Synthesis of a New [3-(4-Chlorophenyl)-4-oxo-1,3-thiazolidin-5-ylidene]acetic Acid Derivative
Abstract
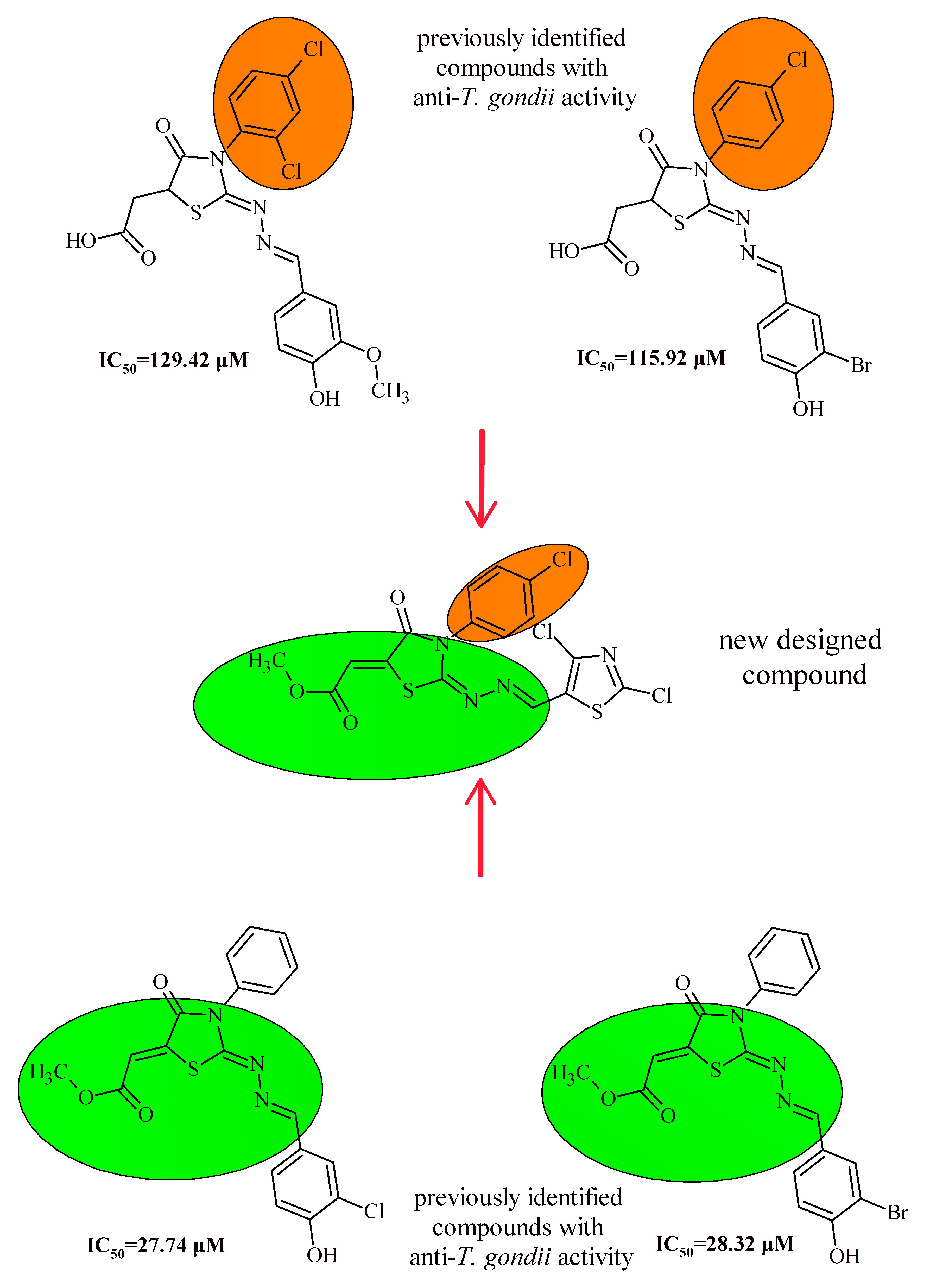
1. Introduction
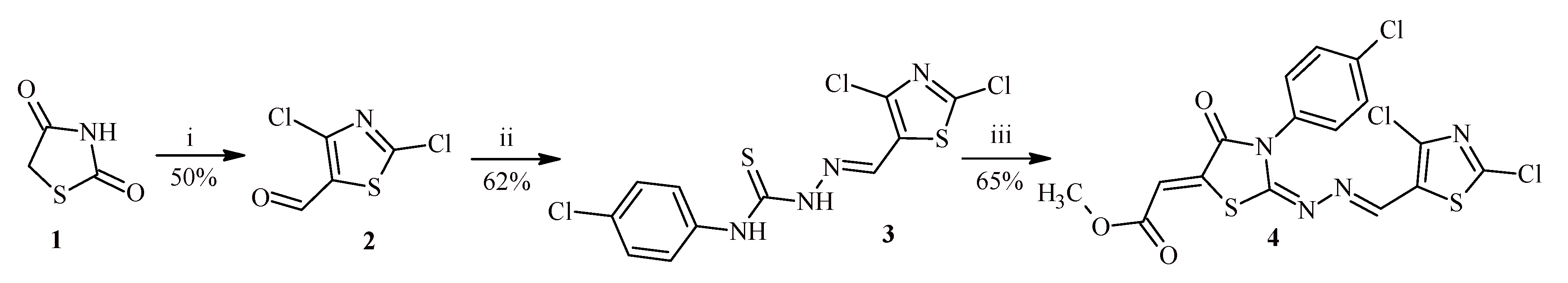
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. 4-(4-Chlorophenyl)-1-(2,4-dichloro-1,3-thiazol-5-yl)methylidene-3-thiosemicarbazide (3)
3.3. Methyl [3-(4-chlorophenyl)-2-{[(2,4-dichloro-1,3-thiazol-5-yl)methylidene]hydrazinylidene}-4-oxo-1,3-thiazolidin-5-ylidene]acetate (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. 5-Ene-4-thiazolidinones—An efficient tool in medicinal chemistry. Eur. J. Med. Chem. 2017, 140, 542–594. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Roman, O.; Lesyk, R. Synthetic approaches, structure activity relationship and biological applications for pharmacologically attractive pyrazole/pyrazoline–thiazolidine-based hybrids. Eur. J. Med. Chem. 2016, 113, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Chadna, N.; Bahia, M.S.; Kaur, M.; Silakari, O. Thiazolidine-2,4-dione derivatives: Programmed chemical weapons for key protein targets of various pathological conditions. Bioorg. Med. Chem. 2015, 23, 2953–2974. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.C.; Gupta, S.J.; Fatima, G.N.; Sonar, P.K.; Verma, A.; Saraf, S.K. 4-Thiazolidinones: The advances continue…. Eur. J. Med. Chem. 2014, 72, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.S.; Vora, D.K.; Ramaa, C.S. Thiazolidine-2,4-diones: Progress towards multifarious applications. Bioorg. Med. Chem. 2013, 21, 1599–1620. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Saraf, S.K. 4-Thiazolidinone—A biologically active scaffold. Eur. J. Med. Chem. 2008, 43, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, R.P.; Carvalho, C.S.; Pessanha, C.S.; de Lima, J.G.; de Faria, A.R.; Alves, A.J.; de Melo, E.J.T.; Goes, A.J.S. Synthesis of thiosemicarbazone and 4-thiazolidinone derivatives and their in vitro anti-Toxoplasma gondii activity. Bioorg. Med. Chem. Lett. 2005, 15, 2575–2578. [Google Scholar] [CrossRef] [PubMed]
- De Aquino, T.M.; Liesen, A.P.; da Silva, R.E.A.; Lima, V.T.; Carvalho, C.S.; de Faria, A.R.; de Araujo, J.M.; de Lima, J.G.; Alves, A.J.; de Melo, E.J.T.; et al. Synthesis, anti-Toxoplasma gondii and antimicrobial activities of benzaldehyde 4-phenyl-3-thiosemicarbazones and 2-[(phenylmethylene)hydrazono]-4-oxo-3-phenyl-5-thiazolidineacetic acids. Bioorg. Med. Chem. 2008, 16, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Liesen, A.P.; de Aquino, T.M.; Carvalho, C.S.; Lima, V.T.; de Araujo, J.M.; de Lima, J.G.; de Faria, A.R.; de Melo, E.J.T.; Alves, A.J.; Alves, E.W.; et al. Synthesis and evaluation of anti-Toxoplasma gondii and antimicrobial activities of thiosemicarbazides, 4-thiazolidinones and 1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010, 45, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Carradori, S.; Secci, D.; Bizzarri, B.; Chimenti, P.; De Monte, C.; Guglielmi, P.; Campestre, C.; Rivanera, D.; Bordon, C.; Jones-Brando, L. Synthesis and biological evaluation of anti-Toxoplasma gondii activity of a novel scaffold of thiazolidinone derivatives. J. Enzyme Inhib. Med. Chem. 2017, 32, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Trotsko, N.; Bekier, A.; Paneth, A.; Wujec, M.; Dzitko, K. Synthesis and in vitro anti-Toxoplasma gondii activity of novel thiazolidin-4-one derivatives. Molecules 2019, 24, 3029. [Google Scholar] [CrossRef] [PubMed]
- Kotlyar, V.N.; Pushkarev, P.A.; Orlov, V.D.; Chernenko, V.N.; Desenko, S.M. Thiazole analogs of chalcones, capable of functionalization at the heterocyclic nucleus. Chem. Heterocycl. Compd. 2010, 46, 334–341. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepański, J.; Tuszewska, H.; Trotsko, N. Synthesis of a New [3-(4-Chlorophenyl)-4-oxo-1,3-thiazolidin-5-ylidene]acetic Acid Derivative. Molbank 2020, 2020, M1150. https://doi.org/10.3390/M1150
Szczepański J, Tuszewska H, Trotsko N. Synthesis of a New [3-(4-Chlorophenyl)-4-oxo-1,3-thiazolidin-5-ylidene]acetic Acid Derivative. Molbank. 2020; 2020(3):M1150. https://doi.org/10.3390/M1150
Chicago/Turabian StyleSzczepański, Jacek, Helena Tuszewska, and Nazar Trotsko. 2020. "Synthesis of a New [3-(4-Chlorophenyl)-4-oxo-1,3-thiazolidin-5-ylidene]acetic Acid Derivative" Molbank 2020, no. 3: M1150. https://doi.org/10.3390/M1150
APA StyleSzczepański, J., Tuszewska, H., & Trotsko, N. (2020). Synthesis of a New [3-(4-Chlorophenyl)-4-oxo-1,3-thiazolidin-5-ylidene]acetic Acid Derivative. Molbank, 2020(3), M1150. https://doi.org/10.3390/M1150




