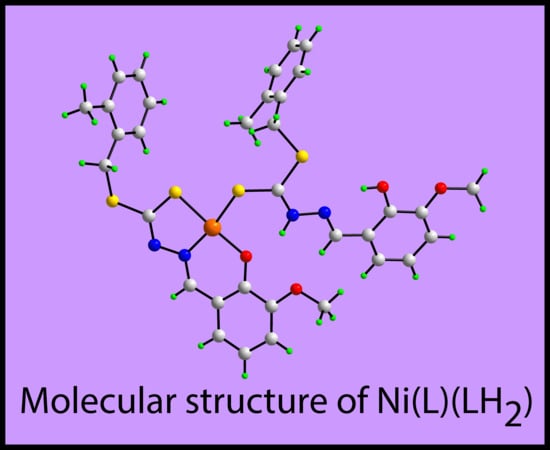
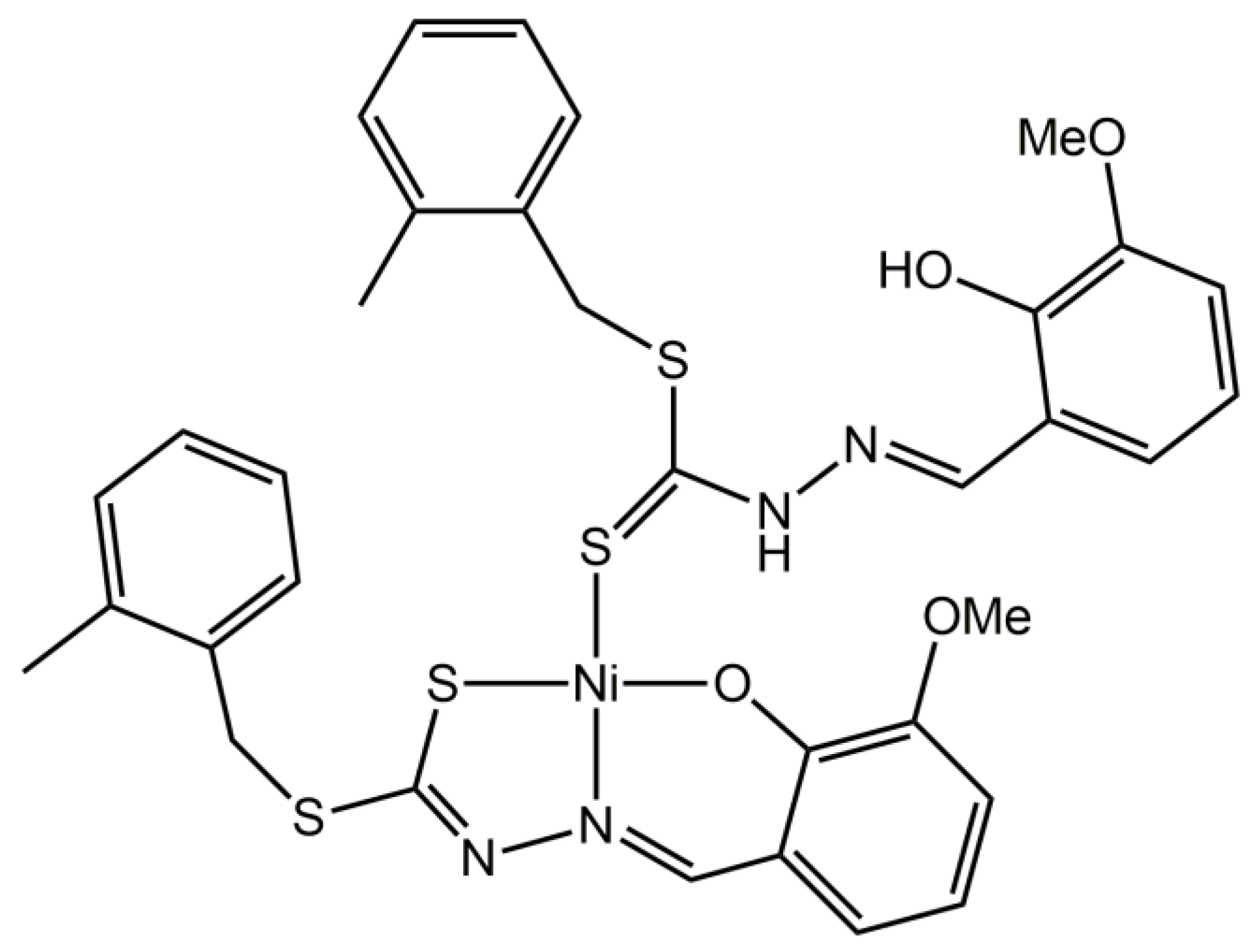
A Ternary Nickel(II) Schiff Base Complex Containing Di-Anionic and Neutral Forms of a Dithiocarbazate Schiff Base
Abstract
:1. Introduction
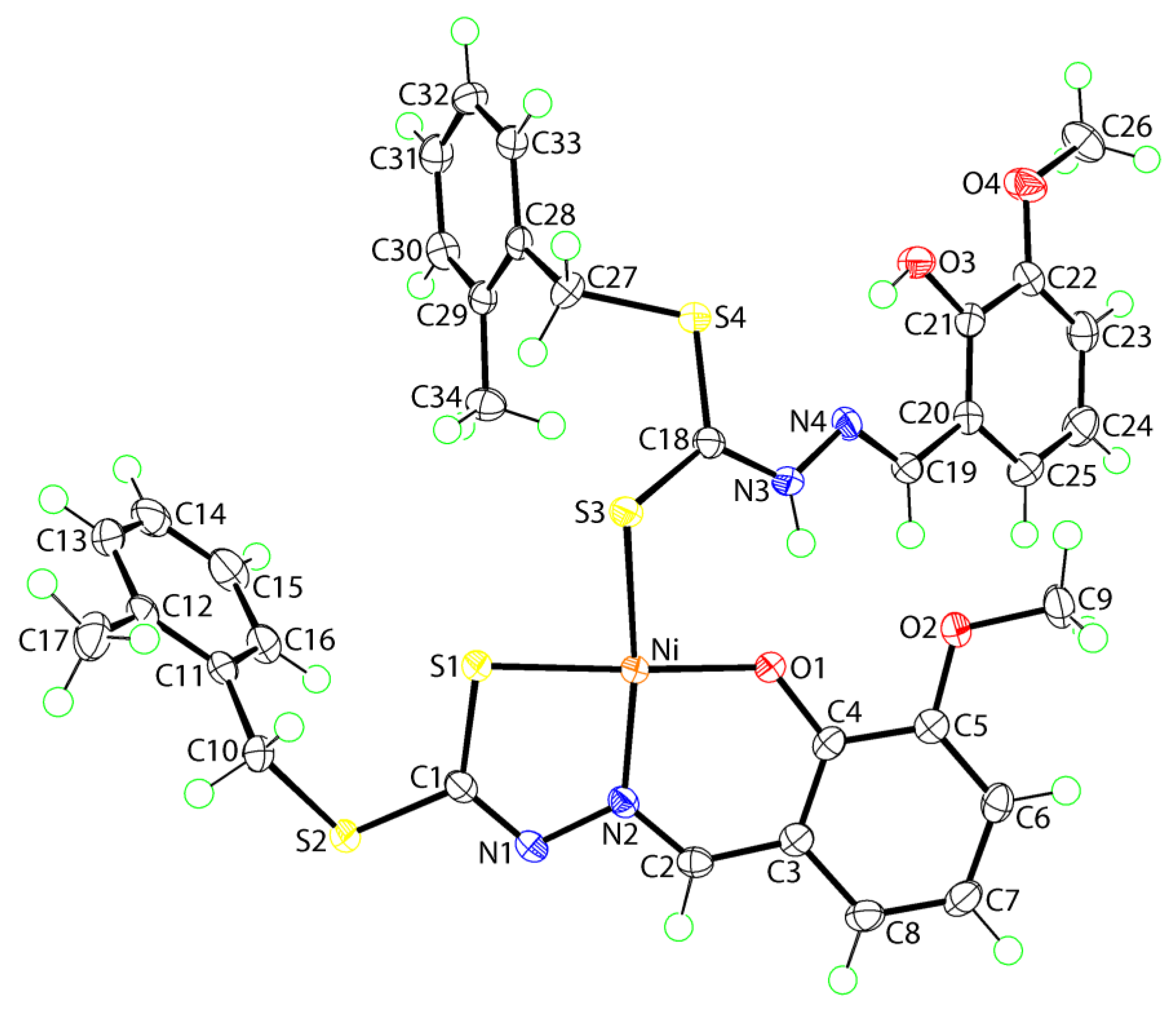
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization of 1
3.3. Crystallography
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, M.A.; Livingstone, S.E. Metal complexes of sulphur-nitrogen chelating agents. Inorg. Chim. Acta 1971, 5, 119–123. [Google Scholar] [CrossRef]
- Ali, M.A.; Livingstone, S.E. Metal chelates of dithiocarbazic acid and its derivatives. II. Complexes of the schiff base formed by condensation of S-methyldithiocarbazate with acetone and pyridine-2-aldehyde. Inorg. Chim. Acta 1971, 5, 493–498. [Google Scholar] [CrossRef]
- Mokhtaruddin, N.S.M.; Yusof, E.N.M.; Ravoof, T.B.S.A.; Tiekink, E.R.T.; Veerakumarasivam, A.; Tahir, M.I.M. Unusual saccharin-N,O (carbonyl) coordination in mixed-ligand copper(II) complexes: Synthesis, X-ray crystallography and biological activity. J. Mol. Struct. 2017, 1139, 1–9. [Google Scholar] [CrossRef]
- How, F.N.-F.; Crouse, K.A.; Tahir, M.I.M.; Tarafder, M.T.H.; Cowley, A.R. Synthesis, characterization and biological studies of S-benzyl-β-N-(benzoyl) dithiocarbazate and its metal complexes. Polyhedron 2008, 27, 3325–3329. [Google Scholar] [CrossRef]
- Akbar Ali, M.; Huq Mirza, A.; Wei, L.K.; Bernhardt, P.V.; Atchade, O.; Song, X.; Eng, G.; May, L. Synthesis and characterization of pentagonal bipyramidal organotin(IV) complexes of 2,6-diacetylpyridine Schiff bases of S-alkyl- and aryldithiocarbazates. J. Coord. Chem. 2010, 63, 1194–1206. [Google Scholar] [CrossRef]
- Ravoof, T.B.S.A.; Crouse, K.A.; Tahir, M.I.M.; How, F.N.F.; Rosli, R.; Watkins, D.J. Synthesis, characterization and biological activities of 3-methylbenzyl 2-(6-methylpyridin-2-ylmethylene)hydrazine carbodithioate and its transition metal complexes. Transit. Met. Chem. 2010, 35, 871–876. [Google Scholar] [CrossRef]
- Omar, S.A.; Ravoof, T.B.S.A.; Tahir, M.I.M.; Crouse, K.A. Synthesis and characterization of mixed-ligand copper(II) saccharinate complexes containing tridentate NNS Schiff bases. X-ray crystallographic analysis of the free ligands and one complex. Transit. Met. Chem. 2013, 39, 119–126. [Google Scholar] [CrossRef]
- Low, M.L.; Maigre, L.; Tahir, M.I.M.; Tiekink, E.R.T.; Dorlet, P.; Guillot, R.; Crouse, K.A. New insight into the structural, electrochemical and biological aspects of macrocyclic Cu(II) complexes derived from S-substituted dithiocarbazate schiff bases. Eur. J. Med. Chem. 2016, 120, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, M.T.H.; Ali, A.M.; Wong, Y.W.; Wong, S.H.; Crouse, K.A. Complexes of transition and nontransition metals of dithiocarbazate ion and their biological activities. Inorg. Met. Org. Chem. 2001, 31, 115–125. [Google Scholar] [CrossRef]
- Yusof, E.N.M.; Latif, M.A.M.; Tahir, M.I.M.; Sakoff, J.A.; Simone, M.I.; Page, A.J.; Veerakumarasivam, A.; Tiekink, E.R.T.; Ravoof, T.B.S.A. o-Vanillin derived Schiff bases and their organotin(IV) compounds: Synthesis, structural characterisation, in-silico studies and cytotoxicity. Int. J. Mol. Sci. 2019, 20, 854. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.V.; Barra, C.V.; Mauro, A.E.; Carlos, I.Z.; Nauton, L.; El Ghozzi, M.; Gautier, A.; Morel, L.; Netto, A.V.G. Synthesis, characterization, X-ray structure, DNA cleavage, and cytotoxic activities of palladium(II) complexes of 4-phenyl-3-thiosemicarbazide and triphenylphosphane. Eur. J. Inorg. Chem. 2013, 4499–4505. [Google Scholar] [CrossRef]
- Malenov, D.P.; Janjić, G.V.; Medaković, V.B.; Hall, M.B.; Zarić, S.D. Noncovalent bonding: Stacking interactions of chelate rings of transition metal complexes. Coord. Chem. Rev. 2017, 345, 318–341. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Supramolecular assembly based on “emerging” intermolecular interactions of particular interest to coordination chemists. Coord. Chem. Rev. 2017, 345, 209–228. [Google Scholar] [CrossRef]
- Ali, M.A.; Guan, T.S.; Bhattacharjee, P.; Butcher, R.J.; Jasinski, J.P.; Li, Y. Nickel(II) and copper(II) complexes of ONS ligands formed from 2-hydroxyacetophenone and S-alkyldithiocarbazates and the X-ray crystal structure of the [Ni(Ap-SMe)py] complex (Ap-SMe = anion of the 2-hydroxyacetophenone Schiff base of S-methyl-dithiocarbazate). Transit. Met. Chem. 1996, 21, 351–357. [Google Scholar] [CrossRef]
- Takjoo, R.; Centore, R. Synthesis, X-ray structure, spectroscopic properties and DFT studies of some dithiocarbazate complexes of nickel(II). J. Mol. Struct. 2013, 1031, 180–185. [Google Scholar] [CrossRef]
- Ali, M.A.; Tan, A.L.; Mirza, A.H.; Santos, J.H.; Abdullah, A.H.H. Synthesis, structural characterization and electrochemical studies of nickel(II), copper(II) and cobalt(III) complexes of some ONS donor ligands derived from thiosemicarbazide and S-alkyl/aryl dithiocarbazates. Transit. Met. Chem. 2012, 37, 651–659. [Google Scholar] [CrossRef]
- Agilent Technologies Inc. CrysAlis PRO; Agilent Technologies Inc.: Santa Clara, CA, USA, 2011. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Brandenburg, K.; Putz, H. DIAMOND; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
Sample Availability: A sample of the complex is not available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusof, E.N.M.; Nasri, N.M.; Ravoof, T.B.S.A.; Tiekink, E.R.T. A Ternary Nickel(II) Schiff Base Complex Containing Di-Anionic and Neutral Forms of a Dithiocarbazate Schiff Base. Molbank 2019, 2019, M1057. https://doi.org/10.3390/M1057
Yusof ENM, Nasri NM, Ravoof TBSA, Tiekink ERT. A Ternary Nickel(II) Schiff Base Complex Containing Di-Anionic and Neutral Forms of a Dithiocarbazate Schiff Base. Molbank. 2019; 2019(2):M1057. https://doi.org/10.3390/M1057
Chicago/Turabian StyleYusof, Enis Nadia Md, Nazhirah Muhammad Nasri, Thahira B. S. A. Ravoof, and Edward R.T. Tiekink. 2019. "A Ternary Nickel(II) Schiff Base Complex Containing Di-Anionic and Neutral Forms of a Dithiocarbazate Schiff Base" Molbank 2019, no. 2: M1057. https://doi.org/10.3390/M1057
APA StyleYusof, E. N. M., Nasri, N. M., Ravoof, T. B. S. A., & Tiekink, E. R. T. (2019). A Ternary Nickel(II) Schiff Base Complex Containing Di-Anionic and Neutral Forms of a Dithiocarbazate Schiff Base. Molbank, 2019(2), M1057. https://doi.org/10.3390/M1057