The Role of Extracellular-Vesicle-Derived miRNAs in Postoperative Organ Dysfunction in Neonates and Infants Undergoing Congenital Cardiac Surgery: An Exploratory Study
Abstract
1. Introduction
2. Results
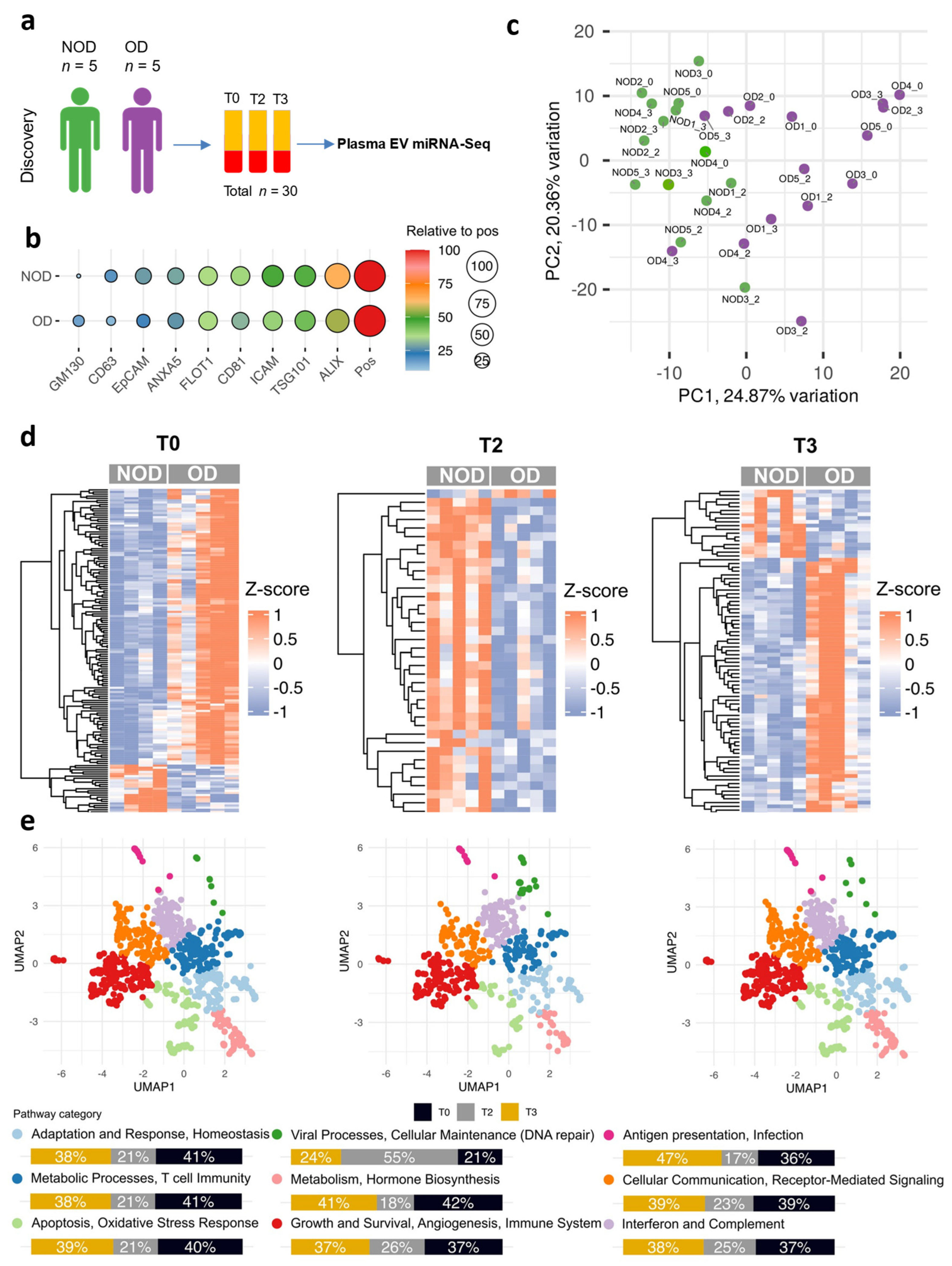
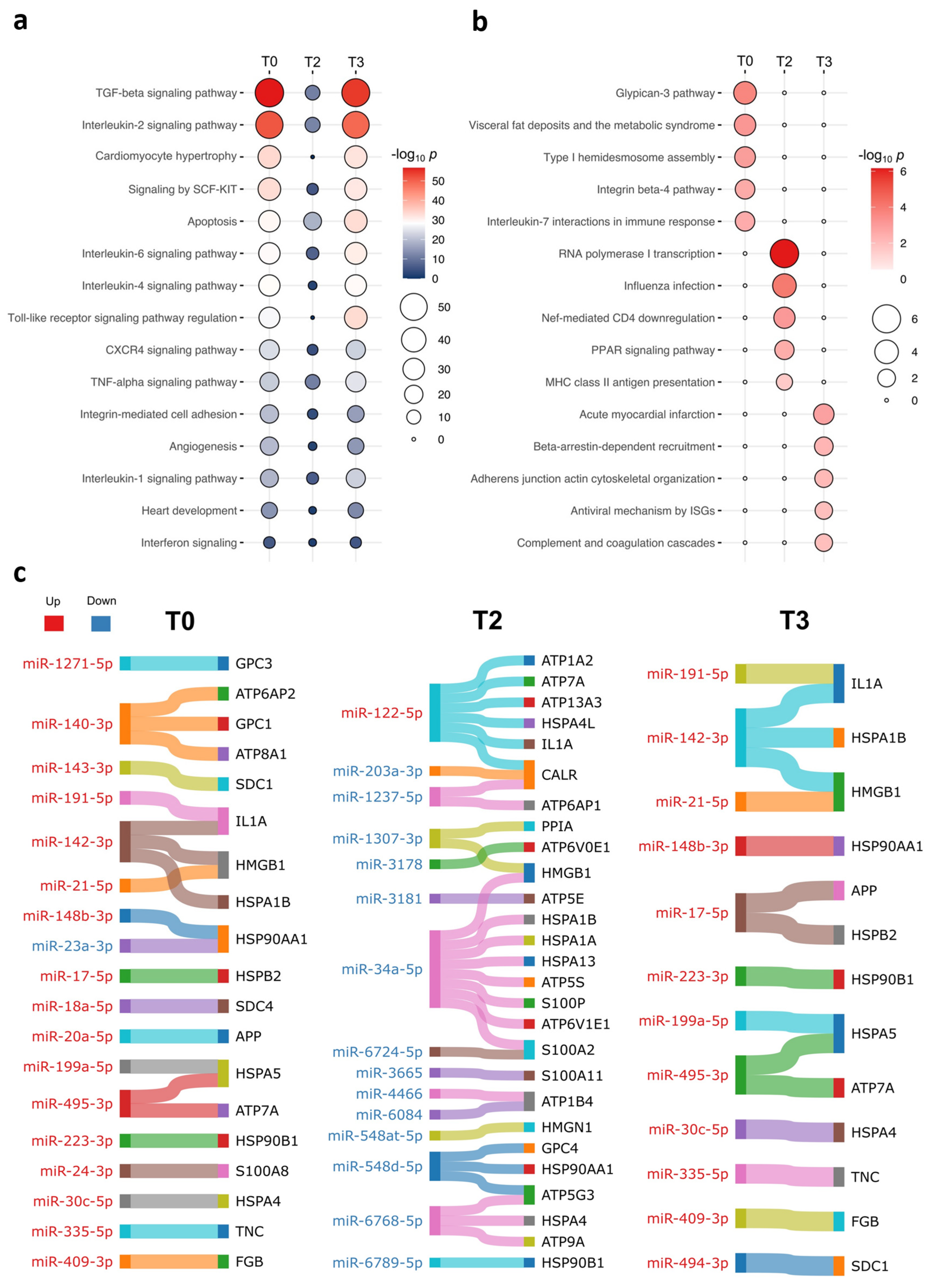
2.1. The Plasma EV miRNAs from Neonates and Infants with Postoperative Organ Dysfunction Distinguished Those from Individuals Without Organ Dysfunction
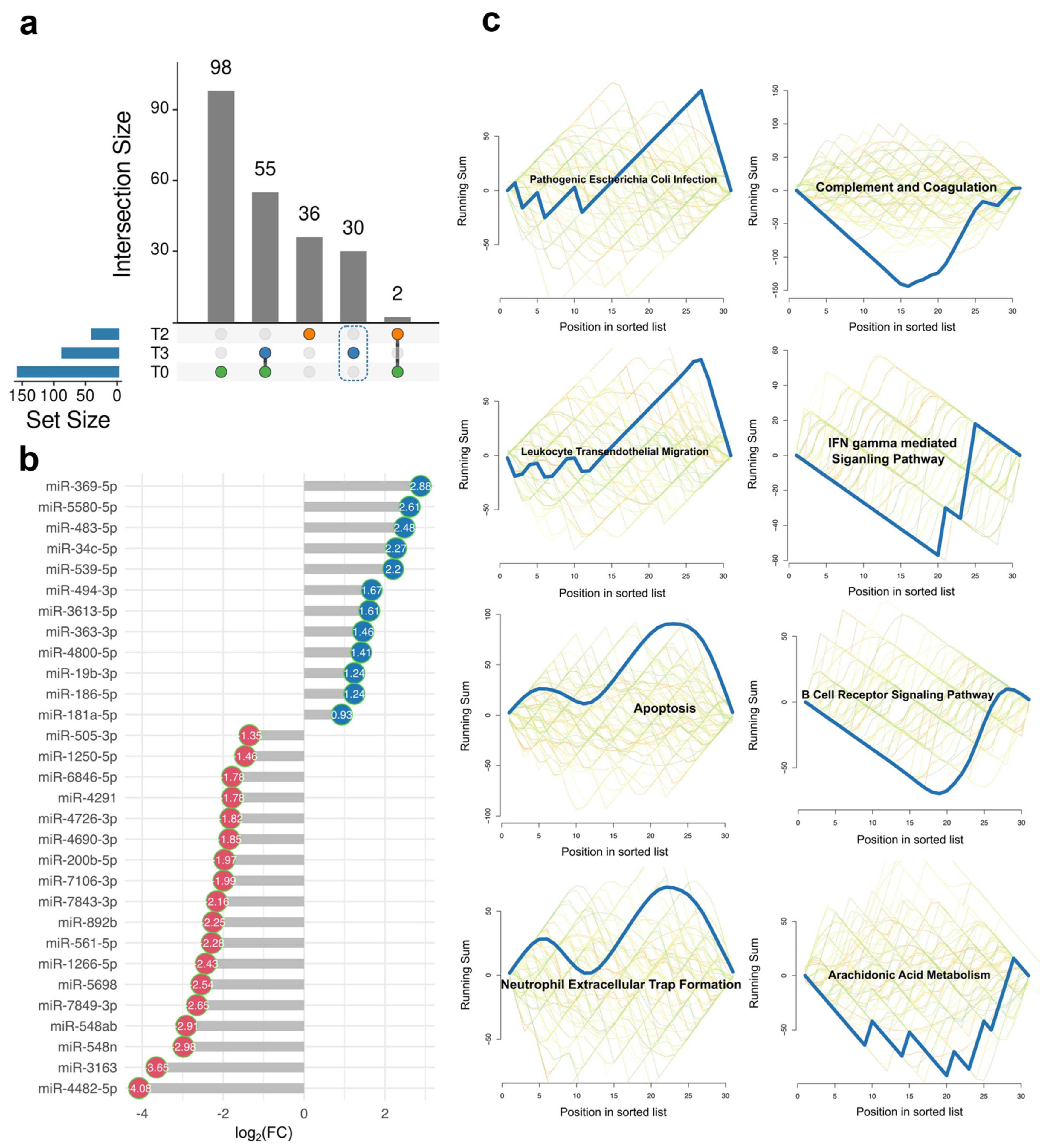
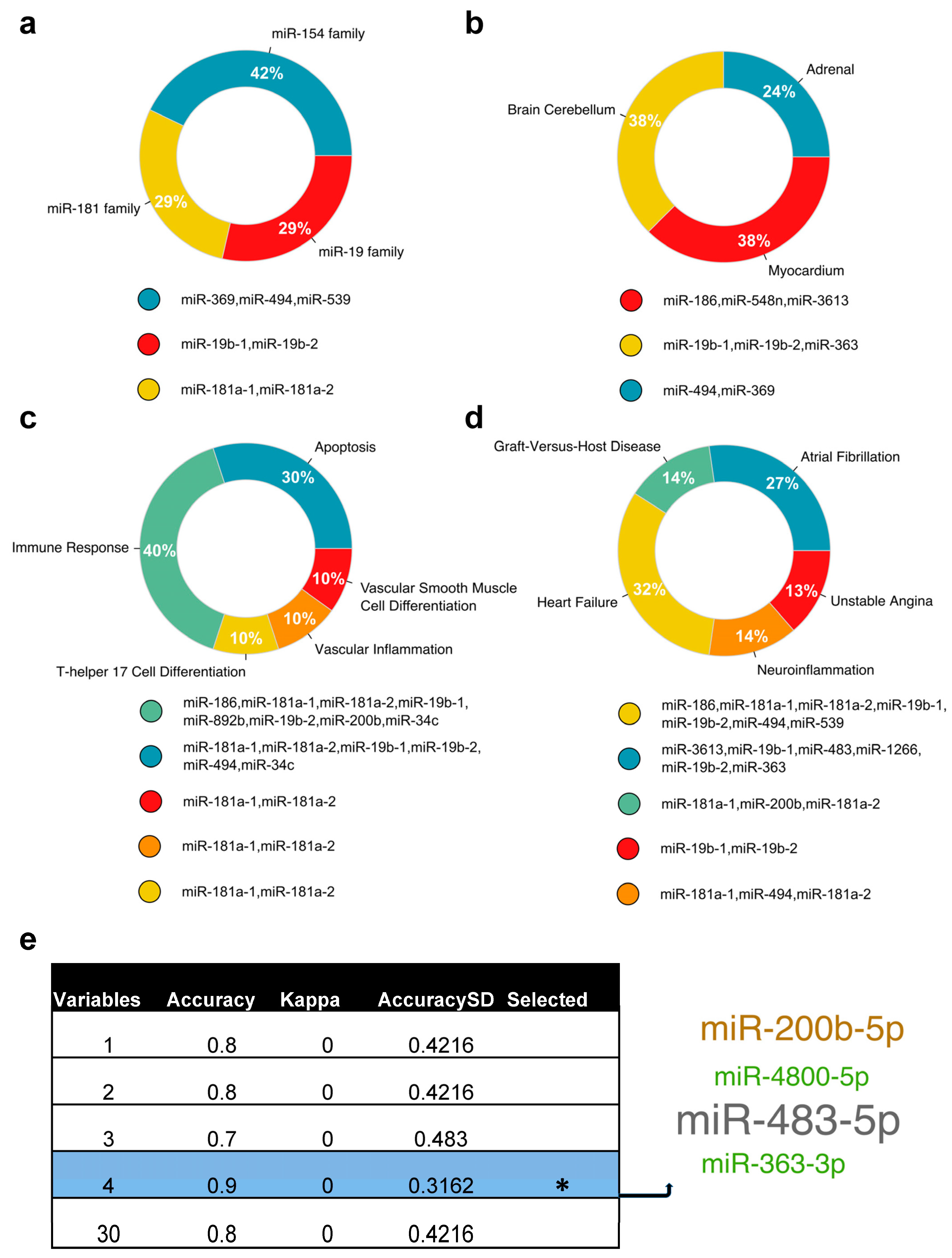
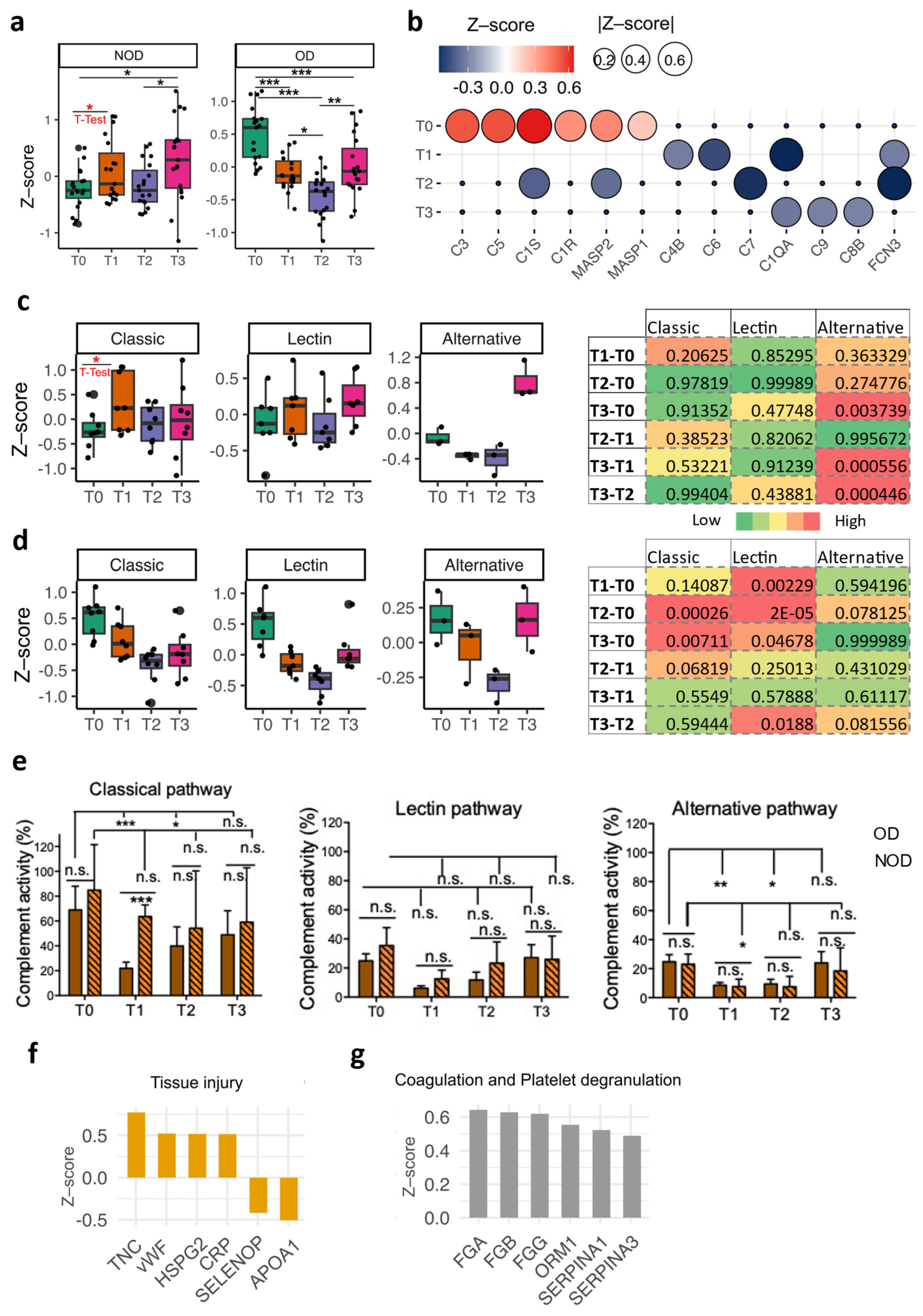
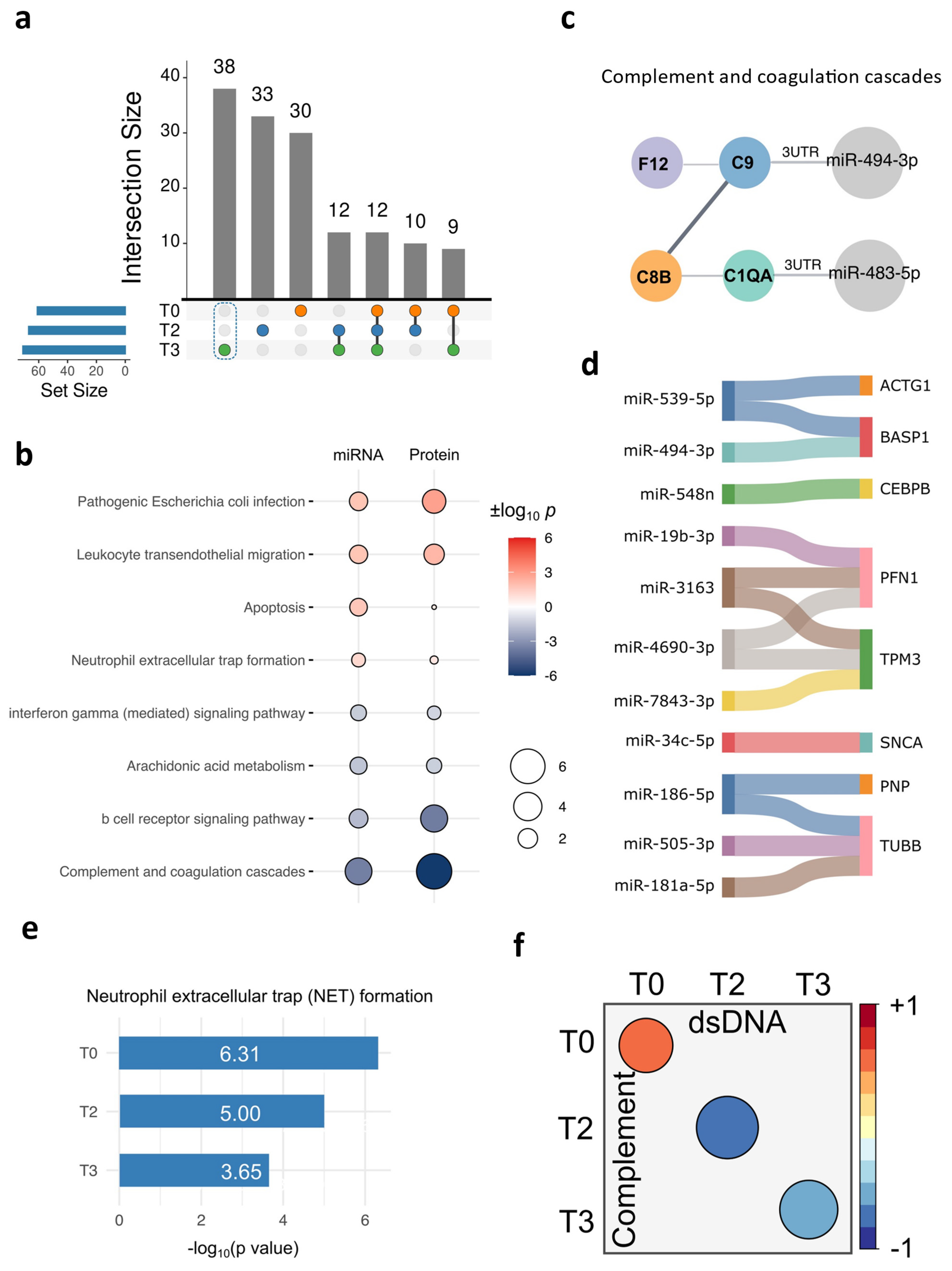
2.2. The Unique DE miRNA Signature at T3 Was Associated with the Complement System
2.3. Delineation of the Complement System Patterns in the Perioperative Period for Congenital Cardiac Patients Using Plasma Proteomics
2.4. Cross-Validation of the Complement Signatures Using the Plasma EV miRNAs at T3
3. Discussion
4. Materials and Methods
4.1. Patient Enrolment and Clinical Data Collection
4.2. Plasma Collection and EV Isolation
4.3. EV RNA Extraction, RNA Library Preparation, and Small RNA-Sequencing
4.4. Bioinformatic Analysis
4.5. Proteomic Sample Preparation
4.6. Nano-HPLC-MS/MS Analysis
4.7. Proteomic Data Analysis
4.8. The Double-Stranded DNA (dsDNA) Assay
4.9. Complement Activity Assays
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Marelli, A.; Gauvreau, K.; Landzberg, M.; Jenkins, K. Sex differences in mortality in children undergoing congenital heart disease surgery: A United States population-based study. Circulation 2010, 122, S234–S240. [Google Scholar] [CrossRef]
- Pasquali, S.K.; Thibault, D.; O′Brien, S.M.; Jacobs, J.P.; Gaynor, J.W.; Romano, J.C.; Gaies, M.; Hill, K.D.; Jacobs, M.L.; Shahian, D.M.; et al. National Variation in Congenital Heart Surgery Outcomes. Circulation 2020, 142, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, G.; Li, Q.; Zuo, Y.; Wang, Q. Clinical characteristics and outcomes of patients who underwent neonatal cardiac surgery: Ten years of experience in a tertiary surgery center. Eur. J. Med. Res. 2024, 29, 144. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.L.; Ridout, D.; Pagel, C.; Wray, J.; Anderson, D.; Barron, D.J.; Cassidy, J.; Davis, P.J.; Rodrigues, W.; Stoica, S.; et al. Incidence and risk factors for important early morbidities associated with pediatric cardiac surgery in a UK population. J. Thorac. Cardiovasc. Surg. 2019, 158, 1185–1196.e7. [Google Scholar] [CrossRef] [PubMed]
- Salvin, J.W.; Scheurer, M.A.; Laussen, P.C.; Wypij, D.; Polito, A.; Bacha, E.A.; Pigula, F.A.; McGowan, F.X.; Costello, J.M.; Thiagarajan, R.R. Blood transfusion after pediatric cardiac surgery is associated with prolonged hospital stay. Ann. Thorac. Surg. 2011, 91, 204–210. [Google Scholar] [CrossRef]
- Redlin, M.; Kukucka, M.; Boettcher, W.; Schoenfeld, H.; Huebler, M.; Kuppe, H.; Habazettl, H. Blood transfusion determines postoperative morbidity in pediatric cardiac surgery applying a comprehensive blood-sparing approach. J. Thorac. Cardiovasc. Surg. 2013, 146, 537–542. [Google Scholar] [CrossRef]
- Iyengar, A.; Scipione, C.N.; Sheth, P.; Ohye, R.G.; Riegger, L.; Bove, E.L.; Devaney, E.J.; Hirsch-Romano, J.C. Association of complications with blood transfusions in pediatric cardiac surgery patients. Ann. Thorac. Surg. 2013, 96, 910–916. [Google Scholar] [CrossRef]
- Faraoni, D.; Nasr, V.G.; DiNardo, J.A. Overall Hospital Cost Estimates in Children with Congenital Heart Disease: Analysis of the 2012 Kid’s Inpatient Database. Pediatr. Cardiol. 2016, 37, 37–43. [Google Scholar] [CrossRef]
- Alashkar Alhamwe, B.; Potaczek, D.P.; Miethe, S.; Alhamdan, F.; Hintz, L.; Magomedov, A.; Garn, H. Extracellular Vesicles and Asthma-More Than Just a Co-Existence. Int. J. Mol. Sci. 2021, 22, 4984. [Google Scholar] [CrossRef]
- Scrimgeour, L.A.; Potz, B.A.; Aboul Gheit, A.; Shi, G.; Stanley, M.; Zhang, Z.; Sodha, N.R.; Ahsan, N.; Abid, M.R.; Sellke, F.W. Extracellular Vesicles Promote Arteriogenesis in Chronically Ischemic Myocardium in the Setting of Metabolic Syndrome. J. Am. Heart Assoc. 2019, 8, e012617. [Google Scholar] [CrossRef]
- Alhamdan, F.; Greulich, T.; Daviaud, C.; Marsh, L.M.; Pedersen, F.; Tholken, C.; Pfefferle, P.I.; Bahmer, T.; Potaczek, D.P.; Tost, J.; et al. Identification of extracellular vesicle microRNA signatures specifically linked to inflammatory and metabolic mechanisms in obesity-associated low type-2 asthma. Allergy 2023, 78, 2944–2958. [Google Scholar] [CrossRef]
- Schindler, V.E.M.; Alhamdan, F.; Preusser, C.; Hintz, L.; Alashkar Alhamwe, B.; Nist, A.; Stiewe, T.; Pogge von Strandmann, E.; Potaczek, D.P.; Tholken, C.; et al. Side-Directed Release of Differential Extracellular Vesicle-associated microRNA Profiles from Bronchial Epithelial Cells of Healthy and Asthmatic Subjects. Biomedicines 2022, 10, 622. [Google Scholar] [CrossRef]
- Jia, W.; Liang, S.; Lin, W.; Li, S.; Yuan, J.; Jin, M.; Nie, S.; Zhang, Y.; Zhai, X.; Zhou, L.; et al. Hypoxia-induced exosomes facilitate lung pre-metastatic niche formation in hepatocellular carcinoma through the miR-4508-RFX1-IL17A-p38 MAPK-NF-kappaB pathway. Int. J. Biol. Sci. 2023, 19, 4744–4762. [Google Scholar] [CrossRef] [PubMed]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.; Kiriakidou, M.; Sharma, A.; Maniataki, E.; Mourelatos, Z. The microRNA world: Small is mighty. Trends Biochem. Sci. 2003, 28, 534–540. [Google Scholar] [CrossRef]
- Mahtal, N.; Lenoir, O.; Tinel, C.; Anglicheau, D.; Tharaux, P.L. MicroRNAs in kidney injury and disease. Nat. Rev. Nephrol. 2022, 18, 643–662. [Google Scholar] [CrossRef]
- Pang, J.K.S.; Phua, Q.H.; Soh, B.S. Applications of miRNAs in cardiac development, disease progression and regeneration. Stem Cell Res. Ther. 2019, 10, 336. [Google Scholar] [CrossRef]
- Das, K.; Rao, L.V.M. The Role of microRNAs in Inflammation. Int. J. Mol. Sci. 2022, 23, 15479. [Google Scholar] [CrossRef]
- Rodriguez-Rius, A.; Lopez, S.; Martinez-Perez, A.; Souto, J.C.; Soria, J.M. Identification of a Plasma MicroRNA Profile Associated With Venous Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1392–1399. [Google Scholar] [CrossRef]
- Luo, M.; Du, M.; Shu, C.; Liu, S.; Li, J.; Zhang, L.; Li, X. The Function of microRNAs in Pulmonary Embolism: Review and Research Outlook. Front. Pharmacol. 2021, 12, 743945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Di, K.; Fan, B.; Wu, J.; Gu, X.; Sun, Y.; Khan, A.; Li, P.; Li, Z. MicroRNAs in extracellular vesicles: Sorting mechanisms, diagnostic value, isolation, and detection technology. Front. Bioeng. Biotechnol. 2022, 10, 948959. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.G.; Roefs, M.T.; de Miguel Perez, D.; Kooijmans, S.A.; de Jong, O.G.; Sluijter, J.P.; Schiffelers, R.M.; Vader, P. Interfering with endolysosomal trafficking enhances release of bioactive exosomes. Nanomedicine 2019, 20, 102014. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, L.; Song, Y.; Zhang, M.; Shan, D.; Liu, Y.; Fang, M.; Lv, F.; Xiao, R.P.; Zhang, Y. beta-arrestin 2 mediates cardiac ischemia-reperfusion injury via inhibiting GPCR-independent cell survival signalling. Cardiovasc. Res. 2017, 113, 1615–1626. [Google Scholar] [CrossRef]
- Aukrust, P.; Gullestad, L.; Lappegard, K.T.; Ueland, T.; Aass, H.; Wikeby, L.; Simonsen, S.; Froland, S.S.; Mollnes, T.E. Complement activation in patients with congestive heart failure: Effect of high-dose intravenous immunoglobulin treatment. Circulation 2001, 104, 1494–1500. [Google Scholar] [CrossRef]
- Zheng, Y.; Gardner, S.E.; Clarke, M.C. Cell death, damage-associated molecular patterns, and sterile inflammation in cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2781–2786. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune. Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Maisat, W.; Hou, L.; Sandhu, S.; Sin, Y.C.; Kim, S.; Van Pelt, H.; Chen, Y.; Emani, S.; Kong, S.W.; Emani, S.; et al. Neutrophil extracellular traps formation is associated with postoperative complications in congenital cardiac surgery. Pediatr. Res. 2024. [Google Scholar] [CrossRef]
- Bruins, P.; te Velthuis, H.; Yazdanbakhsh, A.P.; Jansen, P.G.; van Hardevelt, F.W.; de Beaumont, E.M.; Wildevuur, C.R.; Eijsman, L.; Trouwborst, A.; Hack, C.E. Activation of the complement system during and after cardiopulmonary bypass surgery: Postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation 1997, 96, 3542–3548. [Google Scholar] [CrossRef]
- Chenoweth, D.E.; Cooper, S.W.; Hugli, T.E.; Stewart, R.W.; Blackstone, E.H.; Kirklin, J.W. Complement activation during cardiopulmonary bypass: Evidence for generation of C3a and C5a anaphylatoxins. N. Engl. J. Med. 1981, 304, 497–503. [Google Scholar] [CrossRef]
- Markiewski, M.M.; Lambris, J.D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007, 171, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, P.M. von Willebrand factor: A marker of endothelial damage? Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1359–1362. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Suzuki, H. The Role of Tenascin-C in Tissue Injury and Repair After Stroke. Front. Immunol. 2020, 11, 607587. [Google Scholar] [CrossRef]
- Kinsella, M.G.; Tran, P.K.; Weiser-Evans, M.C.; Reidy, M.; Majack, R.A.; Wight, T.N. Changes in perlecan expression during vascular injury: Role in the inhibition of smooth muscle cell proliferation in the late lesion. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 608–614. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Willing, A.E.; Borlongan, C.V. Apolipoprotein A1 Enhances Endothelial Cell Survival in an In Vitro Model of ALS. Eneuro 2022, 9. [Google Scholar] [CrossRef]
- Cervia-Hasler, C.; Bruningk, S.C.; Hoch, T.; Fan, B.; Muzio, G.; Thompson, R.C.; Ceglarek, L.; Meledin, R.; Westermann, P.; Emmenegger, M.; et al. Persistent complement dysregulation with signs of thromboinflammation in active Long COVID. Science 2024, 383, eadg7942. [Google Scholar] [CrossRef]
- de Bont, C.M.; Boelens, W.C.; Pruijn, G.J.M. NETosis, complement, and coagulation: A triangular relationship. Cell Mol. Immunol. 2019, 16, 19–27. [Google Scholar] [CrossRef]
- Leffler, J.; Martin, M.; Gullstrand, B.; Tyden, H.; Lood, C.; Truedsson, L.; Bengtsson, A.A.; Blom, A.M. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J. Immunol. 2012, 188, 3522–3531. [Google Scholar] [CrossRef]
- Langseth, M.S.; Helseth, R.; Ritschel, V.; Hansen, C.H.; Andersen, G.O.; Eritsland, J.; Halvorsen, S.; Fagerland, M.W.; Solheim, S.; Arnesen, H.; et al. Double-Stranded DNA and NETs Components in Relation to Clinical Outcome After ST-Elevation Myocardial Infarction. Sci. Rep. 2020, 10, 5007. [Google Scholar] [CrossRef]
- Akasaka, Y.; Morimoto, N.; Ishikawa, Y.; Fujita, K.; Ito, K.; Kimura-Matsumoto, M.; Ishiguro, S.; Morita, H.; Kobayashi, Y.; Ishii, T. Myocardial apoptosis associated with the expression of proinflammatory cytokines during the course of myocardial infarction. Mod. Pathol. 2006, 19, 588–598. [Google Scholar] [CrossRef]
- Gao, C.; Qian, H.; Shi, Q.; Zhang, H. MicroRNA-363-3p serves as a diagnostic biomarker of acute myocardial infarction and regulates vascular endothelial injury by targeting KLF2. Cardiovasc. Diagn. Ther. 2020, 10, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, C.; Cui, B.; Liu, Y.; Lin, D.; Zhang, L.; Zhao, L.; Ma, J. Isoflurane Preconditioning May Attenuate Cardiomyocyte Injury Induced by Hypoxia/Reoxygenation Possibly by Regulating miR-363-3p. Neurotox. Res. 2022, 40, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liang, H.; Gao, Z.; Zhang, X.; He, Q.; He, C.; Cai, C.; Chen, J. MiR-483-5p downregulation alleviates ox-LDL induced endothelial cell injury in atherosclerosis. BMC Cardiovasc. Disord. 2023, 23, 521. [Google Scholar] [CrossRef] [PubMed]
- Harling, L.; Lambert, J.; Ashrafian, H.; Darzi, A.; Gooderham, N.J.; Athanasiou, T. Elevated serum microRNA 483-5p levels may predict patients at risk of post-operative atrial fibrillation. Eur. J. Cardiothorac. Surg. 2017, 51, 73–78. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, X.; Ma, Y.; Liu, X.; Peng, Y. Circulating mir-483-5p as a novel diagnostic biomarker for acute coronary syndrome and its predictive value for the clinical outcome after PCI. BMC Cardiovasc. Disord. 2023, 23, 360. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhan, H.; Liu, C.; Zhang, C.; Wei, H.; Li, B.; Zhou, D.; Lu, Y.; Huang, S.; Cheng, J.; et al. Neuroprotective Effect of miR-483-5p Against Cardiac Arrest-Induced Mitochondrial Dysfunction Mediated Through the TNFSF8/AMPK/JNK Signaling Pathway. Cell Mol. Neurobiol. 2023, 43, 2179–2202. [Google Scholar] [CrossRef]
- Michalski, M.; Pagowska-Klimek, I.; Thiel, S.; Swierzko, A.S.; Hansen, A.G.; Jensenius, J.C.; Cedzynski, M. Factors involved in initiation and regulation of complement lectin pathway influence postoperative outcome after pediatric cardiac surgery involving cardiopulmonary bypass. Sci. Rep. 2019, 9, 2930. [Google Scholar] [CrossRef]
- Chen, L.; Duan, H.Z.; Zhang, C.; Li, G.; Wu, Y.T.; Wang, D.; Li, X.Y. Serum complement C1q level is associated with left ventricular hypertrophy induced by coarctation of the aorta: A retrospective observational study. BMC Cardiovasc. Disord. 2022, 22, 367. [Google Scholar] [CrossRef]
- Linders, J.; Madhi, R.; Morgelin, M.; King, B.C.; Blom, A.M.; Rahman, M. Complement component 3 is required for tissue damage, neutrophil infiltration, and ensuring NET formation in acute pancreatitis. Eur. Surg. Res. 2021, 61, 163–176. [Google Scholar] [CrossRef]
- Chen, J.; Losos, M.; Yang, S.; Li, J.; Wu, H.; Cataland, S. Increased complement activation during platelet storage. Transfusion 2017, 57, 2182–2188. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Pettila, V.; Karvonen, M.K.; Jalkanen, J.; Nightingale, P.; Brealey, D.; Mancebo, J.; Ferrer, R.; Mercat, A.; Patroniti, N.; et al. Effect of Intravenous Interferon beta-1a on Death and Days Free From Mechanical Ventilation Among Patients With Moderate to Severe Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2020, 323, 725–733. [Google Scholar] [CrossRef]
- Bengtsson, A.; Lisander, B. Anaphylatoxin and terminal complement complexes in red cell salvage. Acta Anaesthesiol. Scand. 1990, 34, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Konig, G.; Waters, J.H. Washing and filtering of cell-salvaged blood-does it make autotransfusion safer? Transfus. Altern. Transfus. Med. 2012, 12, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Seghaye, M.C.; Strotman, J.M.; Ross, M.P. Multiple system organ failure after open heart surgery in infants and children. Thorac. Cardiovasc. Surg. 1993, 41, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, V.L.; Strotman, J.M.; Ross, M.P. Impact of multiple organ system dysfunction and nosocomial infections on survival of children treated with extracorporeal membrane oxygenation after heart surgery. Crit. Care Med. 2000, 28, 526–531. [Google Scholar] [CrossRef]
- Russell, R.A.; Ghanayem, N.S.; Kuhn, E.M.; Jeffries, H.E.; Scanlon, M.C.; Rice, T.B. Relationship between risk-adjustment tools and the pediatric logistic organ dysfunction score. World J. Pediatr. Congenit. Heart Surg. 2014, 5, 16–21. [Google Scholar] [CrossRef]
- Alhamdan, F.; Yuki, K. Postoperative Organ Dysfunction Risk Stratification Using Extracellular Vesicle-Derived circRNAs in Pediatric Congenital Heart Surgery. Cells 2024, 13, 1417. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Li, J.; Han, X.; Wan, Y.; Zhang, S.; Zhao, Y.; Fan, R.; Cui, Q.; Zhou, Y. TAM 2.0: Tool for MicroRNA set analysis. Nucleic Acids Res 2018, 46, W180–W185. [Google Scholar] [CrossRef]
- Kern, F.; Aparicio-Puerta, E.; Li, Y.; Fehlmann, T.; Kehl, T.; Wagner, V.; Ray, K.; Ludwig, N.; Lenhof, H.P.; Meese, E.; et al. miRTargetLink 2.0-interactive miRNA target gene and target pathway networks. Nucleic Acids Res. 2021, 49, W409–W416. [Google Scholar] [CrossRef]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef] [PubMed]
- Kern, F.; Fehlmann, T.; Solomon, J.; Schwed, L.; Grammes, N.; Backes, C.; Van Keuren-Jensen, K.; Craig, D.W.; Meese, E.; Keller, A. miEAA 2.0: Integrating multi-species microRNA enrichment analysis and workflow management systems. Nucleic Acids Res. 2020, 48, W521–W528. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef]
- Martinod, K.; Fuchs, T.A.; Zitomersky, N.L.; Wong, S.L.; Demers, M.; Gallant, M.; Wang, Y.; Wagner, D.D. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 2015, 125, 1948–1956. [Google Scholar] [CrossRef]

| Organ Dysfunction (OD) | Non-Organ Dysfunction (NOD) | p Value | |
|---|---|---|---|
| Age (mo) | 5.0 ± 5.1 | 5.9 ± 4.7 | 0.70 |
| Male (%) | 36% (4/11) | 44% (4/9) | 0.73 |
| Weight (kg) | 5.4 ± 3.1 | 6.3 ± 2.8 | 0.52 |
| Salvaged RBCs (mL/kg) | 32.7 ± 15.2 | 16.3 ± 11.1 | 0.015 * |
| Platelets (mL/kg) | 26.7 ± 15.7 | 10.9 ± 9.4 | 0.017 * |
| Cryoprecipitate (mL/kg) | 7.7 ± 9.1 | 4.1 ± 6.4 | 0.32 |
| OP time (min) | 470.2. ± 89.9 | 329.9 ± 134.0 | 0.012 * |
| CPB time (min) | 258.0 ± 64.7 | 164.9 ± 78.6 | 0.009 * |
| X-clamp time (min) | 138.3 ± 55.5 | 87.3± 56.9 | 0.06 |
| Circulatory arrest (%) | 36% (4/11) | 33% (3/9) | 0.89 |
| Regional perfusion (%) | 36% (4/11) | 33% (3/9) | 0.89 |
| MV duration (h) | 300.9 ± 296.2 | 50.5 ± 29.3 | 0.02 * |
| ICU stay (h) | 369.0 ± 299.4 | 73.8 ± 58.1 | 0.001 * |
| LOS (d) | 36.2 ± 22.6 | 10.0 ± 6.1 | 0.004 * |
| PELOD-2 POD#0 | 9.3 ± 2.4 | 6.2 ± 1.7 | 0.005 * |
| PELOD-2 POD#1 | 5.0 ± 1.7 | 3.6 ± 2.4 | 0.13 |
| PELOD-2 POD#2 | 5.9 ± 1.6 | 3.1 ± 2.4 | 0.007 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhamdan, F.; Maisat, W.; Higgins, L.; Chen, Y.; Ibla, J.; Yuki, K. The Role of Extracellular-Vesicle-Derived miRNAs in Postoperative Organ Dysfunction in Neonates and Infants Undergoing Congenital Cardiac Surgery: An Exploratory Study. Int. J. Mol. Sci. 2025, 26, 3837. https://doi.org/10.3390/ijms26083837
Alhamdan F, Maisat W, Higgins L, Chen Y, Ibla J, Yuki K. The Role of Extracellular-Vesicle-Derived miRNAs in Postoperative Organ Dysfunction in Neonates and Infants Undergoing Congenital Cardiac Surgery: An Exploratory Study. International Journal of Molecular Sciences. 2025; 26(8):3837. https://doi.org/10.3390/ijms26083837
Chicago/Turabian StyleAlhamdan, Fahd, Wiriya Maisat, LeeAnn Higgins, Yue Chen, Juan Ibla, and Koichi Yuki. 2025. "The Role of Extracellular-Vesicle-Derived miRNAs in Postoperative Organ Dysfunction in Neonates and Infants Undergoing Congenital Cardiac Surgery: An Exploratory Study" International Journal of Molecular Sciences 26, no. 8: 3837. https://doi.org/10.3390/ijms26083837
APA StyleAlhamdan, F., Maisat, W., Higgins, L., Chen, Y., Ibla, J., & Yuki, K. (2025). The Role of Extracellular-Vesicle-Derived miRNAs in Postoperative Organ Dysfunction in Neonates and Infants Undergoing Congenital Cardiac Surgery: An Exploratory Study. International Journal of Molecular Sciences, 26(8), 3837. https://doi.org/10.3390/ijms26083837







