Abstract
Chronic pruritus is a distressing condition associated with various dermatological and systemic diseases, significantly impairing patients’ quality of life. While conventional treatments such as antihistamines and corticosteroids offer relief, their efficacy varies, and long-term use may lead to adverse effects. Emerging evidence suggests that certain vitamins, including vitamin D, vitamin E, vitamin B12, and niacinamide (B3), may play a role in alleviating pruritus through their anti-inflammatory, immune-regulatory, and skin barrier-enhancing properties. However, the effectiveness of these vitamins in managing chronic pruritus remains unclear. This meta-analysis aims to update and expand the evaluation of vitamin supplementation in reducing pruritus severity across different underlying conditions, extending the scope beyond vitamin D to include vitamins B and E. A comprehensive search was performed across PubMed, Embase, Web of Science, and Cochrane Library databases up to January 2025 to identify randomized controlled trials (RCTs) evaluating the effects of vitamin supplementation on chronic pruritus. A total of 21 RCTs (n = 1723) were included in the meta-analysis. Compared to placebo, vitamin supplementation demonstrated a significant reduction in pruritus severity (Standardized Mean Difference [SMD]: −0.578, 95% CI: −0.736 to −0.419, p = 0.000; I2 = 53.630, p = 0.003). Subgroup analysis revealed that topical vitamin B12 and vitamin D3 showed the most pronounced antipruritic effects, particularly in patients with atopic dermatitis and chronic kidney disease-associated pruritus. Sensitivity analysis confirmed the robustness of the findings; however, potential publication bias was suggested by Egger’s regression test (p = 0.00979), indicating that the overall effect may be influenced by small-study effects or underreporting of negative results. This meta-analysis indicates that vitamin B, D, and E supplementation may serve as effective adjunct therapies for managing chronic pruritus. However, the variability among the included studies highlights the necessity for well-structured, long-term RCTs to determine the ideal dosage, treatment duration, and target patient populations that would derive the greatest benefit from vitamin-based interventions.
1. Introduction
Chronic pruritus is a distressing and often intractable symptom that significantly impairs quality of life and is associated with a range of dermatological and systemic disorders, including atopic dermatitis, psoriasis, chronic kidney disease (CKD), cholestatic liver disease, and neuropathic conditions [1,2,3]. While antihistamines, corticosteroids, and immunosuppressants remain the mainstay of treatment, their efficacy is often limited, and long-term use is associated with adverse effects [4,5]. Given these limitations, there is growing interest in alternative and adjunctive therapies, including micronutrient-based interventions.
Recent studies suggest that vitamins play an essential role in modulating pruritus through diverse mechanisms, including immune regulation, skin barrier enhancement, anti-inflammatory activity, and oxidative stress reduction [6,7]. Among them, vitamin D [8,9], vitamin E [10], vitamin B12 [11], and niacinamide (B3) [12] have been explored for their potential antipruritic effects in different conditions. Vitamin D is well-recognized for its immunomodulatory properties and has been implicated in reducing inflammatory responses linked to pruritus in conditions such as atopic dermatitis and CKD-associated pruritus [13,14]. Vitamin E, a potent antioxidant, may alleviate pruritus by reducing oxidative stress and stabilizing cell membranes, particularly in inflammatory skin diseases [15]. Vitamin B12 has demonstrated efficacy as a topical treatment for atopic dermatitis, potentially by inhibiting nitric oxide synthase and thereby mitigating neurogenic inflammation [16]. Similarly, niacinamide, known for its barrier-protective and anti-inflammatory properties, has been investigated for its role in reducing pruritus, especially in barrier-compromised conditions [17].
Despite these promising findings, the efficacy of vitamins in managing chronic pruritus remains inconclusive, as individual studies report varying results depending on the underlying disease, intervention duration, dosage, and mode of administration. To address these gaps, we conducted an updated meta-analysis to systematically evaluate the role of multivitamin approaches in chronic pruritus management. This study synthesizes evidence from randomized controlled trials (RCTs) to assess whether vitamin supplementation is an effective strategy for alleviating pruritus across different dermatological and systemic conditions. The findings of this analysis aim to provide clinically relevant insights into the therapeutic potential of vitamins in dermatology, guiding future research and treatment strategies for chronic pruritus.
2. Results
2.1. Study Search and Characteristics of Included Patients
The search and selection process for trials yielded a comprehensive set of studies for inclusion in this meta-analysis (Figure 1). Our initial search across four databases (PubMed, Embase, Cochrane Library, and Web of Science) along with an additional search using PubMed’s ‘related articles’ feature, yielded a total of 774 trials. After removing duplicates, 402 unique trials remained and were subjected to title and abstract screening, resulting in the exclusion of 358 trials. A detailed full-text review of the 44 remaining trials led to the exclusion of 23 trials for reasons including the absence of a placebo control group (10 trials [18,19,20,21,22,23,24,25,26,27]), being a cohort study (1 trial [28]), involving participants under 18 years old (6 trials [29,30,31,32,33,34]), lack of full-text availability (2 trials [35,36]), combination with other medication (2 trial [37,38]) and outcomes unrelated to the study’s focus (2 trials [39,40]). Ultimately, 21 trials met the inclusion criteria and were included in this meta-analysis [7,8,9,10,11,12,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. Table 1 summarizes the key characteristics of the included trials, which were published between 2004 and 2023. These 21 trials involved a total of 1723 participants, with the number of participants per trial ranging from 11 to 273. The primary objective of all included trials was to assess the potential effects of vitamin D on chronic pruritus.
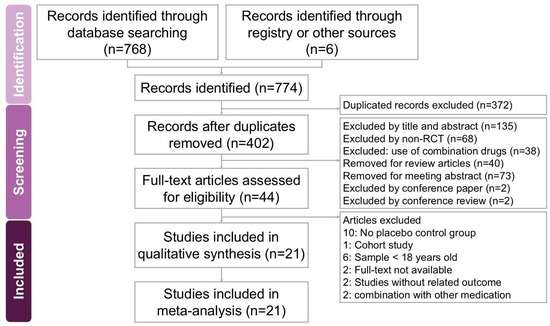
Figure 1.
A flowchart depicting the study selection process for the systematic review and meta-analysis on the effects of vitamin supplementation in managing chronic pruritus across various dermatological conditions. Out of 774 initially identified records, 21 studies met the eligibility criteria and were included in the final analysis.

Table 1.
Characteristics of included studies.
2.2. Quality Assessment
Most of the included trials (Figure 2) were identified as having some concerns of bias, particularly in the selection of reported outcomes. Most studies exhibited a low risk of bias in the randomization process, suggesting that randomization was generally well-executed. However, certain studies, such as Stücker (2004; [7]) and Jung (2015; [9]), lacked sufficient details, resulting in uncertainty in this domain. In terms of deviations from intended interventions, most trials maintained a low risk of bias, indicating that the interventions were administered as planned without substantial deviations that could influence the results. Nevertheless, Jung (2015; [9]) demonstrated a higher risk of bias in this area, which may have impacted its findings. Regarding missing outcome data, the risk of bias varied across studies, with Wohlrab (2014; [12]), Jung (2015; [9]), and Disphanurat (2019; [53]) exhibiting some concerns due to incomplete data reporting. In the assessment of outcome measurement, most trials were considered to have a low risk of bias, but Amestejani (2012; [46]), Jung (2015; [9]), and Nistico (2017; [52]) were flagged as having some concerns, likely due to their open-label study designs. Overall, studies such as Jung (2015; [9]) demonstrated a high risk of bias across multiple domains, which may affect the reliability of their findings. Conversely, other studies, including [49,51,54,55], were categorized as having a low overall risk of bias, suggesting that their results are more reliable and robust. While most studies exhibited a low risk of bias in key areas, some showed high or unclear risks across multiple domains, necessitating careful interpretation of their findings in the broader context of this meta-analysis.
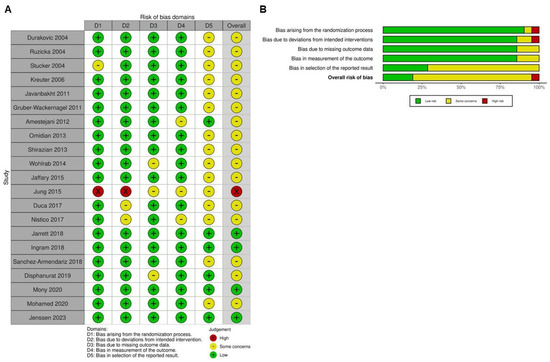
Figure 2.
Evaluation of the methodological quality of the included trials. (A) Individual risk of bias assessment for each selected study, based on the Rob 2.0 tool (https://mcguinlu.shinyapps.io/robvis/). (B) Overall risk of bias summarized as a percentage, considering intention-to-treat and per-protocol analyses. The primary sources of high risk of bias across the studies were deviations from intended interventions, followed by issues related to missing outcome data and deficiencies in the randomization process [7,8,9,10,11,12,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55].
2.3. Impact of Vitamin Supplementation on Chronic Pruritus
As illustrated in Figure 3, the intervention demonstrated a moderate effect in alleviating pruritus among affected patients (overall effect: −0.578, 95% CI: −0.736 to −0.419, p < 0.001; I2 = 53.630%, p = 0.003). Subgroup analysis further indicated a significant reduction in pruritus for those receiving vitamin supplementation compared to placebo (Figure 4A), particularly in cases where the intervention lasted less than 8 weeks (overall effect: −0.681, 95% CI: −0.959 to −0.402, p < 0.001; I2 = 55.737%, p = 0.035). In contrast, patients receiving supplementation for 8 to 12 weeks experienced a moderate effect (overall effect: −0.606, 95% CI: −0.785 to −0.427, p < 0.001; I2 < 0.001%, p = 0.491). Interventions lasting between 12 and 24 weeks did not demonstrate a statistically significant reduction in pruritus (overall effect: −0.466, 95% CI: −1.220 to 0.289, p = 0.226) and were associated with substantial heterogeneity (I2 = 84.007%, p = 0.002). Similarly, interventions exceeding 24 weeks showed no significant effect (overall effect: −0.428, 95% CI: −1.048 to 0.192, p = 0.176), with moderate heterogeneity (I2 = 70.980%, p = 0.063). These results imply that prolonged durations of vitamin supplementation do not necessarily yield greater antipruritic benefits.
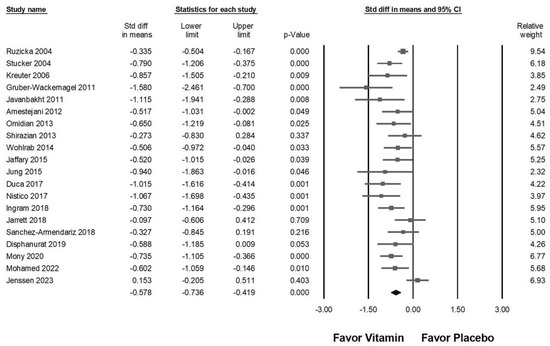
Figure 3.
Displays the overall impact of vitamins on chronic pruritus, as measured by the visual analog scale, compared to placebo [7,8,9,10,11,12,42,43,44,45,46,47,48,49,50,51,52,53,54,55].
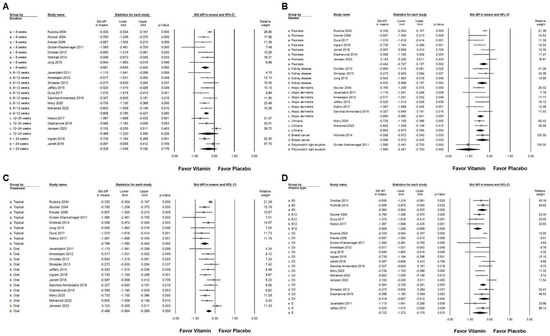
Figure 4.
A forest plot illustrating the subgroup analyses of vitamin supplementation in managing chronic pruritus. (A) Effect of treatment duration, (B) disease, (C) route of administration, and (D) different vitamin types. The squares represent the effect sizes, with horizontal lines indicating the 95% confidence intervals. The diamond symbol at the bottom of each panel summarizes the overall effect size.
2.4. Subgroup Analysis of Vitamin Supplementation Effects on Chronic Pruritus
In the subgroup analysis based on disease diagnosis (Figure 4B), vitamin supplementation demonstrated a small effect in reducing pruritus among patients with psoriasis (overall effect: −0.442, 95% CI: −0.727 to −0.157, p = 0.002; I2 = 68.673%, p = 0.004). A moderate effect was observed in individuals with chronic kidney disease (overall effect: −0.533, 95% CI: −0.898 to −0.167, p = 0.004; I2 < 0.001%, p = 0.418), atopic dermatitis (overall effect: −0.665, 95% CI: −0.890 to −0.441, p < 0.001; I2 = 6.109%, p = 0.377), urticaria (overall effect: −0.683, 95% CI: −0.970 to −0.395, p < 0.001; I2 < 0.001%, p = 0.658), and breast cancer-associated pruritus (overall effect: −0.506, 95% CI: −0.972 to −0.040, p = 0.033; I2 < 0.001%, p > 0.999). Notably, polymorphic light eruption exhibited the most significant response to vitamin supplementation, with a large effect observed (SMD: −1.580, 95% CI: −2.461 to −0.700, p < 0.001; I2 < 0.001%, p > 0.999). The effectiveness of vitamin supplementation varied based on the route of administration (Figure 4C). The topical application resulted in a significant reduction in pruritus compared to placebo (overall effect: −0.786, 95% CI: −1.080 to −0.492, p < 0.001; I2 = 63.314%, p = 0.008). Conversely, oral supplementation exhibited a small effect (overall effect: −0.466, 95% CI: −0.664 to −0.268, p < 0.001; I2 = 47.494%, p = 0.034). Among the various vitamin types (Figure 4D), vitamin D2 showed a small effect (overall effect: −0.419, 95% CI: −0.827 to −0.012, p = 0.044; I2 < 0.001%, p = 0.449). In contrast, moderate effects were observed in patients receiving vitamin B3 (overall effect: −0.564, 95% CI: −0.924 to −0.203, p = 0.002; I2 < 0.001%, p = 0.702), vitamin D3 (overall effect: −0.504, 95% CI: −0.728 to −0.281, p < 0.001; I2 = 63.731%, p = 0.002), and vitamin E (overall effect: −0.722, 95% CI: −1.273 to −0.170, p = 0.010; I2 = 31.703%, p = 0.226). Vitamin B12 supplementation demonstrated the greatest effect in reducing pruritus, with a large effect size (overall effect: −0.909, 95% CI: −1.209 to −0.608, p < 0.001; I2 < 0.001%, p = 0.714). The findings suggest that the efficacy of vitamin supplementation in reducing pruritus varies depending on the disease type, mode of administration, and specific vitamin used.
2.5. Effectiveness of Different Vitamin Types and Administration Routes in Pruritus Management
In the advanced subgroup analysis examining different vitamin types and administration routes (Figure 5), topical vitamin D3 (overall effect: −0.826, 95% CI: −1.392 to −0.259, p = 0.004; I2 = 71.683%, p = 0.014) and topical vitamin B12 (overall effect: −0.909, 95% CI: −1.209 to −0.608, p < 0.001; I2 <0.001%, p = 0.714) exhibited the strongest antipruritic effects. Moderate pruritus reduction was observed with topical vitamin B3 (overall effect: −0.506, 95% CI: −0.972 to −0.040, p = 0.033; I2 < 0.001%, p > 0.999), oral vitamin B3 (overall effect: −0.650, 95% CI: −1.219 to −0.081, p = 0.025; I2 < 0.001%, p > 0.999), and oral vitamin E (overall effect: −0.722, 95% CI: −1.273 to −0.170, p = 0.010; I2 = 31.703%, p = 0.226). On the other hand, oral vitamin D2 (overall effect: −0.419, 95% CI: −0.827 to −0.012, p = 0.044; I2 < 0.001%, p = 0.449) and oral vitamin D3 (overall effect: −0.407, 95% CI: −0.688 to −0.125, p = 0.005; I2 = 64.537%, p = 0.010) were associated with a small yet significant reduction in pruritus. These findings suggest that topical applications of vitamin D3 and B12 may be the most effective in alleviating pruritus, while oral vitamin B3, B12, and E also provide considerable benefits. Conversely, oral vitamins D2 and D3, though effective, demonstrated a comparatively weaker impact on pruritus severity.
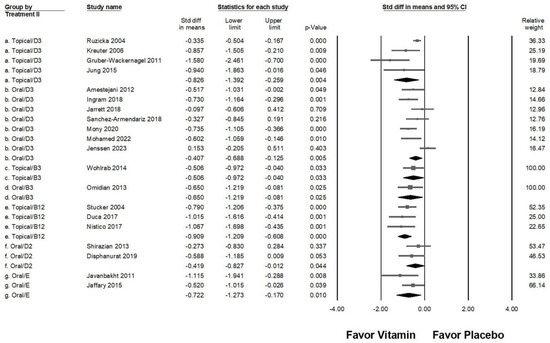
Figure 5.
A forest plot illustrating the combined effects of vitamin supplementation on chronic pruritus, integrating both the route of administration and vitamin type. The squares represent individual study effect sizes, with horizontal lines indicating 95% confidence intervals, while the diamond symbol at the bottom of each panel represents the overall pooled effect size.
2.6. Comparative Efficacy of Vitamin Types, Administration Methods, and Treatment Duration in Pruritus Management
In an in-depth subgroup analysis assessing the effects of various vitamin types, administration routes, and treatment durations (Figure 6), topical vitamin B12 applied for 8–12 weeks (overall effect: −1.015, 95% CI: −1.616 to −0.414, p = 0.001; I2 < 0.001%, p > 0.999) and 12–24 weeks (overall effect: −1.067, 95% CI: −1.698 to −0.435, p = 0.001; I2 < 0.001%, p > 0.999) exhibited a strong antipruritic effect. Likewise, topical vitamin D3 used for less than 8 weeks (overall effect: −0.826, 95% CI: −1.392 to −0.259, p = 0.004; I2 = 71.683%, p = 0.014) resulted in a significant reduction in pruritus severity. Moderate effects were observed with oral vitamin D3 for 8–12 weeks (overall effect: −0.583, 95% CI: −0.809 to −0.357, p < 0.001; I2 < 0.001%, p = 0.645), oral vitamin B3 for less than 8 weeks (overall effect: −0.650, 95% CI: −1.219 to −0.081, p < 0.025; I2 < 0.001%, p > 0.999), oral vitamin E for 8–12 weeks (overall effect: −0.722, 95% CI: −1.273 to −0.170, p = 0.010; I2 = 31.703%, p = 0.226). Additionally, moderate effects were noted for topical vitamin B3 applied for less than 8 weeks (overall effect: −0.506, 95% CI: −0.972 to −0.040, p = 0.033; I2 < 0.001%, p > 0.999) and topical vitamin B12 administered for under 8 weeks (overall effect: −0.790, 95% CI: −1.206 to −0.375, p < 0.001; I2 < 0.001%, p > 0.999). Conversely, oral vitamin D3 administered for 12–24 weeks (overall effect: −0.153, 95% CI: −0.205 to 0.511, p = 0.403; I2 < 0.001%, p > 0.999), oral vitamin D3 was taken for up to 24 weeks (overall effect: −0.428, 95% CI: −1.048 to 0.192, p = 0.176; I2 = 70.980%, p = 0.063), oral vitamin D2 used for 8–12 weeks (overall effect: −0.273, 95% CI: −0.830 to 0.284, p = 0.337; I2 < 0.001%, p > 0.999), and oral vitamin D2 for 12–24 weeks (overall effect: −0.588, 95% CI: −1.185 to −0.009, p = 0.053; I2 < 0.001%, p > 0.999) demonstrated a non-significant effect in reducing pruritus.
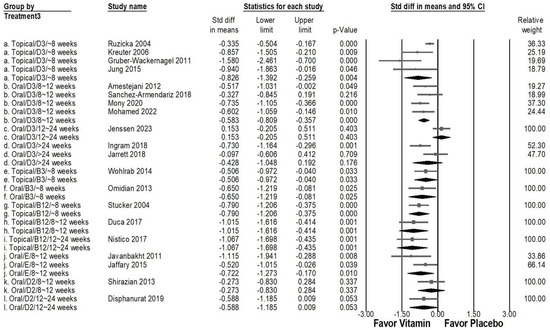
Figure 6.
A forest plot depicting the combined effects of vitamin supplementation on chronic pruritus, considering administration route, treatment duration, and vitamin type. The squares represent effect sizes of individual studies, with horizontal lines indicating 95% confidence intervals, while the diamond symbol at the bottom of each panel represents the overall pooled effect size.
2.7. Impact of Vitamin Supplementation on Skin Lesion Reduction and Inflammatory Cytokine Suppression
Vitamin supplementation significantly reduced skin lesion area, as shown in Figure 7A (overall effect: −0.736, 95% CI: −1.045 to −0.427, p < 0.001; I2 = 83.230%, p < 0.001). Furthermore, vitamins exhibited a moderate inhibitory effect on inflammatory cytokines, including TNF-α (Figure 7B; overall effect: −0.658, 95% CI: −0.945 to −0.371, p < 0.001; I2 < 0.001%, p = 0.518), IL-6 (Figure 7C; overall effect: −0.629, 95% CI: −0.916 to −0.343, p < 0.001; I2 < 0.001%, p = 0.416), and hs-CRP (Figure 7D; overall effect: −0.655, 95% CI: −0.914 to −0.396, p < 0.001; I2 < 0.001%, p = 0.823). These findings suggest that vitamin supplementation may play a role in both reducing lesion severity and modulating inflammatory responses in individuals with chronic pruritus.
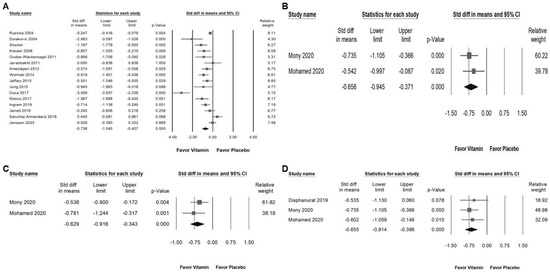
Figure 7.
Presents a forest plot that highlights the effects of vitamin supplementation. The plot is divided into four sections for ease of interpretation: (A) shows the effect on skin lesion area, (B) depicts changes in TNF levels, (C) assesses alterations in IL-6 levels, and (D) evaluates the impact on hs-CRP. The horizontal lines extending from the squares represent the 95% confidence intervals, while the diamond symbols indicate the overall effect sizes.
2.8. Sensitivity Analysis
Among the 20 studies included in Figure 3, one trial that investigated vitamin D3 [54] did not demonstrate a significant effect in relieving chronic pruritus. As a result, this study was excluded from the sensitivity analysis. Figure 8 illustrates the impact of vitamin supplementation on pruritus reduction (overall effect: −0.613, 95% CI: −0.753 to −0.473, p < 0.001; I2 = 34.509%, p = 0.070). The results of the sensitivity analysis remained consistent with the initial findings, reinforcing that vitamin supplementation maintains a moderate effect in alleviating chronic pruritus.
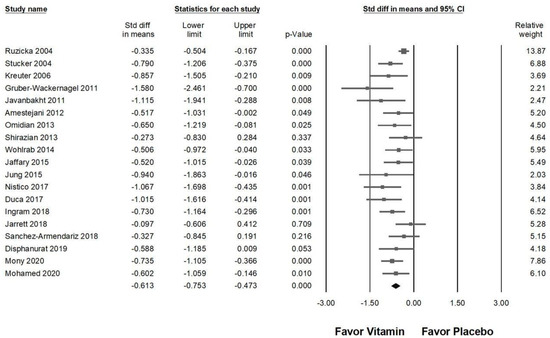
Figure 8.
A sensitivity analysis evaluating the effects of vitamin supplementation on chronic pruritus, measured by the visual analog scale, compared to placebo following the exclusion of Jenssen (2023) [54]. The horizontal lines extending from the squares represent the 95% confidence intervals, while the diamond symbols indicate the overall effect sizes.
2.9. Publishing Bias
Egger’s regression analysis detected significant publication bias in the dataset (p = 0.00979). Figure 9 presents the funnel plots, depicting the SMD values for the effectiveness of vitamin supplementation. The asymmetry observed in the plot further supports the presence of publication bias. Notably, the visible gap in the lower right corner of the funnel plot raises concerns, suggesting that certain studies with less pronounced effects of vitamin supplementation may be unpublished or undiscovered. This potential absence of data could influence the overall interpretation of the meta-analysis findings.
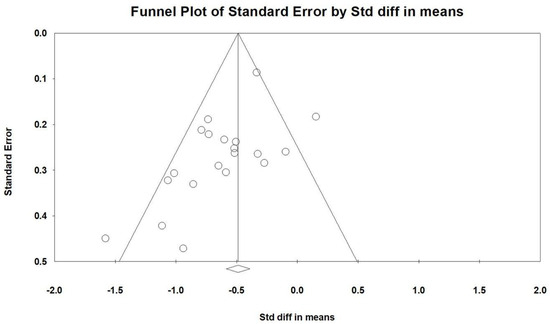
Figure 9.
A funnel plot summarizing the findings from all included studies. The diagonal lines represent confidence intervals around the effect estimates, indicating the expected range for the true effect size. Each circle corresponds to an individual study, with larger circles reflecting studies with greater weight or larger sample sizes. The diamond symbol at the bottom represents the overall pooled effect size, with its center indicating the estimated effect and its width denoting the confidence interval.
3. Discussion
This updated meta-analysis offers a comprehensive synthesis of the current evidence on vitamin supplementation in the management of chronic pruritus, building upon our previously published work that focused solely on vitamin D [14]. Given the growing interest in micronutrients as adjunct therapies for dermatologic and systemic pruritic conditions, we recognized the importance of re-evaluating the literature to include additional vitamins such as B12, E, and niacinamide (B3). These nutrients have demonstrated promising anti-inflammatory properties and potential for enhancing skin barrier function in recent randomized controlled trials. By expanding the focus beyond vitamin D, this study seeks to reflect the evolving therapeutic landscape and address the current gap in consolidated evidence regarding these alternative vitamin-based interventions.
The findings of this study highlight vitamin B supplementation, particularly vitamin B12, as an effective intervention for reducing pruritus severity. Vitamin B12 demonstrated a pronounced antipruritic effect, especially in patients with atopic dermatitis and chronic kidney disease-associated pruritus. This aligns with previous studies [11,52] suggesting that vitamin B12 modulates neuroinflammatory pathways [56] and supports nerve function, potentially reducing itch perception by stabilizing mast cell activity [57] and inhibiting pro-inflammatory cytokines. Its topical use is further supported by favorable tolerability and ease of application, making it a viable option for long-term symptom management in outpatient settings. Niacinamide (vitamin B3) also exhibited a moderate effect in alleviating pruritus, which may be attributed to its anti-inflammatory, antioxidant, and skin barrier-enhancing properties [17]. Niacinamide has been shown to downregulate nuclear factor-kappa B (NF-κB), a key regulator of inflammation, and reduce oxidative stress, both of which are implicated in chronic pruritus pathophysiology [6]. Furthermore, its role in improving epidermal lipid synthesis and skin hydration suggests a potential benefit for pruritus associated with xerosis or barrier dysfunction disorders [6]. Despite these promising findings, the heterogeneity among studies, particularly in dosage, formulation, and duration of supplementation, remains a limitation. Some trials used oral administration, while others applied topical vitamin B12, which may influence bioavailability and therapeutic outcomes. Future research should focus on standardizing treatment protocols and exploring the long-term efficacy of vitamin B supplementation in different pruritic conditions. Additionally, investigating potential synergistic effects with other therapies—such as moisturizers or antihistamines—may further enhance its clinical utility.
Vitamin E has shown moderate efficacy in reducing chronic pruritus, as demonstrated in our meta-analysis. As a potent antioxidant, vitamin E plays a crucial role in maintaining skin integrity, modulating inflammatory responses, and protecting cells from oxidative damage, all of which contribute to its antipruritic effects. Chronic pruritus is often associated with oxidative stress and increased inflammatory mediators, which disrupt the skin barrier and exacerbate itching sensations. The ability of vitamin E to neutralize free radicals and stabilize cell membranes may explain its observed benefits in itch relief [15]. Several studies have reported the effectiveness of vitamin E in alleviating pruritus associated with dermatological and systemic conditions [10,47]. Its anti-inflammatory action, primarily through inhibition of lipid peroxidation and cytokine modulation, may contribute to reduced skin irritation and hypersensitivity responses [58]. Moreover, vitamin E has been found to enhance keratinocyte function and improve the skin’s moisture retention, which is particularly beneficial in conditions such as xerosis-related pruritus [59]. The administration route of vitamin E also plays a role in its effectiveness. While oral supplementation has been widely studied, topical application may offer more targeted benefits, delivering higher concentrations directly to affected areas and bypassing potential limitations of systemic absorption [60]. This suggests that vitamin E could be an effective adjunctive therapy, particularly for patients with inflammatory skin disorders or pruritus linked to oxidative stress.
Vitamin D has been widely studied for its role in skin health and immune regulation, and our meta-analysis further supports its efficacy in reducing chronic pruritus. The findings demonstrate that vitamin D supplementation, particularly in its D3 form, exerts a moderate antipruritic effect, with topical application yielding greater benefits than oral administration. This aligns with previous research [8,9,42] suggesting that vitamin D modulates inflammatory responses, strengthens the skin barrier, and influences neural pathways involved in itch perception [61]. One potential mechanism underlying vitamin D’s antipruritic effects is its ability to regulate immune function, particularly through its influence on T-cell differentiation and cytokine production [34,62]. Chronic pruritus is often associated with an imbalance between pro-inflammatory and anti-inflammatory cytokines, particularly in conditions such as atopic dermatitis, psoriasis, and chronic kidney disease-associated pruritus. Vitamin D has been shown to suppress the production of pro-inflammatory cytokines such as IL-6, TNF-α, and IL-17 while promoting regulatory T-cell activity, thereby reducing skin inflammation and pruritus intensity [34,62]. Although TNF-α and IL-6 are not considered the primary cytokines in classic pruritus pathways, they are part of the broader inflammatory network involved in various pruritic conditions. Vitamin D has been shown to exert immunomodulatory effects by inhibiting Th1 and Th17 responses and promoting Th2 and regulatory T cell development, thereby shifting the immune balance toward an anti-inflammatory state [63]. This is especially relevant in atopic dermatitis, chronic kidney disease-associated pruritus, and other immune-mediated skin disorders, where dysregulated cytokine profiles contribute to itch. Recent literature also highlights vitamin D’s ability to downregulate IL-17A, TNF-α, and IL-6, which indirectly influences neural and immune crosstalk involved in chronic pruritus [64]. These cytokines can sensitize peripheral sensory nerves or enhance pruritogen release, contributing to the itch–scratch cycle. This study also found that both vitamin D2 and D3 were effective in reducing the expression levels of TNF-α, IL-6, and hs-CRP, further supporting their role in mitigating inflammation-driven pruritus. These findings reinforce the notion that vitamin D supplementation, particularly in its active forms, may serve as a valuable adjunct therapy in chronic pruritus management. However, additional well-designed trials are warranted to determine optimal dosing strategies, treatment duration, and patient populations that would benefit most from vitamin D-based interventions. Our findings support the role of vitamin D, particularly topical D3, as an effective intervention for chronic pruritus. Its ability to modulate immune responses, enhance skin barrier function, and suppress inflammatory mediators underscores its therapeutic potential. Given its favorable safety profile and accessibility, vitamin D supplementation may serve as a supportive option for patients with pruritus related to systemic or dermatologic inflammation.
The administration route of vitamin supplementation plays a crucial role in determining its efficacy in alleviating chronic pruritus. Our findings indicate that topical applications, particularly vitamin B12 and D3, demonstrated superior antipruritic effects compared to oral formulations. This observation may be attributed to the direct cutaneous absorption of topically applied vitamins, allowing for localized anti-inflammatory and neuroprotective effects while bypassing metabolic activation and systemic dilution that can occur with oral administration [16]. In contrast, oral administration of vitamin D2 and D3 exhibited only modest effects in pruritus relief. This may be due to variations in bioavailability and the metabolic conversion process, as vitamin D requires activation in the liver and kidneys before exerting its biological effects [5]. Similarly, oral vitamin B3 and vitamin E demonstrated moderate antipruritic benefits, likely due to their systemic anti-inflammatory and antioxidant properties [12]. However, these effects may take longer to manifest compared to topical formulations, where localized delivery allows for a more immediate response. Additionally, treatment duration appears to influence the effectiveness of vitamin supplementation in pruritus management. Our analysis revealed that short-term interventions (<8 weeks) were associated with greater reductions in pruritus, while longer interventions (>12 weeks) demonstrated diminishing effects. This suggests a potential plateau in efficacy, possibly due to receptor downregulation, physiological adaptation, or declining patient adherence over extended periods [15]. Notably, topical vitamin B12 demonstrated sustained efficacy over 8–24 weeks, while vitamin D3 appeared to exert its most pronounced effects within the first eight weeks of treatment. These findings highlight the importance of considering both the administration route and treatment duration when incorporating vitamin supplementation into pruritus management strategies. Future research should focus on optimizing dosage regimens, comparing different formulations, and investigating potential synergistic effects between various vitamins to maximize therapeutic outcomes.
Despite the encouraging results observed, this meta-analysis has several important limitations that warrant careful consideration. First, the included studies exhibited substantial heterogeneity in terms of study design, participant characteristics, underlying etiologies of pruritus, and outcome evaluation methods. Conditions such as atopic dermatitis, chronic kidney disease, and various inflammatory dermatoses differ in their pathophysiology, which may contribute to variable responses to vitamin supplementation. Although subgroup analyses were performed to account for this variability, residual confounding remains a concern and may limit the generalizability of our conclusions. Future trials should consider adopting more standardized diagnostic criteria and patient selection strategies. Second, evidence of publication bias was detected through funnel plot asymmetry and Egger’s regression test. The underreporting of negative or null findings in the literature could have led to an overestimation of the treatment effect. This highlights the need for journals and researchers to promote the dissemination of all findings, regardless of statistical significance, to ensure a more balanced and accurate synthesis of evidence. Third, inconsistencies in dosage, formulation, and mode of administration among the included trials further complicate interpretation. While topical vitamin D3 and B12 demonstrated relatively stronger antipruritic effects, the dosing regimens—ranging in concentration, frequency, and duration—varied widely across studies. This heterogeneity precludes firm conclusions regarding optimal treatment protocols. Larger, well-designed RCTs are needed to determine dose-response relationships and establish evidence-based guidelines for clinical practice. Fourth, most of the included studies had short follow-up periods, typically ranging from 8 to 12 weeks. The limited duration restricts our understanding of the long-term efficacy and safety of vitamin supplementation in managing chronic pruritus. It remains unclear whether initial improvements are maintained over time or whether physiological tolerance may develop. Longitudinal studies with extended follow-up are essential to address these uncertainties. Fifth, most trials relied on subjective measures such as the Visual Analog Scale (VAS) or Numeric Rating Scale (NRS) to assess pruritus severity. Although these tools are widely used and validated, they are inherently prone to patient-reported bias. The inclusion of objective biomarkers, such as inflammatory cytokines or skin histological changes, in future studies could strengthen the accuracy and reproducibility of outcomes. Sixth, there was a general lack of serum vitamin D level monitoring, particularly in studies evaluating topical formulations. Without tracking systemic vitamin D status, it is difficult to establish a clear relationship between serum levels and clinical improvement. Future research should integrate regular biochemical assessments to clarify this potential correlation and better understand the pharmacodynamics of both topical and systemic vitamin D applications. Lastly, although our meta-analysis focused exclusively on randomized controlled trials to enhance methodological rigor, this approach may have inadvertently excluded relevant real-world data from observational studies and clinical case series. Including such data in future syntheses may provide a more comprehensive view of vitamin efficacy in diverse clinical settings. While this meta-analysis supports the potential role of vitamin supplementation in alleviating chronic pruritus, multiple limitations—including heterogeneity, publication bias, inconsistent dosing, short follow-up, reliance on subjective metrics, lack of serum level monitoring, and exclusion of real-world evidence—should be acknowledged. Addressing these gaps in future research will be critical for refining therapeutic strategies and maximizing clinical benefit.
4. Methods and Materials
4.1. Data Sources and Selection Criteria
This study conducted a systematic search for RCTs evaluating the effects of vitamin supplementation on chronic pruritus. The search encompassed PubMed, Embase, Cochrane Library, and Web of Science, covering studies published up to January 2025. A structured search strategy was employed, incorporating key terms such as “vitamin”, “chronic pruritus”, “pruritus”, “itch”, and “chronic itch”, with a focus on human clinical trials. The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [65], ensuring a transparent and comprehensive review process. Additionally, the reference lists of relevant articles were screened to identify further eligible studies. Studies were excluded if they were case reports, technical papers, conference abstracts, reviews, letters, editorials, or laboratory-based research. This meta-analysis is registered with PROSPERO under the identifier CRD42025642182.
4.2. Selection of Studies
The selection of studies was independently carried out by two reviewers, with a third reviewer validating the final decisions to enhance accuracy and minimize bias. To ensure a thorough and in-depth evaluation, full-text versions of all eligible studies were retrieved and carefully examined. A visual representation of the selection process is provided in the PRISMA flow diagram (Figure 1).
4.3. Data Extraction
Data extraction was conducted independently using a standardized template, adhering to the methodological guidelines outlined in the Cochrane Handbook [66]. The collected data encompassed key study details, including author names, publication year and country, inclusion criteria, participant demographics (sample size and age distribution), study design, intervention specifics, and outcome assessments. Additionally, the methods used to evaluate chronic pruritus and associated clinical parameters were recorded to ensure consistency across studies.
4.4. Outcomes
The main outcome measured was the severity of chronic pruritus, evaluated using validated pruritus assessment tools. Secondary outcomes included alterations in skin lesion area and changes in inflammatory cytokine levels, specifically tumor necrosis factor (TNF), interleukin-6 (IL-6), and high-sensitivity C-reactive protein (hsCRP).
4.5. Assessment of Methodological Quality
The quality of the included studies and potential sources of bias were evaluated using the Cochrane Collaboration’s Risk of Bias tool 2.0. Two reviewers independently conducted the assessment, and any disagreements were addressed through discussion with a third reviewer to reach a consensus. Studies were considered to have a high risk of bias if they demonstrated methodological limitations in one or more key areas.
4.6. Statistical Analyses
For each included study, the SMD and 95% confidence intervals (CIs) were calculated to compare outcomes between the vitamin supplementation and placebo groups. A random-effects model was applied to account for variability among studies. All statistical analyses were conducted using Comprehensive Meta-Analysis software (version 3.0 Biostat, Englewood, NJ, USA). To assess heterogeneity, the I2 statistic was used, with values exceeding 50% indicating substantial heterogeneity. Publication bias was evaluated using funnel plots and Egger’s regression test, with statistical significance set at p < 0.05 for most analyses, except for publication bias, where a threshold of p < 0.10 was applied. Additionally, subgroup analyses were conducted to explore potential sources of heterogeneity, while sensitivity analyses systematically excluded individual studies to assess the robustness of the overall findings.
5. Conclusions
This updated meta-analysis highlights the potential of vitamin supplementation, particularly vitamins B12, B3, D, and E, in alleviating chronic pruritus. The findings suggest that topical formulations, especially vitamin B12 and D3, offer superior antipruritic effects compared to oral administration. Shorter treatment durations (8–12 weeks) were associated with more pronounced symptom relief, whereas prolonged interventions did not yield additional benefits. The effectiveness of vitamin supplementation appears to be influenced by factors such as administration route, dosage, and underlying disease conditions. Future well-designed, large-scale RCTs are necessary to establish optimal treatment protocols, refine dosage recommendations, and evaluate long-term efficacy. By integrating vitamin-based interventions into pruritus management strategies, clinicians may offer safer and more effective alternatives for patients suffering from chronic itch conditions.
Author Contributions
W.-H.K.: Data curation and Investigation. K.-S.C.: Data curation and Investigation. M.-H.C.: Data curation. Y.H.: Visualization. R.-Y.T.: Designed research, Conceptualization, Data curation, Investigation, Visualization, Writing—original draft, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Chung Shan Medical University—Yuan-Rung Hospital Research Collaboration Project, Taiwan (Grant No. 1140057).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data included in article.
Acknowledgments
We would like to thank Jhen-You Hu from the Creative Science Experimental Class at Taichung Municipal Taichung Girls’ Senior High School, Taichung, Taiwan, for her valuable assistance in searching for RCTs for this meta-analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Metz, M.; Stander, S. Chronic pruritus—Pathogenesis, clinical aspects and treatment. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.-S.; Chen, S.C.; Osterberg, L.; Brandt, S.; von Grote, E.C.; Meckfessel, M.H. A daily skincare regimen with a unique ceramide and filaggrin formulation rapidly improves chronic xerosis, pruritus, and quality of life in older adults. Geriatr. Nurs. 2018, 39, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Etter, L.; Myers, S.A. Pruritus in systemic disease: Mechanisms and management. Dermatol. Clin. 2002, 20, 459–472. [Google Scholar] [CrossRef]
- Butler, D.C.; Berger, T.; Elmariah, S.; Kim, B.; Chisolm, S.; Kwatra, S.G.; Mollanazar, N.; Yosipovitch, G. Chronic Pruritus: A Review. JAMA 2024, 331, 2114–2124. [Google Scholar] [CrossRef]
- Mettang, T.; Kremer, A.E. Uremic pruritus. Kidney Int. 2015, 87, 685–691. [Google Scholar] [CrossRef]
- Bains, P.; Kaur, M.; Kaur, J.; Sharma, S. Nicotinamide: Mechanism of action and indications in dermatology. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Stücker, M.; Pieck, C.; Stoerb, C.; Niedner, R.; Hartung, J.; Altmeyer, P. Topical vitamin B12—A new therapeutic approach in atopic dermatitis-evaluation of efficacy and tolerability in a randomized placebo-controlled multicentre clinical trial. Br. J. Dermatol. 2004, 150, 977–983. [Google Scholar] [CrossRef]
- Gruber-Wackernagel, A.; Bambach, I.; Legat, F.J.; Hofer, A.; Byrne, S.N.; Quehenberger, F.; Wolf, P. Randomized double-blinded placebo-controlled intra-individual trial on topical treatment with a 1,25-dihydroxyvitamin D3 analogue in polymorphic lighteruption. Br. J. Dermatol. 2011, 165, 152–163. [Google Scholar] [CrossRef]
- Jung, K.E.; Woo, Y.R.; Lee, J.S.; Shin, J.H.; Jeong, J.U.; Koo, D.W.; Bang, K.T. Effect of topical vitamin D on chronic kidney disease-associated pruritus: An open-label pilot study. J. Dermatol. 2015, 42, 800–803. [Google Scholar] [CrossRef]
- Javanbakht, M.H.; Keshavarz, S.A.; Djalali, M.; Siassi, F.; Eshraghian, M.R.; Firooz, A.; Seirafi, H.; Ehsani, A.H.; Chamari, M.; Mirshafiey, A. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J. Dermatol. Treat. 2011, 22, 144–150. [Google Scholar] [CrossRef]
- Del Duca, E.; Farnetani, F.; De Carvalho, N.; Bottoni, U.; Pellacani, G.; Nistico, S.P. Superiority of a vitamin B12-containing emollient compared to a standard emollient in the maintenance treatment of mild-to-moderate plaque psoriasis. Int. J. Immunopathol. Pharmacol. 2017, 30, 439–444. [Google Scholar] [CrossRef]
- Wohlrab, J.; Bangemann, N.; Kleine-Tebbe, A.; Thill, M.; Kummel, S.; Grischke, E.M.; Richter, R.; Seite, S.; Luftner, D. Barrier protective use of skin care to prevent chemotherapy-induced cutaneous symptoms and to maintain quality of life in patients with breast cancer. Breast Cancer Targets Ther. 2014, 6, 115–122. [Google Scholar] [CrossRef]
- Bhan, I.; Dobens, D.; Tamez, H.; Deferio, J.J.; Li, Y.C.; Warren, H.S.; Ankers, E.; Wenger, J.; Tucker, J.K.; Trottier, C.; et al. Nutritional vitamin D supplementation in dialysis: A randomized trial. Clin. J. Am. Soc. Nephrol. 2015, 10, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Li, C.P.; Huang, S.C.; Hsiao, Y.; Tsai, R.Y. Evaluating the Role of Vitamin D in Alleviating Chronic Pruritus: A Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 9983. [Google Scholar] [CrossRef] [PubMed]
- Nachbar, F.; Korting, H.C. The role of vitamin E in normal and damaged skin. J. Mol. Med. 1995, 73, 7–17. [Google Scholar] [CrossRef]
- Brescoll, J.; Daveluy, S. A review of vitamin B12 in dermatology. Am. J. Clin. Dermatol. 2015, 16, 27–33. [Google Scholar] [CrossRef]
- Wohlrab, J.; Kreft, D. Niacinamide—Mechanisms of action and its topical use in dermatology. Ski. Pharmacol. Physiol. 2014, 27, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Alora-Palli, M.B.; Perkins, A.C.; Van Cott, A.; Kimball, A.B. Efficacy and tolerability of a cosmetically acceptable coal tar solution in the treatment of moderate plaque psoriasis: A controlled comparison with calcipotriene (calcipotriol) cream. Am. J. Clin. Dermatol. 2010, 11, 275–283. [Google Scholar] [CrossRef]
- Cassano, N.; Miracapillo, A.; Coviello, C.; Loconsole, F.; Bellino, M.; Vena, G.A. Treatment of psoriasis vulgaris with the two-compound product calcipotriol/betamethasone dipropionate followed by different formulations of calcipotriol. Clin. Drug Investig. 2006, 26, 227–233. [Google Scholar] [CrossRef]
- Guenther, L.C.; Poulin, Y.P.; Pariser, D.M. A comparison of tazarotene 0.1% gel once daily plus mometasone furoate 0.1% cream once daily versus calcipotriene 0.005% ointment twice daily in the treatment of plaque psoriasis. Clin. Ther. 2000, 22, 1225–1238. [Google Scholar] [CrossRef]
- Rorie, A.; Goldner, W.S.; Lyden, E.; Poole, J.A. Beneficial role for supplemental vitamin D3 treatment in chronic urticaria: A randomized study. Ann. Allergy Asthma Immunol. 2014, 112, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Veien, N.K.; Bjerke, J.R.; Rossmann-Ringdahl, I.; Jakobsen, H.B. Once daily treatment of psoriasis with tacalcitol compared with twice daily treatment with calcipotriol. A double-blind trial. Br. J. Dermatol. 1997, 137, 581–586. [Google Scholar] [CrossRef]
- Takahashi, H.; Tsuji, H.; Ishida-Yamamoto, A.; Iizuka, H. Comparison of clinical effects of psoriasis treatment regimens among calcipotriol alone, narrowband ultraviolet B phototherapy alone, combination of calcipotriol and narrowband ultraviolet B phototherapy once a week, and combination of calcipotriol and narrowband ultraviolet B phototherapy more than twice a week. J. Dermatol. 2013, 40, 424–427. [Google Scholar] [CrossRef]
- Juntongjin, P.; Pongprasert, R. Calcipotriol ointment shows comparable efficacy to topical steroids in chronic hand eczema. Dermatol. Ther. 2019, 32, e12956. [Google Scholar] [CrossRef]
- Ozkan, I.; Kose, O.; Ozmen, I.; Arca, E. Efficacy and safety of non-laser, targeted UVB phototherapy alone and in combination with psoralen gel or calcipotriol ointment in the treatment of localized, chronic, plaque-type psoriasis. Int. J. Dermatol. 2012, 51, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Theng, C.T.; Tan, S.H.; Goh, C.L.; Suresh, S.; Wong, H.B.; Machin, D.; Singapore Lichen Planus Study, G. A randomized controlled trial to compare calcipotriol with betamethasone valerate for the treatment of cutaneous lichen planus. J. Dermatolog. Treat. 2004, 15, 141–145. [Google Scholar] [CrossRef]
- Stucker, M.; Memmel, U.; Hoffmann, M.; Hartung, J.; Altmeyer, P. Vitamin B12 cream containing avocado oil in the therapy of plaque psoriasis. Dermatology 2001, 203, 141–147. [Google Scholar] [CrossRef]
- Thaci, D.; Daiber, W.; Boehncke, W.H.; Kaufmann, R. Calcipotriol solution for the treatment of scalp psoriasis: Evaluation of efficacy, safety and acceptance in 3396 patients. Dermatology 2001, 203, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Guttmann-Gruber, C.; Pinon Hofbauer, J.; Tockner, B.; Reichl, V.; Klausegger, A.; Hofbauer, P.; Wolkersdorfer, M.; Tham, K.C.; Lim, S.S.; Common, J.E.; et al. Impact of low-dose calcipotriol ointment on wound healing, pruritus and pain in patients with dystrophic epidermolysis bullosa: A randomized, double-blind, placebo-controlled trial. Orphanet J. Rare Dis. 2021, 16, 473. [Google Scholar] [CrossRef]
- Byremo, G.; Rod, G.; Carlsen, K.H. Effect of climatic change in children with atopic eczema. Allergy 2006, 61, 1403–1410. [Google Scholar] [CrossRef]
- Gooderham, M.; Debarre, J.M.; Keddy-Grant, J.; Xu, Z.; Kurvits, M.; Goodfield, M. Safety and efficacy of calcipotriol plus betamethasone dipropionate gel in the treatment of scalp psoriasis in adolescents 12–17 years of age. Br. J. Dermatol. 2014, 171, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A., Jr.; Ganmaa, D.; Sidbury, R.; Erdenedelger, K.; Radnaakhand, N.; Khandsuren, B. Randomized trial of vitamin D supplementation for winter-related atopic dermatitis in children. J. Allergy Clin. Immunol. 2014, 134, 831–835. [Google Scholar] [CrossRef]
- Sidbury, R.; Sullivan, A.F.; Thadhani, R.I.; Camargo, C.A., Jr. Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: A pilot study. Br. J. Dermatol. 2008, 159, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, P.; Scaparrotta, A.; Rapino, D.; Cingolani, A.; Attanasi, M.; Petrosino, M.I.; Chuang, K.; Di Pillo, S.; Chiarelli, F. Vitamin D supplementation modulates the immune system and improves atopic dermatitis in children. Int. Arch. Allergy Immunol. 2015, 166, 91–96. [Google Scholar] [CrossRef]
- Lebwohl, M.; Menter, A.; Weiss, J.; Clark, S.D.; Flores, J.; Powers, J.; Balin, A.K.; Kempers, S.; Glinert, R.J.; Fleming, T.; et al. Calcitriol 3 microg/g ointment in the management of mild to moderate plaque type psoriasis: Results from 2 placebo-controlled, multicenter, randomized double-blind, clinical studies. J. Drugs Dermatol. 2007, 6, 428–435. [Google Scholar] [PubMed]
- Kircik, L.H.; Schlesinger, T.E.; Tanghetti, E. Efficacy and Safety of Calcipotriene 0.005%/Betamethasone Dipropionate 0.064% Foam with Apremilast for Moderate Plaque Psoriasis. J. Drugs Dermatol. 2020, 19, 874–880. [Google Scholar] [CrossRef]
- Pinter, A.; Reich, A.; Arenberger, P.; Gold, L.S.; Armstrong, A.; Iversen, L.; Praestegaard, M.; Augustin, M. Randomized Phase 3 trial demonstrating high efficacy, favourable safety and convenience of a novel calcipotriol and betamethasone dipropionate cream for the treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2327–2335. [Google Scholar] [CrossRef]
- Jalili, A.; Lebwohl, M.; Stein Gold, L.; Andersen, S.B.; Jensen, K.L.; Pink, A.E.; Segaert, S.; Berg, P.; Calzavara-Pinton, P.G.; de la Cueva Dobao, P.; et al. Itch relief in patients with psoriasis: Effectiveness of calcipotriol plus betamethasone dipropionate foam. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 709–717. [Google Scholar] [CrossRef]
- Akizawa, T.; Ohashi, Y.; Akiba, T.; Suzuki, M.; Nishizawa, Y.; Ogata, E.; Slatopolsky, E.; Kurokawa, K. Dose-response study of 22-oxacalcitriol in patients with secondary hyperparathyroidism. Ther. Apher. Dial. 2004, 8, 480–491. [Google Scholar] [CrossRef]
- Hata, T.R.; Audish, D.; Kotol, P.; Coda, A.; Kabigting, F.; Miller, J.; Alexandrescu, D.; Boguniewicz, M.; Taylor, P.; Aertker, L.; et al. A randomized controlled double-blind investigation of the effects of vitamin D dietary supplementation in subjects with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 781–789. [Google Scholar] [CrossRef]
- Durakovic, C.; Ray, S.; Holick, M.F. Topical paricalcitol (19-nor-1 alpha,25-dihydroxyvitamin D2) is a novel, safe and effective treatment for plaque psoriasis: A pilot study. Br. J. Dermatol. 2004, 151, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, A.; Sommer, A.; Hyun, J.; Brautigam, M.; Brockmeyer, N.H.; Altmeyer, P.; Gambichler, T. 1% pimecrolimus, 0.005% calcipotriol, and 0.1% betamethasone in the treatment of intertriginous psoriasis: A double-blind, randomized controlled study. Arch. Dermatol. 2006, 142, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, T.; Trompke, C. Treatment of scalp psoriasis. An effective and safe tacalcitol emulsion. Hautarzt 2004, 55, 165–170. [Google Scholar] [CrossRef]
- Omidian, M.; Khazanee, A.; Yaghoobi, R.; Ghorbani, A.R.; Pazyar, N.; Beladimousavi, S.S.; Ghadimi, M.; Mohebbipour, A.; Feily, A. Therapeutic effect of oral nicotinamide on refractory uremic pruritus: A randomized, double-blind study. Saudi J. Kidney Dis. Transpl. 2013, 24, 995–999. [Google Scholar] [CrossRef]
- Shirazian, S.; Schanler, M.; Shastry, S.; Dwivedi, S.; Kumar, M.; Rice, K.; Miyawaki, N.; Ghosh, S.; Fishbane, S. The effect of ergocalciferol on uremic pruritus severity: A randomized controlled trial. J. Ren. Nutr. 2013, 23, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Amestejani, M.; Salehi, B.S.; Vasigh, M.; Sobhkhiz, A.; Karami, M.; Alinia, H.; Kamrava, S.K.; Shamspour, N.; Ghalehbaghi, B.; Behzadi, A.H. Vitamin D supplementation in the treatment of atopic dermatitis: A clinical trial study. J. Drugs Dermatol. 2012, 11, 327–330. [Google Scholar]
- Jaffary, F.; Faghihi, G.; Mokhtarian, A.; Hosseini, S.M. Effects of oral vitamin E on treatment of atopic dermatitis: A randomized controlled trial. J. Res. Med. Sci. 2015, 20, 1053–1057. [Google Scholar] [CrossRef]
- Sanchez-Armendariz, K.; Garcia-Gil, A.; Romero, C.A.; Contreras-Ruiz, J.; Karam-Orante, M.; Balcazar-Antonio, D.; Dominguez-Cherit, J. Oral vitamin D3 5000 IU/day as an adjuvant in the treatment of atopic dermatitis: A randomized control trial. Int. J. Dermatol. 2018, 57, 1516–1520. [Google Scholar] [CrossRef]
- Ingram, M.A.; Jones, M.B.; Stonehouse, W.; Jarrett, P.; Scragg, R.; Mugridge, O.; von Hurst, P.R. Oral vitamin D3 supplementation for chronic plaque psoriasis: A randomized, double-blind, placebo-controlled trial. J. Dermatolog. Treat. 2018, 29, 648–657. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Hussein, M.S.; Salah, E.M.; Eldemery, A.; Darwish, M.M.; Ghaith, D.M.; Attala, R.A.; El Borolossy, R. Efficacy and safety of active vitamin D supplementation in chronic spontaneous urticaria patients. J. Dermatolog. Treat. 2022, 33, 427–432. [Google Scholar] [CrossRef]
- Mony, A.; Chandrashekar, L.; Rajappa, M.; Munisamy, M.; Sahoo, J.P.; Selvarajan, S. Effect of vitamin D supplementation on clinical outcome and biochemical profile in South Indian population with vitamin D-deficient chronic urticarial—A randomized double-blind placebo controlled trial. Clin. Chim. Acta 2020, 504, 1–6. [Google Scholar] [CrossRef]
- Nistico, S.P.; Del Duca, E.; Tamburi, F.; Pignataro, E.; De Carvalho, N.; Farnetani, F.; Pellacani, G. Superiority of a vitamin B12-barrier cream compared with standard glycerol-petrolatum-based emollient cream in the treatment of atopic dermatitis: A randomized, left-to-right comparative trial. Dermatol. Ther. 2017, 30, e12523. [Google Scholar] [CrossRef]
- Disphanurat, W.; Viarasilpa, W.; Chakkavittumrong, P.; Pongcharoen, P. The Clinical Effect of Oral Vitamin D2 Supplementation on Psoriasis: A Double-Blind, Randomized, Placebo-Controlled Study. Dermatol. Res. Pract. 2019, 2019, 5237642. [Google Scholar] [CrossRef]
- Jenssen, M.; Furberg, A.S.; Jorde, R.; Wilsgaard, T.; Danielsen, K. Effect of Vitamin D Supplementation on Psoriasis Severity in Patients with Lower-Range Serum 25-Hydroxyvitamin D Levels: A Randomized Clinical Trial. JAMA Dermatol. 2023, 159, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, P.; Camargo, C.A., Jr.; Coomarasamy, C.; Scragg, R. A randomized, double-blind, placebo-controlled trial of the effect of monthly vitamin D supplementation in mild psoriasis. J. Dermatolog. Treat. 2018, 29, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Politis, A.; Olgiati, P.; Malitas, P.; Albani, D.; Signorini, A.; Polito, L.; De Mauro, S.; Zisaki, A.; Piperi, C.; Stamouli, E.; et al. Vitamin B12 levels in Alzheimer’s disease: Association with clinical features and cytokine production. J. Alzheimers Dis. 2010, 19, 481–488. [Google Scholar] [CrossRef]
- Granerus, G.; Lonnqvist, B.; Nystrand, J.; Roupe, G. Serum tryptase measured with B12 and G5 antibody-based immunoassays in mastocytosis patients and its relation to histamine turnover. Br. J. Dermatol. 1998, 139, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef]
- Thiele, J.J.; Hsieh, S.N.; Ekanayake-Mudiyanselage, S. Vitamin E: Critical review of its current use in cosmetic and clinical dermatology. Dermatol. Surg. 2005, 31, 805–813. [Google Scholar] [CrossRef]
- Michalak, M.; Pierzak, M.; Krecisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Brozyna, A.A.; Zmijewski, M.A.; Jozwicki, W.; Jetten, A.M.; Mason, R.S.; Tuckey, R.C.; Elmets, C.A. Vitamin D signaling and melanoma: Role of vitamin D and its receptors in melanoma progression and management. Lab. Investig. 2017, 97, 706–724. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.A.; Toh, J.A.; Vernon, N.; Jariwala, S.P. The role of vitamin D in the immunopathogenesis of allergic skin diseases. Allergy 2012, 67, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, Q.; Zhu, R. Vitamin D and allergic diseases. Front. Immunol. 2024, 15, 1420883. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.-T. Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; p xxviii; 694p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).