Abstract
A minor byproduct in the reaction of (S)-prolinol with thiophosgene in the presence of triethylamine is identified as a novel tricyclic dipyrrolidino-1,3,6-oxadiazocane-2-thione, the first example of such a ring system, and a representative of the uncommon, but useful 1,3,6-oxadiazocanes. A mechanism is proposed for its formation.
1. Introduction
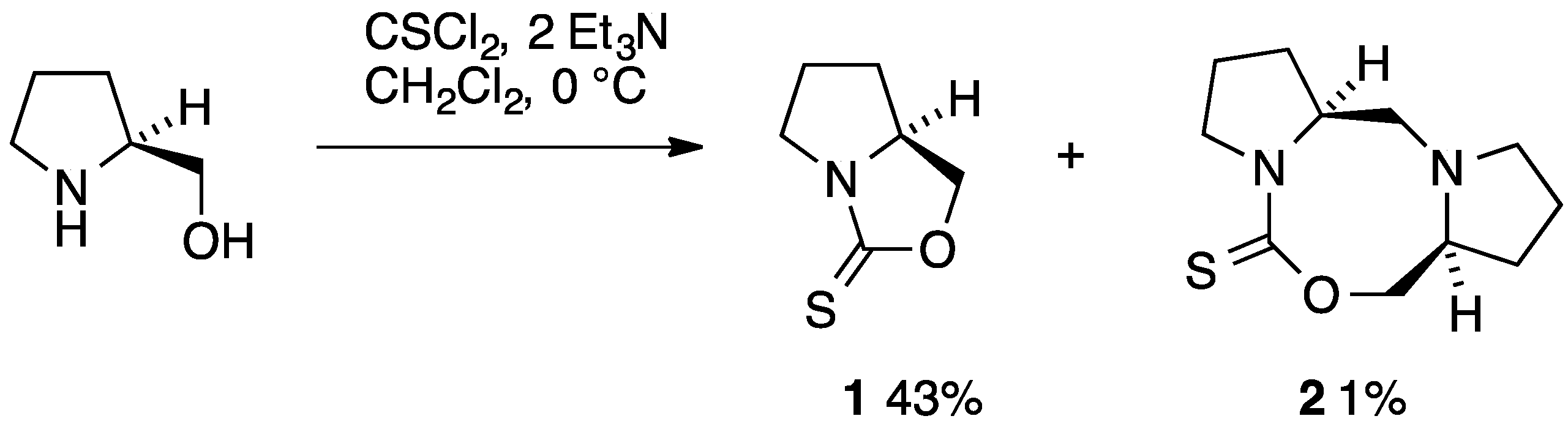
Some years ago we reported synthesis of the simple bicyclic 1,3-oxazolidine-2-thione 1 by reaction of (S)-prolinol with thiophosgene in the presence of triethylamine and its conversion into chiral iminium salts useful for the kinetic resolution of alkoxides [1]. The same compound was reported again recently and was shown to be converted by a ruthenium catalyst into the isomeric 1,3-thiazolidin-2-one [2], also mentioned in our earlier publication [1]. We now describe the isolation and identification of a minor byproduct in the synthesis of 1, which has the novel tricyclic structure 2 featuring a central 1,3,6-oxadiazocane ring.
2. Results
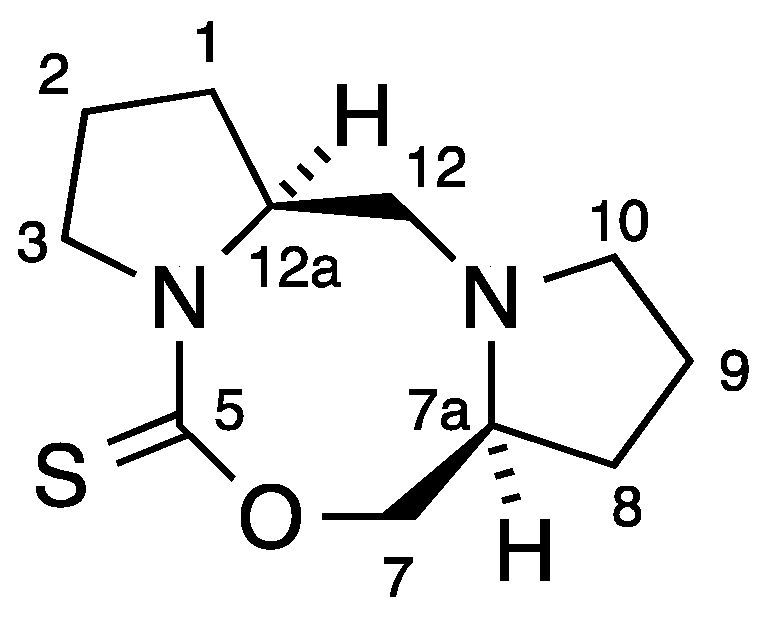
The synthesis of 1 involved slow addition of thiophosgene to a mixture of prolinol and two equivalents of triethylamine in CH2Cl2 at 0 °C (Scheme 1). Chromatographic purification on alumina gave the main product in analytically pure form and moderate yield as fine colourless crystals [1]. In their more recent work, Frost and co-workers conducted the reaction in CHCl3 at room temperature and obtained 1 in high yield by trituration [2] with a good match in spectroscopic properties. However, in the course of repeated chromatographic purifications we noticed a minor product at much higher Rf which was isolated in 1% yield and for which we propose the structure 2 containing the previously unknown 1,3,6-oxadiazocane-2-thione ring system. The 1H-NMR spectrum contained signals for 18 hydrogens, clearly indicating that two inequivalent prolinol-derived fragments were present and this was confirmed by the 13C-NMR spectrum with 11 signals including one C=S at 191 ppm, one CH2O at 79 ppm, two CHNs at 60–65 ppm, three CH2N signals in the range 50–60 ppm and four CH2 signals remote from a heteroatom at 20–30 ppm. Particularly the presence of a third CH2N signal together with only one CH2O and a correct high-resolution mass spectrometry measurement leave little doubt as to the structure of 2. By means of COSY and HSQC NMR studies an almost complete assignment of NMR signals was possible (Figure 1, Table 1).
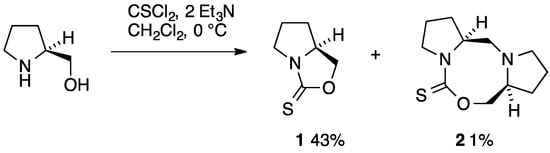
Scheme 1.
Synthetic route to 1 and 2.
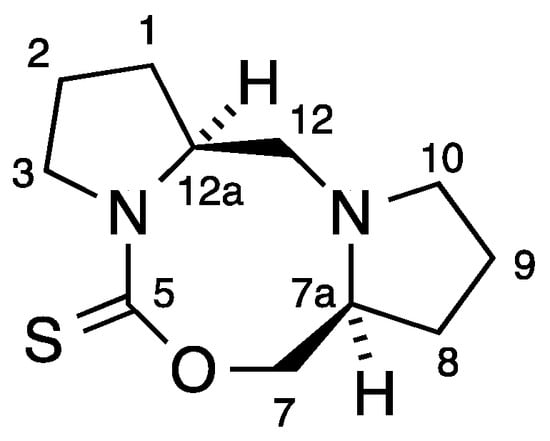
Figure 1.
Numbering system for 2.

Table 1.
NMR assignment for 2.
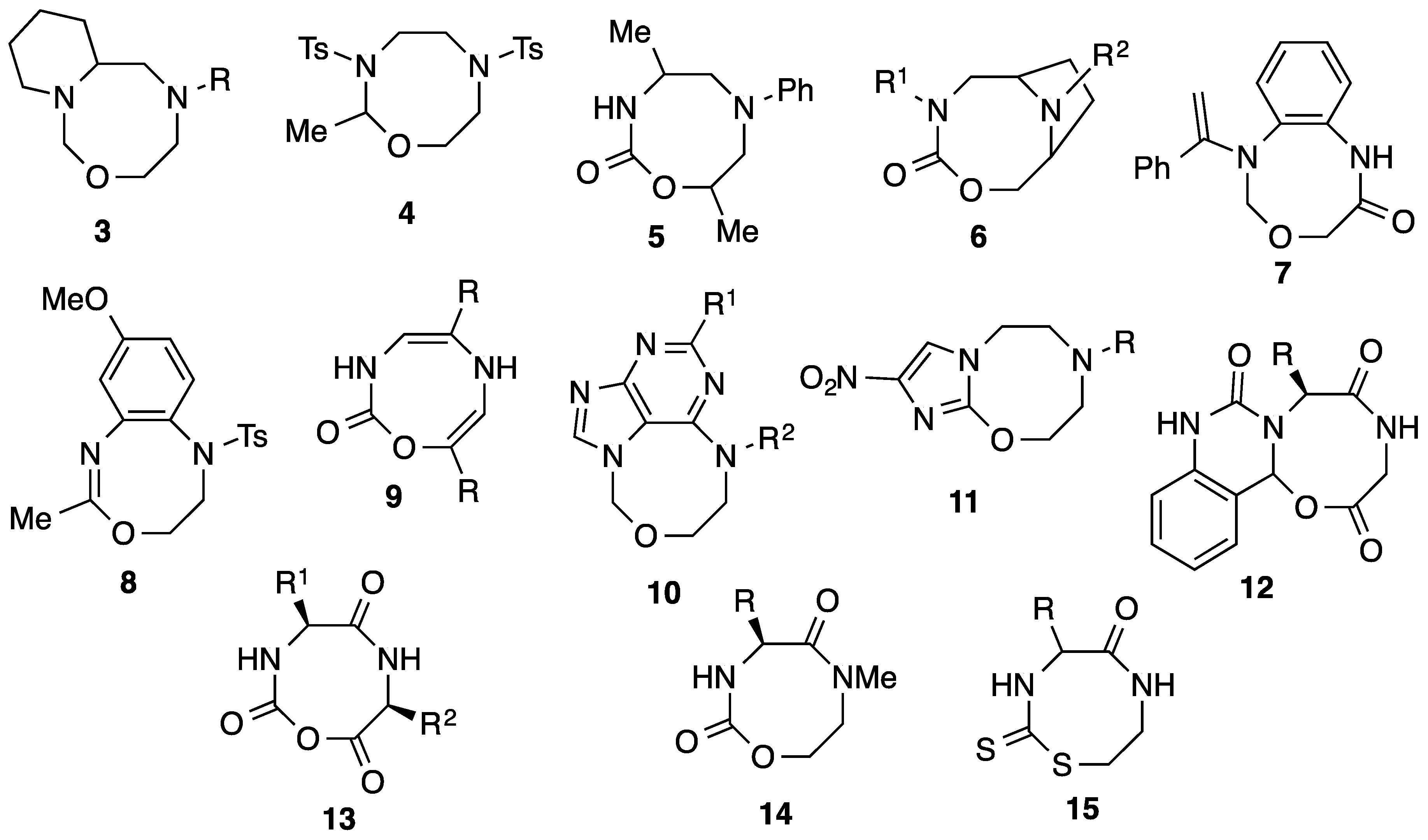
Although it is only formed in low yield, this product is the first 1,3,6-oxadiazocane-2-thione as far as we are aware. Indeed a literature search shows very few publications dealing with 1,3,6-oxadiazocine rings in any state of unsaturation or oxidation. A summary of all such related structures, many of which show potentially useful properties, is shown in Figure 2. NMR studies on the conformation of bicyclic oxadiazocane 3 [3] and oxadiazocanone 5 [4] have appeared and the ditosyl oxadiazocane 4 was obtained as a byproduct in azamacrocycle synthesis [5]. The bridged oxadiazocanones 6 were investigated as potential central nervous system-active agents [6], and the benzoxadiazocinone system 7 was obtained by the photochemical rearrangement of a benzodiazepinone [7]. The unsaturated benzoxadiazocine 8 was prepared as a novel heterocycle [8], and reaction of two equivalents of 2H-azirines with chlorosulfonyl isocyanate gives the oxadiazocinones 9 [9]. Various ring-fused derivatives are also known including the purine-fused compounds 10 [10], and the imidazo[2,1-b] fused compounds 11 which are active against tuberculosis and a range of other tropical diseases [11,12]. Compound 12 is formed as a byproduct in a Pictet Spengler reaction [13]. A range of amino acid-derived oxadiazocanetriones 13 are useful as a component of gel electrolytes [14], and amino acid-derived oxadiazocanediones 14 are active as phospholipase A2 inhibitors [15,16] and against malaria and AIDS [17,18]. Finally, mention could be made of the closely related 1,3,6-thiadiazocane-2-thione, the only similar system as far as we are aware containing a thione [19].
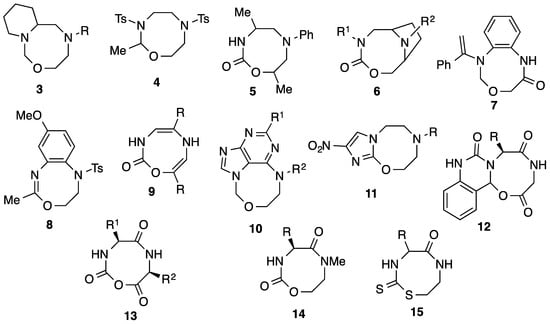
Figure 2.
Related 1,3,6-oxadiazocine ring systems.
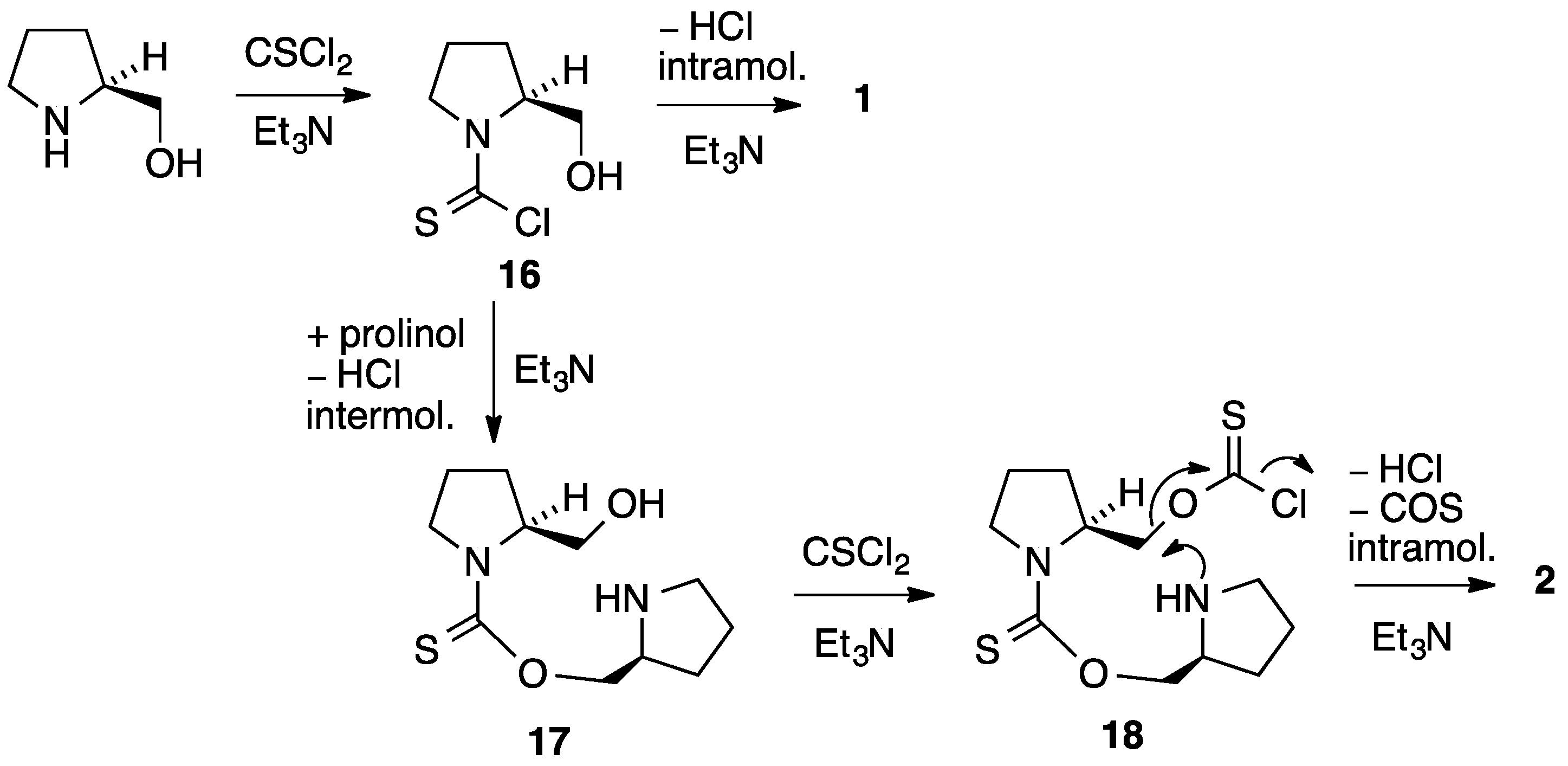
The likely mechanism for formation of 2 is shown in Scheme 2. Initial reaction of prolinol with one molecule of thiophosgene and base gives the thiocarbamoyl chloride 16 and, if this simply loses HCl intramolecularly, the major product 1 is formed. If this intermediate is instead attacked intermolecularly by a second molecule of proline, the thiocarbamate 17 is formed containing a free CH2OH group. This can combine with a second molecule of thiophosgene to give the chlorothioformate 18 and this then undergoes intramolecular attack of the pyrrolidine nitrogen with loss of COS to afford the observed product 2.
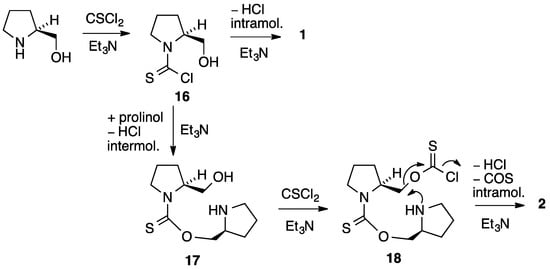
Scheme 2.
Proposed mechanism for the formation of 2.
3. Experimental Section
Octahydro-1H,5H,7H-dipyrrolo[1,2-c:1′,2′-f][1,3,6]oxadiazocine-5-thione (2)
A solution of (S)-prolinol [20] (5.0 g, 50 mmol) and triethylamine (14.0 mL, 10.12 g, 100 mmol) in CH2Cl2 (250 mL) was stirred at 0 °C while a solution of thiophosgene (5.03 mL, 7.59 g, 66 mmol) in CH2Cl2 (100 mL) was added dropwise. The solution was then allowed to warm up to room temperature and stirred for 16 h. The solution was washed with water (2 × 250 mL) and 0.5 M aq. sodium hydroxide (200 mL) and then dried and evaporated to afford a dark coloured oil (6.49 g). This was subjected to column chromatography on alumina (diethyl ether/petroleum, 70:30) to give as the main product at Rf 0.28 (S)-tetrahydro-1H,3H-pyrrolo[1,2-c]oxazole-3-thione 1 (3.11 g, 43%) as colourless crystals, m.p. 58–59 °C. + 69 (c = 1.02, CH2Cl2) (lit. [2] +55.3); 1H-NMR (300 MHz, CDCl3): δ 4.80–4.70 (m, 1H), 4.35–4.20 (m, 2H), 3.95–3.75 (m, 1H), 3.55–3.40 (m, 1H), 2.35–2.00 (m, 3H), 1.85–1.55 (m, 1H) (good agreement with lit. [2]); 13C-NMR (75 MHz, CDCl3): δ 189.5 (C=S), 73.2 (CH2), 63.1 (CH), 47.5 (CH2), 30.8 (CH2), 26.6 (CH2) (good agreement with lit. [2]). Anal. Calcd. for C6H9NOS: C, 50.32; H, 6.34; N, 9.78. Found: C, 50.54; H, 6.36; N, 9.79.
However, this was preceded by a minor component at Rf 0.64, obtained as a pale yellow oil, which proved to be the title compound 2 (56.5 mg, 1%). 1H-NMR (300 MHz, CDCl3): δ 4.60–4.50 (m, 1H), 4.16 (half AB pattern of d, J 12, 5, 1H), 4.08 (half AB pattern of d, J 12, 12, 1H), 3.85–3.75 (m, 1H), 3.72–3.60 (m, 1H), 3.10–2.98 (m, 2H), 2.58–2.54 (m, 1H), 2.53 (d, J 12, 2H), 2.12–2.00 (m, 3H), 1.95–1.65 (m, 4H), 1.48–1.40 (m, 1H); 13C-NMR (75 MHz, CDCl3): δ 190.6 (C=S), 78.9 (CH2O), 62.6 (CHN), 60.0 (CHN), 58.3 (CH2N), 56.7 (CH2N), 50.1 (CH2N), 28.6 (CH2), 27.2 (CH2), 24.4 (CH2), 22.4 (CH2); MS (EI): m/z 226 (M+, 100%), 193 (28), 163 (16), 149 (12), 110 (32), 97 (73), 82 (38), 69 (58), 55 (48). HRMS Calcd. for C11H18N2OS: 226.1140. Found: 226.1142.
Supplementary Materials
The following are available online: http://www.mdpi.com/1422-8599/2018/2/0/s1, Figure S1: 300 MHz 1H-NMR spectrum of 2 in CDCl3, Figure S2: 75 MHz normal and DEPT 13C-NMR spectra of 2 in CDCl3, Figure S3: COSY 2D H-H correlation NMR spectrum of 2, Figure S4: HSQC 2D C-H correlation NMR spectrum of 2, Figure S5: Mass spectrum for 2.
Author Contributions
K.A. performed the experiments; R.A.A. designed the experiments, analysed the data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aitken, R.A.; Ali, K.; Mesher, S.T.E. Kinetic resolution of secondary alcohols using proline-derived bicyclic iminium salts. Tetrahedron Lett. 1997, 38, 4179–4182. [Google Scholar] [CrossRef]
- Mahy, W.; Plucinski, P.; Jover, J.; Frost, C.G. Ruthenium-catalyzed O- to S-alkyl migration: A pseudoreversible Barton-McCombie pathway. Angew. Chem. Int. Ed. 2015, 54, 10944–10948. [Google Scholar] [CrossRef] [PubMed]
- Crabb, T.A.; Jackson, G.C. Proton magnetic resonance studies of compounds with bridgehead nitrogen. XXVIII—Stereochemical studies with Perhydropyrido[1,2-c][1,6,3]dioxazocines and 2-alkylperhydropyrido[1,2-c][1,3,6]oxadiazocines. Org. Magn. Reson. 1975, 7, 488–495. [Google Scholar] [CrossRef]
- Nishiyama, T.; Fujiwara, T.; Miyazaki, S.; Yamada, F. Cis and Trans 4,8-dimethyl-6-phenyl-5,6,7,8-tetrahydro-4H-1,3,6-oxadiazocin-2-ones: Conformational studies and configurational assignments by NMR. J. Heterocycl. Chem. 1992, 29, 1529–1533. [Google Scholar] [CrossRef]
- Pulacchini, S.; Watkinson, M. An Efficient Route to Symmetrically and Unsymmetrically Substituted Azamacrocyclic Ligands. Eur. J. Org. Chem. 2001, 4233–4238. [Google Scholar] [CrossRef]
- Fontanella, L.; Occelli, E. Farmaci potenzialmente attivi sul sistema nervoso centrale derivati del 5-oxa-3,10-diazabiciclo[5.2.1]decano e del 5-oxa-3,10-diazabiciclo[5.2.1]decan-4-one. Farmaco Ed. Scient. 1971, 26, 685–709. (In Italian) [Google Scholar]
- Achour, R.; Essassi, E.M.; Zniber, R.; Desvergne, J.-P.; Bouas-Laurent, H. Réactivité photochimique en solution de 1,5-diazodiazepinones. Bull. Soc. Chim. Belg. 1993, 102, 479–491. (In French) [Google Scholar] [CrossRef]
- Hornyak, G.; Lempert, K. Benzoxadiazocines, benzothiadiazocines and benzotriazocines I: General considerations and the synthesis of two 3,1,6-benzoxadiazocines. Tetrahedron 1983, 39, 479–481. [Google Scholar] [CrossRef]
- Daniel, J.; Dhar, D.N. A facile synthesis of triazocinones and oxadiazocinones. Synth. Commun. 1993, 23, 2151–2157. [Google Scholar] [CrossRef]
- Griengl, H.; Hayden, W.; Plessing, A. C(6)-C(7)-cyclised purines. J. Heterocycl. Chem. 1984, 21, 333–336. [Google Scholar] [CrossRef]
- Musonda, C.C.; Edlin, C.D.; Boyle, G.A. Nitroimidazooxadiazocine Compounds. P.C.T. International Patent Application WO 2013 072903 A1, 23 May 2013. [Google Scholar]
- Sonopo, M.S.; Venter, K.; Boyle, G.; Winks, S.; Marjanovic-Painter, B.; Zeevaart, J.R. Carbon-14 radiolabeling and in vivo biodistribution of a potential anti-TB compound. J. Labelled Compd. Radiopharm. 2015, 58, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Diness, F.; Beyer, J.; Meldal, M. Solid-Phase Synthesis of Terahydro-β-carbolines and Tetrahydroisoquinolines by Stereoselective Intramolecular N-Carbamyliminium Pictet-Spengler Reactions. Chem. Eur. J. 2006, 12, 8056–8066. [Google Scholar] [CrossRef] [PubMed]
- Horikiri, T.; Yoshihiko, K. Gel Electrolyte, Cell and Electrochemical Element. European Patent EP 0996029 A2, 26 April 2000. [Google Scholar]
- Guichard, G.; Lena, G.; Muller, P.; Rognan, D.; Boilard, E.; Lambeau, G. Compositions and Methods for the Inhibition of Phospholipase A2. U.S. Patent 2008 031951 A1, 7 February 2008. [Google Scholar]
- Guichard, G.; Lena, G.; Muller, P.; Rognan, D.; Lambeau, G. Compositions and Methods for the Inhibition of Phospholipase A2. P.C.T. International Patent Application WO 2007 074169 A2, 5 July 2007. [Google Scholar]
- Guichard, G.; Lena, G.; Lallemand, E.; Renia, L. Compositions and Methods for the Treatment of Disease. P.C.T. International Patent Application WO 2007 074170 A2, 5 July 2007. [Google Scholar]
- Guichard, G.; Lena, G.; Lallemand, E.; Renia, L. Aza heterocyclics for the Treatment of Malaria or AIDS. P.C.T. International Patent Application WO 2007 074171 A1, 5 July 2007. [Google Scholar]
- Bao, R.; Huang, W.; Zhen, Y.; He, B. One-pot synthesis of 4-substituted 2-thio-5-oxo-1,3,6-thiadiazaoctane. Synth. Commun. 1990, 20, 2675–2682. [Google Scholar] [CrossRef]
- Enders, D.; Eichenauer, H. Asymmetrische Synthesen via metallierte chirale hydrazone. Enantioselektive Alkylierung von cyclischen Ketonen und Aldehyden. Eur. J. Inorg. Chem. 1979, 112, 2933–2960. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).