(S)-3-(3-((7-Ethynyl-9H-pyrimido[4,5-b]indol-4-yl)amino)piperidin-1-yl)propanenitrile
Abstract
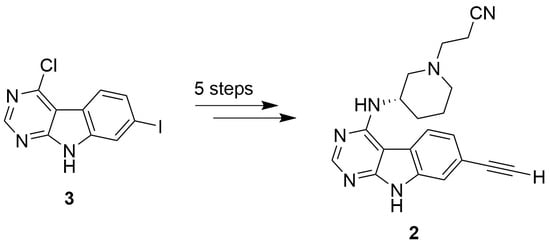
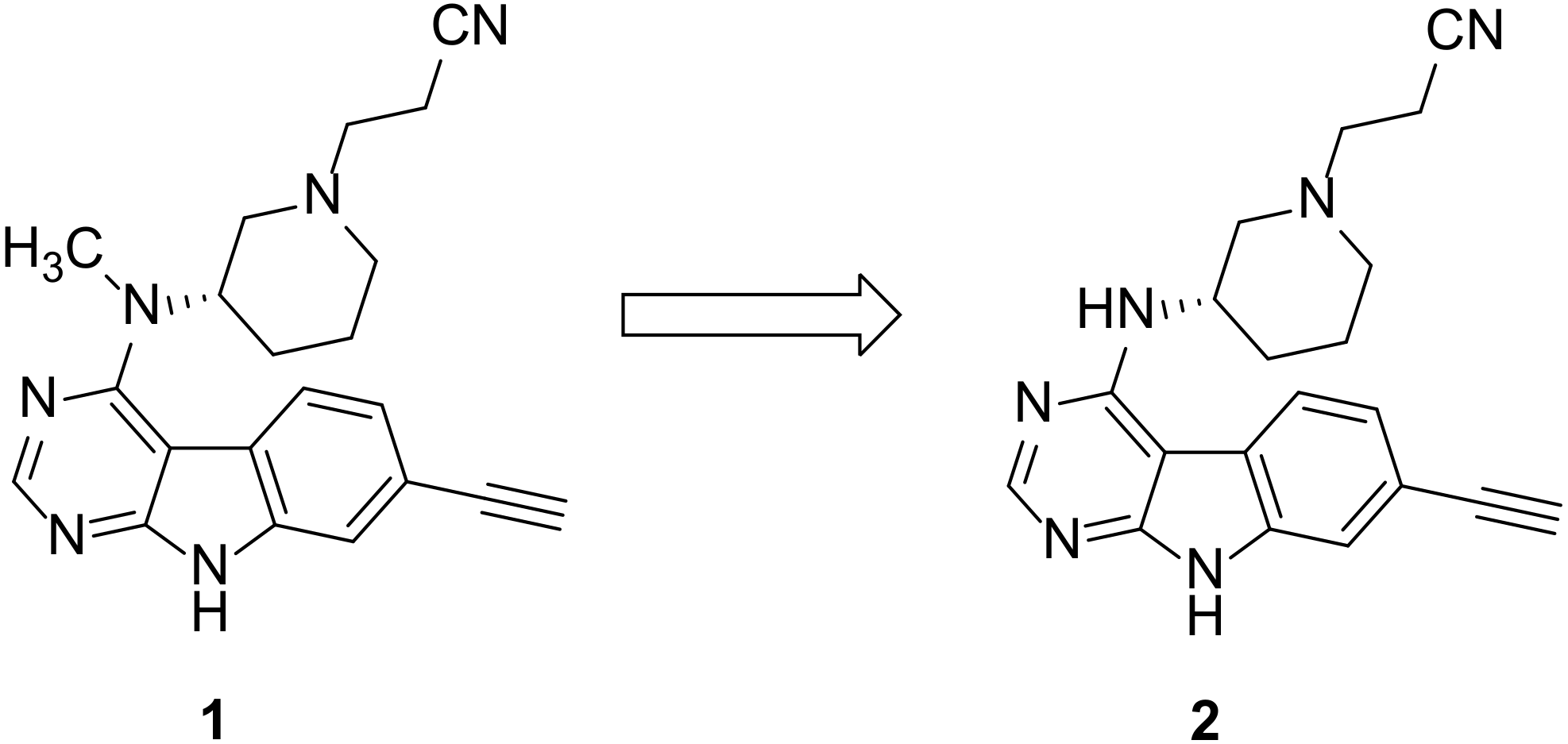
:1. Introduction
2. Results and Discussion
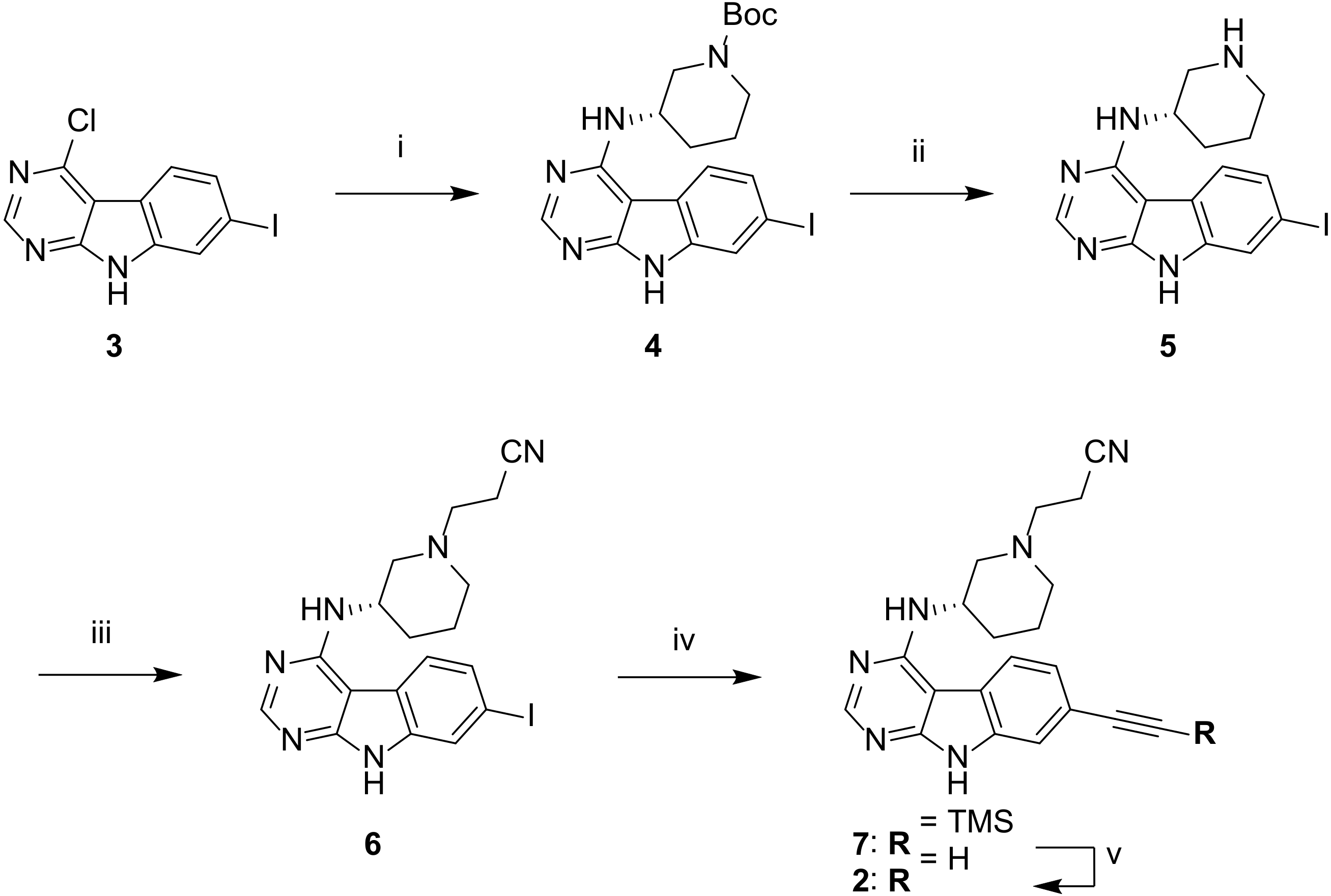
2.1. Chemistry
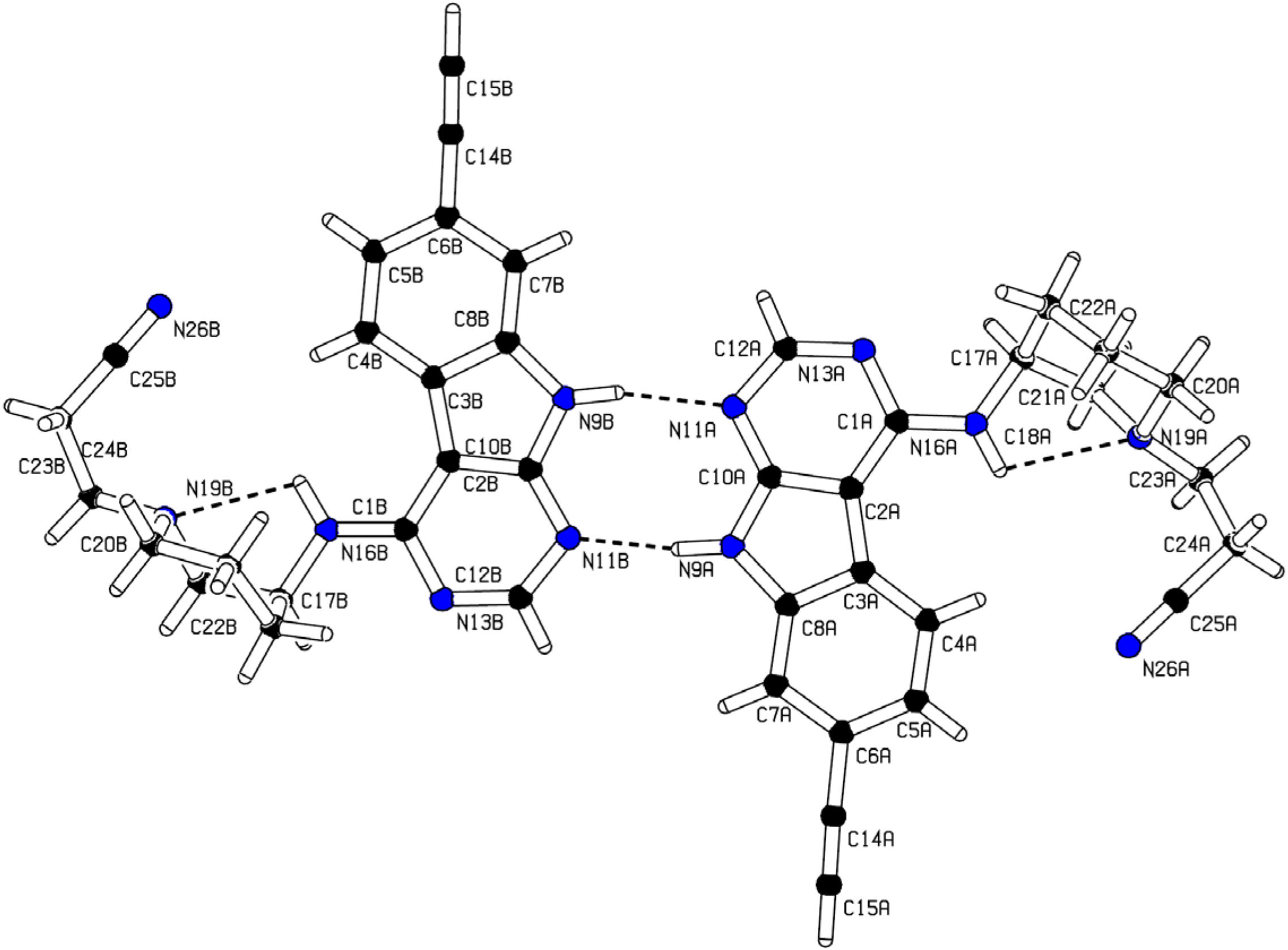
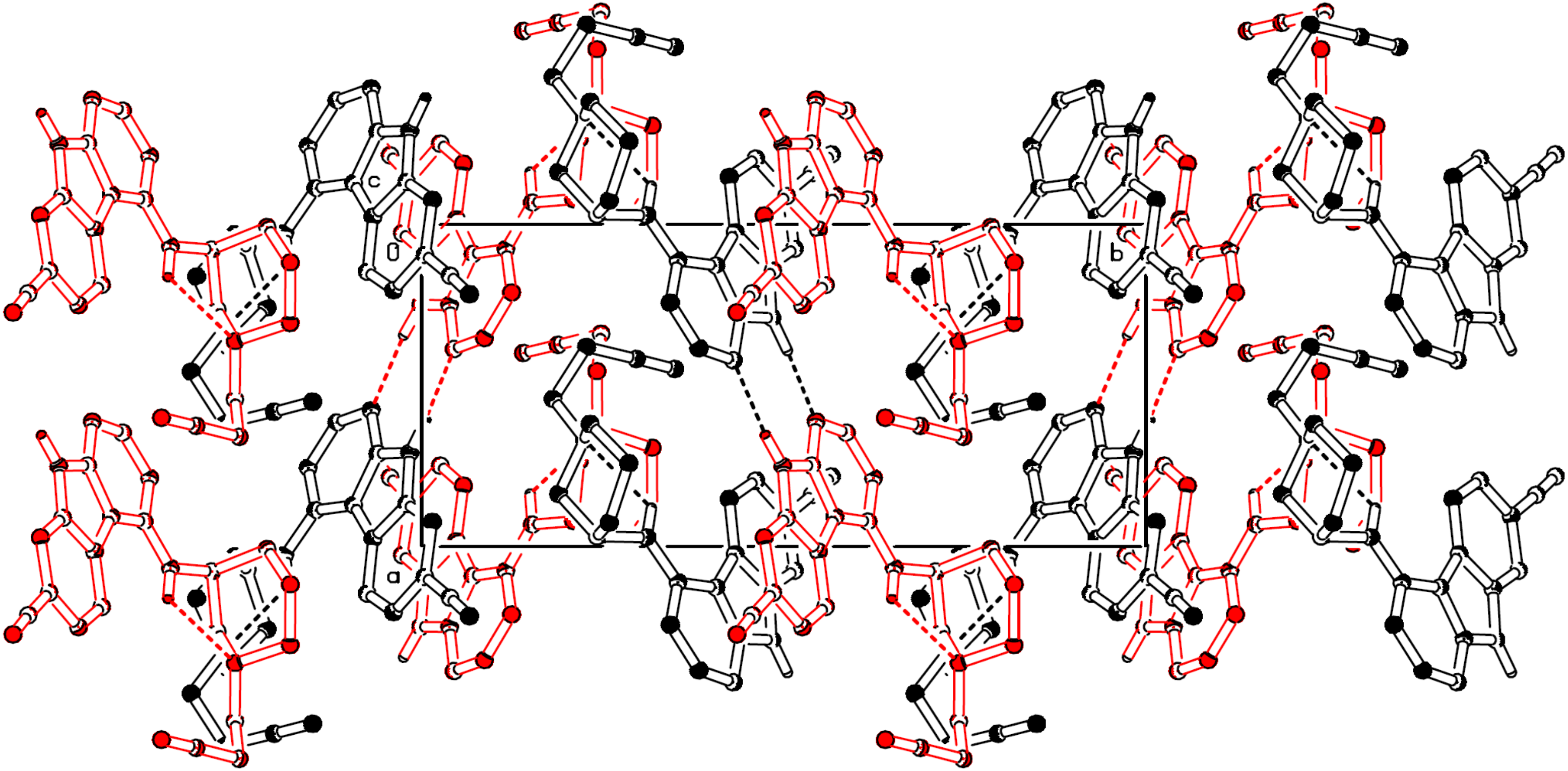
2.2. X-ray Structure
2.3. Biological Evaluation
3. Materials and Methods
3.1. General
3.2. Chemistry
3.2.1. tert-Butyl (S)-3-((7-iodo-9H-pyrimido[4,5-b]indol-4-yl)amino)piperidine-1-carboxylate (4)
3.2.2. (S)-7-Iodo-N-(piperidin-3-yl)-9H-pyrimido[4,5-b]indol-4-amine (5)
3.2.3. (S)-3-(3-((7-Iodo-9H-pyrimido[4,5-b]indol-4-yl)amino)piperidin-1-yl)propanenitrile (6)
3.2.4. (S)-3-(3-((7-((Trimethylsilyl)ethynyl)-9H-pyrimido[4,5-b]indol-4-yl)amino)piperidin-1-yl)propanenitrile (7)
3.2.5. (S)-3-(3-((7-Ethynyl-9H-pyrimido[4,5-b]indol-4-yl)amino)piperidin-1-yl)propanenitrile (2)
3.3. Biological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phukan, S.; Babu, V.; Kannoji, A.; Hariharan, R.; Balaji, V. GSK3β: Role in Therapeutic Landscape and Development of Modulators. Br. J. Pharmacol. 2010, 160, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Arciniegas Ruiz, S.M.; Eldar-Finkelman, H. Glycogen Synthase Kinase-3 Inhibitors: Preclinical and Clinical Focus on CNS-A Decade Onward. Front. Mol. Neurosci. 2022, 14, 792364. [Google Scholar] [CrossRef] [PubMed]
- Andreev, S.; Pantsar, T.; Ansideri, F.; Kudolo, M.; Forster, M.; Schollmeyer, D.; Laufer, S.A.; Koch, P. Design, Synthesis and Biological Evaluation of 7-Chloro-9H-pyrimido[4,5-b]indole-based Glycogen Synthase Kinase-3β Inhibitors. Molecules 2019, 24, 2331. [Google Scholar] [CrossRef] [PubMed]
- Andreev, S.; Pantsar, T.; Tesch, R.; Kahlke, N.; El-Gokha, A.; Ansideri, F.; Grätz, L.; Romasco, J.; Sita, G.; Geibel, C.; et al. Addressing a Trapped High-Energy Water: Design and Synthesis of Highly Potent Pyrimidoindole-Based Glycogen Synthase Kinase-3β Inhibitors. J. Med. Chem. 2022, 65, 1283–1301. [Google Scholar] [CrossRef] [PubMed]
- Andreev, S.; Pantsar, T.; El-Gokha, A.; Ansideri, F.; Kudolo, M.; Anton, D.B.; Sita, G.; Romasco, J.; Geibel, C.; Lämmerhofer, M.; et al. Discovery and Evaluation of Enantiopure 9H-pyrimido[4,5-b]indoles as Nanomolar GSK-3β Inhibitors with Improved Metabolic Stability. Int. J. Mol. Sci. 2020, 21, 7823. [Google Scholar] [CrossRef] [PubMed]
- Thorarensen, A.; Dowty, M.E.; Banker, M.E.; Juba, B.; Jussif, J.; Lin, T.; Vincent, F.; Czerwinski, R.M.; Casimiro-Garcia, A.; Unwalla, R.; et al. Design of a Janus Kinase 3 (JAK3) Specific Inhibitor 1-((2S,5R)-5-((7H-Pyrrolo[2,3-d]pyrimidin-4-yl)amino)-2-methylpiperidin-1-yl)prop-2-en-1-one (PF-06651600) Allowing for the Interrogation of JAK3 Signaling in Humans. J. Med. Chem. 2017, 60, 1971–1993. [Google Scholar] [CrossRef] [PubMed]
| Compound | IC50 (µM ± SEM) a |
|---|---|
| 1 | 1.18 ± 0.03 |
| 2 | 2.24 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreev, S.; Plank, N.; Schollmeyer, D.; Koch, P. (S)-3-(3-((7-Ethynyl-9H-pyrimido[4,5-b]indol-4-yl)amino)piperidin-1-yl)propanenitrile. Molbank 2022, 2022, M1437. https://doi.org/10.3390/M1437
Andreev S, Plank N, Schollmeyer D, Koch P. (S)-3-(3-((7-Ethynyl-9H-pyrimido[4,5-b]indol-4-yl)amino)piperidin-1-yl)propanenitrile. Molbank. 2022; 2022(3):M1437. https://doi.org/10.3390/M1437
Chicago/Turabian StyleAndreev, Stanislav, Nicole Plank, Dieter Schollmeyer, and Pierre Koch. 2022. "(S)-3-(3-((7-Ethynyl-9H-pyrimido[4,5-b]indol-4-yl)amino)piperidin-1-yl)propanenitrile" Molbank 2022, no. 3: M1437. https://doi.org/10.3390/M1437
APA StyleAndreev, S., Plank, N., Schollmeyer, D., & Koch, P. (2022). (S)-3-(3-((7-Ethynyl-9H-pyrimido[4,5-b]indol-4-yl)amino)piperidin-1-yl)propanenitrile. Molbank, 2022(3), M1437. https://doi.org/10.3390/M1437