Abstract
The 11a,12-dihydrobenzo[b]benzo[5,6][1,4]oxazino[2,3-e][1,4]oxazine heterocyclic system has been used in the construction of heteropropellanes, which attracted much attention not only on the possible modification of drugs, but also for novel materials with unusual and important physical properties. In this communication, the reaction of ethyl 2-(hydroxyimino)propanoate 1 with disulfur dichloride and o-aminophenol, which gave ethyl 11a,12-dihydrobenzo[b]benzo[5,6][1,4]oxazino[2,3-e][1,4]oxazine-5a(6H)-carboxylate in moderate yield, was described. The structure of the newly synthesized compound was established by means of elemental analysis, high resolution mass-spectrometry, 1H, 13C NMR and IR spectroscopy, mass-spectrometry and X-ray analysis.
1. Introduction
Disulfur dichloride is a well-known and frequently used sulfurating agent [1,2]. Sometimes it may act as oxidizing agent as well, giving compounds without additional sulfur atoms [3]. It was discovered that by the reaction of substituted acetoximes, including ethyl 2-(hydroxyimino)propanoate, with S2Cl2 4-substituted 1,2,3-dithiazolium salts are formed, which by treatment with oxygen, sulfur and nitrogen nucleophiles afforded 1,2,3-dithiazole 5-ones, 5-thiones and 5-phenylimines, respectively [4]. Recently it was found that, when o-aminophenol was added in the final step, 2-((4-aryl(hetaryl)-5H-1,2,3-dithiazol-5-ylidene)amino)phenols were isolated, although in low yield [5]. Herein, we report the synthesis of previously unknown ethyl 11a,12-dihydrobenzo[b]benzo[5,6][1,4]oxazino[2,3-e][1,4]oxazine-5a(6H)-carboxylate, which unexpectedly formed in the reaction of ethyl 2-(hydroxyimino)propanoate with disulfur dichloride and o-aminophenol.
2. Results and Discussion
Continuing the study of ethyl 2-(hydroxyimino)propanoate reactivity, it was treated with S2Cl2, pyridine in MeCN followed by addition of o-aminophenol. Unexpectedly, a new compound, 1, a yellow solid C17H16N2O4, was being formed instead of the expected ethyl 5-((2-hydroxyphenyl)imino)-5H-1,2,3-dithiazole-4-carboxylate, 2. According to NMR, mass and elemental analysis data compound 1 is formally a product in which two aminophenol molecules are cross-linked by two carbon atoms of ethyl 2-(hydroxyimino)propanoate. Structure 1 was finally proven by X-ray analysis as ethyl 11a,12-dihydrobenzo[b]benzo[5,6][1,4]oxazino[2,3-e][1,4]oxazine-5a(6H)-carboxylate (Scheme 1).
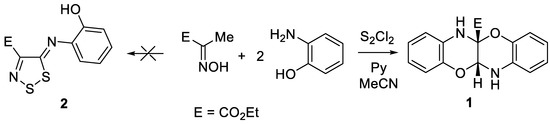
Scheme 1.
Synthesis of ethyl 11a,12-dihydrobenzo[b]benzo[5,6][1,4]oxazino[2,3-e][1,4]oxazine-5a(6H)-carboxylate 1.
It was assumed that ethyl 2-(hydroxyimino)propanoate may be dezoximated by S2Cl2 to ethyl 2-oxopropanoate and then reacted with sulfur containing species, such as S2Cl2 or S8, formed in this reaction, and o-aminophenol to give benzoxazinobenzoxazine 1. Similar transformation was described for 1,2-diphenylethan-1-one oxime (PhCH2C(O)Ph) with the formation of 5a,11a-diphenyl-5a,6,11a,12-tetrahydrobenzo[b]benzo[5,6][1,4]oxazino[2,3-e][1,4]oxazine 3 (Scheme 2) [6].
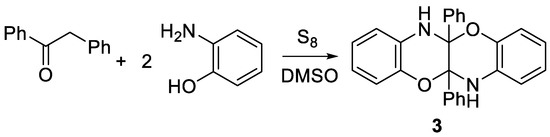
Scheme 2.
Synthesis of 5a,11a-diphenyl-5a,6,11a,12-tetrahydrobenzo[b]benzo[5,6][1,4]oxazino-[2,3-e][1,4]oxazine 3 from 1,2-diphenylethan-1-one oxime.
We checked this possibility and found that ethyl 2-oxopropanoate did not react with S2Cl2 and o-aminophenol. Therefore, the role of ethyl 2-(hydroxyimino)propanoate is crucial for the formation of oxazinooxazine 1, and it is necessary to find another mechanistic explanation for this reaction. So, the mechanism of this transformation is still unclear and requires further investigation.
The structure of oxazinooxazine 1 was confirmed by means of elemental analysis, high resolution mass-spectrometry, 1H, 13C NMR and IR spectroscopy, mass-spectrometry and X-ray analysis (Figure 1) (see Supplementary Materials).
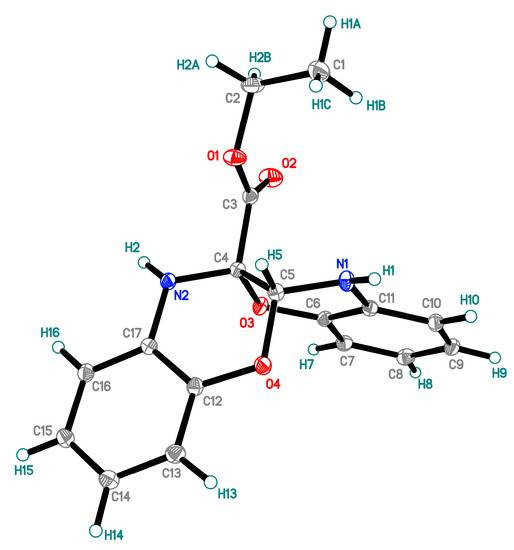
Figure 1.
X-ray structure of ethyl 11a,12-dihydrobenzo[b]benzo[5,6][1,4]oxazino-[2,3-e][1,4]oxazine-5a(6H)-carboxylate 1. Thermal ellipsoids are at 50% probability.
In conclusion, the synthesis of previously unknown ethyl 11a,12-dihydrobenzo[b]benzo[5,6][1,4]oxazino[2,3-e][1,4]oxazine-5a(6H)-carboxylate 1 from ethyl 2-(hydroxyimino)propanoate and disulfur dichloride was developed. The described experimental procedure may serve as an efficient basis for the synthesis of other fused oxazinooxazines. Fused with benzene rings, oxazinooxazines are important heterocyclic scaffold in the construction of heteropropellanes: structurally interesting propeller-like molecules with application in material sciences and medicinal chemistry [7].
3. Experimental Section
3.1. General Information
The solvents and reagents were purchased from commercial sources and used as received. Elemental analysis was performed on a 2400 Elemental Analyzer (Perkin Elmer Inc., Waltham, MA, USA). Melting point was determined on a Kofler hot-stage apparatus and is uncorrected. 1H were taken with a Bruker AM-300 machine (Bruker AXS Handheld Inc., Kennewick, WA, USA) (at frequencies of 300.1) and 13C NMR spectra were taken with a Bruker DRX-500 machine (Bruker AXS Handheld Inc., Kennewick, WA, USA) (125.8 MHz) in DMSO-d6 solution, with TMS as the standard. J values are given in Hz. MS spectrum (EI, 70 eV) was obtained with a Finnigan MAT INCOS 50 instrument (Hazlet, NJ, USA). IR spectrum was measured with a Bruker “Alpha-T” instrument in KBr pellet. High-resolution MS spectrum was measured on a Bruker micrOTOF II instrument (Bruker Daltonik Gmbh, Bremen, Germany) using electrospray ionization (ESI). The measurement was performed in a positive ion mode (interface capillary voltage: 4500 V) or in a negative ion mode (3200 V); mass range was from m/z 50 to m/z 3000 Da; external or internal calibration was performed with Electrospray Calibrant Solution (Fluka). Syringe injection was used for solutions in acetonitrile, methanol, or water (flow rate 3 L/min–1). Nitrogen was applied as a dry gas; interface temperature was set at 180 °C.
Crystal structure determination was performed in the Department of Structural Studies of Zelinsky Institute of Organic Chemistry, Moscow. X-ray diffraction data were collected at 100 K on a Bruker Quest D8 diffractometer (Bruker Corporation, Germany) equipped with a Photon-III area-detector (graphite monochromator, shutterless φ- and ω-scan technique), using Mo Kα-radiation (0.71073 Å). The intensity data were integrated by the SAINT program and were corrected for absorption and decay using SADABS [8]. The structure was solved by direct methods using SHELXS-2013 and refined on F2 using SHELXL-2018. All non-hydrogen atoms were refined with individual anisotropic displacement parameters. The positions of all hydrogen atoms were found from the electron density-difference map; these atoms were refined with individual isotropic displacement parameters. Cambridge Crystallographic Data Centre contains the supplementary crystallographic data for this paper No. CCDC 2012896. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
3.2. Synthesis of Ethyl 11a,12-Dihydrobenzo[b]benzo[5,6][1,4]oxazino[2,3-e][1,4]oxazine-5a(6H)-carboxylate 1
Pyridine (0.24 mL, 3 mmol) was added dropwise at 0–5 °C to a stirred solution of ethyl 2-(hydroxyimino)propanoate (1 mmol) and sulfur monochloride (0.16 mL, 2 mmol) in acetonitrile (10 mL) under inert atmosphere of argon. The mixture was stirred at 0 °C for 15–40 min. Then o-aminophenol (109 mg, 1 mmol) was added, the mixture was stirred at 0 °C for 30 min, and followed by addition of pyridine (0.16 mL, 2 mmol). The reaction mixture was stirred at room temperature for 2 h; filtered and solvents were evaporated. The residue was separated by column chromatography (Silica gel Merck 60, light petroleum and then light petroleum–CH2Cl2 mixtures). Yield 32 mg (11.2%), white crystals, mp 189–191 °C. Rf = 0.56 (CH2Cl2). IR (KBr), ν, cm−1: 3405 (N-H), 3360 (N-H), 3058, 2968, 2929, 2861, 1892, 1743, 1601, 1500, 1431, 1305, 1285, 1261, 1228, 1146, 1113, 1051, 998, 967, 931, 884, 832, 782, 750, 703, 611, 578, 537, 507, 460. 1H NMR (ppm, J/Hz): δ 6.94–6.75 (m, 8H, Ar), 5.51 (br s, 1H), 5.12 (d, 2H, NH, J = 9.17), 4.30–4.23 (m, 2H, CH2), 1.27 (t, 3H, CH3, J = 7.15). 13C NMR (ppm): δ 166.8, 141.9, 141.4, 128.7, 128.2, 122.6, 122.5, 121.3, 121.1, 117.2, 116.9, 115.6, 115.4, 110.4, 76.5, 63.0, 14.0. MS (EI, 70 Ev), m/z (I, %): 312 (M+, 100), 286 (10), 239 (24), 214 (13), 205 (76), 191 (11), 168 (20), 159 (11), 144 (12), 131 (19), 120 (61), 97 (9). HRMS (ESI-TOF): calcd for C17H16N2O4 [M + H]+ 313.1183; found m/z 313.1186. Anal. calcd. for C17H16N2O4: C, 65.38; H, 5.16; N, 8.97; found: C, 65.24; H, 5.02; N, 9.16%.
Crystallographic data are given in Table 1.

Table 1.
Crystal data and structure refinement for compound 1.
Supplementary Materials
The following are available online. CIF file, copies of 1H, 13C NMR, IR and mass-spectra for the compound 1.
Author Contributions
M.A.T. and V.V.P. synthetic experiments; L.S.K. analysis of experimental results and NMR data, O.A.R. writing the paper, supervision and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Ministry of Education and Science of the Russian Federation (FENU-2020-0019 (2020073GZ)) for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Konstantinova, L.S.; Rakitin, O.A. Sulfur monochloride in organic synthesis. Russ. Chem. Rev. 2014, 83, 225–250. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Rakitin, O.A. Design of sulfur heterocycles with sulfur monochloride: Retrosynthetic analysis and prospects. Mendeleev Commun. 2009, 19, 55–61. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Rakitin, O.A. Sulfur monochloride in the synthesis of heterocyclic compounds. Adv. Heterocycl. Chem. 2008, 96, 175–229. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Bol’shakov, O.I.; Obruchnikova, N.V.; Laborie, H.; Tonga, A.; Sopéna, V.; Lanneluc, I.; Picot, L.; Sablé, s.; Thiéry, V.; et al. One-pot synthesis of 5-phenylimino, 5-thione and 5-one-1,2,3-dithiazoles and evaluation of their antimicrobial and antitumor activity. Bioorg. Med. Chem. Lett. 2009, 19, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Baranovsky, I.V.; Konstantinova, L.S.; Tolmachev, M.A.; Popov, V.V.; Lyssenko, K.A.; Rakitin, O.A. Synthesis of 2-((2-(benzo[d]oxazol-2-yl)-2H-imidazol-4-yl)amino)-phenols from 2-((5H-1,2,3-dithiazol-5-ylidene)amino)phenols through Unprecedented Formation of Imidazole Ring from Two Methanimino Groups, N. D. Zelinsky Institute of Organic Chemistry Russian Academy of Sciences: Moscow, Russia, 2020; unpublished work.
- Nguyen, T.B.; Retailleau, P. Sulfur-catalyzed stereo and regioselective synthesis of heteropropellanes via oxidative condensation of cyclohexanones with 2-aminophenols. Adv. Synth. Catal. 2019, 361, 3588–3592. [Google Scholar] [CrossRef]
- Dilmaç, M.; Spuling, E.; de Meijere, A.; Bräse, S. Propellanes—from a chemical curiosity to “explosive” materials and natural products. Angew. Chem. Int. Ed. 2017, 56, 5684–5718. [Google Scholar] [CrossRef] [PubMed]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).