Affinity Electrophoresis of Proteins for Determination of Ligand Affinity and Exploration of Binding Sites
Abstract
1. Introduction
2. Fundamental Principles of AE
3. Methodology of Gel AE
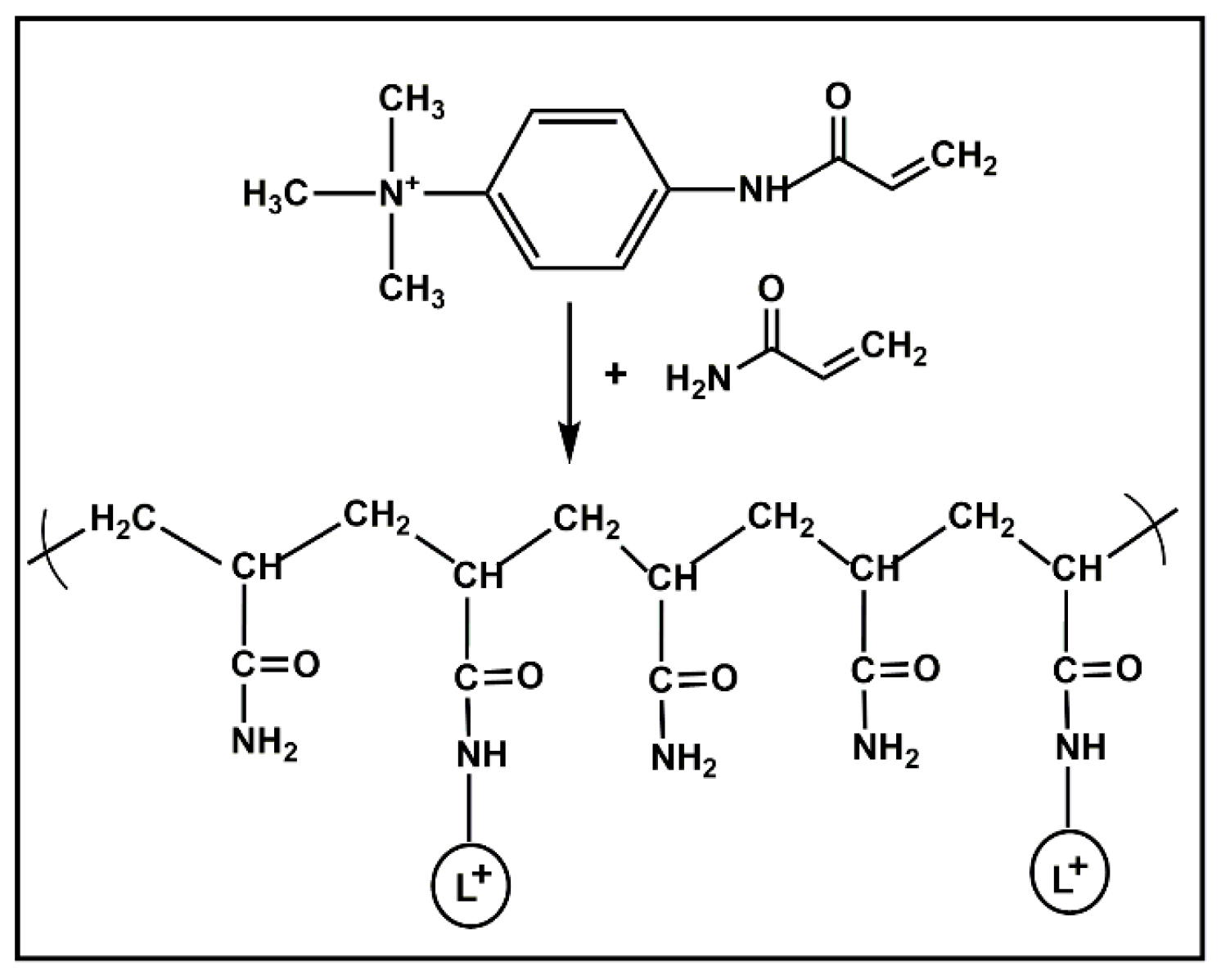
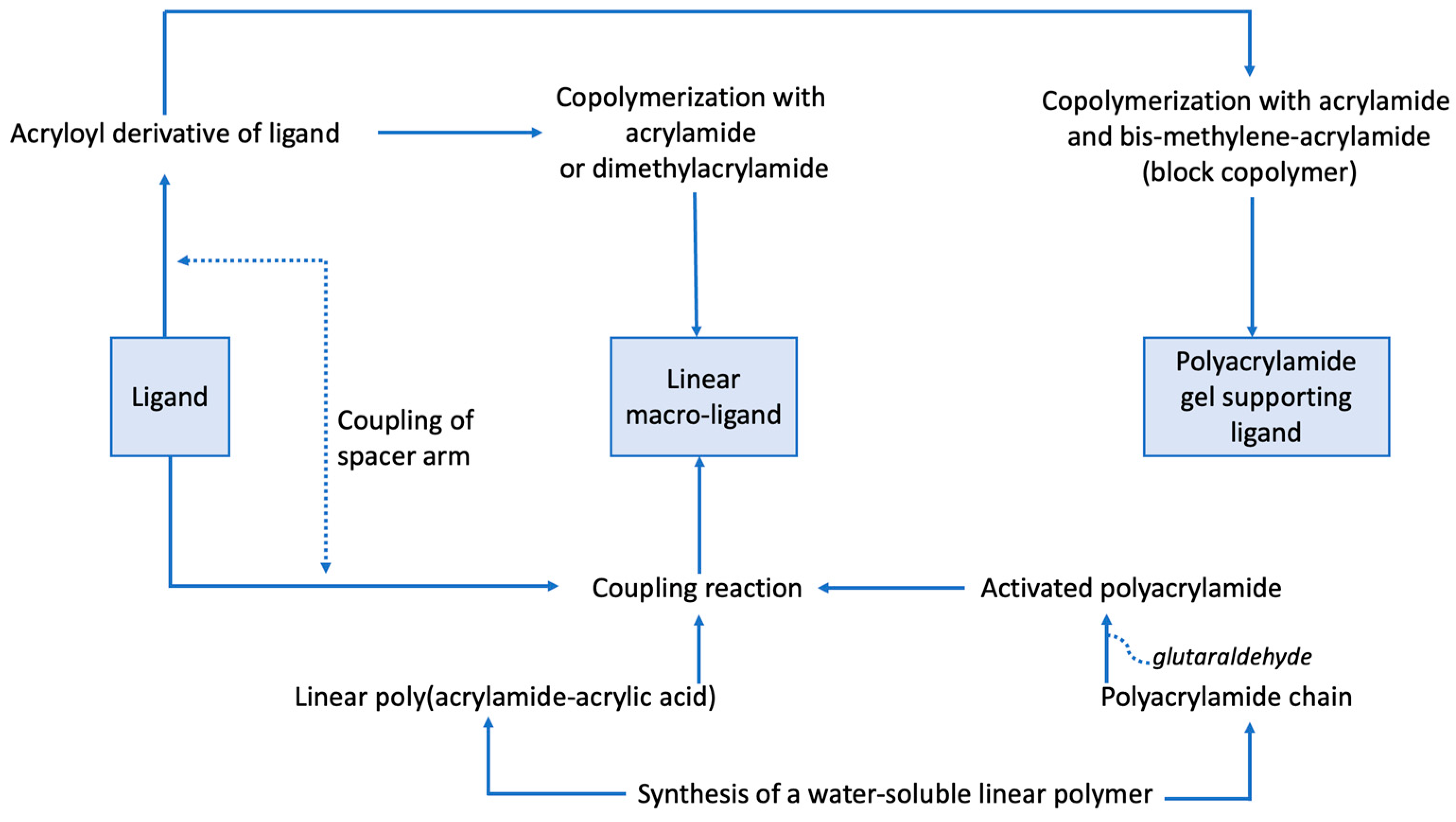
3.1. Immobilization of Ligands in Matrix
3.2. Electrophoretic Conditions
4. Applications of AE
4.1. Quantitative Applications
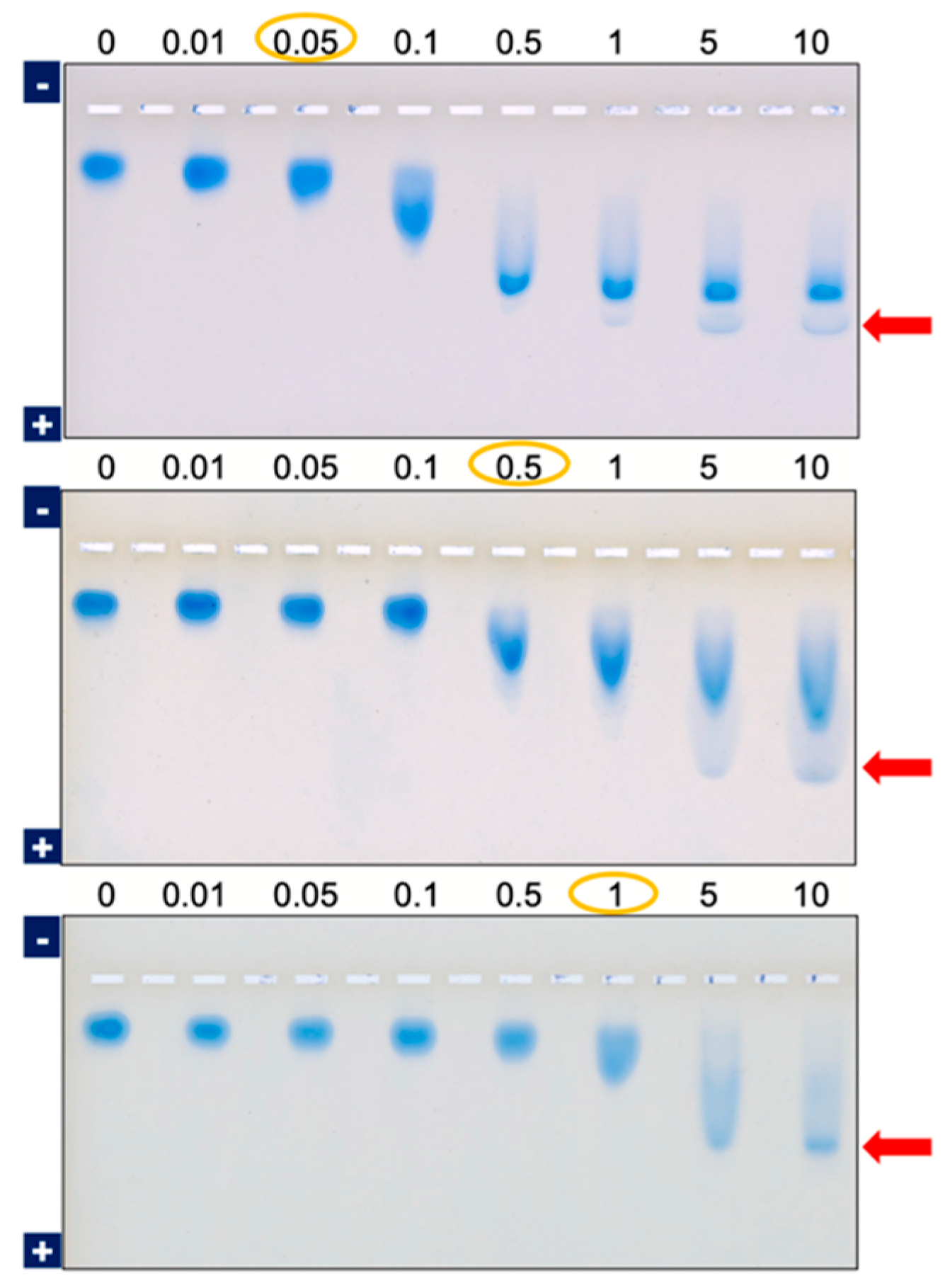
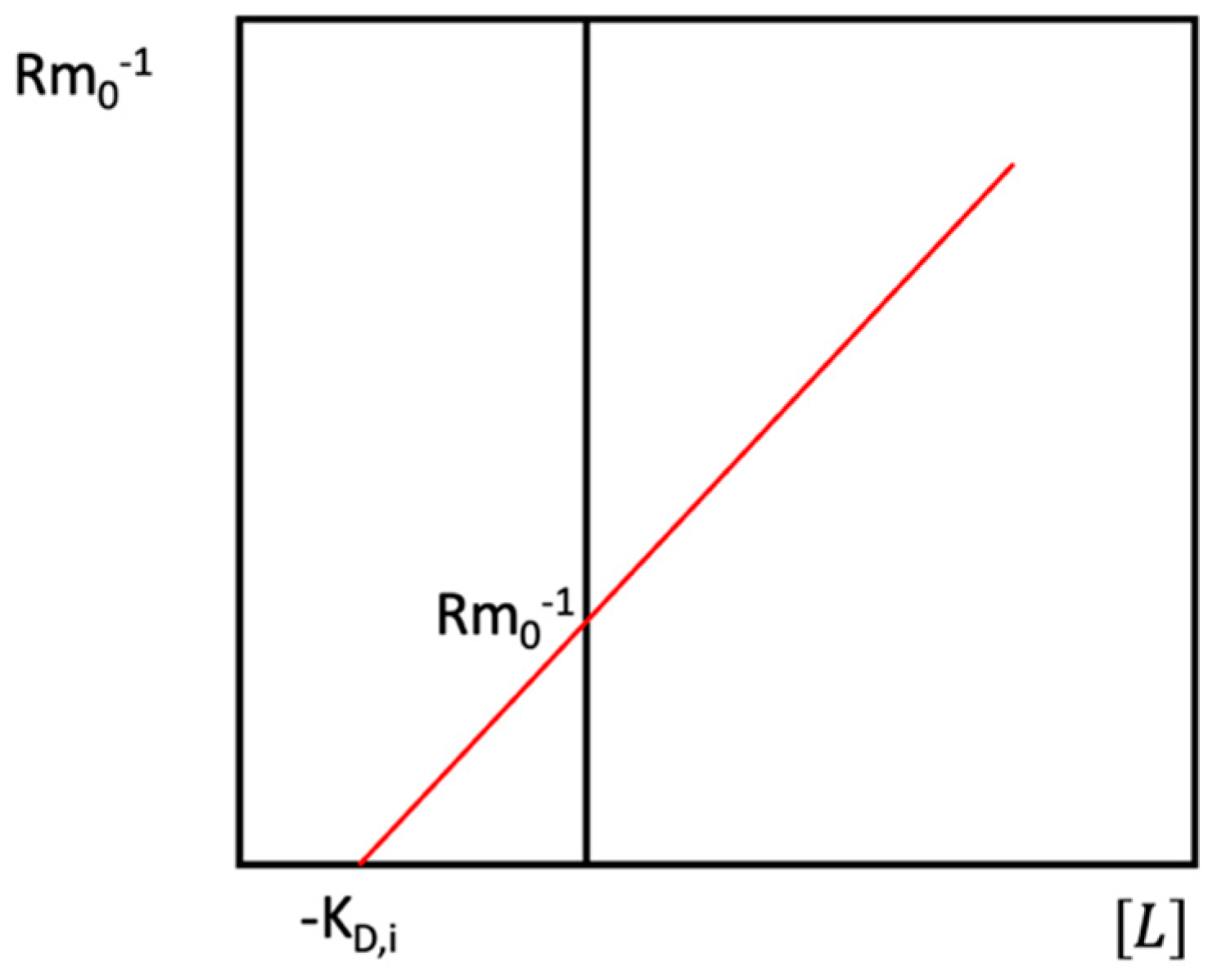
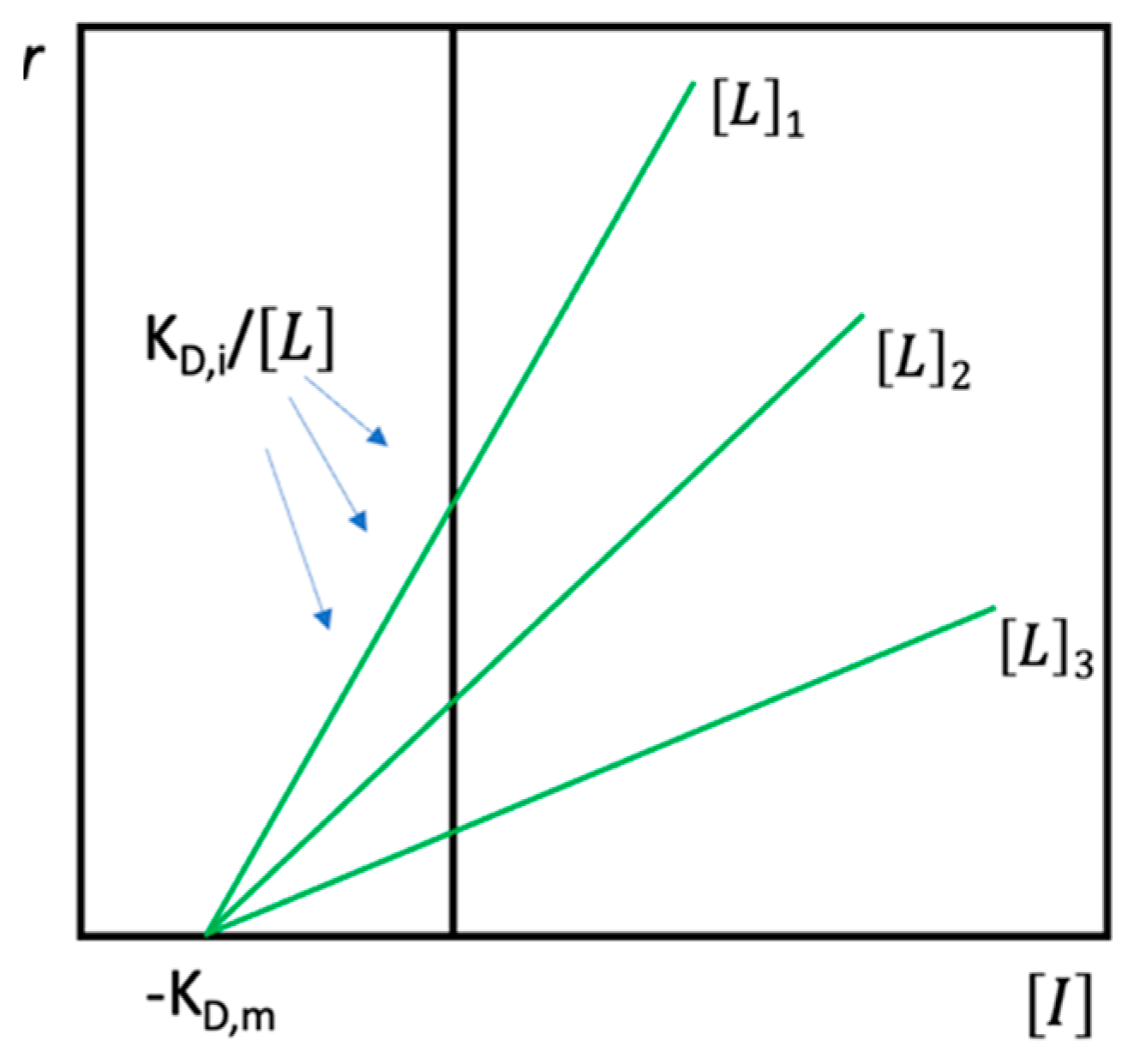
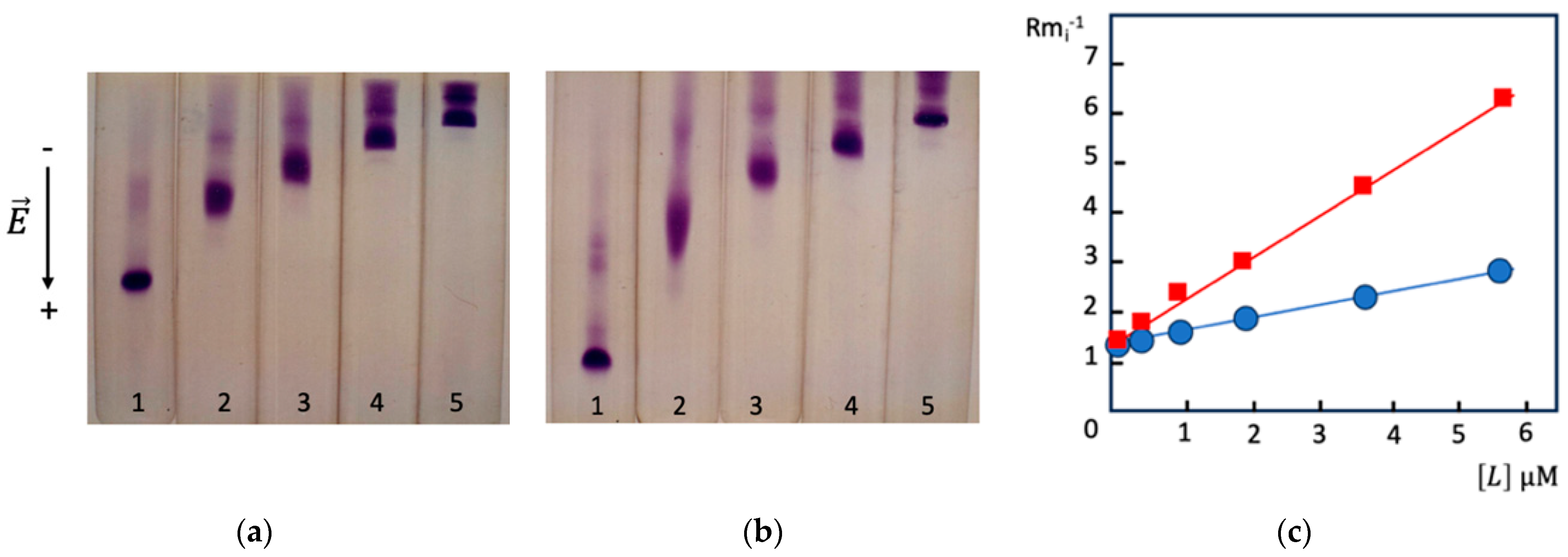
4.1.1. Theoretical Background
4.1.2. Factors Affecting Values of KD in AE
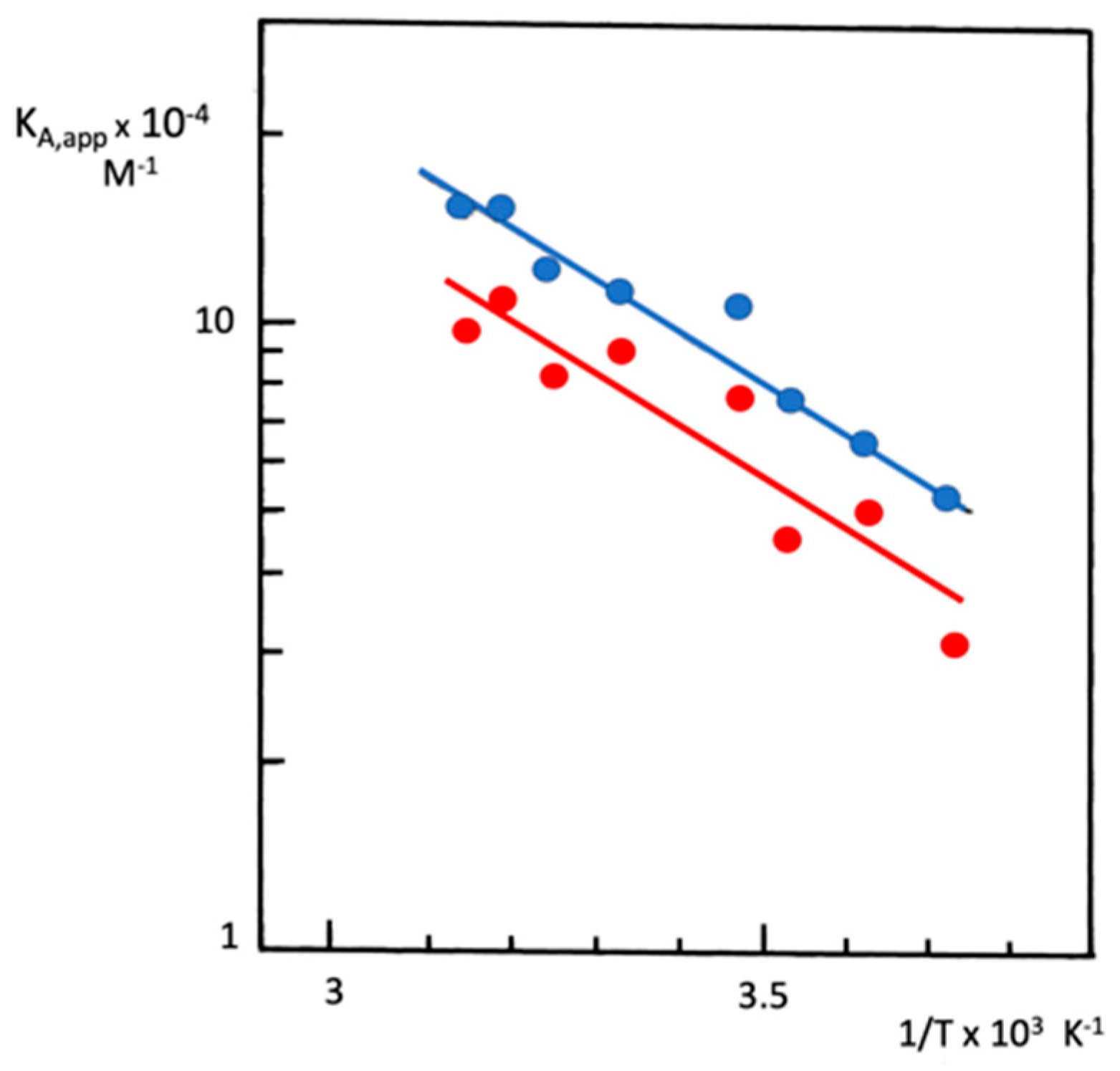
- Temperature
- Hydrostatic pressure
- Solvents, co-solvents, stabilizers, and denaturing agents.
- pH.
- Ionic strength.
- Electric field.
- Gel porosity: interactions between protein and PAG matrix, containing immobilized ligand.
- Protein binding affinity characteristics
4.1.3. Time-Dependent Interactions
4.1.4. Interactions Between Gel Matrix and Macro-Ligand
4.2. Qualitative Applications
4.2.1. Detection of Specific Proteins in Complex Media
4.2.2. Detection of Protein Heterogeneity and Genetic Polymorphism
4.2.3. Detection of Protein/Enzyme Functionality
4.2.4. Study of Protein Chemical Modifications
4.2.5. Study of Supramolecular Conjugates
5. Affinity Capillary Electrophoresis
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Affinity chromatography |
| ACE | Affinity capillary electrophoresis |
| AE | Affinity electrophoresis |
| BChE | Butyrylcholinesterase |
| BSA | Bovine serum albumin |
| ChE | Cholinesterase |
| CZAE | Capillary zone affinity electrophoresis |
| CZE | Capillary zone electrophoresis |
| HSA | Human serum albumin |
| IEF | Isoelectric focusing |
| PAG | Polyacrylamide gel |
| PAGE | Polyacrylamide gel electrophoresis |
| PEG | Polyethylene glycol |
| pI | Isoelectric point |
| SBI | Slow-binding inhibitor |
References
- Masson, P.; Lushchekina, S. Conformational Stability and Denaturation Processes of Proteins Investigated by Electrophoresis under Extreme Conditions. Molecules 2022, 27, 6861. [Google Scholar] [CrossRef] [PubMed]
- Bøg-Hansen, T.C. Crossed Immuno-Affinoelectrophoresis. Anal. Biochem. 1973, 56, 480–488. [Google Scholar] [CrossRef]
- Heegaard, N.H.; Bøg-Hansen, T.C. Affinity Electrophoresis in Agarose Gels. Theory and Some Applications. Appl. Theor. Electrophor. 1990, 1, 249–259. [Google Scholar] [PubMed]
- Kobayashi, Y. Lectin Affinity Electrophoresis. In Lectin; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2014; Volume 1200, pp. 121–129. [Google Scholar] [CrossRef]
- Heegaard, N.H.; Bjerrum, O.J. Affinity Electrophoresis Used for Determination of Binding Constants for Antibody-Antigen Reactions. Anal. Biochem. 1991, 195, 319–326. [Google Scholar] [CrossRef]
- Nakamura, K.; Kashiwagi, S.; Takeo, K. Characterization of the Interaction between Human Plasma Fibronectin and Collagen by Means of Affinity Electrophoresis. J. Chromatogr. A 1992, 597, 351–356. [Google Scholar] [CrossRef]
- Nakamura, K.; Kuwahara, A.; Takeo, K. Study of the Interaction between Phosphorylase and Hydrophobic Groups by Means of Affinity Electrophoresis. J. Chromatogr. A 1979, 171, 89–99. [Google Scholar] [CrossRef]
- Nakamura, K.; Kuwahara, A.; Takeo, K. Study of the Interaction between NADP-Dependent Dehydrogenase and Immobilized Adenosine 2′-Monophosphate by Means of Affinity Electrophoresis. J. Chromatogr. A 1980, 196, 85–99. [Google Scholar] [CrossRef]
- Takeo, K. Affinity Electrophoresis: Principles and Applications. Electrophoresis 1984, 5, 187–195. [Google Scholar] [CrossRef]
- Hořejší, V.; Kocourek, J. [13] Affinity Electrophoresis: Separation of Phytohemagglutinins on O-Glycosyl Polyacrylamide Gels. Methods Enzymol. 1974, 34, 178–181. [Google Scholar]
- Hořejší, V.; Tichá, M.; Tichý, P.; Holý, A. Affinity Electrophoresis: New Simple and General Methods of Preparation of Affinity Gels. Anal. Biochem. 1982, 125, 358–369. [Google Scholar] [CrossRef]
- Hořejší, V. Affinity Electrophoresis. Methods Enzymol. 1984, 104, 275–281. [Google Scholar] [PubMed]
- Hořejší, V.; Ticha’, M. Qualitative and Quantitative Applications of Affinity Electrophoresis for the Study of Protein—Ligand Interactions: A Review. J. Chromatogr. B Biomed. Sci. Appl. 1986, 376, 49–67. [Google Scholar] [CrossRef]
- Galbusera, C.; Chen, D.D. Molecular Interaction in Capillary Electrophoresis. Curr. Opin. Biotechnol. 2003, 14, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Albishri, H.M.; Deeb, S.E.; AlGarabli, N.; AlAstal, R.; Alhazmi, H.A.; Nachbar, M.; El-Hady, D.A.; Wätzig, H. Recent Advances in Affinity Capillary Electrophoresis for Binding Studies. Bioanalysis 2014, 6, 3369–3392. [Google Scholar] [CrossRef] [PubMed]
- Olabi, M.; Stein, M.; Wätzig, H. Affinity Capillary Electrophoresis for Studying Interactions in Life Sciences. Methods 2018, 146, 76–92. [Google Scholar] [CrossRef]
- Le, A.T.H.; Krylova, S.M.; Krylov, S.N. Kinetic Capillary Electrophoresis in Screening Oligonucleotide Libraries for Protein Binders. TrAC Trends Anal. Chem. 2023, 162, 117061. [Google Scholar] [CrossRef]
- Heegaard, N.H.H. Affinity in Electrophoresis. Electrophoresis 2009, 30, S229–S239. [Google Scholar] [CrossRef]
- Kinoshita, E.; Kinoshita-Kikuta, E.; Koike, T. The Cutting Edge of Affinity Electrophoresis Technology. Proteomes 2015, 3, 42–55. [Google Scholar] [CrossRef]
- Kučerová, Z.; Muselová, H.; Přikryl, P.; Tichá, M. Phosphoprotein Electrophoresis in the Presence of Fe(III) Ions. J. Sep. Sci. 2011, 34, 1875–1879. [Google Scholar] [CrossRef]
- Lee, B.-S.; Jayathilaka, L.P.; Huang, J.-S.; Gupta, S. Applications of Immobilized Metal Affinity Electrophoresis. In Electrophoretic Separation of Proteins; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; pp. 371–385. [Google Scholar]
- Pan, Y.; Sackmann, E.K.; Wypisniak, K.; Hornsby, M.; Datwani, S.S.; Herr, A.E. Determination of Equilibrium Dissociation Constants for Recombinant Antibodies by High-Throughput Affinity Electrophoresis. Sci. Rep. 2016, 6, 39774. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Shao, Y.; Zhang, D.; Guo, G.; Wang, X. Analytical Methods for Obtaining Binding Parameters of Drug–Protein Interactions: A Review. Anal. Chim. Acta 2022, 1219, 340012. [Google Scholar] [CrossRef] [PubMed]
- Entlicher, G.; Tichá, M.; KoštíŘ, J.V.; Kocourek, J. Studies on Phytohemagglutinins II. Phytohemagglutinins OfPisum Sativum L. AndLens Esculenta Moench: Specific Interactions with Carbohydrates. Experientia 1969, 25, 17–19. [Google Scholar] [CrossRef]
- Gerbrandy, S.J.; Doorgeest, A. Potato Phosphorylase Isoenzymes. Phytochemistry 1972, 11, 2403–2407. [Google Scholar] [CrossRef]
- Takeo, K.; Nakamura, S. Dissociation Constants of Glucan Phosphorylases of Rabbit Tissues Studied by Polyacrylamide Gel Disc Electrophoresis. Arch. Biochem. Biophys. 1972, 153, 1–7. [Google Scholar] [CrossRef]
- Horejsi, V.; Kocourek, J. Studies on Phytohemagglutinins XII. O-Glycosyl Polyacrylamide Gels for Affinity Chromatography of Phytohemagglutinins. Biochim. Biophys. Acta Gen. Subj. 1973, 297, 346–351. [Google Scholar] [CrossRef]
- Kocourek, J. Synthetic Glycosyl Polymers in Isolation, Characterization and Immobilization of Lectins. Acta Histochem. 1982, 71, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Bosisio, A.B.; Loeherlein, C.; Snyder, R.S.; Righetti, P.G. Titration Curves of Proteins by Combined Isoelectric Focusing-Electrophoresis in Highly Porous Polyacrylamide Matrices. J. Chromatogr. A 1980, 189, 317–330. [Google Scholar] [CrossRef]
- Awada, C.; Sato, T.; Takao, T. Affinity-Trap Polyacrylamide Gel Electrophoresis: A Novel Method of Capturing Specific Proteins by Electro-Transfer. Anal. Chem. 2010, 82, 755–761. [Google Scholar] [CrossRef]
- Archibald, A.L. Further Studies on Bovine Serum Amylase. 2. Affinity Electrophoresis. Anim. Blood Groups Biochem. Genet. 1981, 12, 249–264. [Google Scholar] [CrossRef]
- Brown, S.S.; Kalow, W.; Pilz, W.; Whittaker, M.; Woronic, C.L. The Plasma Cholinesterases: A New Perspective. Adv. Clin. Chem. 1981, 22, 1–123. [Google Scholar]
- Ek, K.; Righetti, P.G. Determination of Protein-ligand Dissociation Constants of Their PH Dependence by Combined Isoelectric Focusing-electrophoresis (Titration Curves): Binding of Phosphorylases a and b to Glycogen. Electrophoresis 1980, 1, 137–140. [Google Scholar] [CrossRef]
- Etzler, M.E.; Gupta, S.; Borrebaeck, C. Carbohydrate Binding Properties of Th Dolichos Biflorus Lectin and Its Subunits. J. Biol. Chem. 1981, 256, 2367–2370. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.L.; Yet, M.-G.; Wold, F. Substrate-Containing Gel Electrophoresis: Sensitive Detection of Amylolytic, Nucleolytic, and Proteolytic Enzymes. Anal. Biochem. 1982, 122, 164–172. [Google Scholar] [CrossRef]
- Karpetsky, T.P.; Davies, G.E.; Shriver, K.K.; Levy, C.C. Use of Polynucleotide/Polyacrylamide-Gel Electrophoresis as a Sensitive Technique for the Detection and Comparison of Ribonuclease Activities. Biochem. J. 1980, 189, 277–284. [Google Scholar] [CrossRef]
- Tichá, M.; Hořejší, V.; Barthová, J. Affinity Electrophoresis of Proteins Interacting with Blue Dextran. Biochim. Biophys. Acta Protein Struct. 1978, 534, 58–63. [Google Scholar] [CrossRef]
- Čeŕovský, V.; Tichá, M.; Turková, J.; Labský, J. Interaction of Trypsin with Immobilized P-Aminobenzamidine Derivatives Studied by Means of Affinity Electrophoresis. J. Chromatogr. A 1980, 194, 175–181. [Google Scholar] [CrossRef]
- Čeřovský, V.; Tichá, M.; Horřejší, V.; Kocourek, J. Studies on Lectins. XLIX. The Use of Glycosyl Derivatives of Dextran T-500 Affinity Electrophoresis of Lectins. J. Biochem. Biophys. Methods 1980, 3, 163–172. [Google Scholar] [CrossRef]
- Chen, J.L.; Morawetz, H. Affinity Electrophoresis in Gels Containing Hydrophobic Substituents. J. Biol. Chem. 1981, 256, 9221–9223. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; De Garilhe, A.P.; Burnat, P. Formes Moleculaires Multiples de La Butyrylcholinesterase Du Plasma Humain. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1982, 701, 269–284. [Google Scholar] [CrossRef]
- Masson, P.; Vallin, P. Possibilitiés d’étude Des Variantes de La Cholinestérase Plasmatique Humaine Par Électrophorèse d’affinité. J. Chromatogr. B Biomed. Sci. Appl. 1983, 273, 289–299. [Google Scholar] [CrossRef]
- Masson, P.; Reybaud, J. Hydrophobic Interaction Electrophoresis under High Hydrostatic Pressure: Study of the Effects of Pressure upon the Interaction of Serum Albumin with a Long-chain Aliphatic Ligand. Electrophoresis 1988, 9, 157–161. [Google Scholar] [CrossRef]
- Masson, P.; Marnot, B.; Lombard, J.-Y.; Morelis, P. Etude Électrophorétique de La Butyrylcholinestérase Agée Après Inhibition Par Le Soman. Biochimie 1984, 66, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.; Hattesohl, I.; Schuetz, H.-J.; Meyer, G. Polyethylene Glycol Derivatives of Base and Sequence Specific DNA Ligands: DNA Interaction and Application for Base Specific Separation of DNA Fragments by Gel Electrophoresis. Nucleic Acids Res. 1981, 9, 95–120. [Google Scholar] [CrossRef]
- Masson, P. Structural and Functional Investigations of Cholinesterases by Means of Affinity Electrophoresis. Cell. Mol. Neurobiol. 1991, 11, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Ornstein, L. Disc electrophoresis-i background and theory. Ann. N. Y. Acad. Sci. 1964, 121, 321–349. [Google Scholar] [CrossRef] [PubMed]
- Hořejší, V. Some Theoretical Aspects of Affinity Electrophoresis. J. Chromatogr. A 1979, 178, 1–13. [Google Scholar] [CrossRef]
- Hor̄ejši, V.; Datta, T.K.; Tichá, M. Affinity Electrophoresis in an Isotachophoretic Discontinuous Buffer System. J. Chromatogr. A 1982, 241, 395–398. [Google Scholar] [CrossRef]
- Ek, K.; Gianazza, E.; Righetti, P.G. Affinity Titration Curves Determination of Dissociation Constants of Lectin-Sugar Complexes and of Their PH-Dependence by Isoelectric Focusing Electrophoresis. Biochim. Biophys. Acta Protein Struct. 1980, 626, 356–365. [Google Scholar] [CrossRef]
- Righetti, P.G.; Chillemi, F. Isoelectric Focusing of Peptides. J. Chromatogr. A 1978, 157, 243–251. [Google Scholar] [CrossRef]
- Shimura, K.; Kasai, K. Affinophoresis of Trypsins. J. Biochem. 1982, 92, 1615–1622. [Google Scholar] [CrossRef]
- Akuta, T.; Ura, T.; Oikawa, T.; Tomioka, Y.; Eguchi, A.; Arakawa, T. Effects of Sodium Dodecyl Sulfate, Sarkosyl and Sodium Lauroyl Glutamate on the Structure of Proteins Monitored by Agarose Native Gel Electrophoresis and Circular Dichroism. Biophys. Chem. 2024, 314, 107316. [Google Scholar] [CrossRef]
- Hořejši, V.; Tichá, M.; Kocourek, J. Studies on Lectins XXXI. Determination of Dissociation Constants of Lectin Sugar Complexes by Means of Affinity Electrophoresis. Biochim. Biophys. Acta Gen. Subj. 1977, 499, 290–300. [Google Scholar] [CrossRef]
- Hořejší, V. Separation of Ricinus Communis Lectins by Affinity Chromatography. J. Chromatogr. A 1979, 169, 457–458. [Google Scholar] [CrossRef]
- Hořejší, V.; Tichá, M. Theory of Affinity Electrophoresis. J. Chromatogr. A 1981, 216, 43–62. [Google Scholar] [CrossRef]
- Takeo, K.; Fujimoto, M.; Kuwahara, A.; Suzuno, R.; Nakamura, K. Calculation of the Thermodynamic Constants of Concanavalin A-Carbohydrate Interactions by Means of Affinity Electrophoresis. In Electrophoresis ’81: Advanced Methods, Biochemical and Clinical Applications: Proceedings of the Third International Conference on Electrophoresis; Allen, R.C., Allen, R.C., Arnaud, P., Eds.; Walter de Gruyter GmbH: Berlin, Germany, 1981; pp. 33–40. [Google Scholar]
- Takeo, K. Advances in Affinity Electrophoresis. J. Chromatogr. A 1995, 698, 89–105. [Google Scholar] [CrossRef]
- Masson, P.; Gouet, P.; Clery, C. Pressure and Propylene Carbonate Denaturation of Native and “Aged” Phosphorylated Cholinesterase. J. Mol. Biol. 1994, 238, 466–478. [Google Scholar] [CrossRef]
- Masson, P.; Cléry, C.; Guerra, P.; Redslob, A.; Albaret, C.; Fortier, P.L. Hydration Change during the Aging of Phosphorylated Human Butyrylcholinesterase: Importance of Residues Aspartate-70 and Glutamate-197 in the Water Network as Probed by Hydrostatic and Osmotic Pressures. Biochem. J. 1999, 343 Pt 2, 361–369. [Google Scholar] [CrossRef]
- Masson, P.; Nachon, F.; Lockridge, O. Structural Approach to the Aging of Phosphylated Cholinesterases. Chem. Biol. Interact. 2010, 187, 157–162. [Google Scholar] [CrossRef]
- Masson, P. Electrophoresis of Proteins under High Hydrostatic Pressure. In High-Pressure Techniques in Chemistry and Physics; Holzapfel, W.H., Isaacs, N.S., Eds.; Oxford University Press: New York, NY, USA, 1997; pp. 353–373. ISBN 0-19-855811-2. [Google Scholar]
- Morild, E. The Theory of Pressure Effects on Enzymes. Adv. Protein Chem. 1981, 34, 93–166. [Google Scholar]
- Mozhaev, V.V.; Heremans, K.; Frank, J.; Masson, P.; Balny, C. High Pressure Effects on Protein Structure and Function. Proteins Struct. Funct. Genet. 1996, 24, 81–91. [Google Scholar] [CrossRef]
- Masson, P.; Arciero, D.M.; Hooper, A.B.; Balny, C. Electrophoresis at Elevated Hydrostatic Pressure of the Multiheme Hydroxylamine Oxidoreductase. Electrophoresis 1990, 11, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal Structure of Human Butyrylcholinesterase and of Its Complexes with Substrate and Products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Balny, C. Conformational Plasticity of Butyrylcholinesterase as Revealed by High Pressure Experiments. Biochim. Biophys. Acta—Protein Struct. Mol. Enzymol. 1990, 1041, 223–231. [Google Scholar] [CrossRef]
- Hauzer, K.; Tichá, M.; Hořejší, V.; Kocourek, J. Studies on Lectins XLIV. The PH Dependence of Lectin Interactions with Sugars as Determined by Affinity Electrophoresis. Biochim. Biophys. Acta—Gen. Subj. 1979, 583, 103–109. [Google Scholar] [CrossRef]
- Maurer, H.R.; Allen, R.C. Useful Buffer and Gel Systems for Polyacrylamide Gel Electrophoresis. Clin. Chem. Lab. Med. 1972, 10, 220–225. [Google Scholar] [CrossRef][Green Version]
- McLellan, T. Electrophoresis Buffers for Polyacrylamide Gels at Various PH. Anal. Biochem. 1982, 126, 94–99. [Google Scholar] [CrossRef]
- Bahga, S.S.; Bercovici, M.; Santiago, J.G. Ionic Strength Effects on Electrophoretic Focusing and Separations. Electrophoresis 2010, 31, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Allison, S.A.; Cottet, H. Extracting Information from the Ionic Strength Dependence of Electrophoretic Mobility by Use of the Slope Plot. Anal. Chem. 2012, 84, 9422–9430. [Google Scholar] [CrossRef]
- Turkova, R.; Ticha, M.; Kocourek, J. Studies on Lectins. J. Chromatogr. A 1980, 192, 408–412. [Google Scholar] [CrossRef]
- Radić, Z.; Kirchhoff, P.D.; Quinn, D.M.; McCammon, J.A.; Taylor, P. Electrostatic Influence on the Kinetics of Ligand Binding to Acetylcholinesterase. J. Biol. Chem. 1997, 272, 23265–23277. [Google Scholar] [CrossRef]
- Kuo, S.-M.; Yang, P.-K. Factors Altering the Affinity of Protein–Ligand Binding in an External Electrostatic Field. Bioelectrochemistry 2015, 104, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bekard, I.; Dunstan, D.E. Electric Field Induced Changes in Protein Conformation. Soft Matter 2014, 10, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-H.; Avila, L.Z.; Gao, J.; Whitesides, G.M. Affinity Capillary Electrophoresis. Acc. Chem. Res. 1995, 28, 461–468. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishio, I.; Sun, S.-T.; Ueno-Nishio, S. Collapse of Gels in an Electric Field. Science 1982, 218, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Matoušek, V.; Hořesjši, V. Affinity Electrophoresis: A Theoretical Study of the Effects of the Kinetics of Protein—Ligand Complex Formation and Dissociation Reactions. J. Chromatogr. A 1982, 245, 271–290. [Google Scholar] [CrossRef]
- Smith, S.S.; Gilroy, T.E.; Ferrari, F.A. The Influence of Agarose-DNA Affinity on the Electrophoretic Separation of DNA Fragments in Agarose Gels. Anal. Biochem. 1983, 128, 138–151. [Google Scholar] [CrossRef]
- Masson, P.; Marnot, B. Électrophorèse d’affinité En Gel de Polyacrylamide. J. Chromatogr. A 1985, 328, 135–144. [Google Scholar] [CrossRef]
- Rodbard, D.; Chrambach, A. Unified Theory for Gel Electrophoresis and Gel Filtration. Proc. Natl. Acad. Sci. USA 1970, 65, 970–977. [Google Scholar] [CrossRef]
- Smerkolj, J.; Stojan, J.; Bavec, A.; Goličnik, M. Substrate-Dependent Inactivation of Recombinant Paraoxonase 1 during Catalytic Dihydrocoumarin Turnover and the Protective Properties of Surfactants. Chem. Biol. Interact. 2023, 382, 110563. [Google Scholar] [CrossRef]
- Goliĉnik, M.; Stojan, J. Slow-binding Inhibition: A Theoretical and Practical Course for Students. Biochem. Mol. Biol. Educ. 2004, 32, 228–235. [Google Scholar] [CrossRef]
- Denizot, F.C.; Delaage, M.A. Statistical Theory of Chromatography: New Outlooks for Affinity Chromatography. Proc. Natl. Acad. Sci. USA 1975, 72, 4840–4843. [Google Scholar] [CrossRef]
- Masson, P. Time-Dependent Kinetic Complexities in Cholinesterase-Catalyzed Reactions. Biochemistry 2012, 77, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Shaihutdinova, Z.; Masson, P. Pre-Steady-State and Steady-State Kinetic Analysis of Butyrylcholinesterase-Catalyzed Hydrolysis of Mirabegron, an Arylacylamide Drug. Molecules 2024, 29, 2356. [Google Scholar] [CrossRef]
- Frieden, C. Slow Transitions and Hysteretic Behavior in Enzymes. Annu. Rev. Biochem. 1979, 48, 471–489. [Google Scholar] [CrossRef]
- Masson, P.; Lushchekina, S.V. Slow-Binding Inhibition of Cholinesterases, Pharmacological and Toxicological Relevance. Arch. Biochem. Biophys. 2016, 593, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Chiantore, O.; Trossarelli, L.; Guaita, M. On the Conformational Characteristics of Acrylamide Polymers. Die Makromol. Chem. 1982, 183, 2257–2263. [Google Scholar] [CrossRef]
- Tichá, M.; Barthová, J.; Labský, J.; Semanský, M. Determination of the Interaction of Lactate Dehydrogenase with High-Molecular-Weight Derivatives of Amp by Affinity Electrophoresis. J. Chromatogr. A 1980, 194, 183–189. [Google Scholar] [CrossRef]
- Gelfi, C.; Righetti, P.G. Polymerization Kinetics of Polyacrylamide Gels I. Effect of Different Cross-linkers. Electrophoresis 1981, 2, 213–219. [Google Scholar] [CrossRef]
- Adinolfi, A.; Hopkinson, D.A. Affinity Electrophoresis of Human Alcohol Dehydrogenase (ADH) Isozymes. Ann. Hum. Genet. 1979, 43, 109–119. [Google Scholar] [CrossRef]
- Somani, B.; Ambade, V.; Arora, M. Polyacrylamide Gel Affinity Electrophoresis for Separation of Enzyme Isoforms. Med. J. Armed Forces India 2003, 59, 125–127. [Google Scholar] [CrossRef]
- Souza, R.L.R.; Furtado, L.; Diniz, A.C.P.; Silva, A.C.D.; Kaiss, J.; Petzl-Erler, M.L.; Chautard-Freire-Maia, E.A. Studies on a Heterologous Complex Formed by Human Butyrylcholinesterase. Biochem. Genet. 2003, 41, 141–150. [Google Scholar] [CrossRef]
- Masson, P. A Naturally Occurring Molecular Form of Human Plasma Cholinesterase Is an Albumin Conjugate. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1989, 998, 258–266. [Google Scholar] [CrossRef]
- Masson, P.; Froment, M.T.; Audras, J.C.; Renault, F. Molecular Characterization of the C5 Human Plasma Cholinesterase Variant. In Cholinesterases Structure, Function, Mechanism, Genetics, and Cell Biology; Massoulie, J., Bacou, F., Barnard, E., Chatonnet, A., Doctor, B.P., Quinn, D.M., Eds.; American Chemical Society: Washington, DC, USA, 1991; ISBN 0841220085/9780841220089. [Google Scholar]
- Schopfer, L.; Delacour, H.; Masson, P.; Leroy, J.; Krejci, E.; Lockridge, O. The C5 Variant of the Butyrylcholinesterase Tetramer Includes a Noncovalently Bound 60 KDa Lamellipodin Fragment. Molecules 2017, 22, 1083. [Google Scholar] [CrossRef] [PubMed]
- Colton, I.J.; Carbeck, J.D.; Rao, J.; Whitesides, G.M. Affinity Capillary Electrophoresis: A Physical-organic Tool for Studying Interactions in Biomolecular Recognition. Electrophoresis 1998, 19, 367–382. [Google Scholar] [CrossRef]
- Štěpánová, S.; Kašička, V. Application of Capillary Electromigration Methods for Physicochemical Measurements. In Capillary Electromigration Separation Methods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 547–591. [Google Scholar]
- Štěpánová, S.; Kašička, V. Determination of Physicochemical Parameters of (Bio)Molecules and (Bio)Particles by Capillary Electromigration Methods. J. Sep. Sci. 2024, 47, e2400174. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.A.; Avila, L.Z.; Chu, Y.-H.; Whitesides, G.M. Determination of Binding Constants of Ligands to Proteins by Affinity Capillary Electrophoresis: Compensation for Electroosmotic Flow. Anal. Chem. 1994, 66, 1785–1791. [Google Scholar] [CrossRef]
- Winzor, D.J. Measurement of Binding Constants by Capillary Electrophoresis. J. Chromatogr. A 1995, 696, 160–163. [Google Scholar] [CrossRef]
- Dubský, P.; Dvořák, M.; Ansorge, M. Affinity Capillary Electrophoresis: The Theory of Electromigration. Anal. Bioanal. Chem. 2016, 408, 8623–8641. [Google Scholar] [CrossRef]
- Kim, H.S.; Austin, J.; Hage, D.S. Identification of Drug-Binding Sites on Human Serum Albumin Using Affinity Capillary Electrophoresis and Chemically Modified Proteins as Buffer Additives. Electrophoresis 2002, 23, 956–963. [Google Scholar] [CrossRef]
- Tanaka, Y.; Terabe, S. Estimation of Binding Constants by Capillary Electrophoresis. J. Chromatogr. B 2002, 768, 81–92. [Google Scholar] [CrossRef]
- Newman, C.I.D.; Collins, G.E. Advances in CE for Kinetic Studies. Electrophoresis 2008, 29, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Gstöttner, C.; Knaupp, A.; Vidarsson, G.; Reusch, D.; Schlothauer, T.; Wuhrer, M.; Domínguez-Vega, E. Affinity Capillary Electrophoresis—Mass Spectrometry Permits Direct Binding Assessment of IgG and FcγRIIa in a Glycoform-Resolved Manner. Front. Immunol. 2022, 13, 980291. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Klag, M.; Kózka, G.; Gołąb, M.; Woźniakiewicz, M. The First Online Capillary Electrophoresis-Microscale Thermophoresis (CE-MST) Method for the Analysis of Dynamic Equilibria—The Determination of the Acidity Constant of Fluorescein Isothiocyanate. Molecules 2022, 27, 5010. [Google Scholar] [CrossRef] [PubMed]
- Rochu, D.; Masson, P. Multiple Advantages of Capillary Zone Electrophoresis for Exploring Protein Conformational Stability. Electrophoresis 2002, 23, 189–202. [Google Scholar] [CrossRef]
- Bastos, M.; Abian, O.; Johnson, C.M.; Ferreira-da-Silva, F.; Vega, S.; Jimenez-Alesanco, A.; Ortega-Alarcon, D.; Velazquez-Campoy, A. Isothermal Titration Calorimetry. Nat. Rev. Methods Prim. 2023, 3, 17. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, Y. Comparative Analysis of the Techniques for the Determination of Binding Affinity between a Small Molecule Inhibitor and a Protein Target. bioRxiv 2024. [Google Scholar] [CrossRef]
- Sekyonda, Z.; An, R.; Avanaki, A.; Fraiwan, A.; Gurkan, U.A. A Novel Approach for Glycosylated Hemoglobin Testing Using Microchip Affinity Electrophoresis. IEEE Trans. Biomed. Eng. 2023, 70, 1473–1480. [Google Scholar] [CrossRef]
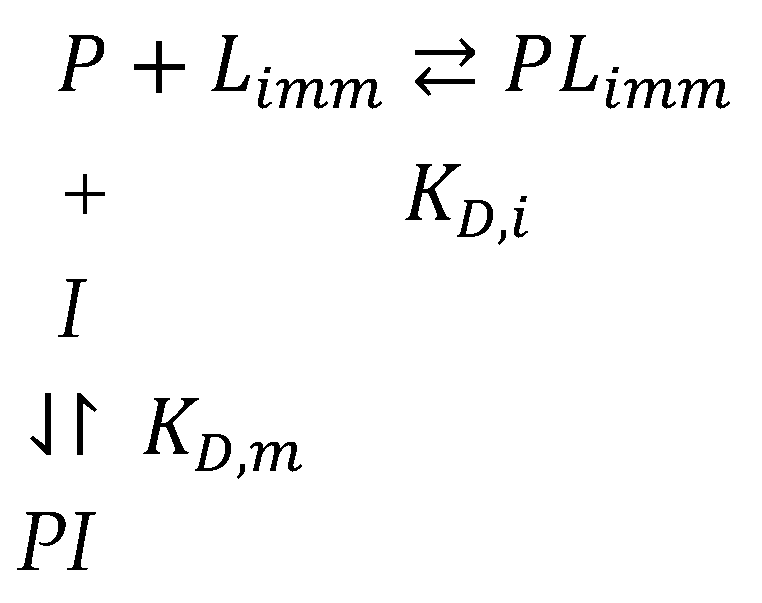
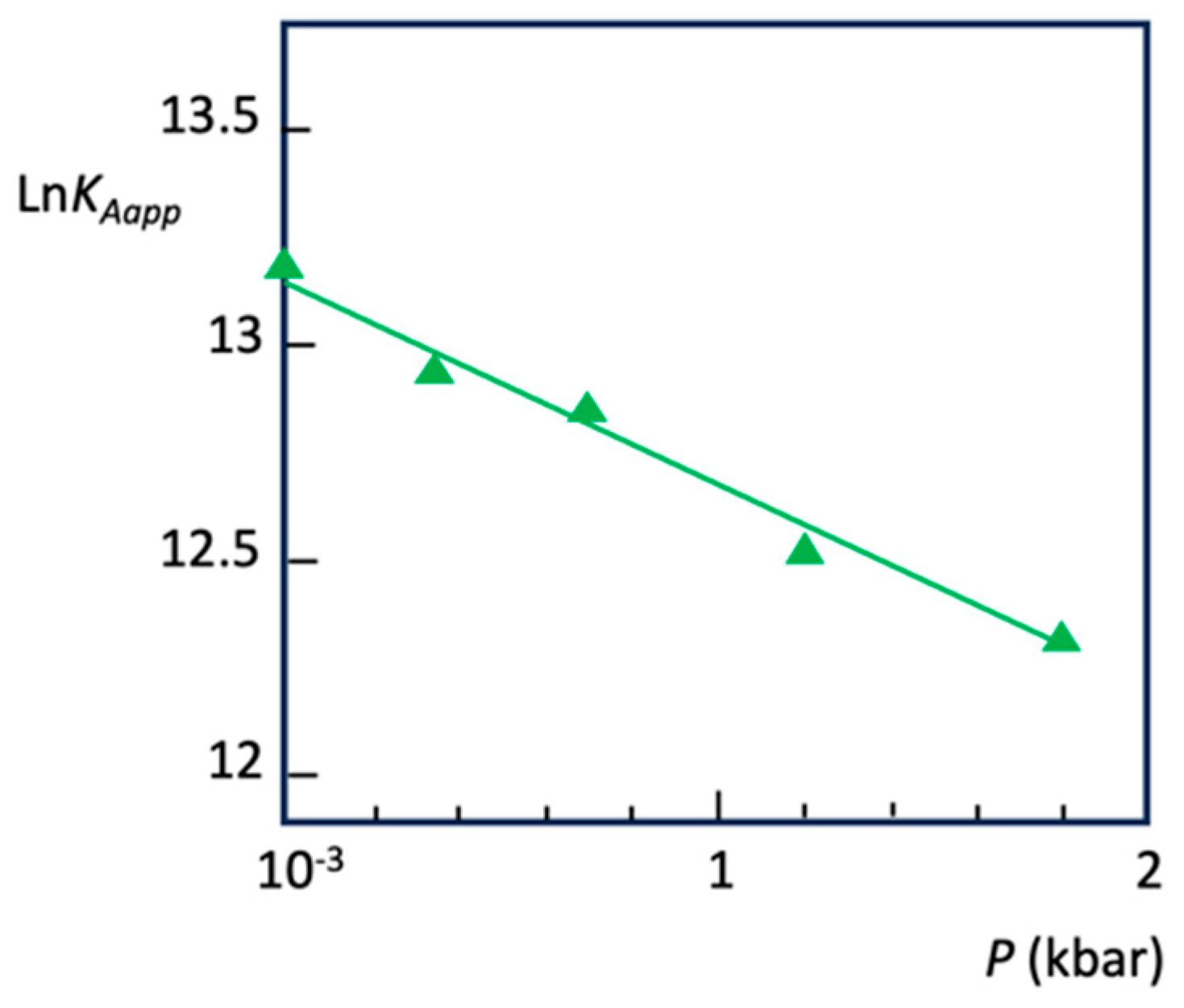
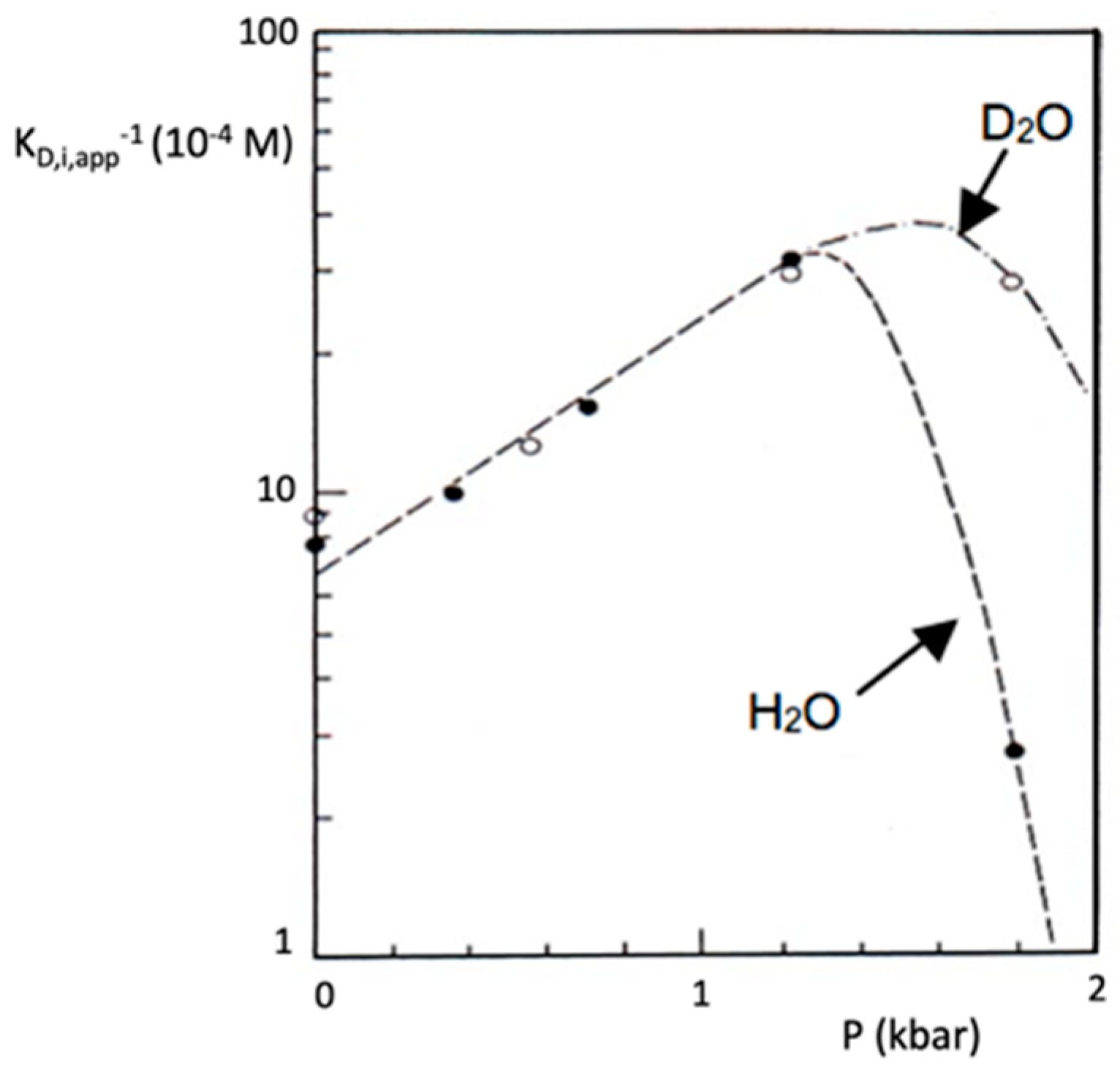
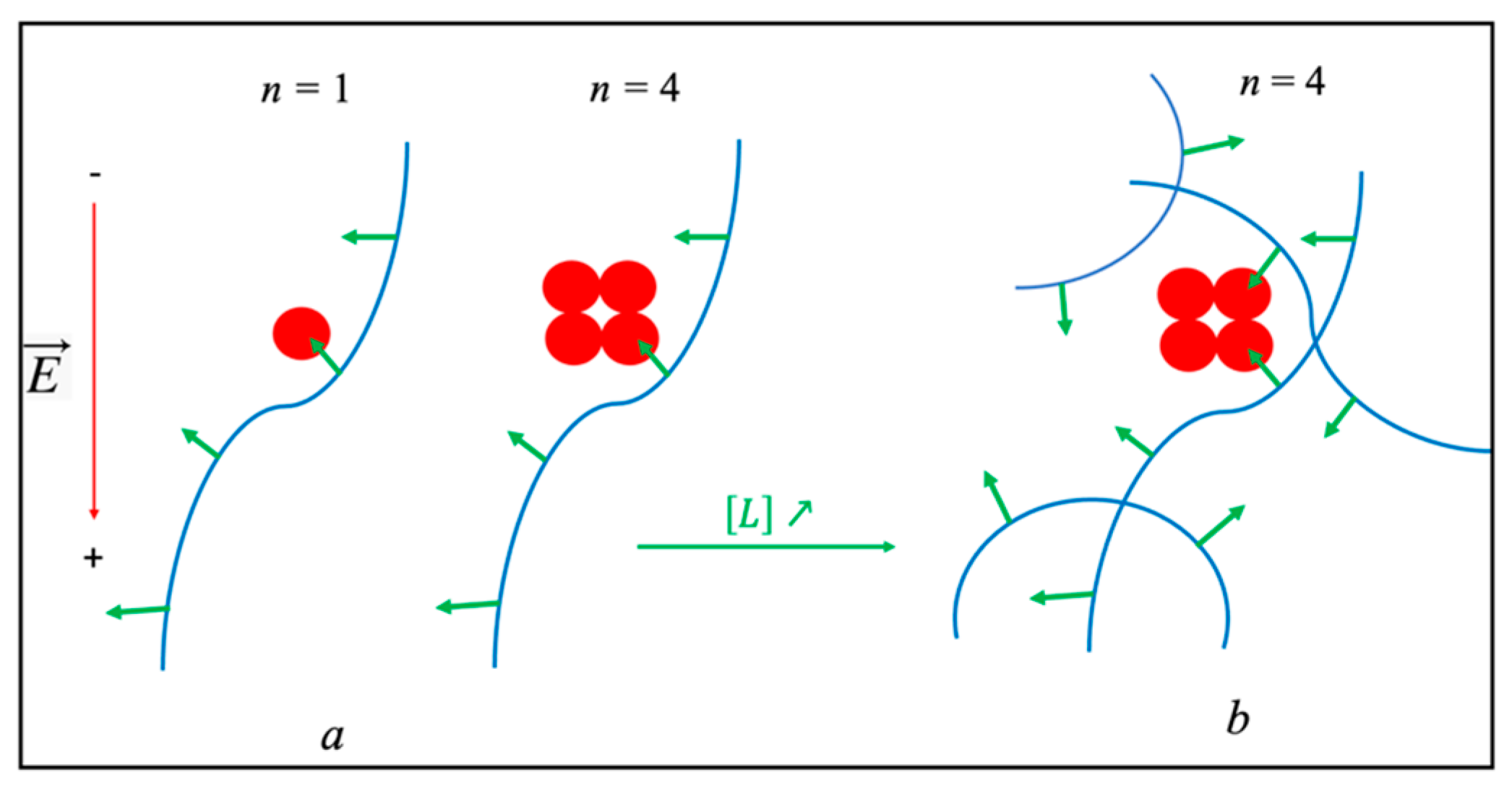
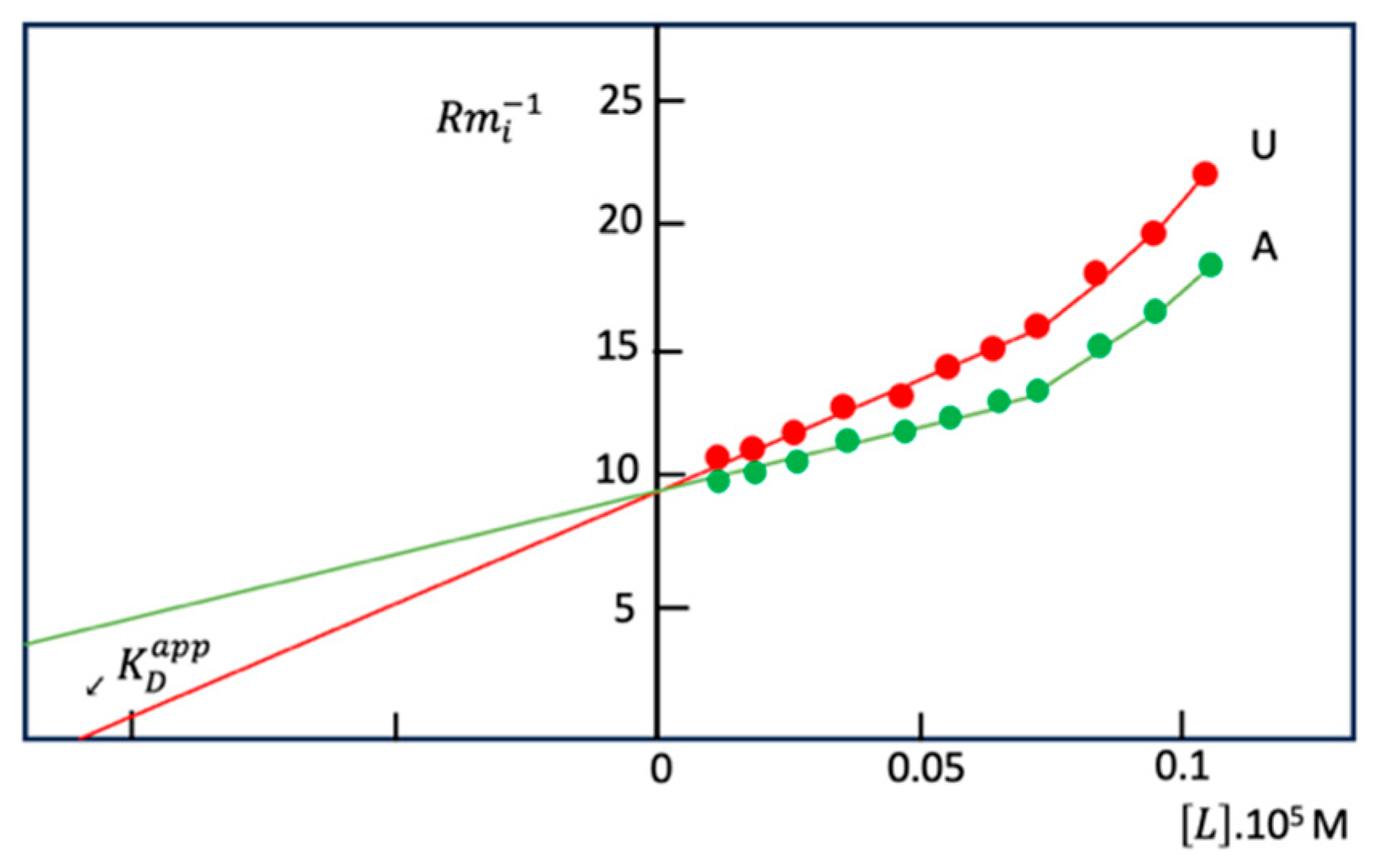



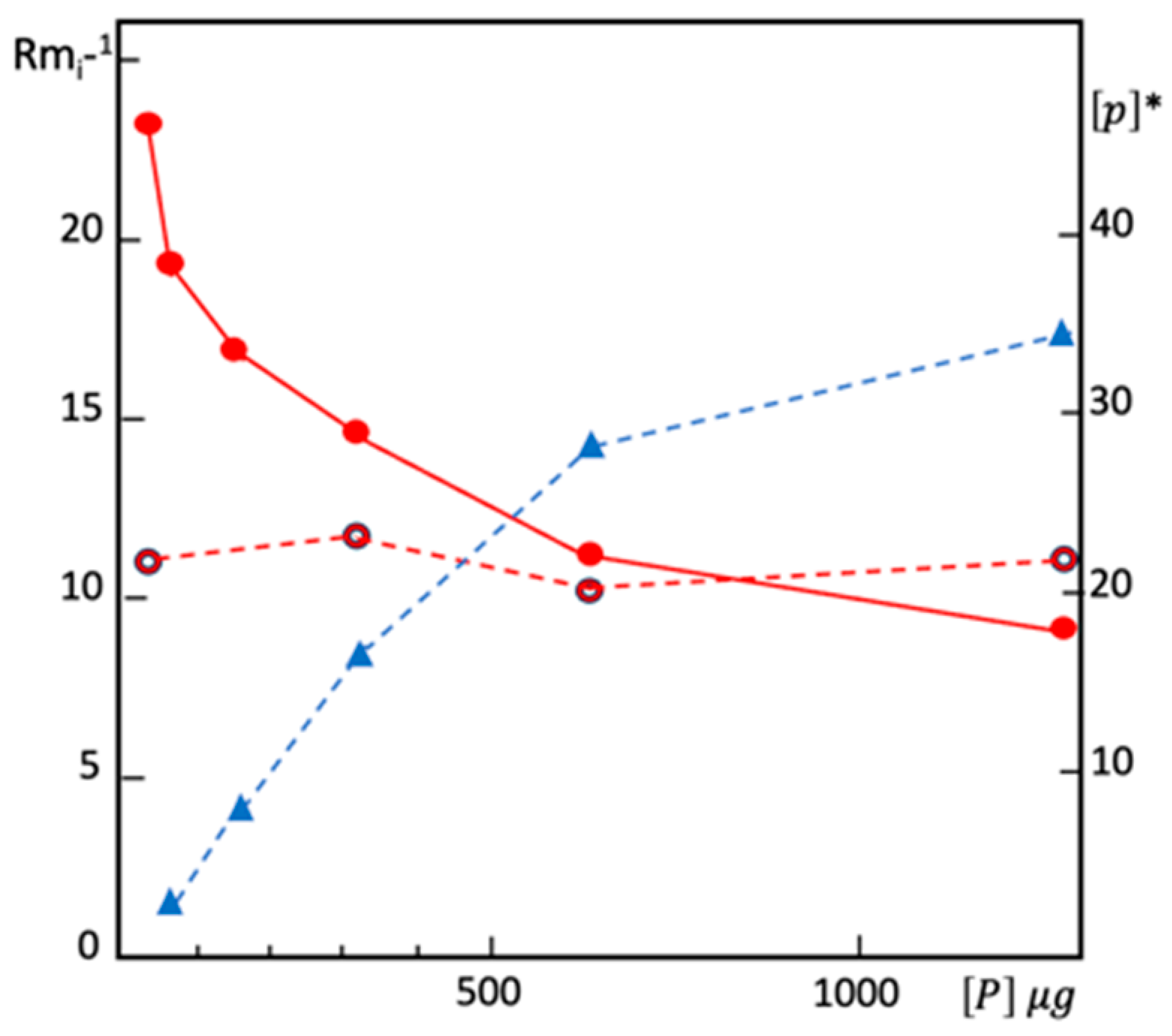
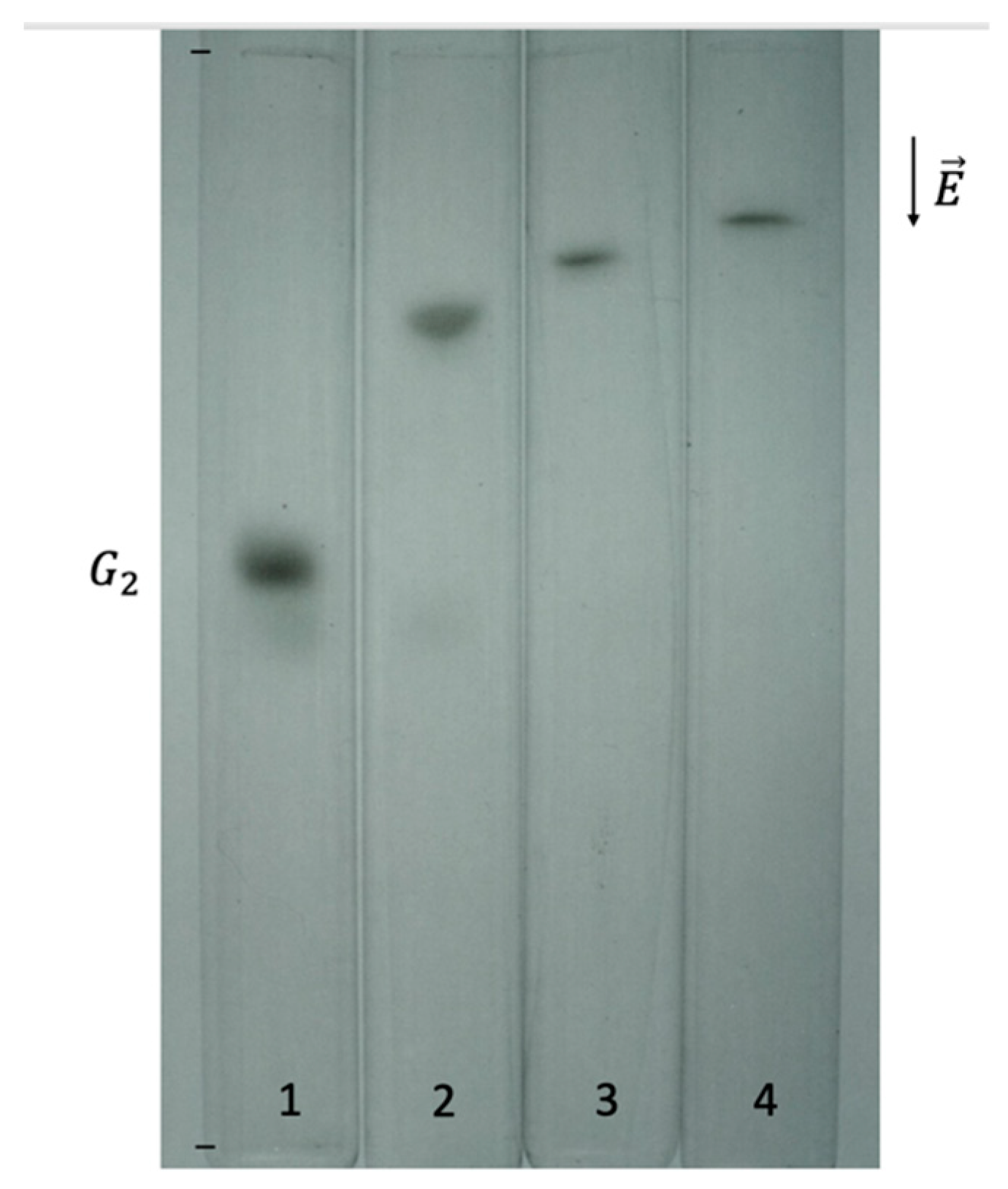
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masson, P.; Pashirova, T. Affinity Electrophoresis of Proteins for Determination of Ligand Affinity and Exploration of Binding Sites. Int. J. Mol. Sci. 2025, 26, 3409. https://doi.org/10.3390/ijms26073409
Masson P, Pashirova T. Affinity Electrophoresis of Proteins for Determination of Ligand Affinity and Exploration of Binding Sites. International Journal of Molecular Sciences. 2025; 26(7):3409. https://doi.org/10.3390/ijms26073409
Chicago/Turabian StyleMasson, Patrick, and Tatiana Pashirova. 2025. "Affinity Electrophoresis of Proteins for Determination of Ligand Affinity and Exploration of Binding Sites" International Journal of Molecular Sciences 26, no. 7: 3409. https://doi.org/10.3390/ijms26073409
APA StyleMasson, P., & Pashirova, T. (2025). Affinity Electrophoresis of Proteins for Determination of Ligand Affinity and Exploration of Binding Sites. International Journal of Molecular Sciences, 26(7), 3409. https://doi.org/10.3390/ijms26073409






