Abstract
N-aminorhodanine as well as isatin are highly solicited motifs known for their wide potential for biological activity. The objective of this work was to synthesize hybrid molecules as kinase inhibitors from these two motifs. In order to study the reactivity of the two active centers in aminorhodanine (N-amino group and the 5-methylene group) toward two carbonyl groups (aromatic aldehyde and ketone of isatin), we decided to carry out a one-pot multi-component reaction by simultaneously introducing aminorhodanine, isatin, and an aromatic aldehyde in ethanol in the presence of AcOEt. Under these conditions, this reaction led to a single adduct. The reaction product structure was confirmed by 1H, 13C-NMR, X-ray single crystal analysis, and high-resolution mass HRMS analysis. As a result, the method used has been very effective and totally stereo- and regioselective.
1. Introduction
The combination of two or more targeted therapeutic pharmacophores with different mechanisms of action into a single so-called hybrid molecule is one of the alternatives of conventional treatments of complex diseases that are multifactorial disorders involving various potential targets associated with their pathogenesis. Among the most requested pharmacophores in recent years due to their broad spectrum of biological activity are isatin and rhodamine, which share certain bioactivities such as the inhibition of kinases [1,2,3]. This is why there is interest in combining these two pharmacophores in hybrid molecules to study the evolution of their kinase inhibitory potency. Several numbers of successful isatin–rhodanine examples of pharmacophore hybridization strategies for drug design, discovery, and development have been reported [4,5,6,7]. Some of these have shown potent inhibitor activity against the UDP-Galactopyranose mutase UGM [8], β-lactamase infectious [9], and histone acetyltransferases [5] (a wide range of diseases).
Arylidenerhodanines, due to the Michael acceptor functionality, are often claimed as pan assay interference compounds (PAINS) mainly in high throughput screening (HTS) campaigns [10,11]. Despite this, medicinal chemists have not lost interest in them as evidenced by numerous publications in these last few years [12], as the relationships between the inactivity, specific activity, and artificial activity of ligands with potential liabilities are often highly complex and difficult to unravel [13]. Therefore, such protein-ligand complex must be more rigorously evaluated using two or more experimental methods to avoid false results.
In light of these findings, we were interested in the design, synthesis, and evaluation of a new series of hybrid molecules by combining the two pharmacophoric elements: N-aminorhodanine and isatin, which are present in a number of natural and synthetic agents. In this paper, we describe efforts toward the preparation and identification of new of isatin–aminorhodanine hybrid molecules: (5-[(Z)-5-chloro-2-oxoindolin-3-ylidene]-3-{(E)-[(4-hydroxyphenyl)imino]methyl}-2-thioxothiazolidin-4-one) via the one-pot multi-component reaction to later evaluate their kinase inhibitor activity.
2. Results and Discussion
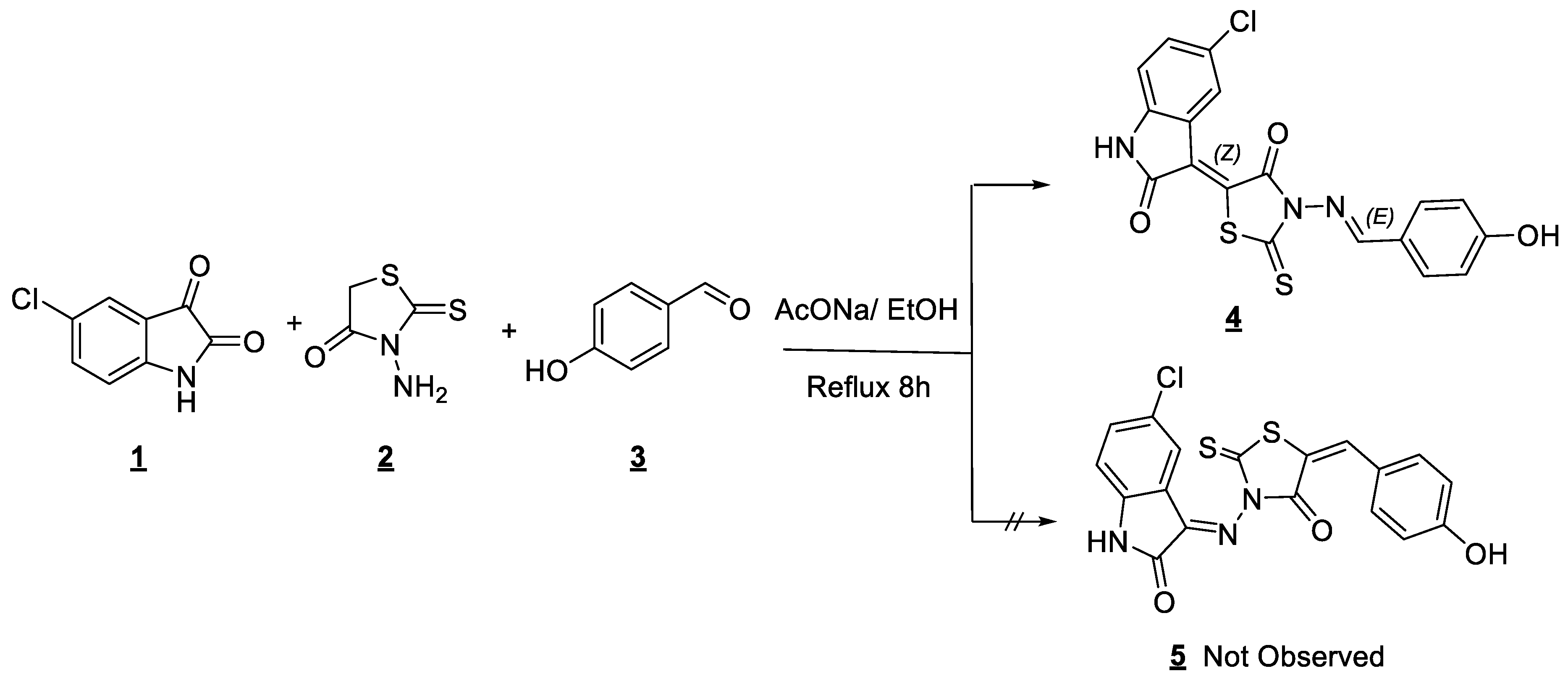
Oxindolinylidene-iminorhodanine 4 was prepared as single adduct by the one-pot multi-component reaction from 5-chloroisatin 1, N-aminorhodanine 2, 4-hydroxybenzaldehyde 3 by conventional refluxing in ethanol in the presence of sodium acetate (Scheme 1). The product was obtained pure in 70% of yield by simple washing and filtration from ethanol.
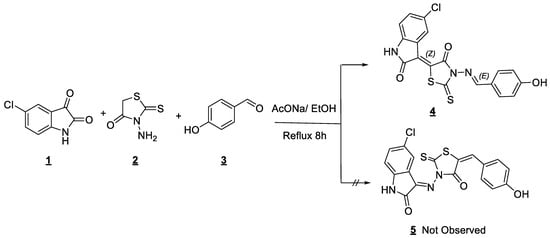
Scheme 1.
Synthesis of 5-[(Z)-5-chloro-2-oxoindolin-3-ylidene]-3-{(E)-[(4-hydroxyphenyl)imino]methyl}-2-thioxothiazolidin-4-one by conventional reflux.
The structure of the resulting compound was defined using 1H, 13C-NMR spectroscopy; HRMS, and X-ray single crystal analysis (see Supplementary Materials for original spectra).
The 1H-NMR spectra showed seven aromatic hydrogens of the two benzene rings in addition to the imine proton in the same region at 6.94–8.75 ppm. We also noted that no proton of the CH2 or NH2 group of the amino rhodanine appeared between 4 and 6 ppm, which means that aminorhodanine undergoes an addition on these two active centers.
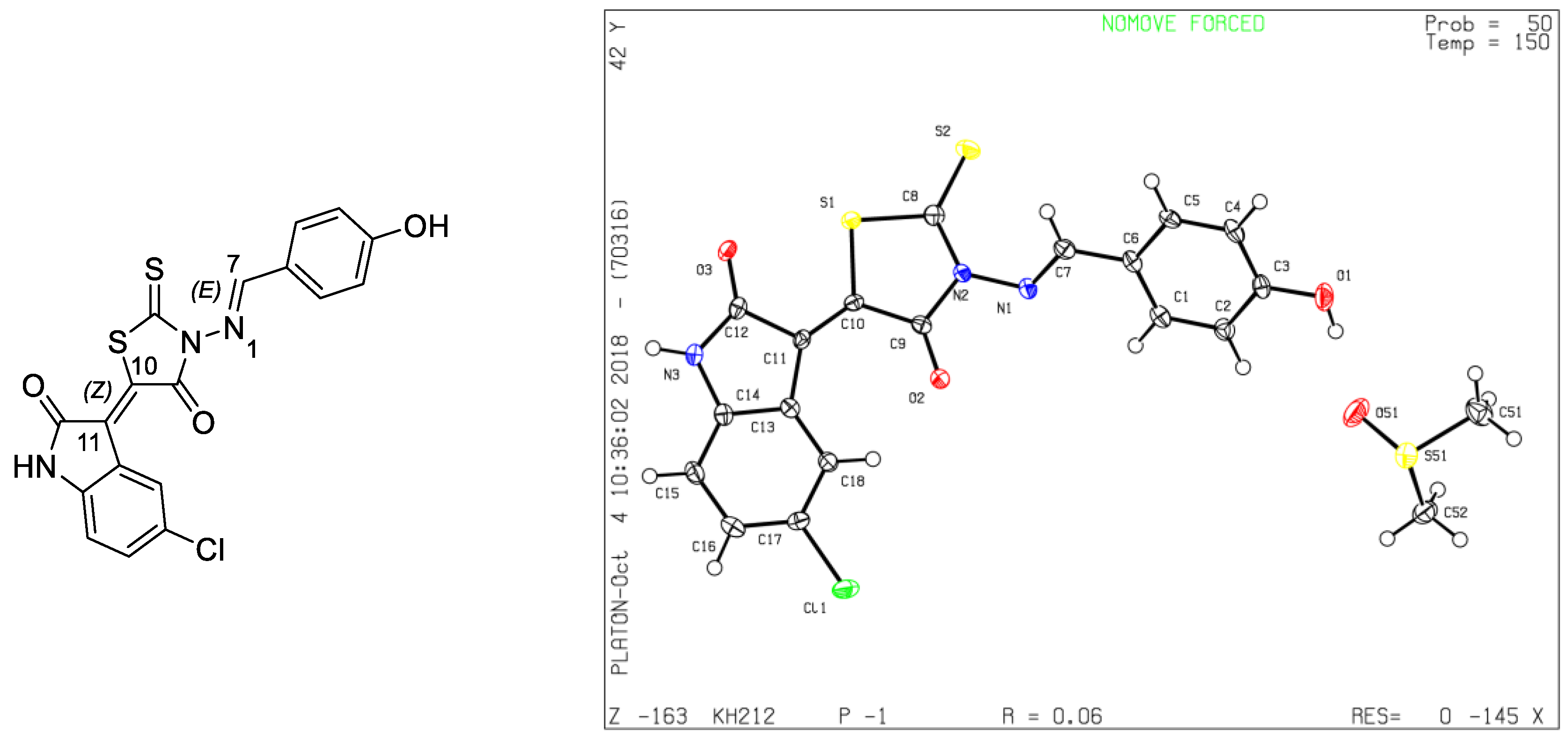
Single crystals of 4 suitable for X-ray diffraction [14] were grown via slow evaporation in DMSO-cyclohexane at room temperature. All crystallographic data and refinement details are presented in Table 1 and the X-ray diffraction structure of 4 is shown in Figure 1, which confirms that isatin was added to the CH2 group by a Knoevenagel reaction, while the aldehyde was fixed on the primary amine NH2 group forming a Schiff base. Through this analysis, we were also able to establish the stereochemistry of this molecule. Figure 1 shows that the double bond between C(10) and C(11) is of a Z-configuration, while that between N(1) and C(7) is of an E-configuration.

Table 1.
Crystal data and structure refinement details.
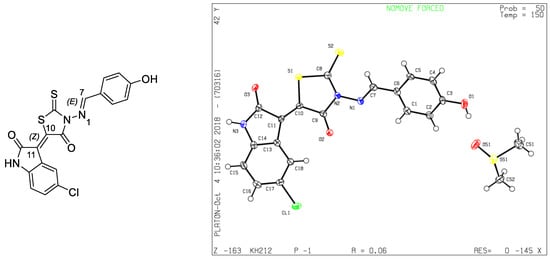
Figure 1.
General view of molecule 4 with atomic numbering.
3. Materials and Methods
3.1. General Information
All reagents were purchased from Acros (Geel, Belgium), Aldrich (Saint Louis, MI, USA), and were used without further purification. Melting points were determined on a Kofler melting point apparatus (Wagner & Munz, Munich, Germany), and were uncorrected. 1H-NMR spectra were recorded on a BRUKER AC 300 P (300 MHz) spectrometer (Bruker, Bremen, Germany), and 13C-NMR spectra on a BRUKER AC 300 P (75 MHz, Bruker) spectrometer. Chemical shifts were expressed in parts per million downfield from tetramethylsilane as an internal standard. Data are given in the following order: δ value, multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad), number of protons, and coupling constants J is given in Hz. High-resolution mass spectra (HMRS) were recorded on a Bruker Micro-Tof-Q II (Bruker) or on a Waters Q-Tof 2 at the CRMPO (Centre Régional de Mesures Physiques de l’Ouest, Rennes, France) using positive ion Electro-Spray Ionization (ESI, Waters, Manchester, UK). For the X-ray diffraction experiment, an appropriate single crystal of the title compound was selected and x-ray-data were collected using a STOE IPDS II single crystal X-ray diffractometer equipped with a two-dimensional image plate detector and with graphite monochromated MoKα radiation (λ = 0.71073 Å). The SHELXS [15] program was used to solve the structure by direct methods and the SHELXL [16] program was used for refinement through the full-matrix least-squares method. These two programs were implemented in WinGX [17] software. All atoms, except for the hydrogen atoms, were refined anisotropically. Hydrogen atoms were fixed geometrically at their calculated positions. Reflections with were used in the structure determination. Structural results are presented using the Mercury program [18].
General Procedure for Synthesis and Characterization of 4
A mixture of aminorhodanine (0.503 g, 3.4 mmol), 5-chloroisatin (0.617 g, 3.4 mmol), and of 4-hydroxybenzaldehyde (0.415 g, 3.4 mmol) in 10 mL of ethanol was refluxed for 8 h. The reaction mixture was allowed to cool down at room temperature and the crude product was purified by recrystallization from ethanol to furnish compound 4 (0.98 g, 70% yield) as a fair brown solid (mp > 300 °C). The structure of 4 was confirmed by the reported spectral analysis as follows: 1H-NMR (300 MHz, DMSO-d6) δ 11.42 (s, 1H, NH), 10.52 (s, 1H, OH), 8.73 (d, J = 11.9 Hz, 2H, =CHar, =CH), 7.82 (d, J = 8.2 Hz, 2H, =CHar), 7.46 (d, J = 7.5 Hz, 1H, =CHar), 6.96 (m, 3H, =CHar). 13C-NMR (75 MHz, DMSO) δ 191.2, 171.7, 168.2, 163.2, 162.8, 143.8, 132.8, 132.3, 131.9, 127.5, 126.5, 124.4, 123.1, 121.5, 116.6, and 112.6. HRMS (ESI) [M + Na]+ for: (C18H10N4O5NaS2) m/z, z = 1, calcd: 448.99848 m/z found: 448.9986 [M + K]+ (C18H10N4O5S2K) m/z, z = 1 , calcd: 464.97242 m/z found: 464.9721.
4. Conclusions
This result leads us to conclude that by using these conditions, the one-pot multi-components reaction is totally regio-selective in favor of the Knoevenagel condensation between CH2 in aminorhodanine with isatin and the formation of the Schiff base between NH2 of aminorhodanine and 4-hydroxybenzaldehyde and is totally stereo-selective, leading to a single stereoisomer Z-E (Figure 1).
Supplementary Materials
The following are available online: 1H, 13C-NMR and crystallographic data for 4 in crystallographic information file (CIF) format. CCDC 1871619 also contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via: http://www.ccdc.cam.ac.uk/conts/retrieving.html.
Author Contributions
All authors contributed equally to this work. Synthesis and spectroscopic characterization was carried out by K.K., A.S., and S.S.-B. The manuscript was written by A.S.
Funding
This research received no external funding.
Acknowledgments
The authors are thankful to Vincent Dorcet and the Centre de DIFfractométrie X, University of Rennes1 France for performing the X-ray crystallography analysis.
Conflicts of Interest
The authors have no conflicts of interest.
References and Note
- Lockman, J.W.; Reeder, M.D.; Robinson, R.; Ormonde, P.A.; Cimbora, D.M.; Williams, B.L.; Willardsen, J.A. Oxindole derivatives as inhibitors of TAK1 kinase. Bioorg. Med. Chem. Lett. 2011, 21, 1724–1727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Di, H.; Hanson, P.J.; Li, H.; Guo, H.; Ye, X.; Hemida, M.G.; Wang, L.; Tong, Y.; Qiu, Y.; et al. Antiviral Activity of an Isatin Derivative via Induction of PERK-Nrf2-Mediated Suppression of Cap-Independent Translation. ACS Chem. Biol. 2014, 9, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Bharti, S.K.; Budhwani, A.K. 3D-QSAR and virtual screening studies of thiazolidine-2,4-dione analogs: Validation of experimental inhibitory potencies towards PIM-1 kinase. J. Mol. Struct. 2017, 1133, 278–293. [Google Scholar] [CrossRef]
- Hueso-Falcón, I.; Amesty, Á.; Anaissi-Afonso, L.; Lorenzo-Castrillejo, I.; Machín, F.; Estévez-Braun, A. Synthesis and biological evaluation of naphthoquinone-coumarin conjugates as topoisomerase II inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. 5-Ene-4-thiazolidinones–An efficient tool in medicinal chemistry. Eur. J. Med. Chem. 2017, 140, 542–594. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Ge, H.; Song, M.; Chen, J.H.; Zhou, H.; Qi, Q.; Wang, F.; Ma, X.; Yang, X.; Zhang, G.; et al. Discovery of Imigliptin, a Novel Selective DPP-4 Inhibitor for the Treatment of Type 2 Diabetes. ACS Med. Chem. Lett. 2014, 5, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tawa, G.; Wallqvist, A. Classification of scaffold-hopping approaches. Drug Discov. Today 2012, 17, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.E.; May, J.F.; Kiessling, L.L. Chemical Probes of UDP-Galactopyranose Mutase. Chem. Biol. 2006, 13, 825–837. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Furdas, S.D.; Shekfeh, S.; Kannan, S.; Sippl, W.; Jung, M. Rhodanine carboxylic acids as novel inhibitors of histone acetyltransferases. MedChemComm 2012, 3, 305–311. [Google Scholar] [CrossRef]
- Baell, J.B. Observations on screening-based research and some concerning trends in the literature. Future Med. Chem. 2010, 2, 1529–1546. [Google Scholar] [CrossRef] [PubMed]
- Glaser, J.; Holzgrabe, U. Focus on PAINS: False friends in the quest for selective anti-protozoal lead structures from Nature? Med. Chem. Comm. 2016, 7, 214–223. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. Recent developments with rhodanine as a scaffold for drug discovery. Expert Opin. Drug Discov. 2017, 12, 1233–1252. [Google Scholar] [CrossRef] [PubMed]
- Gilberg, E.; Gütschow, M.; Bajorath, J. X-ray Structures of Target–Ligand Complexes Containing Compounds with Assay Interference Potential. J. Med. Chem. 2018, 61, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- The supplementary crystallographic data have been deposited at the Cambridge Crystallographic Data Centre (CCDC), 12 Union ROAD, Cambridge CB2 1EZ (UK), Tel.: (þ44) 1223/336 408, fax: (þ44) 1223/336 033, E-mail: Deposit@ccdc.cam.ac.uk, World Wide Web: http://www.ccdc.cam.ac.uk (deposition No. CCDC 1871619).
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Mercury—Crystal Structure Visualisation, Exploration and Analysis Made Easy. Available online: https://www.ccdc.cam.ac.uk/solutions/csd-system/Components/Mercury/ (accessed on 11 January 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).