Special Issue “Depression: From Molecular Basis to Therapy”
Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
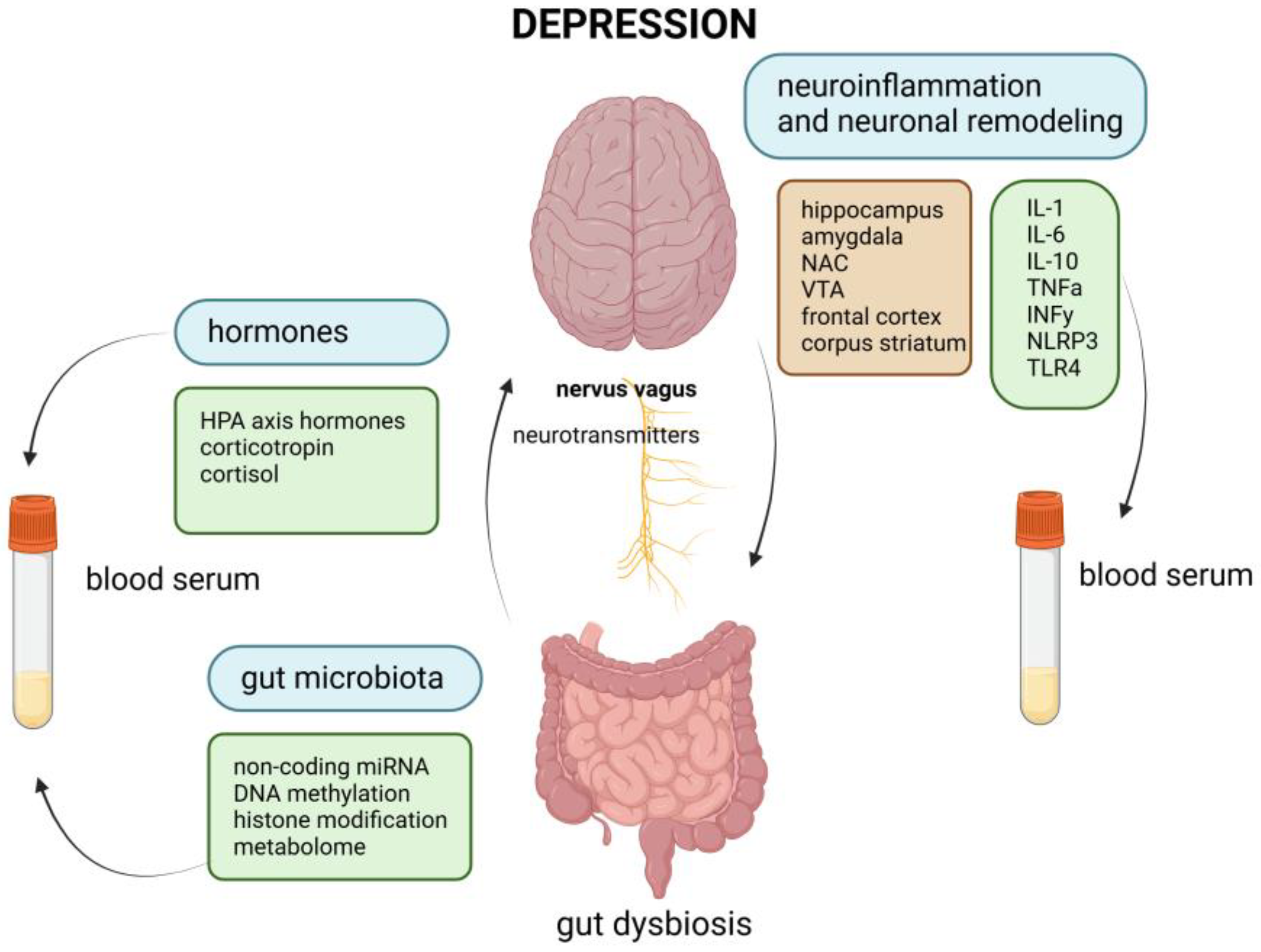
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open-access journals |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
References
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.; Luca, M.; Kasper, S.; Pecorino, B.; Zohar, J.; Souery, D.; Montgomery, S.; Ferentinos, P.; Rujescu, D.; Messina, A.; et al. Anhedonia is associated with a specific depression profile and poor antidepressant response. Int. J. Neuropsychopharmacol. 2024, 27, pyae055. [Google Scholar] [CrossRef] [PubMed]
- WHO. Mental Health Atlas. Available online: https://www.who.int/teams/mental-health-and-substance-use/data-research/mental-health-atlas (accessed on 28 March 2025).
- Danielsson, K.; Ahlborg, M.; Mortazavi, R.; Jarbin, H.; Larsson, I. Depression in adolescence and the understanding of health-A phenomenographic study. PLoS ONE 2025, 20, e0318061. [Google Scholar] [CrossRef] [PubMed]
- Overall, J.E. Methodologic issues in the epidemiology of treatment resistant depression. Contribution to epidemiology. Pharmakopsychiatr. Neuropsychopharmakol. 1974, 7, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Lucido, M.J.; Dunlop, B.W. Emerging Medications for Treatment-Resistant Depression: A Review with Perspective on Mechanisms and Challenges. Brain Sci. 2025, 15, 161. [Google Scholar] [CrossRef] [PubMed]
- Kverno, K.S.; Mangano, E. Treatment-resistant depression: Approaches to treatment. J. Psychosoc. Nurs. Ment. Health Serv. 2021, 59, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kavakbasi, E.; Van Assche, E.; Schwarte, K.; Hohoff, C.; Baune, B.T. Long-Term Immunomodulatory Impact of VNS on Peripheral Cytokine Profiles and Its Relationship with Clinical Response in Difficult-to-Treat Depression (DTD). Int. J. Mol. Sci. 2024, 25, 4196. [Google Scholar] [CrossRef] [PubMed]
- Zvozilova, A.; Bukatova, S.; Koprdova, R.; Mach, M. Evaluation of New Approaches to Depression Treatment Using an Animal Model of Pharmacoresistant Depression. Int. J. Mol. Sci. 2024, 25, 5265. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, N.; Karasova, M.; Jendzelovska, Z.; Majerník, M.; Kolesarova, M.; Kecsey, D.; Jendzelovsky, R.; Bohus, P.; Kiskova, T. Gyrophoric Acid, a Secondary Metabolite of Lichens, Exhibits Antidepressant and Anxiolytic Activity In Vivo in Wistar Rats. Int. J. Mol. Sci. 2024, 25, 11840. [Google Scholar] [CrossRef] [PubMed]
- White, A.G.; Elias, E.; Orozco, A.; Robinson, S.A.; Manners, M.T. Chronic Stress-Induced Neuroinflammation: Relevance of Rodent Models to Human Disease. Int. J. Mol. Sci. 2024, 25, 5085. [Google Scholar] [CrossRef] [PubMed]
- Kukucka, T.; Ferencova, N.; Visnovcova, Z.; Ondrejka, I.; Hrtanek, I.; Kovacova, V.; Macejova, A.; Mlyncekova, Z.; Tonhajzerova, I. Mechanisms Involved in the Link between Depression, Antidepressant Treatment, and Associated Weight Change. Int. J. Mol. Sci. 2024, 25, 4511. [Google Scholar] [CrossRef] [PubMed]
- Averina, O.V.; Poluektova, E.U.; Zorkina, Y.A.; Kovtun, A.S.; Danilenko, V.N. Human Gut Microbiota for Diagnosis and Treatment of Depression. Int. J. Mol. Sci. 2024, 25, 5782. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hsieh, Y.-R.; Lai, W.-D.; Tung, T.-H.; Chen, Y.-X.; Yang, C.-H.; Fang, Y.-C.; Huang, S.-Y. Melatonin Ameliorates Neuropsychiatric Behaviors, Gut Microbiome, and Microbiota-Derived Metabolites in Rats with Chronic Sleep Deprivation. Int. J. Mol. Sci. 2023, 24, 16820. [Google Scholar] [CrossRef] [PubMed]
- Dallaspezia, S.; Cardaci, V.; Mazza, M.G.; De Lorenzo, R.; Rovere Querini, P.; Colombo, C.; Benedetti, F. Higher Seasonal Variation of Systemic Inflammation in Bipolar Disorder. Int. J. Mol. Sci. 2024, 25, 4310. [Google Scholar] [CrossRef] [PubMed]
- Quiccione, M.S.; Tirozzi, A.; Cassioli, G.; Morelli, M.; Costanzo, S.; Pepe, A.; Bracone, F.; Magnacca, S.; Cerletti, C.; Licastro, D.; et al. Are Methylation Patterns in the KALRN Gene Associated with Cognitive and Depressive Symptoms? Findings from the Moli-sani Cohort. Int. J. Mol. Sci. 2024, 25, 10317. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiskova, T.; Bednarova, A. Special Issue “Depression: From Molecular Basis to Therapy”. Int. J. Mol. Sci. 2025, 26, 3844. https://doi.org/10.3390/ijms26083844
Kiskova T, Bednarova A. Special Issue “Depression: From Molecular Basis to Therapy”. International Journal of Molecular Sciences. 2025; 26(8):3844. https://doi.org/10.3390/ijms26083844
Chicago/Turabian StyleKiskova, Terezia, and Aneta Bednarova. 2025. "Special Issue “Depression: From Molecular Basis to Therapy”" International Journal of Molecular Sciences 26, no. 8: 3844. https://doi.org/10.3390/ijms26083844
APA StyleKiskova, T., & Bednarova, A. (2025). Special Issue “Depression: From Molecular Basis to Therapy”. International Journal of Molecular Sciences, 26(8), 3844. https://doi.org/10.3390/ijms26083844





