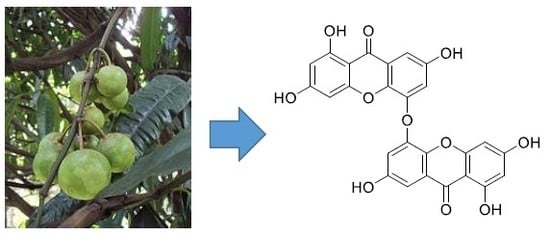
5,5′-Oxybis(1,3,7-trihydroxy-9H-xanthen-9-one): A New Xanthone from the Stem Bark of Garcinia porrecta (Clusiaceae)
Abstract
1. Introduction
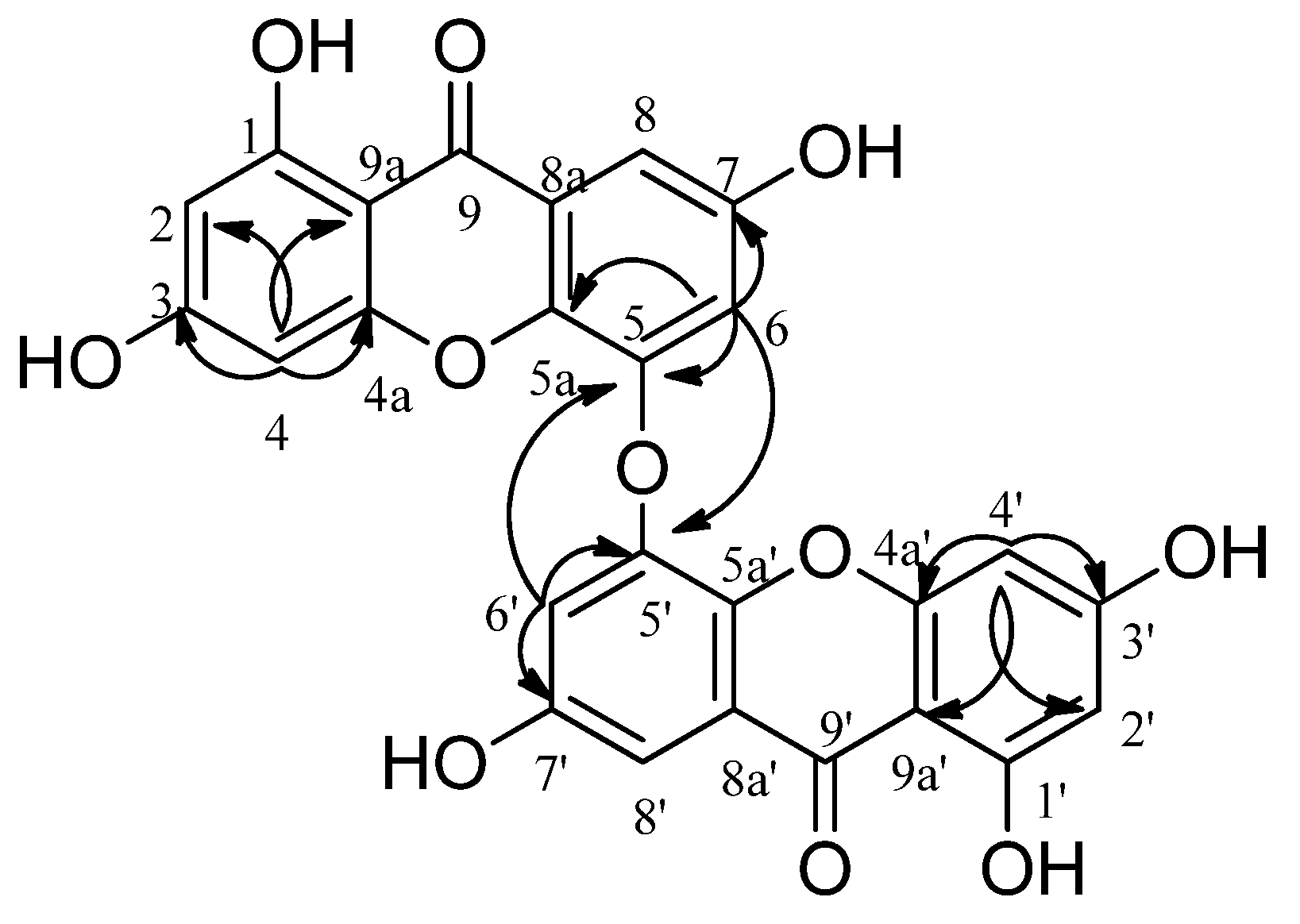
2. Results
Extraction and Isolation
3. Discussion
| Position | 1 | Gentisein [14] | ||
|---|---|---|---|---|
| δH [∑H, mult., J (Hz)] | δC (mult.) | δH [∑H, mult., J (Hz)] | δC | |
| 1 | 163.53 (s) | 162.5 | ||
| 2 | 6.22 (1H, s) | 97.69 (d) | 6.2 (1H, d, 1.7) | 97.8 |
| 3 | 163.22 (s) | 165.5 | ||
| 4 | 6.37 (1H, s) | 93.55 (d) | 6.3 (1H, d, 1.7) | 93.8 |
| 4a | 157.97 (s) | 157.6 | ||
| 5 | 143.38 (s) | 7.4 (1H, d, 9.0) | 119.1 | |
| 5a | 153.58 (s) | 149.1 | ||
| 6 | 7.53 (1H, s) | 108.21 (d) | 7.3 (1H, dd, 9.0, 2.8) | 124.5 |
| 7 | 151.67 (s) | 153.8 | ||
| 8 | 6.91 (1H, s) | 102.51 (d) | 7.4 (1H, d, 2.8) | 108.0 |
| 8a | 112.81 (s) | 120.5 | ||
| 9 | 179.60 (s) | 179.7 | ||
| 9a | 102.19 (s) | 102.0 | ||
| 1′ | 164.74 (s) | |||
| 2′ | 6.22 (1H, s) | 97.63 (d) | ||
| 3′ | 164.79 (s) | |||
| 4′ | 6.37 (1H, s) | 93.48 (d) | ||
| 4a′ | 157.96 (s) | |||
| 5′ | 143.38 (s) | |||
| 5a′ | 153.55 (s) | |||
| 6′ | 7.53 (1H, s) | 108.2 (d) | ||
| 7′ | 151.65 (s) | |||
| 8′ | 6.91 (1H, s) | 102.51 (d) | ||
| 8a′ | 122.77 (s) | |||
| 9′ | 179.53 (s) | |||
| 9a′ | 102.16 (s) | |||
| 1,1′-OH | 13.21 (1H, s) | |||
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sari, A.C.; Elya, B.; Katrin. Antioxidant activity and lipoxygenase enzyme inhibition assay with total flavonoid assay of Garcinia porrecta laness. stem bark extracts. Pharm. J. 2017, 9, 257–266. [Google Scholar] [CrossRef]
- Brito, L.C.; Berenger, A.L.R.; Figueiredo, M.R. An overview of anticancer activity of garcinia and hypericum. Food Chem. Toxicol. 2017, 109, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Kardono, L.B.S.; Hanafi, M.; Sherley, G.; Kosela, S.; Harrison, L.J. Bioactive constituents of Garcinia porrecta and Garcinia parvifolia grown in Indonesia. J. Biol. Sci. 2006, 9, 483–486. [Google Scholar]
- Putri, I.P. Effectivity of xanthone of mangosteen (Garcinia mangostana) rind as anticancer. J. Major. 2015, 4, 33–38. [Google Scholar]
- Chin, Y.W.; Kinghorn, A.D. Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Mini Rev. Org. Chem. 2008, 5, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Suksamrarn, S.; Komutiban, O.; Ratananukul, P.; Chimnoi, N.; Lartpornmatulee, N.; Suksamrarn, A. Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem. Pharm. Bull. 2006, 54, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Abdallah, H.M.; El-Halawany, A.M.; Radwan, M.F.; Shehata, I.A.; Al-Harshany, E.; Zayed, M.F.; Mohamed, G.A. Garcixanthones b an c, new xanthones from the pericarps of Garcinia mangostana and their cytotoxic activity. Phytochem. Lett. 2018, 25, 12–16. [Google Scholar] [CrossRef]
- Subarnas, A.; Diantinil, A.; Abdulah, R.; Zuhrotun, A.; Nugraha, P.A.; Hadisaputri, Y.E.; Puspitasari, I.M.; Yamazaki, C.; Kuwano, H.; Koyama, H. Apoptosis-mediated antiproliferative activity of friedolanostane triterpenoid isolated from the leaves of Garcinia celebica against MCF7 human breast cancer cell lines. Biomed. Rep. 2016, 4, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.Q.; Bui, A.; Nguyen, K.T.; Nguyen, V.T.; Trinh, B.T.D.; Nguyen, L.D. A depsidone and six triterpenoids from the bark of Garcinia celebica. Tetrahedron Lett. 2016, 57, 2524–2529. [Google Scholar] [CrossRef]
- Ritthiwigrom, T.; Laphookhieo, S.; Pyne, S.G. Chemical constituents and biological activities of Garcinia cowa Roxb. J. Sci. Technol. 2013, 7, 212–231. [Google Scholar]
- Shiono, Y.; Miyazani, N.; Murayama, T.; Koseki, T.; Harizon; Katja, D.G.; Supratman, U.; Nakata, J.; Kakihara, Y.; Saeki, M.; et al. GSK-3β inhibitory activities of novel dichloresorcinol derivatives from Cosmospora vilior isolated from a mangrove plant. Phytochem. Lett. 2016, 18, 122–127. [Google Scholar] [CrossRef]
- Susilawati, Y.; Nugraha, R.; Muhtadi, A.; Soetardjo, S.; Supratman, U. (S)-2-Methyl-2-(4-methylpent-3-enyl)-6-(propan-2-ylidene)-3,4,6,7-tetra-hydropyrano [4,3-g]chromen-9(2H)-one. Molbank 2015, 2015, M855. [Google Scholar] [CrossRef]
- Prakash, O.; Sing, R.; Kumar, S.; Srivastava, S.; Ved, A. Gentiana lutea Linn. (Yellow Gentian): A comprehensive review. J. Ayurdevic Herb. Med. 2017, 3, 175–181. [Google Scholar]
- Ebrahim, W.; El-Neketi, M.; Lewald, L.I.; Orfali, R.S.; Lin, W.; Rehberg, N.; Kalscheuer, R.; Daletos, G.; Proksch, P. Metabolites from the fungal endophyte Aspergillus austroafricanus in axenic culture and in fungul-bacterial mixed cultures. J. Nat. Prod. 2016, 79, 914–922. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safitri, A.N.; Nurlelasari; Mayanti, T.; Darwati; Supratman, U. 5,5′-Oxybis(1,3,7-trihydroxy-9H-xanthen-9-one): A New Xanthone from the Stem Bark of Garcinia porrecta (Clusiaceae). Molbank 2020, 2020, M1153. https://doi.org/10.3390/M1153
Safitri AN, Nurlelasari, Mayanti T, Darwati, Supratman U. 5,5′-Oxybis(1,3,7-trihydroxy-9H-xanthen-9-one): A New Xanthone from the Stem Bark of Garcinia porrecta (Clusiaceae). Molbank. 2020; 2020(3):M1153. https://doi.org/10.3390/M1153
Chicago/Turabian StyleSafitri, Ayu N., Nurlelasari, Tri Mayanti, Darwati, and Unang Supratman. 2020. "5,5′-Oxybis(1,3,7-trihydroxy-9H-xanthen-9-one): A New Xanthone from the Stem Bark of Garcinia porrecta (Clusiaceae)" Molbank 2020, no. 3: M1153. https://doi.org/10.3390/M1153
APA StyleSafitri, A. N., Nurlelasari, Mayanti, T., Darwati, & Supratman, U. (2020). 5,5′-Oxybis(1,3,7-trihydroxy-9H-xanthen-9-one): A New Xanthone from the Stem Bark of Garcinia porrecta (Clusiaceae). Molbank, 2020(3), M1153. https://doi.org/10.3390/M1153