Identifying Aberrant 1CM-Related Pathways by Multi-Omics Analysis and Validating Tumor Inhibitory Effect of One-Carbon Donor Betaine in Gastric Cancer
Abstract
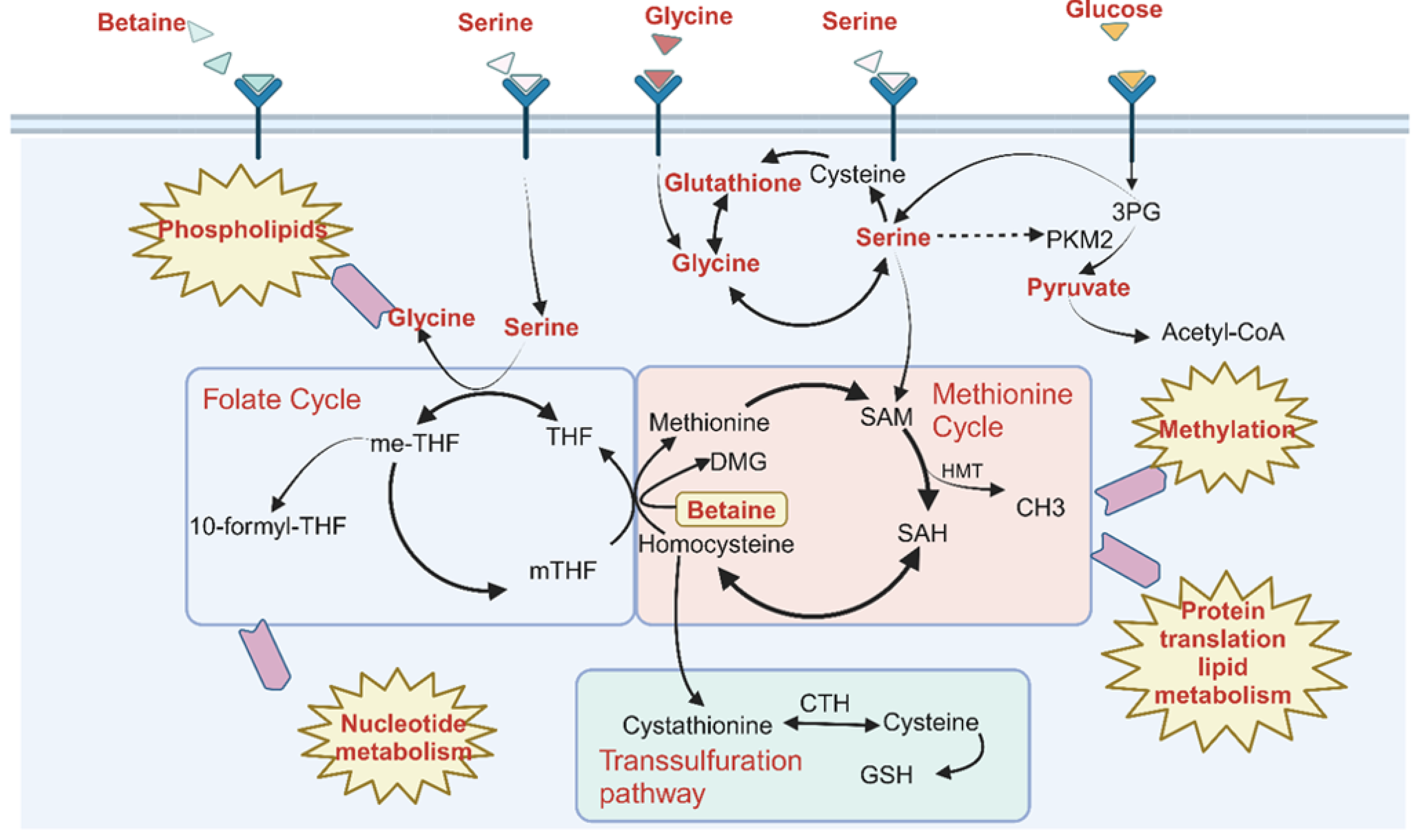
1. Introduction
2. Results
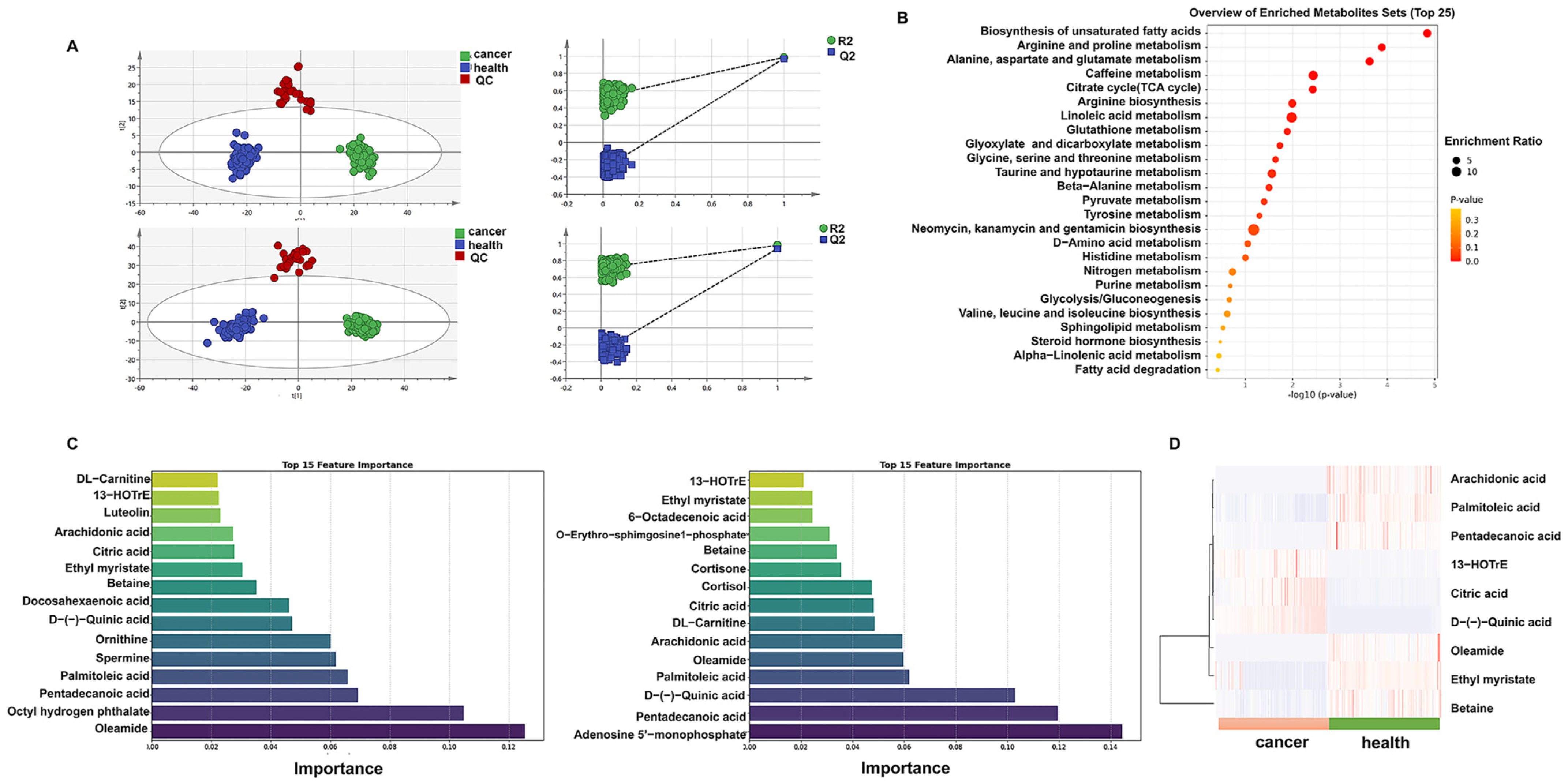
2.1. Plasma Metabolomics Reveals Differential Metabolites Between Gastric Cancer (GC) Patients and Healthy Individuals
2.2. One-Carbon Donor Metabolite Betaine Inhibits the Development and Progression of Gastric Cancer (GC) Cells
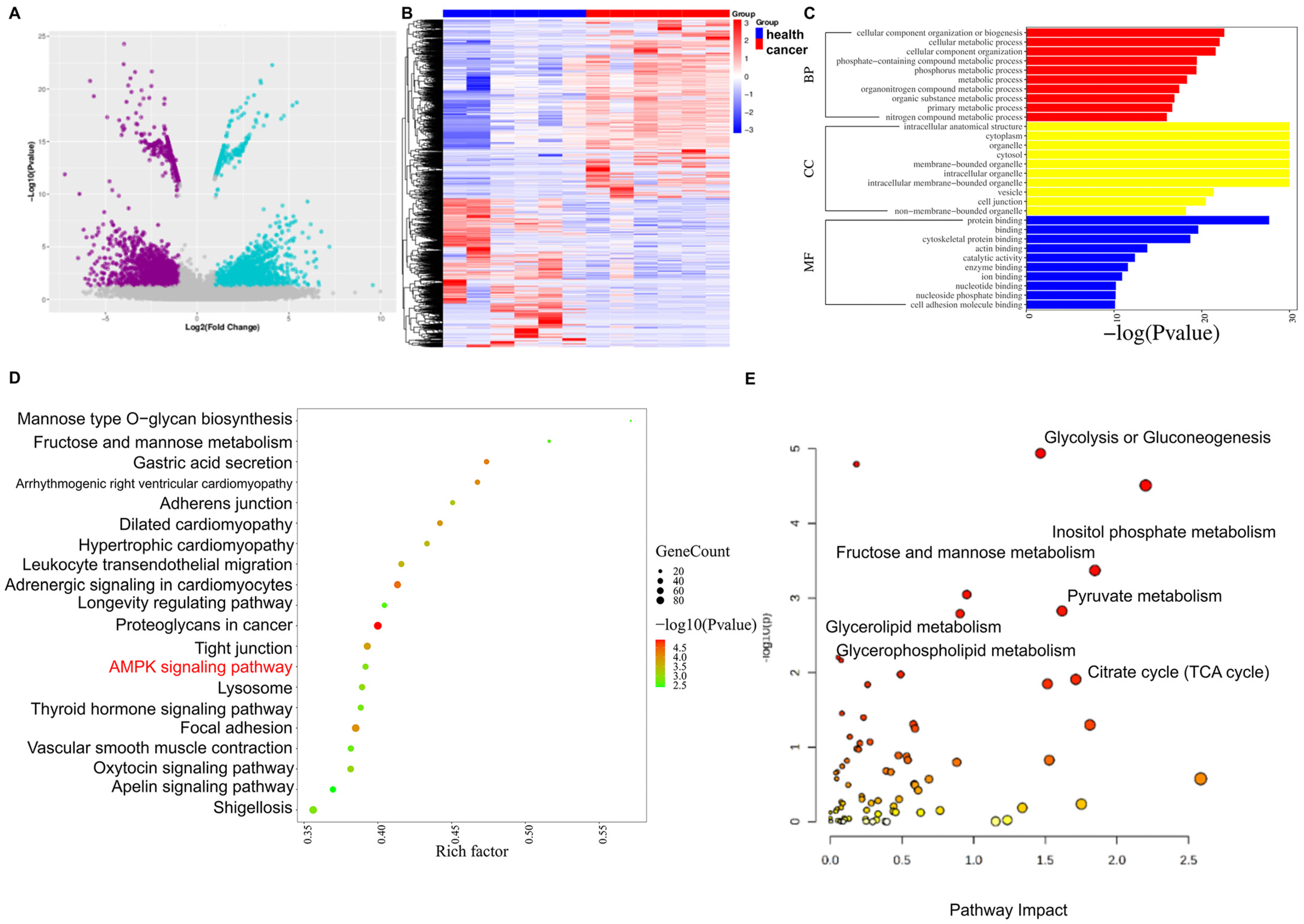
2.3. Tissue Transcriptome and Single-Cell RNA Sequencing, Combined with Metabolomics, Reveal Changes in 1CM-Related Pathways in GC
2.4. One-Carbon Donor Betaine Could Inhibit GC by Activating AMPK Phosphorylation
3. Discussion
4. Materials and Methods
4.1. Patients and Specimens
4.2. Cell Proliferation Assays
4.3. Migration and Invasion Assays
4.4. RNA Sequencing Analysis
4.5. Single-Cell RNA Data Analysis
4.6. Reverse Transcription-Quantitative (RT-q) PCR
4.7. Western Blot Analysis
4.8. Metabolomics Sample Preparation
4.9. Untargeted Metabolomics Analysis
4.10. Data Processing and Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GC | Gastric cancer |
| 1CM | 1-carbon metabolism |
| me-THF | 5,10-Methylenetetrahydrofolate |
| PCA | principal component analysis |
| GEO | Gene Expression Omnibus |
References
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Li, J.; Xu, S.; Zhu, F.; Shen, F.; Zhang, T.; Wan, X.; Gong, S.; Liang, G.; Zhou, Y. Multi-omics Combined with Machine Learning Facilitating the Diagnosis of Gastric Cancer. Curr. Med. Chem. 2024, 31, 6692–6712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, S.; Wang, D.; Liu, S.; Xiao, T.; Gu, W.; Yang, H.; Wang, H.; Yang, M.; Chen, P. Metabolic reprogramming and immune evasion: The interplay in the tumor microenvironment. Biomark. Res. 2024, 12, 96. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Qi, J.; Tian, X.; Dovjak, E.; Zhang, J.; Du, H.; Zhang, N.; Zhao, J.; Zhang, Y.; et al. Comprehensive profiling of lipid metabolic reprogramming expands precision medicine for HCC. Hepatology 2024, 81, 1164–1180. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Hosios, A.M.; Hecht, V.C.; Danai, L.V.; Johnson, M.O.; Rathmell, J.C.; Steinhauser, M.L.; Manalis, S.R.; Vander Heiden, M.G. Amino Acids Rather than Glucose Account for the Majority of Cell Mass in Proliferating Mammalian Cells. Dev. Cell 2016, 36, 540–549. [Google Scholar] [CrossRef]
- Fuller, H.; Zhu, Y.; Nicholas, J.; Chatelaine, H.A.; Drzymalla, E.M.; Sarvestani, A.K.; Julián-Serrano, S.; Tahir, U.A.; Sinnott-Armstrong, N.; Raffield, L.M.; et al. Metabolomic epidemiology offers insights into disease aetiology. Nat. Metab. 2023, 5, 1656–1672. [Google Scholar] [CrossRef]
- Sun, L.; Song, L.; Wan, Q.; Wu, G.; Li, X.; Wang, Y.; Wang, J.; Liu, Z.; Zhong, X.; He, X.; et al. cMyc-mediated activation of serine biosynthesis pathway is critical for cancer progression under nutrient deprivation conditions. Cell Res. 2015, 25, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.C.; Maddocks, O.D.K. Serine and Functional Metabolites in Cancer. Trends Cell Biol. 2017, 27, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xia, Y.; He, F.; Fu, J.; Xin, Z.; Deng, B.; He, L.; Zhou, X.; Ren, W. Serine Supports IL-1β Production in Macrophages Through mTOR Signaling. Front. Immunol. 2020, 11, 1866. [Google Scholar] [CrossRef]
- Sowers, M.L.; Herring, J.; Zhang, W.; Tang, H.; Ou, Y.; Gu, W.; Zhang, K. Analysis of glucose-derived amino acids involved in one-carbon and cancer metabolism by stable-isotope tracing gas chromatography mass spectrometry. Anal. Biochem. 2019, 566, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef]
- Geeraerts, S.L.; Heylen, E.; De Keersmaecker, K.; Kampen, K.R. The ins and outs of serine and glycine metabolism in cancer. Nat. Metab. 2021, 3, 131–141. [Google Scholar] [CrossRef]
- Biffo, S.; Ruggero, D.; Santoro, M.M. The crosstalk between metabolism and translation. Cell Metab. 2024, 36, 1945–1962. [Google Scholar] [CrossRef]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial Effects of Betaine: A Comprehensive Review. Biology 2021, 10, 456. [Google Scholar] [CrossRef]
- Pan, S.; Fan, M.; Liu, Z.; Li, X.; Wang, H. Serine, glycine and one-carbon metabolism in cancer (Review). Int. J. Oncol. 2021, 58, 158–170. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Huang, S.-Y.; Zhong, K.-Y.; Huang, W.-G.; Huang, Z.-H.; He, T.-T.; Yang, M.-T.; Wusiman, M.; Zhou, D.-D.; Chen, S.; et al. Betaine alleviates cognitive impairment induced by homocysteine through attenuating NLRP3-mediated microglial pyroptosis in an m(6)A-YTHDF2-dependent manner. Redox Biol. 2024, 69, 103026. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gao, M.; Qian, Q.; Guo, Z.; Zhu, P.; Wang, X.; Wang, H. Triclosan-induced abnormal expression of miR-30b regulates fto-mediated m(6)A methylation level to cause lipid metabolism disorder in zebrafish. Sci. Total Environ. 2021, 770, 145285. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Szkudelski, T. The anti-diabetic potential of betaine. Mechanisms of action in rodent models of type 2 diabetes. Biomed. Pharmacother. 2022, 150, 112946. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Soto, C.G.; Valenzuela-Soto, E.M. Glycine betaine rather than acting only as an osmolyte also plays a role as regulator in cellular metabolism. Biochimie 2018, 147, 89–97. [Google Scholar] [CrossRef]
- Dahlhoff, C.; Worsch, S.; Sailer, M.; Hummel, B.A.; Fiamoncini, J.; Uebel, K.; Obeid, R.; Scherling, C.; Geisel, J.; Bader, B.L.; et al. Methyl-donor supplementation in obese mice prevents the progression of NAFLD, activates AMPK and decreases acyl-carnitine levels. Mol. Metab. 2014, 3, 565–580. [Google Scholar] [CrossRef]
- Shen, L.; Hu, P.; Zhang, Y.; Ji, Z.; Shan, X.; Ni, L.; Ning, N.; Wang, J.; Tian, H.; Shui, G.; et al. Serine metabolism antagonizes antiviral innate immunity by preventing ATP6V0d2-mediated YAP lysosomal degradation. Cell Metab. 2021, 33, 971–987.e6. [Google Scholar] [CrossRef]
- Sun, W.; Liu, R.; Gao, X.; Lin, Z.; Tang, H.; Cui, H.; Zhao, E. Targeting serine-glycine-one-carbon metabolism as a vulnerability in cancers. Biomark. Res. 2023, 11, 48. [Google Scholar] [CrossRef]
- Wu, G.; Lupton, J.R.; Turner, N.D.; Fang, Y.-Z.; Yang, S. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Lapenna, D. Glutathione and glutathione-dependent enzymes: From biochemistry to gerontology and successful aging. Ageing Res. Rev. 2023, 92, 102066. [Google Scholar] [CrossRef]
- Ghrayeb, A.; Finney, A.C.; Agranovich, B.; Peled, D.; Anand, S.K.; McKinney, M.P.; Sarji, M.; Yang, D.; Weissman, N.; Drucker, S.; et al. Serine synthesis via reversed SHMT2 activity drives glycine depletion and acetaminophen hepatotoxicity in MASLD. Cell Metab. 2024, 36, 116–129.e7. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; DeBerardinis, R.J. Understanding the Intersections between Metabolism and Cancer Biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Yuan, Q.; Deng, D.; Pan, C.; Ren, J.; Wei, T.; Wu, Z.; Zhang, B.; Li, S.; Yin, P.; Shang, D. Integration of transcriptomics, proteomics, and metabolomics data to reveal HER2-associated metabolic heterogeneity in gastric cancer with response to immunotherapy and neoadjuvant chemotherapy. Front. Immunol. 2022, 13, 951137. [Google Scholar] [CrossRef]
- Sun, C.; Wang, A.; Zhou, Y.; Chen, P.; Wang, X.; Huang, J.; Gao, J.; Wang, X.; Shu, L.; Lu, J.; et al. Spatially resolved multi-omics highlights cell-specific metabolic remodeling and interactions in gastric cancer. Nat. Commun. 2023, 14, 2692. [Google Scholar] [CrossRef]
- De Felice, F.; Malerba, S.; Nardone, V.; Salvestrini, V.; Calomino, N.; Testini, M.; Boccardi, V.; Desideri, I.; Gentili, C.; De Luca, R.; et al. Progress and Challenges in Integrating Nutritional Care into Oncology Practice: Results from a National Survey on Behalf of the NutriOnc Research Group. Nutrients 2025, 17, 188. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, J.; Chen, L.; Deng, P.; Bu, Q.; Xiang, P.; Li, M.; Lu, W.; Xu, Y.; Lin, H.; et al. 1H-NMR based metabonomic profiling of human esophageal cancer tissue. Mol. Cancer 2013, 12, 25. [Google Scholar] [CrossRef]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef]
- Barak, A.J.; Beckenhauer, H.C.; Badakhsh, S.; Tuma, D.J. The effect of betaine in reversing alcoholic steatosis. Alcohol. Clin. Exp. Res. 1997, 21, 1100–1102. [Google Scholar] [CrossRef]
- Ji, C.; Kaplowitz, N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 2003, 124, 1488–1499. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Liang, H.; Liu, X.; Lee, J.-S.; Cho, J.Y.; Cheong, J.-H.; Kim, H.; Li, M.; Downey, T.J.; Dyer, M.D.; et al. AMPKα modulation in cancer progression: Multilayer integrative analysis of the whole transcriptome in Asian gastric cancer. Cancer Res. 2012, 72, 2512–2521. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Feng, J.; Jiang, D.; Zhou, X.; Jiang, Q.; Cai, M.; Wang, X.; Shan, T.; Wang, Y. AMPK regulates lipid accumulation in skeletal muscle cells through FTO-dependent demethylation of N(6)-methyladenosine. Sci. Rep. 2017, 7, 41606. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liu, H.; Yang, P.; Zhu, F.; Shen, F.; Liang, G. Identifying Aberrant 1CM-Related Pathways by Multi-Omics Analysis and Validating Tumor Inhibitory Effect of One-Carbon Donor Betaine in Gastric Cancer. Int. J. Mol. Sci. 2025, 26, 3841. https://doi.org/10.3390/ijms26083841
Li J, Liu H, Yang P, Zhu F, Shen F, Liang G. Identifying Aberrant 1CM-Related Pathways by Multi-Omics Analysis and Validating Tumor Inhibitory Effect of One-Carbon Donor Betaine in Gastric Cancer. International Journal of Molecular Sciences. 2025; 26(8):3841. https://doi.org/10.3390/ijms26083841
Chicago/Turabian StyleLi, Jie, Huan Liu, Panpan Yang, Feng Zhu, Fei Shen, and Geyu Liang. 2025. "Identifying Aberrant 1CM-Related Pathways by Multi-Omics Analysis and Validating Tumor Inhibitory Effect of One-Carbon Donor Betaine in Gastric Cancer" International Journal of Molecular Sciences 26, no. 8: 3841. https://doi.org/10.3390/ijms26083841
APA StyleLi, J., Liu, H., Yang, P., Zhu, F., Shen, F., & Liang, G. (2025). Identifying Aberrant 1CM-Related Pathways by Multi-Omics Analysis and Validating Tumor Inhibitory Effect of One-Carbon Donor Betaine in Gastric Cancer. International Journal of Molecular Sciences, 26(8), 3841. https://doi.org/10.3390/ijms26083841





