Abstract
A new onoceranoid triterpenes, namely 3-hydroxy-8,14-secogammacera-7,14-dien-21-one (1), has been isolated from the fruit peels of Lansium domesticum Corr. cv kokossan. The structure of 1 was determined on the basis of spectroscopic data including infrared, 1D and 2D-NMR, as well as high resolution mass spectroscopy analysis. Compound 1 showed a weak activity against MCF-7 breast cancer cell lines.
1. Introduction
Lansium domesticum Corr. (Meliaceae) is a popular plant that widely grows in southeastern Asia [1,2]. L. domesticum Corr. cv kokossan (Meliaceae) is a higher tree growing up to 30 m in height, commonly called “kokosan” in Indonesia [3]. Several bioactive triterpenoids have been isolated from L. domesticum [4,5,6,7,8,9], of which some have shown potential anticancer [10], antibacterial [11], insecticides [12], antimalarial [13], antimutagenic [14,15], and antifeedant activities [16]. Previously, we isolated six triterpenes from the seeds and bark of L. domesticum Corr. cv kokossan [2,16]. In this paper, we report the isolation and structural elucidation of the new onoceranoid triterpenes 1 (Figure 1), along with its cytotoxic activity against MCF-7 breast cancer lines.
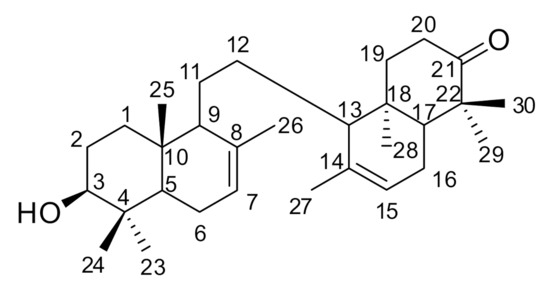
Figure 1.
Chemical Structure of compound 1.
2. Results
Extraction and Isolation
The dried fruit peel (1.7 kg) was extracted with n-hexane, ethyl acetate, and methanol three times, respectively, for 24 h, at room temperature. After solvent removal under reduced pressure, we obtained the n-hexane (86 g), ethyl acetate (110 g), and methanol (75 g) crude extracts. A portion of n-hexane (60 g) was subjected to vacuum liquid chromatography over silica gel using a 10% gradient of n-hexane/EtOAc (10:0–0:10) to afford eight fractions (A–H). Fraction D (6.7 g) was subjected to vacuum liquid chromatography, eluted by a 1% gradient of n-hexane/EtOAc (100:0–85:15) to afford five combined fractions (D1–D5). Fraction D4 was then subjected to silica gel column chromatography, eluted with n-hexane:acetone:methanol (7:2.5:0.5), resulting in four fractions (D4A–D4D). Compound 1 was obtained from fraction D4B as a white crystal (0.27 g).
3-Hydroxy-8,14-secogammacera-7,14-dien-21-one (1), white crystal, m.p. 153–155 °C, [α]20D − 3.0° (c 0.1, MeOH), HR-TOF-MS m/z 463.3569 [M + Na]+ (calcd. for C30H48O2Na, m/z 463.3552); IR (KBr) νmax (cm−1): 3533, 2932, 1700, 1456; 1H-NMR and 13C-NMR showed in the Table 1. Compound 1 was evaluated for its cytotoxic acitivity against MCF-7 breast cancer cell line, and compared to doxorubicin (35.7 µM) as a positive control. Compound 1 exhibited weak activity against MCF-7 with an IC50 value of 717.5 µM.

Table 1.
13C and 1H NMR Spectral Data of Compounds 1 in CDCl3.
3. Discussion
The molecular composition of 1 was proposed as C30H48O2 with seven degrees of unsaturation, based on HR-TOF-MS and NMR spectral data. Mass spectra showed molecular ion peak at m/z 463.3569 [M + Na]+, with calculated m/z 463.3552 for C30H48O2Na. The IR spectrum exhibited bands at νmax (cm−1) 3533 (hydroxy), 2932 (C-H stretching of aliphatic), 1700 (ketone), and 1456 (gem dimethyl).
The 13C NMR data (Table 1) with DEPT and HSQC experiments (Figures S2–S4) revealed the presence of total of 30 carbon signals, which were classified as eight methyls, eight methylenes, seven methines (two olefinic and one oxygenated), and seven quaternary carbons (two olefinic and one carbonyl). The two trisubstituted double bonds and the carbonyl in a system with seven degrees of unsaturation suggested that 1 possess a tetracyclic structure. Previous studies supported that evidence, indicating that compound 1 has an onoceranoid-type triterpenoid [3,14].
The 1H NMR spectrum of 1 (Figure S1) displayed the presence of eight methyl groups at δH (ppm) 1.00 (3H, Me-23), 0.87 (3H, Me-24), 0.99 (3H, Me-25), 1.75 (3H, Me-26), 1.72 (3H, Me-27), 0.76 (3H, Me-28), 1.12 (3H, Me-29), and 1.08 (3H, Me-30). Two olefinic protons (H-7 and H-15) were observed at δH (ppm) 5.45 and 5.42 (each 1H, brs), together with two vinyl methyls proton resonating at δH (ppm) 1.75 (3H, s, H-26) and δH 1.72 (3H, s, H-27), indicating two trisubstituted double bonds of 1. One oxygenated methine signal was also observed at δH 3.29 ppm (1H, dd, H-3).
The structure of 1 was further defined by 1H-1H COSY (correlated spectroscopy) and HMBC (heteronuclear multiple bond connectivity) spectra (Figure 2, Figures S5 and S6). The 1H-1H COSY spectra showed couplings between H-1 (δH 1.14)/H-2 (δH 1.65), H-2 (δH 1.65)/H-3 (δH 3.29), H-9 (δH 1.66)/H-11 (δH 2.10), H-11 (δH 2.10)/H-12 (δH 1.98), H-12 (δH 1.98)/H-13 (δH 1.59), and H-19 (δH 1.51)/H-20 (δH 2.77), supporting the presence of a onoceranoid-type triterpenoid structure. The oxygenated methine bearing a hydroxy group was located at C-3 by the HMBC correlations of H2-1 (δH 1.86), H3-23 (δH 1.00) and H3-24 (δH 0.87) to C-3 (δC 79.1). The Δ7,8 and Δ14,15 double bonds were assigned by the HMBC correlations from H3-26 (δH 1.75) to C-7 (δC 121.7), C-8 (δC 135.4), C-9 (δC 55.3); and H3-27(δH 1.72) to C-13 (δC 56.0), C-14 (δC 134.9), C-15 (δC 122.3). The ketone group was attached to C-21 by the HMBC correlations of H2-20 (δH 2.77) and H3-29 (δH 1.12) to C-21 (δC 217.0). This analysis indicated that compound 1 was similar to 3β-hydroxyonocera-8(26),14-dien-21-one [5], uniquely differing on the double bond position in the B ring. Based on the coupling constants of H-3 (dd, J = 11.4; 4.3 Hz), the configuration of the hydroxyl group at C-3 was indicated in the β-equatorial position [5,17]. From the analyses, compound 1 was determined as 3-hydroxy-8,14-secogammacera-7,14-dien-21-one.
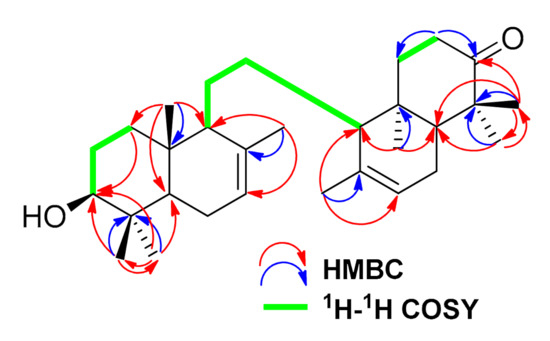
Figure 2.
1H-1H COSY and HMBC correlations of compound 1.
4. Materials and Methods
4.1. General Experimental Procedures
Mass spectrum was recorded on a waters Xevo QTOFMS (Waters, Milford, MA, USA). IR spectrum was measured on a One Perkin Elmer infrared-100 (Waltham, MA, USA) in KBr. NMR data were recorded on a Brucker spectrometer (Billerica, MA, USA) at 400 MHz for 1H and 120 MHz for 13C using TMS as an internal standard. Chromatographic separations were carried out on silica gel G60 (Merck, Darmstadt, Germany) and RP18 (Merck). TLC plates were precoated with silica gel GF254 (Merck, 0.25 mm) and detection was achieved by spraying with 10% (v/v) H2SO4 in ethanol, followed by heating.
4.2. Plant Material
The fruit peels of L. domesticum Corr. cv kokossan (Meliaceae) was collected from Cililin, West Java, Indonesia in April 2018. The plant was identified and deposited in The Herbarium of Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Indonesia (Identification Number: 195/HB/08/2018).
4.3. Cytotoxic Bioassay
Cytotoxicity of the compound against MCF-7 human breast cancer cells was measured using MTT (Methyl Thiazoldiphenyl-Tetrazoliumbromide) assay. Stock culture was grown in flasks, containing Roswell Park Memorial Institute (RPMI) medium, respectively supplemented with 10% (v/v) feta bovine serum (FBS) and 1% (v/v) penicillin-streptomicin as an antibiotic. The culture was incubated at 37 °C for 24 h. The medium was changed, and tumor cells were detached and seeded in 96-well microliter plates. After, 24 h, compounds were added to the wells. After 48 h, cell viability was determined by measuring the metabolic conversion of yellow salt or 3-(4,5-dimethyltiazol-2-yl)-2,5-diphenyltetrazolium bromide to its insoluble formazan, which has a purple color product resulting from reduction in viable cells. Insoluble formazan was diluted with DMSO. The MTT assay results were read using spectrophotometer UV at 450 nm. Compound 1 was evaluated at eight concentrations (15.9; 34.0; 70.3; 140.7; 283.6; 567.3; 1134.6; 2269.1 µM) in 100% DMSO with the final concentration of DMSO of 2.5% (v/v) in each well. Doxorubicin was used as a positive control. IC50 values were calculated by the linear regression method using Microsoft Excel software.
5. Conclusions
A new onoceranoid triterpene, namely 3-hydroxy-8,14-secogammacera-7,14-dien-21-one (1), was isolated from fruit peels of L. domesticum Corr. cv kokossan (Meliaceae). This compound exhibited weak cytotoxic activity against MCF-7 human breast cancer cell lines with IC50 value of 717.5 µM.
Supplementary Materials
The following are available online, Figure S1. 1H-NMR spectrum of 1 (500 MHz in CDCl3), Figure S2. 13C-NMR spectrum of 1 (125 MHz in CDCl3), Figure S3. DEPT-135°_spectrum of 1 (125 MHz in CDCl3), Figure S4. HSQC Spectrum of 1, Figure S5. 1H-1H COSY spectrum of 1, Figure S6. HMBC spectrum of 1, Figure S7. Infrared Spectrum of 1 (in KBr), Figure S8. HR-TOF-MS Spectrum of 1, Figure S9. TLC Profile of 1.
Author Contributions
Conceptualization, T.M. and U.S.; methodology, N.K.P. and Z.; software validation, S.F.; formal analysis, S.F.; investigation, R.M. and J.A.A.; resources, T.M. and M.Y.; data curation, N.K.P. and Z.; writing—original draft preparation, Z.; writing—review and editing, J.A.A. and M.Y.; visualization, R.M.; supervision, T.M., M.Y., and U.S.; project administration, N.K.P.; funding acquisition T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PTM, Ministry of Research, Technology and Higher Education, Indonesia, grant number: 1827/UN6.3.1/LT/2020 (T.M.).
Acknowledgments
We thank to Joko Kusmoro, M.P. at Jatinangor Herbarium for identification of the plant material, Ahmad Darmawan at the Research Center for Chemistry, Indonesian Science Institute, for performing the NMR measurements, Kansi at the Center Laboratory of Universitas Padjadjaran for performing the HR-TOF-MS measurements, Tenny Putri Wikayani and Nurul Qomarilla at Cells and Tissues Culture Laboratory, Faculty of Medicine, Universitas Padjadjaran for the cytotoxic assay.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Techavuthiporn, C. Langsat–Lansium Domesticum. Exotic Fruits Reference Guides; Rodrigues, S., de Brito, E.S., Silva, E.d.O., Eds.; Academic Press: London, UK, 2018; pp. 279–283. [Google Scholar]
- Mayanti, T.; Tjokronegoro, R.; Supratman, U.; Mukhtar, M.R.; Awang, K.; Hadi, A.H.A. Antifeedant triterpenoids from the seeds and bark of Lansium domesticum cv kokossan (Meliaceae). Molecules 2011, 16, 2785–2795. [Google Scholar] [CrossRef]
- Dong, S.H.; Zhang, C.R.; Dong, L.; Wu, Y.; Yue, J.M. Onoceranoid-type triterpenoids from Lansium domesticum. J. Nat. Prod. 2011, 74, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, M.; Nishide, H.; Hayashi, Y.; Kosela, S. The structure of lansioside A: A novel triterpene glycoside with amino-sugar from Lansium domesticum. Tetrahedron Lett. 1982, 23, 1349–1350. [Google Scholar] [CrossRef]
- Nishizawa, M.; Nishide, H.; Kosela, S.; Hayashi, Y. Structure of lansiosides: Biologically active new triterpene glycosides from Lansium domesticum. J. Org. Chem. 1983, 48, 4462–4466. [Google Scholar] [CrossRef]
- Tanaka, T.; Ishibashi, M.; Fujimoto, H.; Okuyama, E.; Koyano, T.; Kowiyhayakorn, T.; Hayashi, M.; Komiyama, K. New onoceranoid constituents from Lansium domesticum. J. Nat. Prod. 2002, 65, 1709–1711. [Google Scholar] [CrossRef] [PubMed]
- Habaguchi, K.; Watanabe, M.; Nakadaira, Y.; Nakanishi, K.; Kaing, A.K.; Lim, F.L. The full structures of lansic acid and its minor congener, an unsymmetric onoceradienedione. Tetrahedron Lett. 1986, 34, 3731–3734. [Google Scholar] [CrossRef]
- Mayanti, T.; Supratman, U.; Mukhtar, M.R.; Awang, K.; Ng, S.W. Kokosanolide from the seed of Lansium domesticum Corr. Acta Crystallogr. 2009, E65, o750. [Google Scholar]
- Supratman, U.; Mayanti, T.; Awang, K.; Mukhtar, M.R.; Ng, S.W. 14-Hydroxy-8,14-secogammacera-7-ene-3,21-dione from the bark of Lansium domesticum Corr. Acta Crystallogr. 2010, E66, o1621. [Google Scholar]
- Manosroi, A.; Jantrawut, P.; Sainakham, M.; Manosroi, W.; Manosroi, J. Anticaner activities of the extract from longkong (Lansium domesticum) young fruits. Pharm. Biol. 2012, 50, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Labrador, P.; Rideout, J.A. Antimicrobial terpenoid from Lansium domesticum. Philipp. Agric. Sci. 2006, 89, 101–105. [Google Scholar]
- Leatemia, J.A.; Isman, M.B. Insecticidal activity of crude seed extracts of Annona. spp., Lansium domesticum and Sandoricum koetjape against lepidopteran larvae. Phytopatasitica 2004, 32, 30–37. [Google Scholar] [CrossRef]
- Saewan, N.; Sutherland, J.D.; Chantrapromma, K. Antimalarial tetranortriterpenoids from the seed of Lansium domesticum Corr. Phytrochemistry 2006, 67, 2288–2293. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kitagawa, T.; Teo, S.; Anai, Y.; Ikeda, R.; Imahori, D.; Ahmad, H.S.; Watanabe, T. Structures and antimutagenic effects of onoceranoid-type triterpenoids from the leaves of Lansium domesticum. J. Nat. Prod. 2018, 81, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kitagawa, T.; Ohta, T.; Yoshida, T.; Imahori, D.; Teo, S.; Ahmad, H.S.; Watanabe, T. Structures of triterpenoids from the leaves of Lansium domesticum. J. Nat. Med. 2019, 73, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Mayanti, T.; Sianturi, J.; Harneti, D.; Darwati; Supratman, U.; Rosli, M.M.; Fun, H.K. 9,19-Cyclolanost-24-en-3-one,21,23-epoxy-21,22-dihydroxy (21R, 22S, 23S) from the leaves of Lansium domesticum Corr cv kokossan. Molbank 2015, 2015, M880. [Google Scholar] [CrossRef]
- Hou, Y.; Cao, S.; Brodie, P.J.; Miller, J.S.; Birkinshaw, C.; Andrianjafy, M.N.; Andriantsiferana, R.; Rasamison, V.E.; Tendyke, K.; Shen, Y.; et al. Euphane triterpenoids of Cassipourea lanceolata from the Madagascar rainforest. Phytochemistry 2010, 71, 669–674. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1 are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).