Effect of a Laparoscopic Donor Nephrectomy in Healthy Living Kidney Donors on the Acute Phase Response Using Either Propofol or Sevoflurane Anesthesia
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
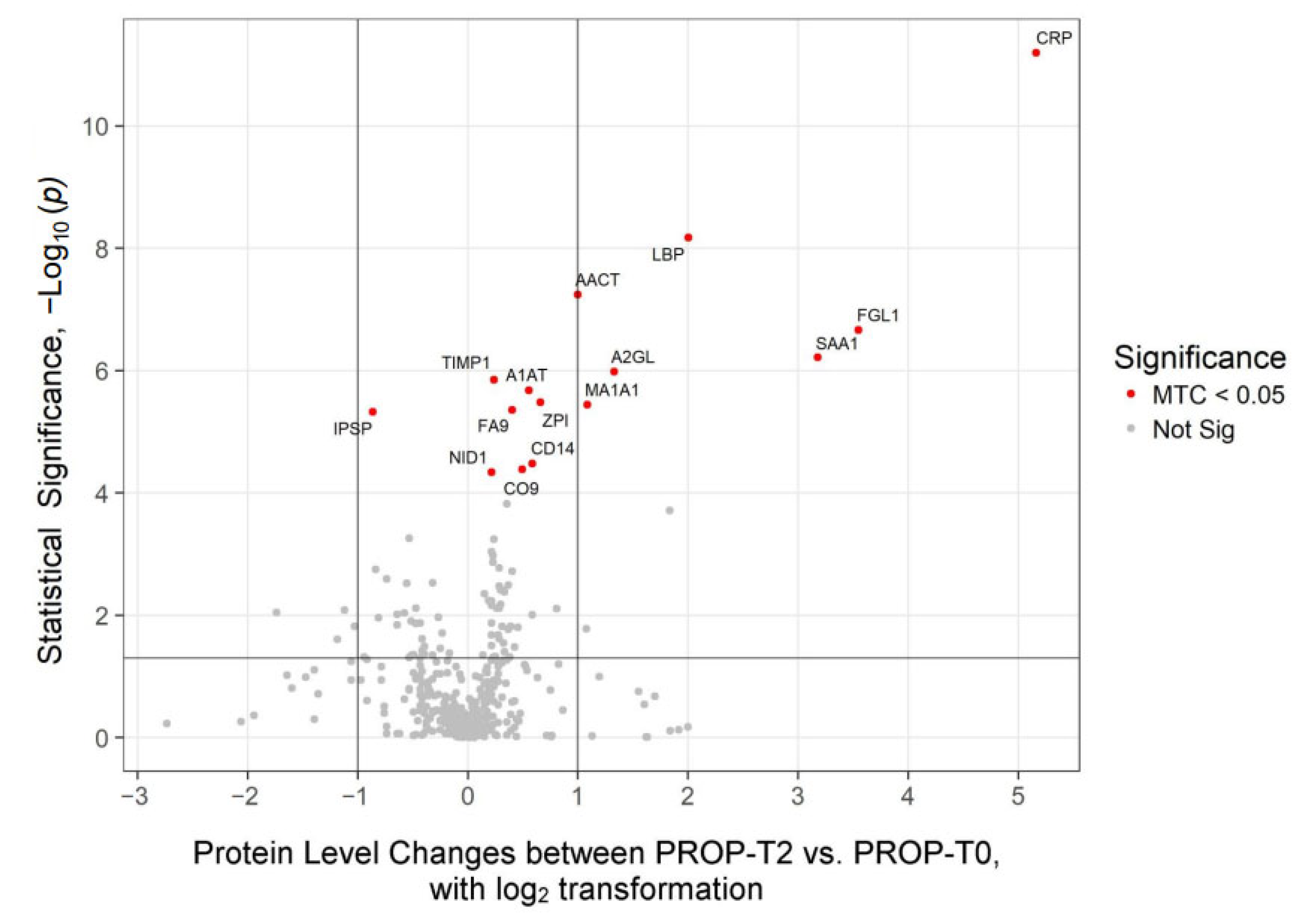
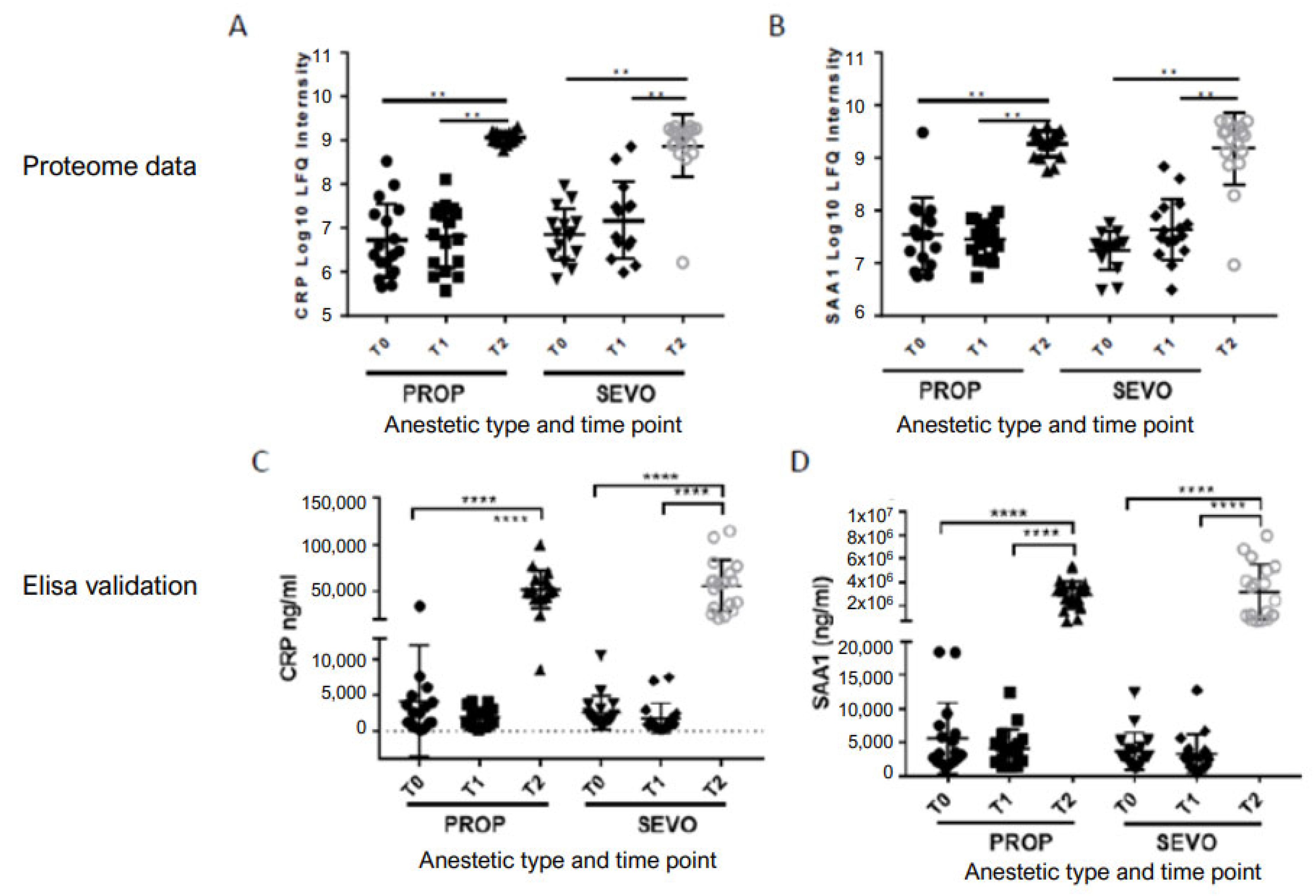
2.2. Proteomics Profiles Dependent of Anesthetic Regimen
2.3. Proteomics Profiles Independent of Anesthetic Regimen
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Surgery and Anesthesia
4.3. Samples Acquisition
4.4. Sample Preparation
4.5. Mass Spectrometry Analysis
4.6. Data Analysis and Statistics
4.7. ELISA
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nepogodiev, D.; Martin, J.; Biccard, B.; Makupe, A.; Bhangu, A. Global burden of postoperative death. Lancet 2019, 393, 401. [Google Scholar] [CrossRef]
- Kim, M.; Wall, M.M.; Li, G. Risk Stratification for Major Postoperative Complications in Patients Undergoing Intra-abdominal General Surgery Using Latent Class Analysis. Anesth. Analg. 2018, 126, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, V.A.; Hazuda, H.P.; Cornell, J.E.; Pederson, T.; Bradshaw, P.T.; Mulrow, C.D.; Page, C.P. Functional independence after major abdominal surgery in the elderly. J. Am. Coll. Surg. 2004, 199, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Ivascu, R.; Torsin, L.I.; Hostiuc, L.; Nitipir, C.; Corneci, D.; Dutu, M. The Surgical Stress Response and Anesthesia: A Narrative Review. J. Clin. Med. 2024, 13, 3017. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Chalidis, B.; Hall, G.M. Surgical stress response. Injury 2006, 37, S3–S9. [Google Scholar] [CrossRef]
- Dobson, G.P. Addressing the Global Burden of Trauma in Major Surgery. Front. Surg. 2015, 2, 43. [Google Scholar] [CrossRef]
- Margraf, A.; Ludwig, N.; Zarbock, A.; Rossaint, J. Systemic Inflammatory Response Syndrome After Surgery: Mechanisms and Protection. Anesth. Analg. 2020, 131, 1693–1707. [Google Scholar] [CrossRef]
- Mutoh, M.; Takeyama, K.; Nishiyama, N.; Kunishima, Y.; Matsukawa, M.; Takahashi, S.; Hotta, H.; Itoh, N.; Tsukamoto, T. Systemic inflammatory response syndrome in open versus laparoscopic adrenalectomy. Urology 2004, 64, 422–425. [Google Scholar] [CrossRef]
- Viikinkoski, E.; Aittokallio, J.; Lehto, J.; Ollila, H.; Relander, A.; Vasankari, T.; Jalkanen, J.; Gunn, J.; Jalkanen, S.; Airaksinen, J.; et al. Prolonged Systemic Inflammatory Response Syndrome After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2024, 38, 709–716. [Google Scholar] [CrossRef]
- Wigmore, T.J.; Mohammed, K.; Jhanji, S. Long-term Survival for Patients Undergoing Volatile versus IV Anesthesia for Cancer Surgery: A Retrospective Analysis. Anesthesiology 2016, 124, 240. [Google Scholar] [CrossRef]
- Rollins, K.E.; Tewari, N.; Ackner, A.; Awwad, A.; Madhusudan, S.; Macdonald, I.A.; Fearon, K.C.; Lobo, D.N. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin. Nutr. 2016, 35, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Khuri, S.F.; Healey, N.A.; Hossain, M.; Birjiniuk, V.; Crittenden, M.D.; Josa, M.; Treanor, P.R.; Najjar, S.F.; Kumbhani, D.J.; Henderson, W.G. Intraoperative regional myocardial acidosis and reduction in long-term survival after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2005, 129, 372–381. [Google Scholar] [CrossRef]
- Simegn, G.D.; Bayable, S.D.; Fetene, M.B. Prevention and management of perioperative hypothermia in adult elective surgical patients: A systematic review. Ann. Med. Surg. 2021, 72, 103059. [Google Scholar] [CrossRef]
- Rampersad, C.; Patel, P.; Koulack, J.; McGregor, T. Back-to-back comparison of mini-open vs. laparoscopic technique for living kidney donation. Can. Urol. Assoc. J. 2016, 10, 253–257. [Google Scholar] [CrossRef]
- Agabiti, N.; Stafoggia, M.; Davoli, M.; Fusco, D.; Barone, A.P.; Perucci, C.A. Thirty-day complications after laparoscopic or open cholecystectomy: A population-based cohort study in Italy. BMJ Open 2013, 3, e001943. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.F.; Rocco, P.R.M.; Pelosi, P. Immunomodulators in anesthesia. Curr. Opin. Anaesthesiol. 2021, 34, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Motayagheni, N.; Phan, S.; Eshraghi, C.; Nozari, A.; Atala, A. A Review of Anesthetic Effects on Renal Function: Potential Organ Protection. Am. J. Nephrol. 2017, 46, 380–389. [Google Scholar] [CrossRef]
- Sugasawa, Y.; Yamaguchi, K.; Kumakura, S.; Murakami, T.; Suzuki, K.; Nagaoka, I.; Inada, E. Effects of sevoflurane and propofol on pulmonary inflammatory responses during lung resection. J. Anesth. 2012, 26, 62–69. [Google Scholar] [CrossRef]
- Luo, C.; Yuan, D.; Li, X.; Yao, W.; Luo, G.; Chi, X.; Li, H.; Irwin, M.G.; Xia, Z.; Hei, Z. Propofol Attenuated Acute Kidney Injury after Orthotopic Liver Transplantation via Inhibiting Gap Junction Composed of Connexin 32. Anesthesiology 2015, 122, 72–86. [Google Scholar] [CrossRef]
- Yang, S.; Chou, W.-P.; Pei, L. Effects of propofol on renal ischemia/reperfusion injury in rats. Exp. Ther. Med. 2013, 6, 1177–1183. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, D.; Lei, L.; Jia, Y.; Zhou, H.; Yang, B. Propofol Prevents Renal Ischemia-Reperfusion Injury via Inhibiting the Oxidative Stress Pathways. Cell Physiol. Biochem. 2015, 37, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, A.; Marseu, K.; Slinger, P.; McRae, K.; Kim, H.; Guan, Z.; Hwang, D.M.; Liu, M.; Keshavjee, S.; Cypel, M. Sevoflurane Attenuates Ischemia-Reperfusion Injury in a Rat Lung Transplantation Model. Ann. Thorac. Surg. 2017, 103, 1578–1586. [Google Scholar] [CrossRef]
- Kotani, Y.; Pruna, A.; Turi, S.; Borghi, G.; Lee, T.C.; Zangrillo, A.; Landoni, G.; Pasin, L. Propofol and survival: An updated meta-analysis of randomized clinical trials. Crit. Care 2023, 27, 139. [Google Scholar] [CrossRef] [PubMed]
- Kampman, J.M.; Hermanides, J.; Hollmann, M.W.; Gilhuis, C.N.; Bloem, W.A.; Schraag, S.; Pradelli, L.; Repping, S.; Sperna Weiland, N.H. Mortality and morbidity after total intravenous anaesthesia versus inhalational anaesthesia: A systematic review and meta-analysis. eClinicalMedicine 2024, 72, 102636. [Google Scholar] [CrossRef]
- Bang, J.-Y.; Lee, J.; Oh, J.; Song, J.G.; Hwang, G.S. The Influence of Propofol and Sevoflurane on Acute Kidney Injury After Colorectal Surgery: A Retrospective Cohort Study. Anesth. Analg. 2016, 123, 363–370. [Google Scholar] [CrossRef]
- Mantovani, A.; Garlanda, C. Humoral Innate Immunity and Acute-Phase Proteins. N. Engl. J. Med. 2023, 388, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, A.; Sawamura, T.; Chen, C.-H.; Kraler, S.; Vdovenko, D.; Lüscher, T.F. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1): A crucial driver of atherosclerotic cardiovascular disease. Eur. Heart J. 2021, 42, 1797–1807. [Google Scholar] [CrossRef]
- Sereda, A.P.; Rukina, A.N.; Trusova, Y.V.; Dzhavadov, A.A.; Cherny, A.A.; Bozhkova, S.A.; Shubnyakov, I.I.; Tikhilov, R.M. Dynamics of C-reactive protein level after orthopedic surgeries. J. Orthop. 2023, 47, 1–7. [Google Scholar] [CrossRef]
- Rosa Neto, N.S.; de Carvalho, J.F.; Shoenfeld, Y. Screening tests for inflammatory activity: Applications in rheumatology. Mod. Rheumatol. 2009, 19, 469–477. [Google Scholar] [CrossRef]
- Lundin, E.S.; Wodlin, N.B.; Nilsson, L.; Theodorsson, E.; Ernerudh, J.; Kjølhede, P. Markers of tissue damage and inflammation after robotic and abdominal hysterectomy in early endometrial cancer: A randomised controlled trial. Sci. Rep. 2020, 10, 7226. [Google Scholar] [CrossRef]
- Neumaier, M.; Scherer, M.A. C-reactive protein levels for early detection of postoperative infection after fracture surgery in 787 patients. Acta Orthop. 2008, 79, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Sack, G.H. Serum Amyloid A (SAA) Proteins BT—Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and other Body Fluid Proteins; Hoeger, U., Harris, J.R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 421–436. [Google Scholar]
- Baranova, I.N.; Souza, A.C.P.; Bocharov, A.V.; Vishnyakova, T.G.; Hu, X.; Vaisman, B.L.; Amar, M.J.; Chen, Z.; Remaley, A.T.; Patterson, A.P.; et al. Human SR-BII mediates SAA uptake and contributes to SAA pro-inflammatory signaling in vitro and in vivo. PLoS ONE 2017, 12, e0175824. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Okuda, Y.; Takasugi, K.; Itoh, K.; Igari, J. Relative serum amyloid A (SAA) values: The influence of SAA1 genotypes and corticosteroid treatment in Japanese patients with rheumatoid arthritis. Ann. Rheum. Dis. 2001, 60, 124–127. [Google Scholar] [CrossRef]
- Eklund, K.K.; Niemi, K.; Kovanen, P.T. Immune functions of serum amyloid A. Crit. Rev. Immunol. 2012, 32, 335–348. [Google Scholar] [CrossRef]
- Linke, R.P.; Meinel, A.; Chalcroft, J.P.; Urieli-Shoval, S. Serum amyloid A (SAA) treatment enhances the recovery of aggravated polymicrobial sepsis in mice, whereas blocking SAA’s invariant peptide results in early death. Amyloid 2017, 24, 149–150. [Google Scholar] [CrossRef]
- Wierdak, M.; Pisarska, M.; Kuśnierz-Cabala, B.; Witowski, J.; Major, P.; Ceranowicz, P.; Budzyński, A.; Pędziwiatr, M. Serum Amyloid A as an Early Marker of Infectious Complications after Laparoscopic Surgery for Colorectal Cancer. Surg. Infect. 2018, 19, 622–628. [Google Scholar] [CrossRef]
- He, Y.; Ma, C.; Xing, J.; Wang, S.; Ji, C.; Han, Y.; Zhang, J. Serum amyloid a protein as a potential biomarker in predicting acute onset and association with in-hospital death in acute aortic dissection. BMC Cardiovasc. Disord. 2019, 19, 282. [Google Scholar] [CrossRef]
- Meng, L.; Song, Z.; Liu, A.; Dahmen, U.; Yang, X.; Fang, H. Effects of Lipopolysaccharide-Binding Protein (LBP) Single Nucleotide Polymorphism (SNP) in Infections, Inflammatory Diseases, Metabolic Disorders and Cancers. Front. Immunol. 2021, 12, 681810. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.-H.; Jin, L.J. The role of lipopolysaccharide-binding protein in innate immunity: A revisit and its relevance to oral/periodontal health. J. Periodontal Res. 2014, 49, 1–9. [Google Scholar] [CrossRef]
- Prucha, M.; Herold, I.; Zazula, R.; Dubska, L.; Dostal, M.; Hildebrand, T.; Hyanek, J. Significance of lipopolysaccharide-binding protein (an acute phase protein) in monitoring critically ill patients. Crit. Care 2003, 7, R154. [Google Scholar] [CrossRef]
- Brănescu, C.; Şerban, D.; Şavlovschi, C.; Dascălu, A.M.; Kraft, A. Lipopolysaccharide binding protein (L.B.P.)—An inflammatory marker of prognosis in the acute appendicitis. J. Med. Life 2012, 5, 342–347. [Google Scholar] [PubMed]
- Turgunov, Y.; Ogizbayeva, A.; Shakeyev, K.; Mugazov, M.; Akhmaltdinova, L.; Nuraly, S.; Rudolf, V. The dynamics of the lipopolysaccharide-binding protein (LBP) level in assessing the risk of adverse outcomes in operated colorectal cancer patients. Asian J. Surg. 2024, 47, 3435–3441. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ukomadu, C. Fibrinogen-like protein 1, a hepatocyte derived protein is an acute phase reactant. Biochem. Biophys. Res. Commun. 2008, 365, 729–734. [Google Scholar] [CrossRef]
- Rijken, D.C.; Dirkx, S.P.G.; Luider, T.M.; Leebeek, F.W.G. Hepatocyte-derived fibrinogen-related protein-1 is associated with the fibrin matrix of a plasma clot. Biochem. Biophys. Res. Commun. 2006, 350, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, J.; Zhang, S.; Qin, X.; Jin, W.; Sun, L.; Li, F.; Cheng, Y. Serological cytokine profiles of cardiac rejection and lung infection after heart transplantation in rats. J. Cardiothorac. Surg. 2019, 14, 26. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, R.; Tang, Q.; Yu, Y.; Huang, Q.; Chen, Y.; Wang, G.; Wang, X. Leucine-rich alpha-2-glycoprotein-1 is up-regulated in colorectal cancer and is a tumor promoter. Onco Targets Ther. 2018, 11, 2745–2752. [Google Scholar] [CrossRef]
- Fujimoto, M.; Hosono, Y.; Serada, S.; Suzuki, Y.; Ohkawara, T.; Murata, O.; Quick, A.; Suzuki, K.; Kaneko, Y.; Takeuchi, T.; et al. Leucine-rich α2-glycoprotein as a useful biomarker for evaluating disease activity in rheumatoid arthritis. Mod. Rheumatol. 2024, 34, 1072–1075. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, J.; Xie, Z.; Wang, J.; Ho, C.K.; Zhang, Y.; Li, Q. Mechanical strain promotes skin fibrosis through LRG-1 induction mediated by ELK1 and ERK signalling. Commun. Biol. 2019, 2, 359. [Google Scholar] [CrossRef]
- Banfi, C.; Parolari, A.; Brioschi, M.; Barcella, S.; Loardi, C.; Centenaro, C.; Alamanni, F.; Mussoni, L.; Tremoli, E. Proteomic Analysis of Plasma from Patients Undergoing Coronary Artery Bypass Grafting Reveals a Protease/Antiprotease Imbalance in Favor of the Serpin α1-Antichymotrypsin. J. Proteome Res. 2010, 9, 2347–2357. [Google Scholar] [CrossRef]
- Kellici, T.F.; Pilka, E.S.; Bodkin, M.J. Small-molecule modulators of serine protease inhibitor proteins (serpins). Drug Discov. Today 2021, 26, 442–454. [Google Scholar] [CrossRef]
- Dai, E.; Guan, H.; Liu, L.; Little, S.; McFadden, G.; Vaziri, S.; Cao, H.; Ivanova, I.A.; Bocksch, L.; Lucas, A. Serp-1, a Viral Anti-inflammatory Serpin, Regulates Cellular Serine Proteinase and Serpin Responses to Vascular Injury. J. Biol. Chem. 2003, 278, 18563–18572. [Google Scholar] [CrossRef] [PubMed]
- Bergin, D.A.; Hurley, K.; McElvaney, N.G.; Reeves, E.P. Alpha-1 Antitrypsin: A Potent Anti-Inflammatory and Potential Novel Therapeutic Agent. Arch. Immunol. Ther. Exp. 2012, 60, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Radovani, B.; Gudelj, I. N-Glycosylation and Inflammation; the Not-So-Sweet Relation. Front. Immunol. 2022, 13, 893365. [Google Scholar] [CrossRef]
- Nieuwenhuijs-Moeke, G.J.; Nieuwenhuijs, V.B.; Seelen, M.A.J.; Berger, S.P.; van den Heuvel, M.C.; Burgerhof, J.G.M.; Ottens, P.J.; Ploeg, R.J.; Leuvenink, H.G.D.; Struys, M.M.R.F. Propofol-based anaesthesia versus sevoflurane-based anaesthesia for living donor kidney transplantation: Results of the VAPOR-1 randomized controlled trial. Br. J. Anaesth. 2017, 118, 720–732. [Google Scholar] [CrossRef]
- Davis, S.; Charles, P.D.; He, L.; Mowlds, P.; Kessler, B.M.; Fischer, R. Expanding Proteome Coverage with CHarge Ordered Parallel Ion aNalysis (CHOPIN) Combined with Broad Specificity Proteolysis. J. Proteome Res. 2017, 16, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ideh, R.C.; Gitau, E.; Thézénas, M.L.; Jallow, M.; Ebruke, B.; Chimah, O.; Oluwalana, C.; Karanja, H.; Mackenzie, G.; et al. Discovery and Validation of Biomarkers to Guide Clinical Management of Pneumonia in African Children. Clin. Infect. Dis. 2014, 58, 1707–1715. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bandla, C.; Kundu, D.J.; Kamatchinathan, S.; Bai, J.; Hewapathirana, S.; John, N.S.; Prakash, A.; Walzer, M.; Wang, S.; et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 2025, 53, D543–D553. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| PROP n = 19 | SEVO n = 17 | p | |
|---|---|---|---|
| Baseline characteristics | |||
| Age y | 49.6 (13.2) | 33.8 (10.6) | 0.297 |
| Male n (%) | 10 (53%) | 9 (53%) | >0.999 |
| BMI kg/m2 | 25.9 (3.6) | 27.4 (3.5) | 0.233 |
| ASA I/II | 15/4 | 11/6 | 0.463 |
| mGFR mL/kg | 125 (98–140) | 107 (97–132) | 0.364 |
| Smoking n (%) | 7 (37%) | 5 (30%) | 0.728 |
| CCI | 1 (0–2) | 1 (0–1) | 0.620 |
| Cardiovascular comorbidity n (%) | 1 (5%) | 5 (29%) | 0.080 |
| MAP baseline mmHg | 93 (90–96) | 94 (85–104) | 0.863 |
| Perioperative data | |||
| Duration procedure min | 230 (28.4) | 245 (42.5) | 0.196 |
| Amount of fluid mL/kg BW | 61.5 (10.0) | 60.2 (11.7) | 0.730 |
| BIS | 39 (7) | 45 (6) | 0.012 |
| Blood sample clip renal artery | |||
| pH | 7.41 (0.04) | 7.39 (0.04) | 0.146 |
| PaO2 kPa | 20.3 (4.9) | 20.4 (4.7) | 0.955 |
| Hemoglobin mmol/L | 7.2 (0.9) | 7.3 (1.0) | 0.725 |
| Lactate mmol/L | 1.3 (0.5) | 1.7 (0.7) | 0.044 |
| Anesthetics/analgesics | |||
| Propofol Cet (μg/mL) | 3.3 (0.5) | - | - |
| Sevoflurane EtC | - | 1.53 (0.14) | - |
| Remifentanil Cet (ng/mL) | 3.2 (0.86) | 2.6 (0.63) | 0.020 |
| Vasoactive medication | |||
| Ephedrine n (%) | 13 (68%) | 17 (100%) | 0.020 |
| Dose mg | 15 (10–20) | 15 (10–30) | 0.207 |
| Phenylephrine n (%) | 2 (11%) | 1 (6%) | >0.999 |
| Dose μg | 150 (100–200) | 100 | n too small |
| Other medication | |||
| Piritramide intraoperative mg | 8 (7.0–9.0) | 7.5 (7.0–8.5) | 0.901 |
| Piritramide postoperative mg | 12 (10.0–16.0) | 11 (8.5–19.0) | 0.688 |
| Ondansetron intraoperative n (%) | 2 (11%) | 8 (47%) | 0.025 |
| Ondansetron PACU n (%) | 8 (42%) | 3 (18%) | 0.156 |
| Dexamethasone n (%) | 2 (11%) | 3 (18%) | 0.650 |
| Droperidol n (%) | 1 (5%) | 1 (6%) | >0.999 |
| Postoperative complications | 2 (10%) | 0 (0%) | 0.487 |
| LOH d | 5 (5–7) | 5 (5–7.5) | 0.912 |
| UniProt ID | Gene | Protein | PROP (T2-vs-T0) | SEVO (T2-vs-T0) | ||||
|---|---|---|---|---|---|---|---|---|
| MTC Level | p-Value | Fold Change | MTC Level | p-Value | Fold Change | |||
| P02763 | A1AG1 | Alpha-1-acid glycoprotein 1 | - | - | - | p < 0.05 | 4.60 × 10−5 | 1.52 |
| P01009 | A1AT | Alpha-1-antitrypsin | p < 0.01 | 2.10 × 10−6 | 1.47 | p < 0.01 | 1.10 × 10−5 | 1.76 |
| P02750 | A2GL | Leucine-rich alpha-2-glycoprotein | p < 0.01 | 1.00 × 10−6 | 2.51 | p < 0.01 | 3.20 × 10−7 | 3.04 |
| P01011 | AACT | Alpha-1-antichymotrypsin | p < 0.01 | 5.70 × 10−8 | 2 | p < 0.01 | 3.50 × 10−7 | 2.07 |
| P02760 | AMBP | Protein AMBP | - | - | - | p < 0.01 | 2.60 × 10−6 | 1.51 |
| P61769 | B2MG | Beta-2-microglobulin | - | - | - | p < 0.01 | 6.00 × 10−6 | 1.67 |
| P08571 | CD14 | Monocyte differentiation antigen CD14 | p < 0.05 | 3.30 × 10−5 | 1.5 | p < 0.05 | 1.30 × 10−4 | 1.38 |
| P02748 | CO9 | Complement component C9 | p < 0.05 | 4.10 × 10−5 | 1.41 | p < 0.01 | 2.20 × 10−6 | 1.41 |
| P02741 | CRP | C-reactive protein | p < 0.01 | 6.40 × 10−12 | 35.83 | p < 0.01 | 9.90 × 10−7 | 70.09 |
| P00740 | FA9 | Coagulation factor IX | p < 0.01 | 4.40 × 10−6 | 1.32 | - | - | - |
| Q08830 | FGL1 | Fibrinogen-like protein 1 | p < 0.01 | 2.20 × 10−7 | 11.69 | p < 0.01 | 4.50 × 10−7 | 10.69 |
| P18065 | IBP2 | Insulin-like growth factor-binding protein 2 | - | - | - | p < 0.01 | 4.70 × 10−7 | 1.73 |
| P22692 | IBP4 | Insulin-like growth factor-binding protein 4 | - | - | - | p < 0.01 | 1.30 × 10−5 | 1.76 |
| P05154 | IPSP | Plasma serine protease inhibitor | p < 0.01 | 4.80 × 10−6 | −1.82 | p < 0.01 | 9.50 × 10−6 | −1.89 |
| P18428 | LBP | Lipopolysaccharide-binding protein | p < 0.01 | 6.60 × 10−9 | 4.01 | p < 0.01 | 6.80 × 10−6 | 4.04 |
| P33908 | MAN1A1 | Mannosyl-oligosaccharide 1,2-alpha-mannosidase IA | p < 0.01 | 3.60 × 10−6 | 2.12 | - | - | - |
| P14543 | NID1 | Nidogen-1 | p < 0.05 | 4.60 × 10−5 | 1.16 | - | - | - |
| P41222 | PTGDS | Prostaglandin-H2 D-isomerase | - | - | - | p < 0.01 | 2.60 × 10−6 | 1.73 |
| P0DJI8 | SAA1 | Serum amyloid A-1 protein | p < 0.01 | 6.10 × 10−7 | 9.05 | p < 0.01 | 1.20 × 10−5 | 110.69 |
| P0DJI9 | SAA2 | Serum amyloid A-2 protein | - | - | - | p < 0.01 | 1.40 × 10−5 | 48.67 |
| P01033 | TIMP1 | Metalloproteinase inhibitor 1 | p < 0.01 | 1.40 × 10−6 | 1.18 | - | - | - |
| Q9UK55 | ZPI | Protein Z-dependent protease inhibitor | p < 0.01 | 3.30 × 10−6 | 1.58 | p < 0.01 | 2.00 × 10−6 | 1.45 |
| ALL T2-T0 | ALL T2-T1 | ALL T1-T0 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UniPro ID | Gene | Protein | MTC | p-Value | Fold Change | Direction | MTC | p-Value | Fold Change | Direction | MTC | p-Value | Fold Change | Direction |
| P02741 | CRP | C-reactive protein | p < 0.01 | 2.7 × 10−16 | 46.42 | UP | p < 0.01 | 2.4 × 10−14 | 22.61 | UP | - | - | - | - |
| P01011 | AACT | Alpha-1-antichymotrypsin | p < 0.01 | 3.4 × 10−14 | 2.03 | UP | p < 0.01 | 2.9 × 10−13 | 1.93 | UP | - | - | - | - |
| P18428 | LBP | Lipopolysaccharide-binding protein | p < 0.01 | 1.5 × 10−13 | 4.04 | UP | p < 0.01 | 1.8 × 10−9 | 3.73 | UP | - | - | - | - |
| Q08830 | FGL1 | Fibrinogen-like protein 1 | p < 0.01 | 1.6 × 10−13 | 11.76 | UP | p < 0.01 | 6.8 × 10−11 | 8.48 | UP | - | - | - | - |
| P02750 | A2GL | Leucine-rich alpha-2-glycoprotein | p < 0.01 | 7.2 × 10−13 | 2.74 | UP | p < 0.01 | 7.9 × 10−12 | 2.63 | UP | - | - | - | - |
| Q9UK55 | ZPI | Protein Z-dependent protease inhibitor | p < 0.01 | 3.1 × 10−11 | 1.52 | UP | p < 0.01 | 3.5 × 10−8 | 1.41 | UP | - | 4.3 × 10−2 | 1.08 | UP |
| P0DJI8 | SAA1 | Serum amyloid A-1 protein | p < 0.01 | 5.7 × 10−11 | 17.69 | UP | p < 0.01 | 2.2 × 10−11 | 32.42 | UP | - | - | - | - |
| P05154 | IPSP | Plasma serine protease inhibitor | p < 0.01 | 1.0 × 10−10 | 1.85 | DOWN | p < 0.01 | 8.1 × 10−9 | 1.69 | DOWN | - | - | - | - |
| P01009 | A1AT | Alpha-1-antitrypsin | p < 0.01 | 3.2 × 10−10 | 1.61 | UP | p < 0.01 | 1.2 × 10−11 | 2.09 | UP | p < 0.05 | 8.8 × 10−5 | 1.3 | DOWN |
| P02748 | CO9 | Complement component C9 | p < 0.01 | 4.2 × 10−10 | 1.41 | UP | p < 0.01 | 3.1 × 10−7 | 1.25 | UP | - | 9.3 × 10−3 | 1.13 | UP |
| P00740 | FA9 | Coagulation factor XI | p < 0.01 | 6.7 × 10−9 | 1.32 | UP | - | 1.5 × 10−2 | 1.1 | UP | p < 0.05 | 2.8 × 10−5 | 1.2 | UP |
| P0DJI9 | SAA2 | Serum amyloid A-2 protein | p < 0.01 | 9.1 × 10−9 | 4.58 | UP | p < 0.01 | 2.7 × 10−9 | 16.47 | UP | - | - | - | - |
| P33908 | MAN1A1 | Mannosyl-oligosaccharide 1,2-alhpa-mannosidase IA | p < 0.01 | 1.0 × 10−8 | 1.93 | UP | p < 0.05 | 6.2 × 10−5 | 1.63 | UP | - | - | - | - |
| P08571 | CD14 | Monocyte differentiation antigen CD14 | p < 0.01 | 1.1 × 10−8 | 1.44 | UP | p < 0.01 | 1.2 × 10−6 | 1.38 | UP | - | - | - | - |
| P18065 | IBP2 | Insulin-like growth factor-binding protein 2 | p < 0.01 | 1.2 × 10−8 | 1.38 | UP | p < 0.01 | 2.9 × 10−7 | 1.47 | UP | - | 2.6 × 10−2 | 1.06 | DOWN |
| P15169 | CBPN | Carboxy peptidase N catalytic chain | p < 0.01 | 1.9 × 10−6 | 1.25 | UP | - | 8.0 × 10−4 | 1.22 | UP | - | - | - | - |
| P02760 | AMBP | Protein AMBP | p < 0.01 | 2.9 × 10−6 | 1.4 | UP | p < 0.05 | 2.6 × 10−5 | 1.22 | UP | - | - | - | - |
| P01033 | TIMP1 | Metalloproteinase inhibitor 1 | p < 0.01 | 3.2 × 10−6 | 1.23 | UP | p < 0.01 | 1.4 × 10−5 | 1.24 | UP | - | - | - | - |
| P36955 | PEDF | Pigment epithelium-derived factor | p < 0.01 | 3.3 × 10−6 | 1.27 | UP | p < 0.01 | 1.1 × 10−6 | 1.21 | UP | - | - | - | - |
| P05019 | IGF1 | Insulin-like growth factor I | p < 0.01 | 8.5 × 10−6 | 1.25 | UP | - | - | - | - | - | 1.3 × 10−2 | 1.14 | UP |
| P04004 | VTNC | Vitronectin | p < 0.01 | 1.4 × 10−5 | 1.22 | UP | - | - | - | - | - | 2.0 × 10−2 | 1.13 | UP |
| P49747 | COMP | Cartilage oligomeric matrix protein | p < 0.01 | 1.5 × 10−5 | 1.47 | DOWN | - | 1.6 × 10−2 | 1.2 | DOWN | - | 7.1 × 10−4 | 1.2 | DOWN |
| P00748 | FA12 | Coagulation factor XII | p < 0.05 | 2.6 × 10−5 | 1.32 | DOWN | - | 1.5 × 10−3 | 1.27 | DOWN | - | - | - | - |
| P02787 | TRFE | Serotransferrin | p < 0.05 | 3.2 × 10−5 | 1.54 | DOWN | - | - | - | - | - | 7.0 × 10−3 | 1.3 | DOWN |
| P02763 | A1AG1 | Alpha-1-acid glycoprotein 1 | p < 0.05 | 3.4 × 10−5 | 1.38 | UP | p < 0.01 | 3.3 × 10−10 | 1.7 | UP | - | 2.6 × 10−2 | 1.23 | DOWN |
| Q86UD1 | OAF | Out at first protein homolog | p < 0.05 | 4.1 × 10−5 | 1.87 | UP | p < 0.01 | 8.1 × 10−6 | 1.67 | UP | - | - | - | - |
| P02649 | APOE | Apolipoprotein E | p < 0.05 | 8.0 × 10−5 | 1.25 | UP | - | - | - | - | p < 0.01 | 6.5 × 10−6 | 1.31 | UP |
| P22792 | CPN2 | Carboxy peptidase N subunit 2 | p < 0.05 | 8.3 × 10−5 | 1.16 | UP | - | - | - | - | - | - | - | - |
| Q92820 | GGH | Gamma-glutamyl hydrolase | - | 1.5 × 10−4 | 1.15 | UP | - | - | - | - | p < 0.05 | 9.0 × 10−5 | 1.15 | UP |
| P14543 | NID1 | Nidogen-1 | - | 2.5 × 10−4 | 1.05 | UP | p < 0.01 | 3.2 × 10−10 | 1.08 | UP | - | - | - | - |
| P05160 | F13B | Coagulation factor XIII B chain | - | 1.9 × 10−3 | 1.39 | DOWN | p < 0.05 | 7.9 × 10−6 | 1.59 | DOWN | - | - | - | - |
| P22352 | GPX3 | Glutathione peroxidase 3 | - | 2.8 × 10−3 | 1.2 | UP | - | - | - | - | p < 0.01 | 6.5 × 10−6 | 1.3 | UP |
| Q06033 | ITIH3 | Inter-alpha-trypsin inhibitor heavy chain H3 | - | 1.2 × 10−2 | 1.17 | UP | p < 0.01 | 1.9 × 10−7 | 1.19 | UP | - | - | - | - |
| P22105 | TENX | Tenascin-X | - | 4.0 × 10−2 | 1.14 | UP | - | - | - | - | p < 0.05 | 2.7 × 10−5 | 1.35 | UP |
| O75636 | FCN3 | Ficolin-3 | - | - | - | - | - | 4.2 × 10−3 | 1.19 | DOWN | p < 0.05 | 5.0 × 10−5 | 1.26 | UP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brattinga, B.; Huang, H.; Maslau, S.; Thorne, A.M.; Hunter, J.; Knight, S.; Struys, M.M.R.F.; Leuvenink, H.G.D.; de Bock, G.H.; Ploeg, R.J.; et al. Effect of a Laparoscopic Donor Nephrectomy in Healthy Living Kidney Donors on the Acute Phase Response Using Either Propofol or Sevoflurane Anesthesia. Int. J. Mol. Sci. 2025, 26, 5196. https://doi.org/10.3390/ijms26115196
Brattinga B, Huang H, Maslau S, Thorne AM, Hunter J, Knight S, Struys MMRF, Leuvenink HGD, de Bock GH, Ploeg RJ, et al. Effect of a Laparoscopic Donor Nephrectomy in Healthy Living Kidney Donors on the Acute Phase Response Using Either Propofol or Sevoflurane Anesthesia. International Journal of Molecular Sciences. 2025; 26(11):5196. https://doi.org/10.3390/ijms26115196
Chicago/Turabian StyleBrattinga, Baukje, Honglei Huang, Sergei Maslau, Adam M. Thorne, James Hunter, Simon Knight, Michel M. R. F. Struys, Henri G. D. Leuvenink, Geertruida H. de Bock, Rutger J. Ploeg, and et al. 2025. "Effect of a Laparoscopic Donor Nephrectomy in Healthy Living Kidney Donors on the Acute Phase Response Using Either Propofol or Sevoflurane Anesthesia" International Journal of Molecular Sciences 26, no. 11: 5196. https://doi.org/10.3390/ijms26115196
APA StyleBrattinga, B., Huang, H., Maslau, S., Thorne, A. M., Hunter, J., Knight, S., Struys, M. M. R. F., Leuvenink, H. G. D., de Bock, G. H., Ploeg, R. J., Kessler, B. M., & Nieuwenhuijs-Moeke, G. J. (2025). Effect of a Laparoscopic Donor Nephrectomy in Healthy Living Kidney Donors on the Acute Phase Response Using Either Propofol or Sevoflurane Anesthesia. International Journal of Molecular Sciences, 26(11), 5196. https://doi.org/10.3390/ijms26115196







