The Relevance of Endothelial Dysfunction Biomarkers in Thalassemia Patients and Healthy Individuals: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
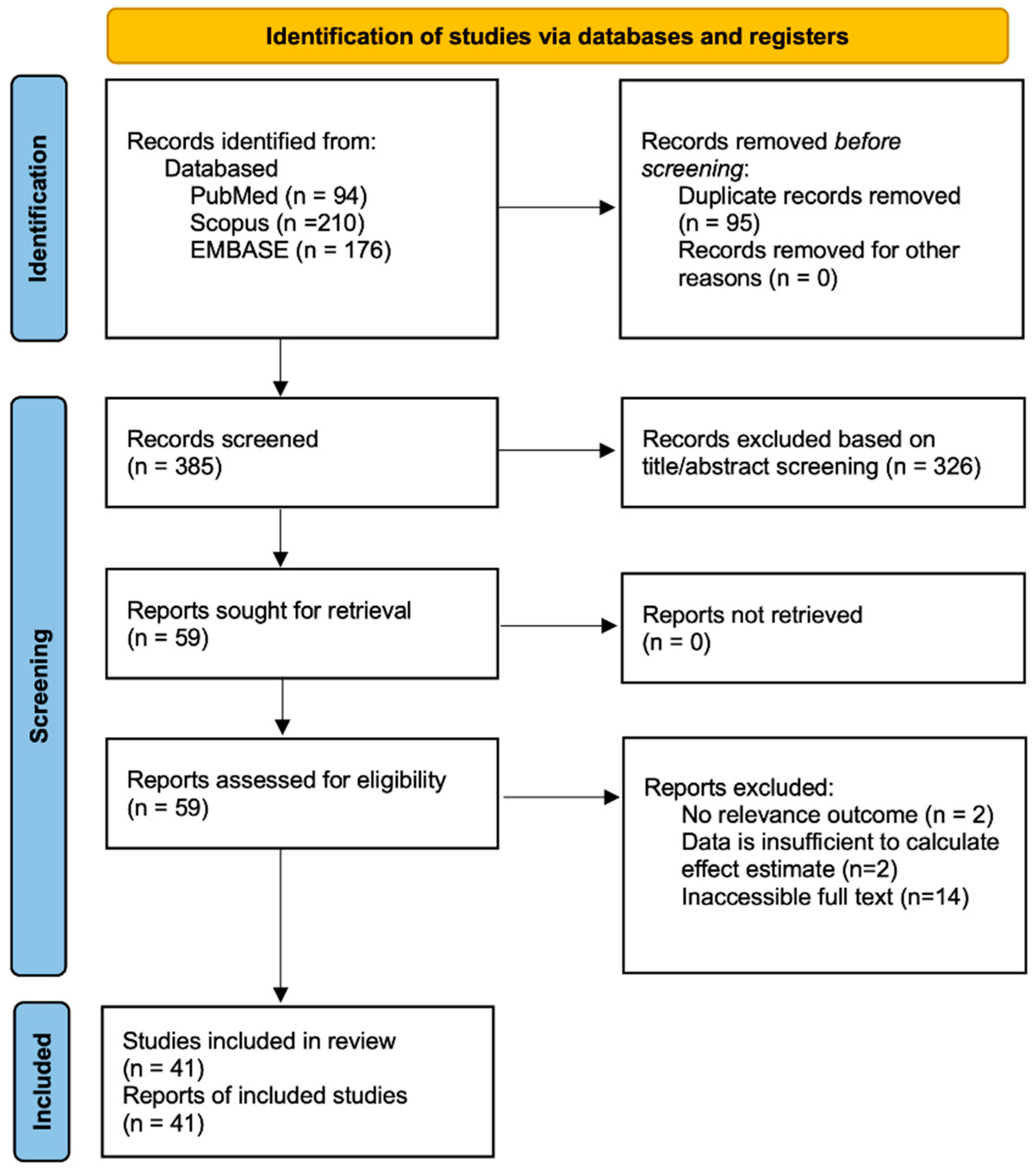
2.1. Literature Search and Study Characteristics
2.2. Quality Assessment
2.3. Study Characteristics
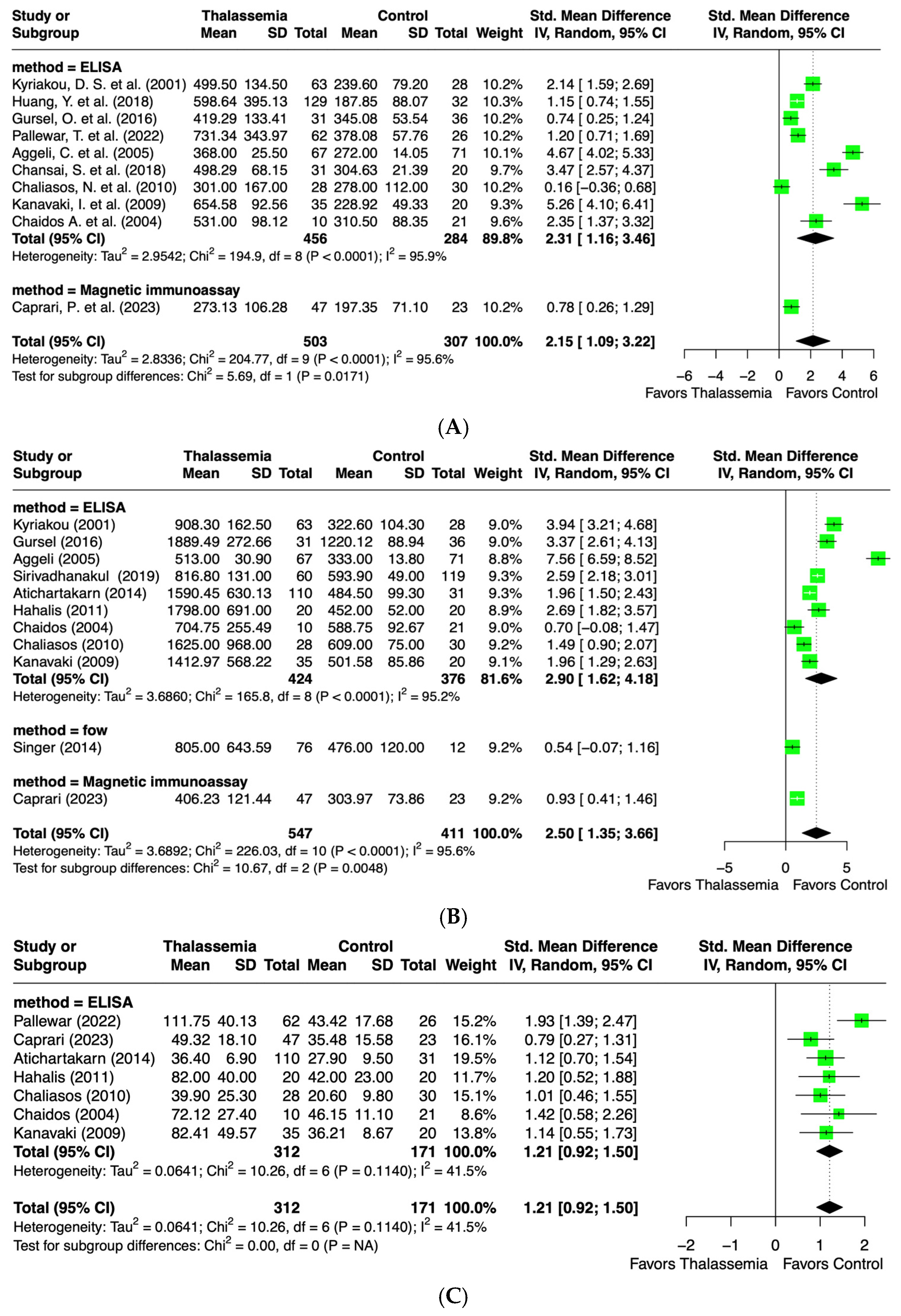
2.4. Meta-Analysis: Endothelial Biomarkers Level
2.4.1. Intercellular Adhesion Molecule-1 (ICAM-1) Levels
2.4.2. Vascular Cell Adhesion Molecule-1 (VCAM-1) Levels
2.4.3. E-Selectin Levels
2.4.4. P-Selectin Levels
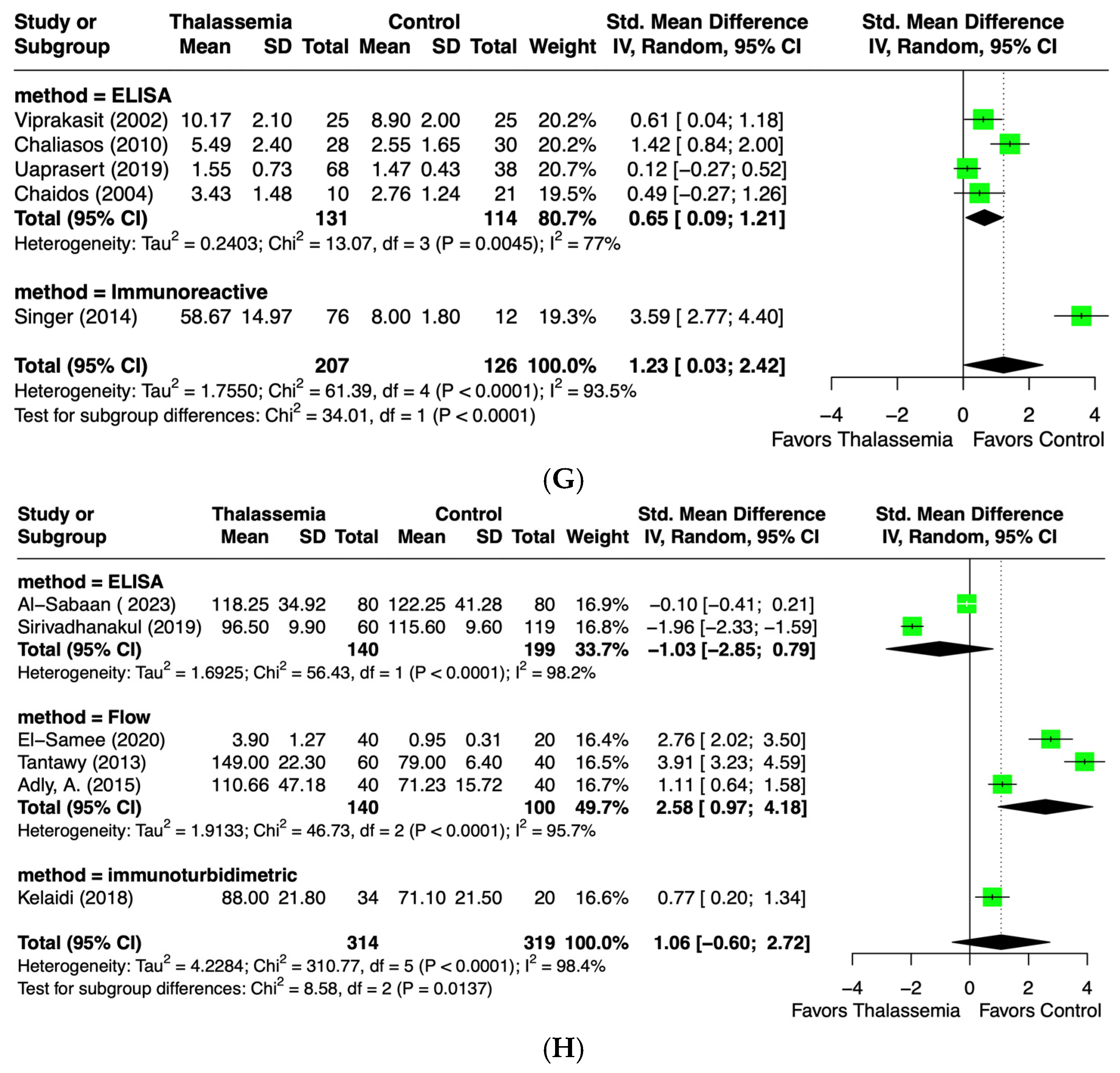
2.4.5. Nitric Oxide (NO) Levels
2.4.6. Asymmetric Dimethylarginine (ADMA) Levels
2.4.7. Endothelin-1 (ET-1) Levels
2.4.8. Von Willebrand Factor (vWF) Levels
2.4.9. Endothelial Microparticles (EMPs)

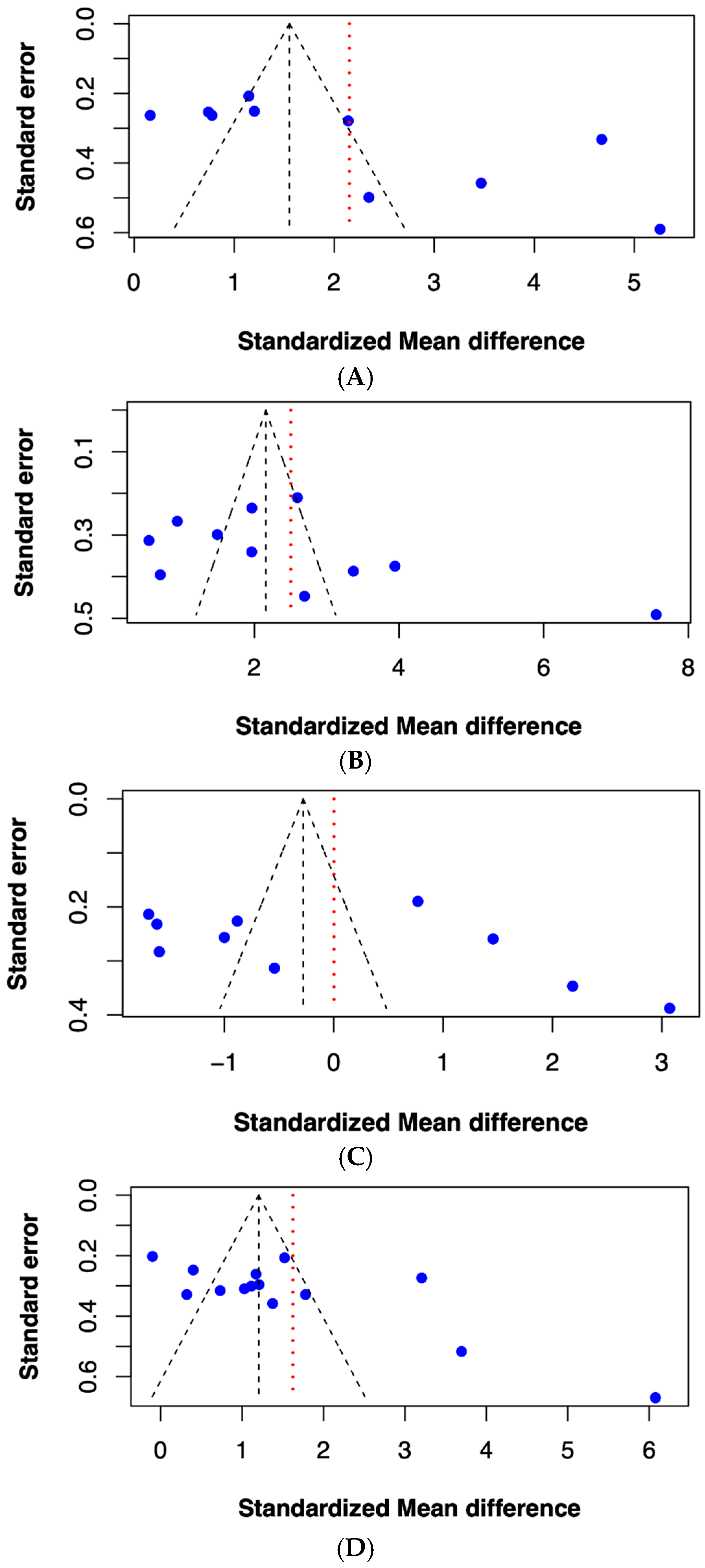
2.5. Publication Bias of Included Studies
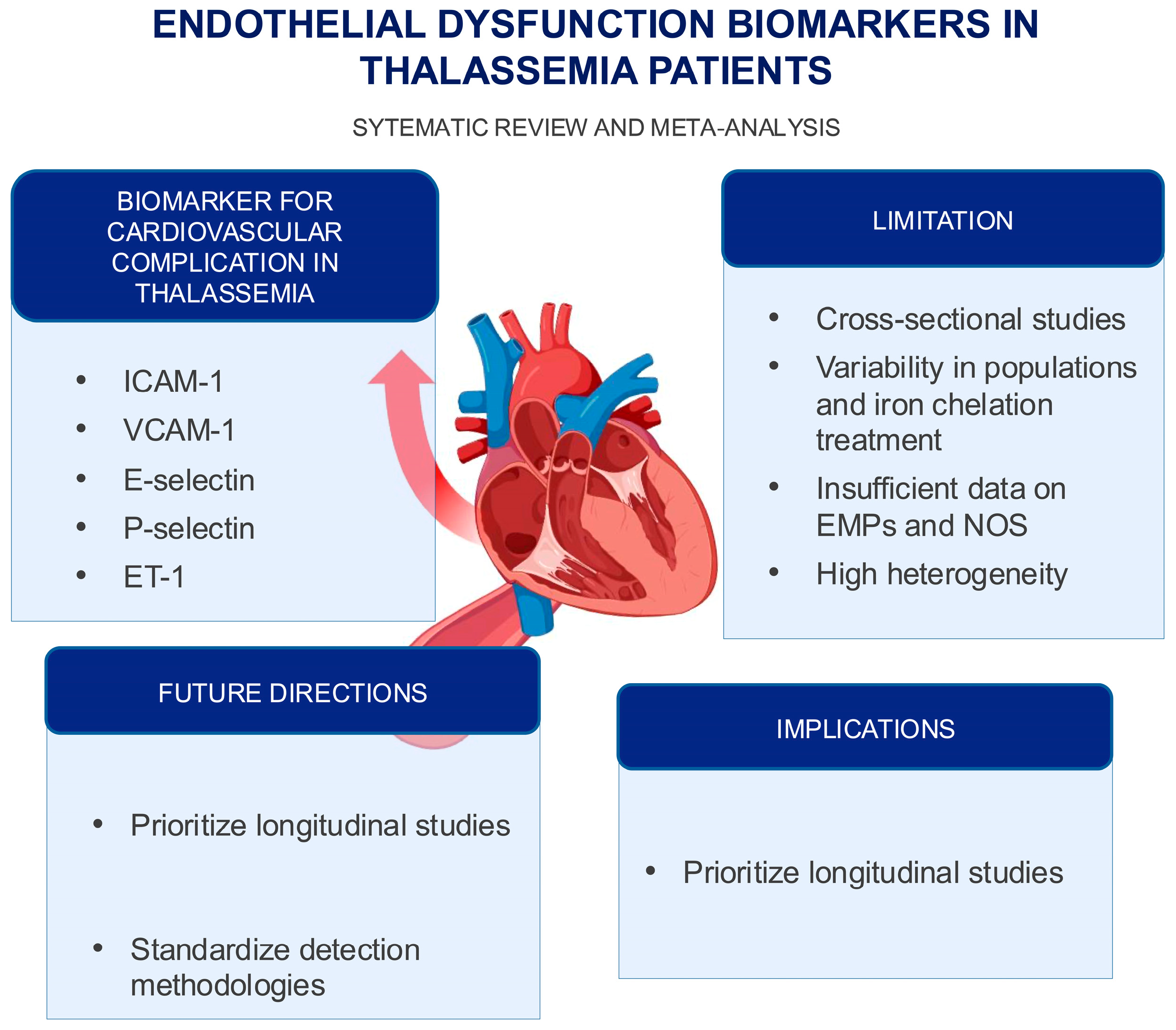
3. Discussion
4. Materials and Methods
4.1. Information Source and Search Strategy
4.2. Study Selection
4.3. Data Extraction
4.4. Quality Assessment
4.5. Statistical Analysis and Publication Bias
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ICAM-1 | intercellular adhesion molecule-1 |
| VCAM-1 | vascular cell adhesion molecule-1 |
| vWF | von Willebrand factor |
| EMPs | endothelial microparticles |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| ADMA | asymmetric form of dimethylarginine |
| ET-1 | endothelin-1 |
| SMD | standardized mean differences |
| CIs | confidence intervals |
References
- Taher, A.T.; Weatherall, D.J.; Cappellini, M.D. Thalassaemia. Lancet 2018, 391, 155–167. [Google Scholar] [CrossRef]
- Kattamis, A.; Kwiatkowski, J.L.; Aydinok, Y. Thalassaemia. Lancet 2022, 399, 2310–2324. [Google Scholar] [CrossRef] [PubMed]
- Akiki, N.; Hodroj, M.H.; Bou-Fakhredin, R.; Matli, K.; Taher, A.T. Cardiovascular Complications in β-Thalassemia: Getting to the Heart of It. Thalass. Rep. 2023, 13, 38–50. [Google Scholar] [CrossRef]
- Tantawy, A.A.G.; Tadros, M.A.R.; Adly, A.A.M.; Ismail, E.A.R.; Ibrahim, F.A.; Salah Eldin, N.M.; Hussein, M.M.; Alfeky, M.A.; Ibrahim, S.M.; Hashem, M.A.; et al. Endothelin-1 gene polymorphism (G8002A) and endothelial monocyte-activating polypeptide II: Role in vascular dysfunction in pediatric patients with β-thalassemia major. Cytokine 2023, 161, 156048. [Google Scholar] [CrossRef]
- Motta, I.; Mancarella, M.; Marcon, A.; Vicenzi, M.; Cappellini, M.D. Management of age-associated medical complications in patients with β-thalassemia. Expert Rev. Hematol. 2020, 13, 85–94. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, V.; Kumari, P.; Singh, R.; Chopra, H.; Emran, T.B. Novel insights on the role of VCAM-1 and ICAM-1: Potential biomarkers for cardiovascular diseases. Ann. Med. Surg. 2022, 84, 104802. [Google Scholar] [CrossRef] [PubMed]
- Habas, K.; Shang, L. Alterations in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells. Tissue Cell 2018, 54, 139–143. [Google Scholar] [CrossRef]
- VISCHERUM von Willebrand factor, endothelial dysfunction, and cardiovascular disease. J. Thromb. Haemost. 2006, 4, 1186–1193. [CrossRef]
- Fonseca, F.A.H.; Izar, M.C.O. Chapter 17—Endothelial Biomarkers. In Endothelium and Cardiovascular Diseases; Da Luz, P.L., Libby, P., Chagas, A.C.P., Laurindo, F.R.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 229–233. [Google Scholar]
- Balta, S. Endothelial Dysfunction and Inflammatory Markers of Vascular Disease. Curr. Vasc. Pharmacol. 2021, 19, 243–249. [Google Scholar] [CrossRef]
- Giaid, A.; Yanagisawa, M.; Langleben, D.; Michel, R.P.; Levy, R.; Shennib, H.; Kimura, S.; Masaki, T.; Duguid, W.P.; Stewart, D.J. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1993, 328, 1732–1739. [Google Scholar] [CrossRef]
- Nishiyama, S.K.; Zhao, J.; Wray, D.W.; Richardson, R.S. Vascular function and endothelin-1: Tipping the balance between vasodilation and vasoconstriction. J. Appl. Physiol. 2017, 122, 354–360. [Google Scholar] [CrossRef]
- Klaihmon, P.; Lertthammakiat, S.; Anurathapan, U.; Pakakasama, S.; Sirachainan, N.; Hongeng, S.; Pattanapanyasat, K. Activated platelets and leukocyte activations in young patients with β-thalassemia/HbE following bone marrow transplantation. Thromb. Res. 2018, 169, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Kumar, A.; Batra, H.S.; Bandyopadhyay, S.; Kapoor, R. Are thalassemia patients oxidatively challenged? Med. J. Armed Forces India 2019, 75, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Helmi, N.; Choudhry, H.; Qari, M.; Kumosani, T.A.; Al-Malki, A.L.; Moselhy, S.S.; Kumosani, A.T. Association of serum asymmetric dimethyl-arginine and troponin I levels as a risk of myocardial infarction in thalassemia. Afr. Health Sci. 2018, 18, 720–726. [Google Scholar] [CrossRef]
- El-shanshory, M.; Badraia, I.; Donia, A.; El-Kady, N.; Mabrouk, M. Asymmetric dimethylarginine levels in children with β-thalassemia and their correlations to tricuspid regurgitant jet velocity. Pediatr. Blood Cancer 2014, 61, 1540–1543. [Google Scholar]
- Kyriakou, D.S.; Alexandrakis, M.G.; Kyriakou, E.S.; Liapi, D.; Kourelis, T.V.; Passam, F.; Papadakis, A. Activated peripheral blood and endothelial cells in thalassemia patients. Ann. Hematol. 2001, 80, 577–583. [Google Scholar] [PubMed]
- Huang, Y.; Long, Y.; Deng, D.; Liu, Z.; Liang, H.; Sun, N.; Xu, Y.; Lai, Y.; Cheng, P. Alterations of anticoagulant proteins and soluble endothelial protein C receptor in thalassemia patients of Chinese origin. Thromb. Res. 2018, 172, 61–66. [Google Scholar] [CrossRef]
- Viprakasit, V.; Kankirawatana, S.; Akarasereenont, P.; Durongpisitkul, K.; Chotewuttakorn, S.; Tanphaichitr, V.S. Baseline levels of plasma endothelin-1 (ET-1) and changes during transfusion in thalassemic patients. Am. J. Hematol. 2002, 70, 260–262. [Google Scholar] [CrossRef]
- Suvachananonda, T.; Wankham, A.; Srihirun, S.; Tanratana, P.; Unchern, S.; Fucharoen, S.; Chuansumrit, A.; Sirachainan, N.; Sibmooh, N. Decreased nitrite levels in erythrocytes of children with β-thalassemia/hemoglobin E. Nitric Oxide 2013, 33, 1–5. [Google Scholar] [CrossRef]
- Chamchoi, A.; Srihirun, S.; Paiboonsukwong, K.; Sriwantana, T.; Sathavorasmith, P.; Pattanapanyasat, K.; Hirsch, R.E.; Schechter, A.N.; Sibmooh, N. Decreased nitrite reductase activity of deoxyhemoglobin correlates with platelet activation in hemoglobin E/ß-thalassemia subjects. PLoS ONE 2018, 13, e0203955. [Google Scholar] [CrossRef]
- Satitthummanid, S.; Uaprasert, N.; Songmuang, S.B.; Rojnuckarin, P.; Tosukhowong, P.; Sutcharitchan, P.; Srimahachota, S. Depleted nitric oxide and prostaglandin E(2) levels are correlated with endothelial dysfunction in β-thalassemia/HbE patients. Int. J. Hematol. 2017, 106, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Manakeng, K.; Prasertphol, P.; Phongpao, K.; Chuncharunee, S.; Tanyong, D.; Worawichawong, S.; Svasti, S.; Chaichompoo, P. Elevated levels of platelet- and red cell-derived extracellular vesicles in transfusion-dependent β-thalassemia/HbE patients with pulmonary arterial hypertension. Ann. Hematol. 2019, 98, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Gursel, O.; Tapan, S.; Sertoglu, E.; Taşçılar, E.; Eker, I.; Ileri, T.; Uysal, Z.; Kurekci, A.E. Elevated plasma asymmetric dimethylarginine levels in children with beta-thalassemia major may be an early marker for endothelial dysfunction. Hematology 2018, 23, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.T.; Kuypers, F.; Fineman, J.; Gildengorin, G.; Larkin, S.; Sweeters, N.; Rosenfeld, H.; Kurio, G.; Higa, A.; Jeng, M.; et al. Elevated tricuspid regurgitant jet velocity in subgroups of thalassemia patients: Insight into pathophysiology and the effect of splenectomy. Ann. Hematol. 2014, 93, 1139–1148. [Google Scholar] [CrossRef]
- Pallewar, T.S.; Sharma, K.; Sharma, S.; Chandra, J.; Nangia, A. Endothelial Activation Markers in Polytransfused Children with Beta Thalassemia: Study from a Tertiary Care Centre in India. Indian J. Hematol. Blood Transfus. 2022, 38, 178–183. [Google Scholar] [CrossRef]
- Aggeli, C.; Antoniades, C.; Cosma, C.; Chrysohoou, C.; Tousoulis, D.; Ladis, V.; Karageorga, M.; Pitsavos, C.; Stefanadis, C. Endothelial dysfunction and inflammatory process in transfusion-dependent patients with beta-thalassemia major. Int. J. Cardiol. 2005, 105, 80–84. [Google Scholar] [CrossRef]
- Al-Sabaan, K.; Al-Awadhi, A. Evaluating von Willebrand factor and ADAMTS13 levels in thalassemia major patients and assessing a possible association with Thrombospondin-1. Int. J. Lab. Hematol. 2023, 45, 945–952. [Google Scholar] [CrossRef]
- Chansai, S.; Fucharoen, S.; Fucharoen, G.; Jetsrisuparb, A.; Chumpia, W. Elevations of Thrombotic Biomarkers in Hemoglobin H Disease. Acta Haematol. 2018, 139, 47–51. [Google Scholar] [CrossRef]
- Tantawy, A.; Adly, A.; Ismail, I. Endothelial nitric oxide synthase gene introne4 VNTR polymorphism in sickle cell disease and transfusion-dependent b-Thalassemia major: Relation to cardio-vascular complications. Haematologica 2013, 98, 174–175. [Google Scholar]
- Chamchoi, A.; Srihirun, S.; Paiboonsukwong, K.; Sriwantana, T.; Kongkaew, P.; Fucharoen, S.; Pattanapanyasat, K.; Sibmooh, N. Hemoglobin-bound platelets correlate with the increased platelet activity in hemoglobin E/β-thalassemia. Int. J. Lab. Hematol. 2020, 42, 518–525. [Google Scholar] [CrossRef]
- Caprari, P.; Profumo, E.; Massimi, S.; Buttari, B.; Riganò, R.; Regine, V.; Gabbianelli, M.; Rossi, S.; Risoluti, R.; Materazzi, S.; et al. Hemorheological profiles and chronic inflammation markers in transfusion-dependent and non-transfusion- dependent thalassemia. Front. Mol. Biosci. 2022, 9, 1108896. [Google Scholar] [CrossRef]
- Abd El-Samee, H.; Bassiouny, N.; Nabih, N. Impact of activated monocyte and endothelial dysfunction on coagulopathy in Egyptian adult beta thalassemic patients. Hematol. Rep. 2020, 12, 8365. [Google Scholar] [CrossRef] [PubMed]
- Ruf, A.; Pick, M.; Deutsch, V.; Patscheke, H.; Goldfarb, A.; Rachmilewitz, E.A.; Guillin, M.C.; Eldor, A. In-vivo platelet activation correlates with red cell anionic phospholipid exposure in patients with beta-thalassaemia major. Br. J. Haematol. 1997, 98, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Sirivadhanakul, P.; Chuansumrit, A.; Songdej, D.; Kadegasem, P.; Wongwerawattanakoon, P.; Sirachainan, N. Increased endothelial activation in α-thalassemia disease. Ann. Hematol. 2019, 98, 1593–1602. [Google Scholar] [CrossRef]
- Atichartakarn, V.; Chuncharunee, S.; Archararit, N.; Udomsubpayakul, U.; Aryurachai, K. Intravascular hemolysis, vascular endothelial cell activation and thrombophilia in splenectomized patients with hemoglobin E/β-thalassemia disease. Acta Haematol. 2014, 132, 100–107. [Google Scholar] [CrossRef] [PubMed]
- El-Hady, S.B.; Farahat, M.H.; Atfy, M.; Elhady, M.A. Nitric oxide metabolites and arginase I levels in β-thalassemic patients: An Egyptian study. Ann. Hematol. 2012, 91, 1193–1200. [Google Scholar] [CrossRef]
- Naithani, R.; Chandra, J.; Bhattacharjee, J.; Verma, P.; Narayan, S. Peroxidative stress and antioxidant enzymes in children with beta-thalassemia major. Pediatr. Blood Cancer 2006, 46, 780–785. [Google Scholar] [CrossRef]
- Srihirun, S.; Tanjararak, N.; Chuncharunee, S.; Sritara, P.; Kaewvichit, R.; Fucharoen, S.; Pattanapanyasat, K.; Sibmooh, N. Platelet hyperactivity in thalassemia patients with elevated tricuspid regurgitant velocity and the association with hemolysis. Thromb. Res. 2015, 135, 121–126. [Google Scholar] [CrossRef]
- Chanpeng, P.; Svasti, S.; Paiboonsukwong, K.; Smith, D.R.; Leecharoenkiat, K. Platelet proteome reveals specific proteins associated with platelet activation and the hypercoagulable state in β-thalassmia/HbE patients. Sci. Rep. 2019, 9, 6059. [Google Scholar] [CrossRef]
- Kelaidi, C.; Kattamis, A.; Apostolakou, F.; Poziopoulos, C.; Lazaropoulou, C.; Delaporta, P.; Kanavaki, I.; Papassotiriou, I. PlGF and sFlt-1 levels in patients with non-transfusion-dependent thalassemia: Correlations with markers of iron burden and endothelial dysfunction. Eur. J. Haematol. 2018, 100, 630–635. [Google Scholar] [CrossRef]
- Gursel, O.; Kurekci, A.E.; Tascilar, E.; Ileri, T.; Altun, D.; Tapan, S.; Kurt, I.; Kocaoglu, M.; Aydin, A.; Okutan, V.; et al. Premature atherosclerosis in children with β-thalassemia major. J. Pediatr. Hematol. Oncol. 2012, 34, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Hahalis, G.; Kalogeropoulos, A.; Terzis, G.; Tselepis, A.D.; Kourakli, A.; Mylona, P.; Grapsas, N.; Alexopoulos, D. Premature atherosclerosis in non-transfusion-dependent β-thalassemia intermedia. Cardiology 2011, 118, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, Z.N.; Al-Mudallal, S.S.; Hameed, B.M. Role of red blood cells “annexin V” and platelets “P-selectin” in patients with thalassemia. Hematol. Oncol. Stem Cell Ther. 2019, 12, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Chaliasos, N.; Challa, A.; Hatzimichael, E.; Koutsouka, F.; Bourantas, D.K.; Vlahos, A.P.; Siamopoulou, A.; Bourantas, K.L.; Makis, A. Serum adipocytokine and vascular inflammation marker levels in Beta-thalassaemia major patients. Acta Haematol. 2010, 124, 191–196. [Google Scholar] [CrossRef]
- Kanavaki, I.; Makrythanasis, P.; Lazaropoulou, C.; Tsironi, M.; Kattamis, A.; Rombos, I.; Papassotiriou, I. Soluble endothelial adhesion molecules and inflammation markers in patients with beta-thalassemia intermedia. Blood Cells Mol. Dis. 2009, 43, 230–234. [Google Scholar] [CrossRef]
- Fayed, M.A.; Abdel-Hady, H.E.; Hafez, M.M.; Salama, O.S.; Al-Tonbary, Y.A. Study of platelet activation, hypercoagulable state, and the association with pulmonary hypertension in children with β-thalassemia. Hematol. Oncol. Stem Cell Ther. 2018, 11, 65–74. [Google Scholar] [CrossRef]
- Aygüneş, U.; Can, Ü.; Doğan, M.; Keçeli, M.; Eker, H. The Effect of plasma endocan and asymmetric dimethyl arginine levels on endothelial and cardiac functions in children with beta thalassemia major. Authorea 2024, 1–8. [Google Scholar] [CrossRef]
- Bayraktar, N.; Erkurt, M.A.; Aydoğdu, İ.; Başaran, Y. The levels of nitric oxide in beta-thalassemia minor. Turk. J. Haematol. 2008, 25, 187–189. [Google Scholar]
- Uaprasert, N.; Satitthummanid, S.; Akkawat, B.; Sutcharitchan, P.; Rojnuckarin, P. Vascular and hemostatic alterations associated with pulmonary hypertension in β-thalassemia hemoglobin E patients receiving regular transfusion and iron chelation. Thromb. Res. 2019, 174, 104–112. [Google Scholar] [CrossRef]
- Abo-Elwafa, H.A.; Youseff, L.M.; Mahmoud, R.A.; Elbadry, M.I.; Tawfeek, A.; Aziz, S.P. Venous Thromboembolism Risk Assessment among Beta-thalassemia Patients. J. Appl. Hematol. 2023, 14, 230–235. [Google Scholar] [CrossRef]
- Adly, A.A.M.; El-Sherif, N.H.; Ismail, E.A.R.; El-Zaher, Y.A.; Farouk, A.; El-Refaey, A.M.; Wahba, M.S. Vascular dysfunction in patients with young β-thalassemia: Relation to cardiovascular complications and subclinical atherosclerosis. Clin. Appl. Thromb. Hemost. 2015, 21, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Chaidos, A.; Makis, A.; Hatzimichael, E.; Tsiara, S.; Gouva, M.; Tzouvara, E.; Bourantas, K.L. Treatment of beta-thalassemia patients with recombinant human erythropoietin: Effect on transfusion requirements and soluble adhesion molecules. Acta Haematol. 2004, 111, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Sherief, L.M.; Dawood, O.; Ali, A.; Sherbiny, H.S.; Kamal, N.M.; Elshanshory, M.; Alazez, O.A.; Alhady, M.A.; Nour, M.; Mokhtar, W.A. Premature atherosclerosis in children with beta-thalassemia major: New diagnostic marker. BMC Pediatr. 2017, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, A.A.; Adly, A.A.; Ismail, E.A.; Habeeb, N.M. Flow cytometric assessment of circulating platelet and erythrocytes microparticles in young thalassemia major patients: Relation to pulmonary hypertension and aortic wall stiffness. Eur. J. Haematol. 2013, 90, 508–518. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Sterne, J.A.; Gavaghan, D.; Egger, M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 2000, 53, 1119–1129. [Google Scholar] [CrossRef]
- Sajadi Hezaveh, Z.; Azarkeivan, A.; Janani, L.; Hosseini, S.; Shidfar, F. The effect of quercetin on iron overload and inflammation in β-thalassemia major patients: A double-blind randomized clinical trial. Complement. Ther. Med. 2019, 46, 24–28. [Google Scholar] [CrossRef]
- Kohgo, Y.; Ikuta, K.; Ohtake, T.; Torimoto, Y.; Kato, J. Body iron metabolism and pathophysiology of iron overload. Int. J. Hematol. 2008, 88, 7–15. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 2011, 15, 1607–1638. [Google Scholar] [CrossRef]
- Galkina, E.; Ley, K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2292–2301. [Google Scholar] [CrossRef]
- Hurtado, J.; Sellak, H.; Joseph, G.; Lewis, C.V.; Naudin, C.R.; Garcia, S.; Wodicka, J.R.; Archer, D.R.; Taylor, W.R. Accelerated atherosclerosis in beta-thalassemia. Am. J. Physiol.-Heart Circ. Physiol. 2023, 325, H1133–H1143. [Google Scholar] [CrossRef]
- Farmakis, D.; Giakoumis, A.; Angastiniotis, M.; Eleftheriou, A. The changing epidemiology of the ageing thalassaemia populations: A position statement of the Thalassaemia International Federation. Eur. J. Haematol. 2020, 105, 16–23. [Google Scholar] [CrossRef]
- Goel, S.; Miller, A.; Agarwal, C.; Zakin, E.; Acholonu, M.; Gidwani, U.; Sharma, A.; Kulbak, G.; Shani, J.; Chen, O. Imaging modalities to identity inflammation in an atherosclerotic plaque. Radiol. Res. Pract. 2015, 2015, 410967. [Google Scholar] [CrossRef] [PubMed]
- Buzelé, R.; Barbier, L.; Sauvanet, A.; Fantin, B. Medical complications following splenectomy. J. Visc. Surg. 2016, 153, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.L.; Guo, T.; Zhu, D.L.; Zheng, S.; Han, D.D.; Chen, Y. Risk factors of portal vein thrombosis after splenectomy in patients with liver cirrhosis. Hepatoma Res. 2020, 6, 37. [Google Scholar] [CrossRef]
- Ray, A.; Ch Maharana, K.; Meenakshi, S.; Singh, S. Endothelial dysfunction and its relation in different disorders: Recent update. Health Sci. Rev. 2023, 7, 100084. [Google Scholar] [CrossRef]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, 10, 2303259. [Google Scholar] [CrossRef]
- Minneci, P.C.; Deans, K.J.; Zhi, H.; Yuen, P.S.T.; Star, R.A.; Banks, S.M.; Schechter, A.N.; Natanson, C.; Gladwin, M.T.; Solomon, S.B. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J. Clin. Investig. 2005, 115, 3409–3417. [Google Scholar] [CrossRef]
- Morris, C.R.; Gladwin, M.T.; Kato, G.J. Nitric oxide and arginine dysregulation: A novel pathway to pulmonary hypertension in hemolytic disorders. Curr. Mol. Med. 2008, 8, 620–632. [Google Scholar] [CrossRef][Green Version]
- Østergaard, L.; Stankevicius, E.; Andersen, M.R.; Eskildsen-Helmond, Y.; Ledet, T.; Mulvany, M.J.; Simonsen, U. Diminished NO release in chronic hypoxic human endothelial cells. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H2894–H2903. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Tajima, S.; Yoshida, S.; Yamano, N.; Kihira, Y.; Ishizawa, K.; Aihara, K.; Tomita, S.; Tsuchiya, K.; Tamaki, T. Deferoxamine promotes angiogenesis via the activation of vascular endothelial cell function. Atherosclerosis 2011, 215, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Sydow, K.; Münzel, T. ADMA and oxidative stress. Atheroscler. Suppl. 2003, 4, 41–51. [Google Scholar] [CrossRef]
- Davids, M.; van Hell, A.J.; Visser, M.; Nijveldt, R.J.; van Leeuwen, P.A.; Teerlink, T. Role of the human erythrocyte in generation and storage of asymmetric dimethylarginine. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1762–H1770. [Google Scholar] [CrossRef]
- Sibal, L.; Agarwal, S.C.; Home, P.D.; Boger, R.H. The Role of Asymmetric Dimethylarginine (ADMA) in Endothelial Dysfunction and Cardiovascular Disease. Curr. Cardiol. Rev. 2010, 6, 82–90. [Google Scholar] [CrossRef]
- Ramzy, D.; Rao, V.; Tumiati, L.C.; Xu, N.; Sheshgiri, R.; Miriuka, S.; Delgado, D.H.; Ross, H.J. Elevated endothelin-1 levels impair nitric oxide homeostasis through a PKC-dependent pathway. Circulation 2006, 114, I-319–I-326. [Google Scholar] [CrossRef] [PubMed]
- Kourembanas, S.; McQuillan, L.P.; Leung, G.K.; Faller, D.V. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J. Clin. Investig. 1993, 92, 99–104. [Google Scholar] [CrossRef]
- Yamashita, K.; Discher, D.J.; Hu, J.; Bishopric, N.H.; Webster, K.A. Molecular Regulation of the Endothelin-1 Gene by Hypoxia: Contributions of Hypoxia-Inducible Factor-1, Activator Protein-1, GATA-2, and p300/CBP*. J. Biol. Chem. 2001, 276, 12645–12653. [Google Scholar] [CrossRef]
- Böhm, F.; Pernow, J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 2007, 76, 8–18. [Google Scholar] [CrossRef]
- Bourque, S.L.; Davidge, S.T.; Adams, M.A. The interaction between endothelin-1 and nitric oxide in the vasculature: New perspectives. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 300, R1288–R1295. [Google Scholar] [CrossRef]
- Sabatier, F.; Camoin-Jau, L.; Anfosso, F.; Sampol, J.; Dignat-George, F. Circulating endothelial cells, microparticles and progenitors: Key players towards the definition of vascular competence. J. Cell. Mol. Med. 2009, 13, 454–471. [Google Scholar] [CrossRef] [PubMed]
- Giró, O.; Jiménez, A.; Pané, A.; Badimon, L.; Ortega, E.; Chiva-Blanch, G. Extracellular vesicles in atherothrombosis and cardiovascular disease: Friends and foes. Atherosclerosis 2021, 330, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Helbing, T.; Olivier, C.; Bode, C.; Moser, M.; Diehl, P. Role of microparticles in endothelial dysfunction and arterial hypertension. World J. Cardiol. 2014, 6, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Matthew, J.P.; Joanne, E.M.; Patrick, M.B.; Isabelle, B.; Tammy, C.H.; Cynthia, D.M.; Larissa, S.; Jennifer, M.T.; Elie, A.A.; Sue, E.B.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar]
- Martin, J.D.; Marnie, L.B.; Hywel, C.W.; Rachel, S.D. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar]
- Rovito, M.J.; Bruzzone, A.; Lee, E.; López Castillo, H.; Talton, W.; Taliaferro, L.; Falk, D. Assessing Health-Related Quality of Life Among Survivors of Testicular Cancer: A Systematic Review. Am. J. Men’s Health 2021, 15, 1557988320982184. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
| Study ID | Study Group | Group Size (N) | Age (Years) | Serum Ferritin (ng/mL) | ICAM-1 (ng/mL) | VCAM-1 (ng/mL) | Nitric Oxide (µM) | ADMA (µmol/L) | EMP (%) | vWF | P-Selectin | E-Selectin (ng/mL) | Endothelin-1 (pg/mL) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Klaihmon [13] | β-thal/HbE | 87 | 12.8 ± 4.9 | BMT = 1795.1 | 19.48 ± 11.27% | |||||||||
| Healthy control | 20 | 12.4 ± 3.9 | ND | 7.41 ± 1.86% | |||||||||||
| 2 | Banerjee [14] | Thalassemia | 30 | ND | 200–870 | 167.0 ± 48.0 | |||||||||
| Healthy control | 30 | ND | 19.04–143.84 | 57.0 ± 14.0 | |||||||||||
| 3 | Helmi [15] | Thalassemia | 20 | ND | ND | 0.048 ± 0.014 | |||||||||
| Healthy control | 20 | ND | ND | 0.017 ± 0.004 | |||||||||||
| 4 | El-shanshory [16] | β-TM | 30 | 6–18 | 600–5000 | 0.62 ± 0.10 | |||||||||
| Healthy control | 30 | 100–150 | 0.60 ± 0.14 | ||||||||||||
| 5 | Kyriakou [17] | Thalassemia | 63 | 24.3 (10–56) | TM = 4186 TI = 356 β-thalassemia carriers = 85 | 499.5 ± 134.5 | 0.91 ± 0.16 | ||||||||
| Healthy control | 18 | 28 (10–60) | ND | 239.6 ± 79.2 | 0.32 ± 0.10 | ||||||||||
| 6 | Huang [18] | Thalassemia | β-TM | 67 | 9.5 ± 4.6 | 3814 (380–15,960) | 808.1 ± 614.6 | ||||||||
| β-TI | 22 | 14.5 ± 7.8 | 2552 (126–12,169) | 629.6 ± 334.8 | |||||||||||
| α-TI | 33 | 18.2 ± 14.2 | 544 (51–11,110) | 613.1 ± 351.9 | |||||||||||
| α + β | 7 | 8.5 ± 2.9 | 2915 (358–6446) | 343.9 ± 118.9 | |||||||||||
| Healthy control | 32 | 17.5 ± 13.3 | ND | 187.8 ± 88.1 | |||||||||||
| 7 | Viprakasit [19] | β-thalassemia/HbE | 25 | 5–15 | ND | 10.17± 2.1 | |||||||||
| Healthy control | 25 | 5–15 | ND | 8.9 ± 2.0 | |||||||||||
| 8 | Suvachananonda [20] | β-thalassemia/HbE | 38 | 12.0 ± 1.9 | Mild = 708.8 ± 276.4 Moderate = 1953.0 ± 307.3 Severe = 2162.0 ± 360.4 | 23.9 ± 2.7 | |||||||||
| Healthy control | 20 | 11.2 ± 0.1 | ND | 18.8 ± 1.2 | |||||||||||
| 9 | Chamchoi [21] | β-thal/HbE | NSP | 58 | ND | 633.1 (336.8 ± 1552.0) | 0.23 ± 0.95 | 4.8 ± 1.5% | |||||||
| SP | 23 | ND | 1428.0 (606.5 ± 2575.0) | 0.22 ± 0.95 | 11.1 ± 2.9% | ||||||||||
| Healthy control | 47 | ND | ND | 0.164 ± 0.41 | 1.9 ± 0.6% | ||||||||||
| 10 | Satitthummanid [22] | β-thal/HbE | 43 | 34.63 ± 9.7 | 3090.2 ± 3297 | 117.2 ± 27.3 | |||||||||
| Healthy control | 43 | 36.69 ± 8.6 | ND | 135.8 ± 11.3 | |||||||||||
| 11 | Manakeng [23] | β-thal/HbE | PAH | 11 | 39 ± 11 | 769 ± 791 | 13.45 ± 16.88% | ||||||||
| No PAH | 14 | 37 ± 12 | 1072 ± 981 | 11.69 ± 13.54% | |||||||||||
| Healthy control | 15 | 28 ± 5 | 62 ± 50 | 8.22 ± 9.05% | |||||||||||
| 12 | Gursel [24] | β-TM | 31 | 8.5 ± 0.1 | 2235 (390–5119) | 419.29 ± 133.41 | 1889.49 ± 272.66 | 0.89 ± 1.09 | 192.51 ± 52.97 ng/mL | ||||||
| Healthy control | 36 | 8.0 ± 0.3 | 32 (18–90) | 345.08 ± 53.54 | 1220.12 ± 88.94 | 0.50 ± 0.21 | 173.42 ± 41.57 ng/mL | ||||||||
| 13 | Singer [25] | Thalassemia | TM | 41 | 27.5 ± 9.8 | TRV < 2.5 m/s = 1872 (2534) TRV > 2.5 m/s = 486 (355) | 1043 ± 840 | 9.1 ± 12 | 35 ± 13 ng/mL | 38 ± 29 | |||||
| TI | 27 | 30.5 ± 12.7 | 692 ± 600 | 4.9 ± 2.5 | 30 ±12 ng/mL | 65 ± 45 | |||||||||
| Hb-Cs | 8 | 23.2 ± 3.7 | 680 ± 422 | 4.0 ±1.9 | 33 ± 7 ng/mL | 73 ± 49 | |||||||||
| Healthy control | 12 | 20 ± 35 | ND | 476 ±120 | 7.2 ± 2 | 25 ± 5 ng/mL | 8 ±1.8 | ||||||||
| 14 | Pallewar [26] | β-thalassemia major | 62 | 10.7 ± 3.1 | 3168.70 ± 1361.30 | 731.34 ± 343.97 | 111.75 ± 40.13 | ||||||||
| Healthy control | 26 | 9.6 ± 3.5 | ND | 378.08 ± 57.76 | 43.42 ± 17.68 | ||||||||||
| 15 | Aggeli [27] | β-TM | 67 | 24.6 ± 0.7 | 1108 ± 38.17 pmol/L | 368 ± 25.5 | 513 ± 30.9 | ||||||||
| Healthy control | 71 | 25.63 ± 0.49 | 312 ± 15.09 pmol/L | 272 ±14.1 | 333 ± 13.8 | ||||||||||
| 16 | Al-Sabaan [28] | β-TM | 80 | ND | ND | 118.25 ± 34.92 U/dL | |||||||||
| Healthy control | 80 | ND | ND | 122.25 ± 41.28 U/dL | |||||||||||
| 17 | Chansai [29] | β-thal | NSP | 20 | 14.9 ± 1.0 | 876 (734.91–1229.39) | 520.82 ± 55.22 | ||||||||
| SP | 11 | 16.5 ± 1.7 | 1133 (880–1473.82) | 475.75 ± 79.0 | |||||||||||
| Healthy control | 20 | 21.4 ± 0.5 | ND | 304.63 ± 21.39 | |||||||||||
| 18 | Tantawy [30] | β-TM | 60 | 10.5 ± 3.9 | 2774.5 ± 988.9 | 149 ± 22.3% | |||||||||
| Healthy control | 40 | 9.7 ± 3.1 | 176 ± 53 | 79 ± 6.4% | |||||||||||
| 19 | Chamchoi [31] | β-thal/HbE | NSP | 16 | 34 (24–40) | 629 (339–1458) | 5.33 ± 3.00% | ||||||||
| SP | 10 | 24 (18–28) | 1298 (216–2625) | 6.13 ± 3.70% | |||||||||||
| Healthy control | 22 | 27 (25–31) | 45 (28–97) | 2.82 ± 2.14% | |||||||||||
| 20 | Caprari [32] | Thalassemia | 47 | 39 ± 15 | 662 (67–4438) | 273.13 ± 106.28 | 406.23 ± 121.44 | 49.32 ± 18.1 | |||||||
| Healthy control | 23 | 40 ± 11 | ND | 197.35 ± 71.10 | 303.97 ± 73.86 | 35.48 ± 15.58 | |||||||||
| 21 | El-Samee [33] | β-thalassemia | 40 | 23 ± 4.0 | ND | 3.90 ± 1.27 IU/dL | |||||||||
| Healthy control | 20 | 23.3 ± 4.6 | ND | 0.95 ± 0.31 IU/dL | |||||||||||
| 22 | Ruf [34] | β-TM | 30 | 24 (12–42) | ND | 4.9 ± 3.5% | |||||||||
| Healthy control | 25 | 24 (15–40) | ND | 1.7 ± 0.5% | |||||||||||
| 23 | Sirivadhanakul [35] | α-thal | Non-deletion | 31 | 12.9 ± 4.8 | 209.2 (103.0–353.6) | 816.8 ± 131.0 | 96.5 ± 9.9% | |||||||
| Deletion | 29 | 13.3 ± 4.4 | 64.5 (41.1–103.1) | ||||||||||||
| Healthy control | 119 | 13.6 ± 3.0 | 56.6 (41.1–77.0) | 593.9 ± 49.0 | 115.6 ± 9.6% | ||||||||||
| 24 | Atichartakarn [36] | β-thal/HbE | SP | 61 | 29.2 ± 10.5 | 1892.1 (97.9–9520) | 1575.9 ± 557.4 | 113.4 ± 31.2 ng/mL | 43.3 ± 21.5 | ||||||
| NSP | 49 | 33.9 ± 11.4 | 1050 (17.2–7813) | 1605 ± 696.3 | 56.4 ± 32.1 ng/mL | 29.5 ± 18.4 | |||||||||
| Healthy control | 31 | ND | 10–300 | 484.5 ± 99.3 | ND | 27.9 ± 9.5 | |||||||||
| 25 | El-Hady [37] | β-thalassemia | 109 | 7.25 ± 2.42 | 1788.32 ± 362.59 | 24.08 ± 8.86 | |||||||||
| Healthy control | 19 | 7.05 ± 2.48 | 37.4 ± 20 | 33 ± 8.9 | |||||||||||
| 26 | Naithani [38] | TD-β-thal | 50 | 10.2 ± 4.6 | 3709 ± 1625 | 25.1 ± 14.3 | |||||||||
| Healthy control | 30 | 9.0 ± 4.91 | 131 ± 117 | 8.1 ± 3.7 | |||||||||||
| 27 | Srihirun [39] | β-thal/HbE | 27 | 32.2 ± 5.42 | TRV < 2.5 m/s = 1414 TRV > 2.5 m/s = 587.7 | 11.97 ± 8.37% | |||||||||
| Healthy control | 15 | 25.25 ± 0.9 | ND | 2.46 ± 1.19% | |||||||||||
| 28 | Chanpeng [40] | β-thal/HbE | NSP | 15 | 28.7 ± 6.2 | 512.2 ± 319.6 | 17.7 ± 4.2% | ||||||||
| SP | 8 | 34.0 ± 8.8 | 1757 ± 382.8 | 6.4 ± 1.6% | |||||||||||
| Healthy control | 20 | 28.0 ± 6.1 | 76.2 ± 37.8 | 3.1 ± 0.7% | |||||||||||
| 29 | Kelaidi [41] | β-thalassemia | 34 | 37.6 ± 12.9 | 789.9 ± 514.5 | 88.0 ± 21.8 IU/dL | |||||||||
| Healthy control | 20 | Age matched | ND | 71.1 ± 21.5 IU/dL | |||||||||||
| 30 | Gursel [42] | β-TM | 31 | 10.84 ± 4.38 | 2235 (390–5119) | 699.1± 223.2 | |||||||||
| Healthy control | 36 | 10.06 ± 4.38 | 32 (18–90) | 1020.5 ± 175.8 | |||||||||||
| 31 | Hahalis [43] | β-TI | 20 | 35.9 ± 11.6 | 1568 ± 1344 | 1798 ± 691 | 82 ± 40 | ||||||||
| Healthy control | 20 | 34.5 ± 5.4 | ND | 452 ± 52 | 42 ± 23 | ||||||||||
| 32 | Mahdi [44] | β-thal | minor | 10 | ND | ND | 5.25 ± 3.912% | ||||||||
| intermedia | 10 | ND | ND | 87.20 ± 8.091% | |||||||||||
| major | 30 | ND | ND | 73.20 ± 12.548% | |||||||||||
| Healthy control | 10 | ND | ND | 4.76 ± 0.823% | |||||||||||
| 33 | Chaliasos [45] | β-TM | 28 | 25.2 ± 13.0 | 1286 ± 839 | 301 ± 167 | 1625 ± 968 | 39.9 ± 25.3 | 5.49 ± 2.40 | ||||||
| Healthy control | 30 | Age matched | ND | 278 ± 112 | 609 ± 75 | 20.6 ± 9.8 | 2.55 ± 1.65 | ||||||||
| 34 | Kanavaki [46] | β-TI | 35 | 34.7 ± 15.9 | 650.6 ± 523.7 | 654,580 ± 9256 | 1412.97 ± 568.22 (mg/L) | 119.86 ± 72.64 mg/L | 82,410 ± 49,570 | ||||||
| Healthy control | 20 | Age matched | ND | 228,920 ± 49,330 | 501.58 ± 85.86 (mg/L) | 54.07 ± 6.66 mg/L | 36,210 ± 8670 | ||||||||
| 35 | Fayed [47] | β-thalassemia | 36 | 9.9 ± 4.7 | 1623 ± 1038 | 2.337 ± 0.57 ng/mL | |||||||||
| Healthy control | 20 | 90.4 ± 23.2 | 1.467 ± 0.25 ng/mL | ||||||||||||
| 36 | Aygüneş [48] | β-thalassemia major | 39 | 6.85 ± 0.49 | 1394.7 ± 1389.6 | 3.34 ± 0.85 | |||||||||
| Healthy control | 39 | 6.77 ± 4.84 | 103.3 ± 41.1 | 2.25 ± 1.28 | |||||||||||
| 37 | Bayraktar [49] | β-thalassemia minor | 60 | 26 | ND | 425.48 ± 73.79 | |||||||||
| Healthy control | 60 | 24 | ND | 859.75 ± 352.86 | |||||||||||
| 38 | Uaprasert [50] | β-thal/HbE | 68 | 34.1 ± 12.6 | 2918.7 ± 2732.9 | 132.4 ± 32.5 | 29.75 ± 15.43 ng/mL | 1.55 ± 0.73 | |||||||
| Healthy control | 38 | 37.2 ± 8.6 | 152.5 ± 128.9 | 178.2 ± 17.6 | 31.18 ± 11.32 ng/mL | 1.47 ± 0.43 | |||||||||
| 39 | Abo-Elwafa [51] | β-thalassemia | 75 | 11.94 ± 9.27 | ND | 26.28 ± 18.01% | |||||||||
| Healthy control | 50 | 12.94 ± 10.45 | ND | 4.78 ± 2.27% | |||||||||||
| 40 | Adly [52] | β-thalassemia | 40 | 15.4 ± 3.9 | 2094.5 (1340–3582) | 1.97 ± 1.21 | 110.66 ± 47.18% | ||||||||
| Healthy control | 40 | 14.7 ± 2.1 | ND | 1.05 ± 0.46 | 71.23 ± 15.72% | ||||||||||
| 41 | Chaidos [53] | β-thalassemia | 10 | 29.9 ± 7.8 | 2300 (500–4964) | 531± 98.12 | 704.75 ± 255.49 | 72.12 ± 27.4 | 3.43 ± 1.48 | ||||||
| Healthy control | 21 | ND | 310.5 ± 88.35 | 588.75 ± 92.67 | 46.15 ± 11.1 | 2.76 ± 1.24 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuljerm, H.; Maneekesorn, S.; Thorup, G.; Nantakool, S.; Charoenkwan, P.; Rerkasem, K. The Relevance of Endothelial Dysfunction Biomarkers in Thalassemia Patients and Healthy Individuals: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 3842. https://doi.org/10.3390/ijms26083842
Chuljerm H, Maneekesorn S, Thorup G, Nantakool S, Charoenkwan P, Rerkasem K. The Relevance of Endothelial Dysfunction Biomarkers in Thalassemia Patients and Healthy Individuals: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(8):3842. https://doi.org/10.3390/ijms26083842
Chicago/Turabian StyleChuljerm, Hataichanok, Supawadee Maneekesorn, Gabriel Thorup, Sothida Nantakool, Pimlak Charoenkwan, and Kittipan Rerkasem. 2025. "The Relevance of Endothelial Dysfunction Biomarkers in Thalassemia Patients and Healthy Individuals: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 8: 3842. https://doi.org/10.3390/ijms26083842
APA StyleChuljerm, H., Maneekesorn, S., Thorup, G., Nantakool, S., Charoenkwan, P., & Rerkasem, K. (2025). The Relevance of Endothelial Dysfunction Biomarkers in Thalassemia Patients and Healthy Individuals: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(8), 3842. https://doi.org/10.3390/ijms26083842






