-2-yl)vinyl)anthracene: Aggregation-induced emission, mechanochromic luminescence, and reversible volatile acids-amines switching. Dye. Pigment. 2018, 149, 407–414. [Google Scholar] [CrossRef]
- Mathur, R.; Negi, K.S.; Shrivastava, R.; Nair, R. Recent developments in the nanomaterial-catalyzed green synthesis of structurally diverse 1,4-dihydropyridines. RSC Adv. 2021, 11, 1376–1393. [Google Scholar] [CrossRef] [PubMed]
- Klusa, V. Atypical 1,4-dihydropyridine derivatives, an approach to neuroprotection and memory enhancement. Pharmacol. Res. 2016, 113, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Hyvönen, Z.; Plotniece, A.; Reine, I.; Chekavichus, B.; Duburs, G.; Urtti, A. Novel cationic amphiphilic 1,4-dihydropyridine derivatives for DNA delivery. Biochim. Biophys. Acta-Biomembr. 2000, 1509, 451–466. [Google Scholar] [CrossRef]
- Pajuste, K.; Hyvonen, Z.; Petrichenko, O.; Kaldre, D.; Rucins, M.; Cekavicus, B.; Ose, V.; Skrivele, B.; Gosteva, M.; Morin-Picardat, E.; et al. Gene delivery agents possessing antiradical activity: Self-assembling cationic amphiphilic 1,4-dihydropyridine derivatives. New J. Chem. 2013, 37, 3062–3075. [Google Scholar] [CrossRef]
- Petrichenko, O.; Rucins, M.; Vezane, A.; Timofejeva, I.; Sobolev, A.; Cekavicus, B.; Pajuste, K.; Plotniece, M.; Gosteva, M.; Kozlovska, T.; et al. Studies of the physicochemical and structural properties of self-assembling cationic pyridine derivatives as gene delivery agents. Chem. Phys. Lipids 2015, 191, 25–37. [Google Scholar] [CrossRef]
- Petrichenko, O.; Plotniece, A.; Pajuste, K.; Rucins, M.; Dimitrijevs, P.; Sobolev, A.; Sprugis, E.; Cēbers, A. Evaluation of physicochemical properties of amphiphilic 1,4-dihydropyridines and preparation of magnetoliposomes. Nanomaterials 2021, 11, 593. [Google Scholar] [CrossRef]
- Niemirowicz-Laskowska, K.; Głuszek, K.; Piktel, E.; Pajuste, K.; Durnaś, B.; Król, G.; Wilczewska, A.Z.; Janmey, P.A.; Plotniece, A.; Bucki, R. Bactericidal and immunomodulatory properties of magnetic nanoparticles functionalized by 1,4-dihydropyridines. Int. J. Nanomed. 2018, 13, 3411–3424. [Google Scholar] [CrossRef]
- Apsite, G.; Timofejeva, I.; Vezane, A.; Vigante, B.; Rucins, M.; Sobolev, A.; Plotniece, M.; Pajuste, K.; Kozlovska, T.; Plotniece, A. Synthesis and comparative evaluation of novel cationic amphiphile C12-Man-Q as an efficient DNA delivery agent in vitro. Molecules 2018, 23, 1540. [Google Scholar] [CrossRef]
- Rucins, M.; Smits, R.; Sipola, A.; Vigante, B.; Domracheva, I.; Turovska, B.; Muhamadejev, R.; Pajuste, K.; Plotniece, M.; Sobolev, A.; et al. Pleiotropic Properties of Amphiphilic Dihydropyridines, Dihydropyridones, and Aminovinylcarbonyl Compounds. Oxid. Med. Cell. Longev. 2020, 2020, 8413713. [Google Scholar] [CrossRef]
- Rucins, M.; Dimitrijevs, P.; Pajuste, K.K.; Petrichenko, O.; Jackevica, L.; Gulbe, A.; Kibilda, S.; Smits, K.; Plotniece, M.; Tirzite, D.; et al. Contribution of Molecular Structure to Self-Assembling and Biological Properties of Bifunctional Lipid-Like 4-(N-Alkylpyridinium)-1,4-Dihydropyridines. Pharmaceutics 2019, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Rucins, M.; Kaldre, D.; Pajuste, K.; Fernandes, M.A.S.; Vicente, J.A.F.; Klimaviciusa, L.; Jaschenko, E.; Kanepe-Lapsa, I.; Shestakova, I.; Plotniece, M.; et al. Synthesis and studies of calcium channel blocking and antioxidant activities of novel 4-pyridinium and/or N-propargyl substituted 1,4-dihydropyridine derivatives. Comptes Rendus Chim. 2014, 17, 69–80. [Google Scholar] [CrossRef]
- Rucins, M.; Kaukulis, M.; Plotniece, A.; Pajuste, K.; Pikun, N.; Sobolev, A. 1,1′-{[3,5-Bis(dodecyloxycarbonyl)-4-(naphthalen-2-yl)-1,4-dihydropyridine-2,6-diyl]bis(methylene)}bis{4-[(E)-2-(naphthalen-2-yl)vinyl]pyridin-1-ium} dibromide. Molbank 2022, 2022, M1396. [Google Scholar] [CrossRef]
- Charcosset, C.; Juban, A.; Valour, J.P.; Urbaniak, S.; Fessi, H. Preparation of liposomes at large scale using the ethanol injection method: Effect of scale-up and injection devices. Chem. Eng. Res. Des. 2015, 94, 508–515. [Google Scholar] [CrossRef]
- Rucins, M.; Pajuste, K.; Sobolev, A.; Plotniece, M.; Pikun, N.; Pajuste, K.; Plotniece, A. Data for the synthesis and characterisation of 2,6-di(bromomethyl)-3,5-bis(alkoxycarbonyl)-4-aryl-1,4-dihydropyridines as important intermediates for synthesis of amphiphilic 1,4-dihydropyridines. Data Br. 2020, 30, 105532. [Google Scholar] [CrossRef]
- Ramsay, W.J.; Szczypiński, F.T.; Weissman, H.; Ronson, T.K.; Smulders, M.M.J.; Rybtchinski, B.; Nitschke, J.R. Designed enclosure enables guest binding within the 4200 Å3 cavity of a self-assembled cube. Angew. Chem.-Int. Ed. 2015, 54, 5636–5640. [Google Scholar] [CrossRef]
- Pajuste, K.; Plotniece, A.; Kore, K.; Intenberga, L.; Cekavicus, B.; Kaldre, D.; Duburs, G.; Sobolev, A. Use of pyridinium ionic liquids as catalysts for the synthesis of 3,5-bis(dodecyloxycarbonyl)-1,4-dihydropyridine derivative. Cent. Eur. J. Chem. 2011, 9, 143–148. [Google Scholar] [CrossRef]
- Chibowski, E.; Szcześ, A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption 2016, 22, 755–765. [Google Scholar] [CrossRef]
- Rucins, M.; Petricenko, O.; Pajuste, K.; Plotniece, M.; Pajuste, K.; Gosteva, M.; Cekavicus, B.; Sobolev, A.; Plotniece, A. Studies of Preparation and Stability of Liposomes Formed by 1,1′-[(3,5-Didodecyloxycarbo nyl-4-phenyl-1,4-dihydropyridine-2,6-diyl)-dimethylen]bispyridinium Dibromide. Adv. Mater. Res. 2013, 787, 157–162. [Google Scholar] [CrossRef]
- Rucins, M.; Pajuste, K.; Plotniece, A.; Pikun, N.; Rodik, R.; Vyshnevskiy, S.; Sobolev, A. Synthesis and Characterisation of 1,1′-{[3,5-Bis(dodecyloxy-carbonyl)-4-(thiophen-3-yl)-1,4-dihydropyridine-2,6-diyl]bis-(methylene)}bis(pyridin-1-ium) Dibromide. Molbank 2022, 2022, M1311. [Google Scholar] [CrossRef]
| Entry | Condition, Days | Zav DH, nm | PDI | D, nm (%) | Zpot, mV |
|---|---|---|---|---|---|
| 1 | 0 * | 225 ± 4.0 | 0.51 ± 0.02 | 346 (87); 49 (9); 12 (4) | 41.6 ± 3.2 |
| 2 | 7 ** | 141 ± 3.0 | 0.49 ± 0.03 | 244 (90); 27 (10) | 34.5 ± 3.5 |
| 3 | 30 *** | 135 ± 3.0 | 0.47 ± 0.01 | 209 (90); 23 (10) | 32.8 ± 1.8 |
| Entry | Compound | P, mN/m | Cs−1, mN/m | MMA, Å2 |
|---|---|---|---|---|
| 1 | 4 | 51.24 ± 0.49 | 100.99 ± 8.84 | 117.53 ± 3.65 |
| 2 | 5 | 45.28 ± 0.65 | 87.20 ± 8.84 | 88.72 ± 2.14 |
| 3 | 5 * | 46.62 ± 0.02 | 15.65 ± 1.24 | 82.77 ± 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozolins, R.; Plotniece, M.; Pajuste, K.; Putralis, R.; Pikun, N.; Sobolev, A.; Plotniece, A.; Rucins, M. 1,1′-{[3,5-Bis((dodecyloxycarbonyl)-4-phenyl-1,4-dihydropyridine-2,6-diyl]bis(methylene)}bis[4-(anthracen-9-yl)pyridin-1-ium] Dibromide. Molbank 2022, 2022, M1438. https://doi.org/10.3390/M1438
Ozolins R, Plotniece M, Pajuste K, Putralis R, Pikun N, Sobolev A, Plotniece A, Rucins M. 1,1′-{[3,5-Bis((dodecyloxycarbonyl)-4-phenyl-1,4-dihydropyridine-2,6-diyl]bis(methylene)}bis[4-(anthracen-9-yl)pyridin-1-ium] Dibromide. Molbank. 2022; 2022(3):M1438. https://doi.org/10.3390/M1438
Chicago/Turabian StyleOzolins, Reinis, Mara Plotniece, Karlis Pajuste, Reinis Putralis, Nadiia Pikun, Arkadij Sobolev, Aiva Plotniece, and Martins Rucins. 2022. "1,1′-{[3,5-Bis((dodecyloxycarbonyl)-4-phenyl-1,4-dihydropyridine-2,6-diyl]bis(methylene)}bis[4-(anthracen-9-yl)pyridin-1-ium] Dibromide" Molbank 2022, no. 3: M1438. https://doi.org/10.3390/M1438
APA StyleOzolins, R., Plotniece, M., Pajuste, K., Putralis, R., Pikun, N., Sobolev, A., Plotniece, A., & Rucins, M. (2022). 1,1′-{[3,5-Bis((dodecyloxycarbonyl)-4-phenyl-1,4-dihydropyridine-2,6-diyl]bis(methylene)}bis[4-(anthracen-9-yl)pyridin-1-ium] Dibromide. Molbank, 2022(3), M1438. https://doi.org/10.3390/M1438






