Hyperthermophilic L-Asparaginase from Thermococcus sibiricus and Its Double Mutant with Increased Activity: Insights into Substrate Specificity and Structure
Abstract
1. Introduction
2. Results
2.1. Comparison of the Main Enzymatic Properties of TsAI Forms
2.1.1. Substrate Specificity
2.1.2. Kinetic Characteristics
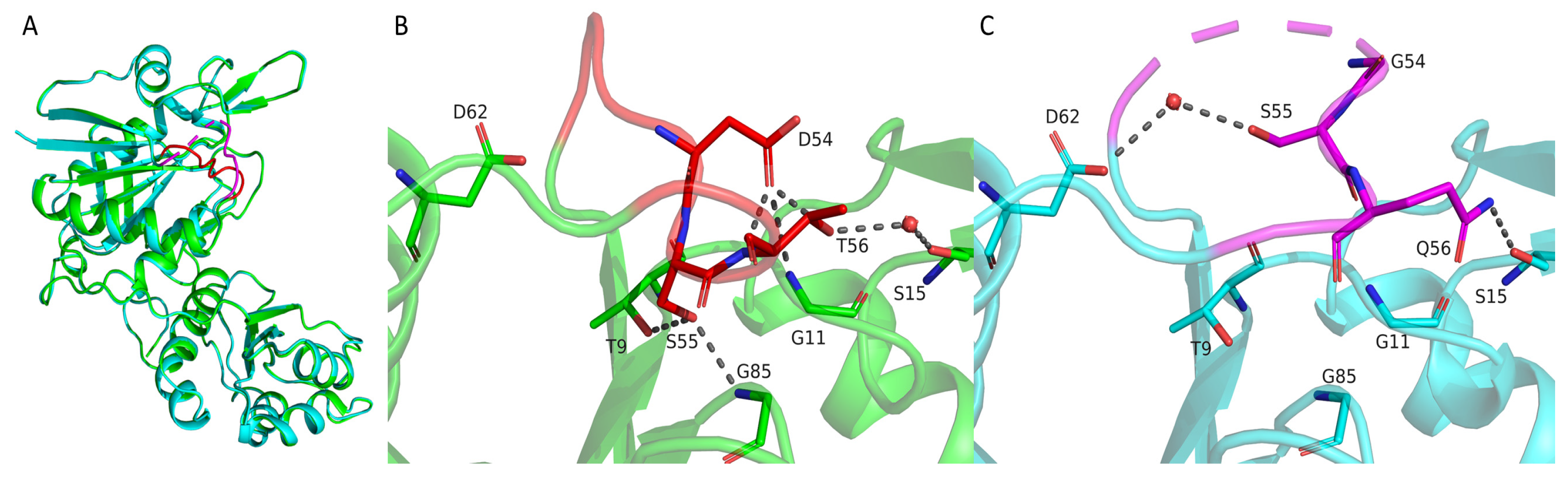
2.2. Overall Crystal Structure of the Wild-Type TsAI and Structural Comparison with Type I and Type II L-Asparaginases
2.3. Structural Differences Between Wild-Type TsAI and Mutant TsAID54G/T56Q That Affect Their Activity and Stability
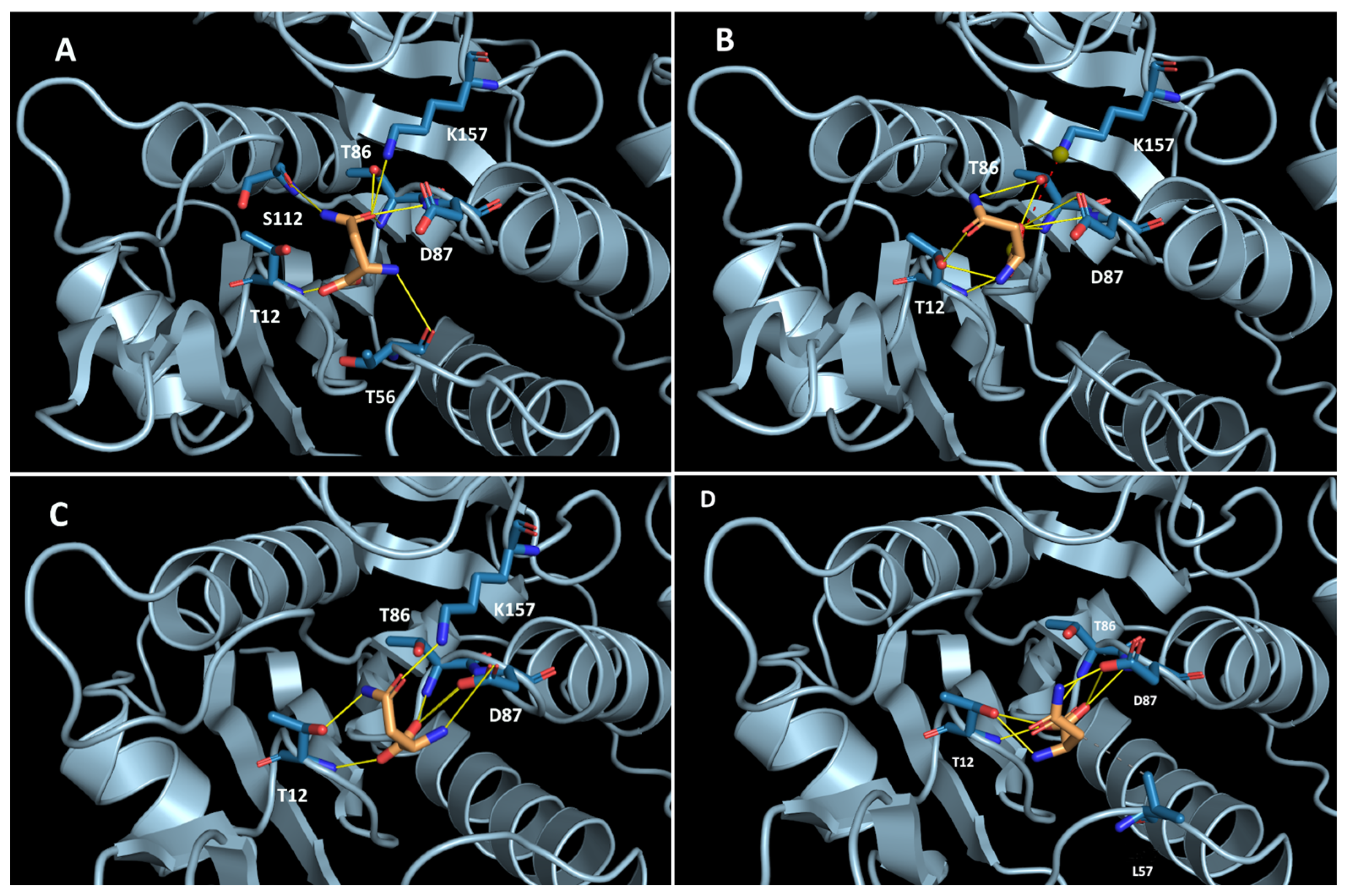
2.4. Elucidation of Substrate-Enzyme Interaction and Stereoselectivity of TsAI Forms Using Molecular Docking Analysis
3. Discussion
3.1. Engineering of Improved L-ASNase Variants Using Nature-Inspired Mutagenesis
3.2. The Flexibility of the Loop M51-L57 (TsAI) Is Essential for Thermo-ASNases Activity
3.3. Thermo-ASNase Features in Substrate Specificity: Biological Significance and Practical Importance
3.4. The Potential Implications of Research Outcomes
4. Materials and Methods
4.1. Protein Expression and Purification
4.2. Measurement of Enzyme Activity and Kinetic Parameters
4.3. Crystallization, Data Collection, and Structure Refinement
4.4. Molecular Docking
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vimal, A.; Kumar, A. Biotechnological Production and Practical Application of L-Asparaginase Enzyme. Biotechnol. Genet. Eng. Rev. 2017, 33, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.; Taurino, G.; Bianchi, M.G.; Kilberg, M.S.; Bussolati, O. Asparagine Synthetase in Cancer: Beyond Acute Lymphoblastic Leukemia. Front. Oncol. 2020, 9, 1480. [Google Scholar] [CrossRef]
- Shrivastava, A.; Khan, A.A.; Khurshid, M.; Kalam, M.A.; Jain, S.K.; Singhal, P.K. Recent Developments in L-Asparaginase Discovery and Its Potential as Anticancer Agent. Crit. Rev. Oncol. Hematol. 2016, 100, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dumina, M.V.; Eldarov, M.A.; Zdanov, D.D.; Sokolov, N.N. L-Asparaginases of Extremophilic Microorganisms in Biomedicine. Biochem. Suppl. Ser. B Biomed. Chem. 2020, 14, 277–296. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Deng, P.; He, Z.; Qin, F.; Chen, Q.; Wang, Z.; Pan, H.; Chen, J.; Zeng, M. Research Progress on Generation, Detection and Inhibition of Multiple Hazards—Acrylamide, 5-Hydroxymethylfurfural, Advanced Glycation End Products, Methylimidazole—In Baked Goods. Food Chem. 2024, 431, 137152. [Google Scholar] [CrossRef]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.C.; Riediker, S. Acrylamide from Maillard Reaction Products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef]
- Sajed, M.; un Naeem, S.; Rashid, N. L-Asparaginases from Hyperthermophilic Archaea and Their Applications. In Microbial Extremozymes; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Dumina, M.; Zhgun, A. Thermo-L-Asparaginases: From the Role in the Viability of Thermophiles and Hyperthermophiles at High Temperatures to a Molecular Understanding of Their Thermoactivity and Thermostability. Int. J. Mol. Sci. 2023, 24, 2674. [Google Scholar] [CrossRef]
- Dumina, M.; Zhgun, A.; Pokrovskaya, M.; Aleksandrova, S.; Zhdanov, D.; Sokolov, N.; El’darov, M. A Novel L-asparaginase from Hyperthermophilic Archaeon Thermococcus Sibiricus: Heterologous Expression and Characterization for Biotechnology Application. Int. J. Mol. Sci. 2021, 22, 9894. [Google Scholar] [CrossRef]
- Dumina, M.; Zhdanov, D.; Zhgun, A.; Pokrovskaya, M.; Aleksandrova, S.; Veselovsky, A.; El’darov, M. Enhancing the Catalytic Activity of Thermo-Asparaginase from Thermococcus Sibiricus by a Double Mesophilic-like Mutation in the Substrate-Binding Region. Int. J. Mol. Sci. 2023, 24, 9632. [Google Scholar] [CrossRef]
- Swain, A.L.; Jaskolski, M.; Housset, D.; Rao, J.K.; Wlodawer, A. Crystal Structure of Escherichia Coli L-Asparaginase, an Enzyme Used in Cancer Therapy. Proc. Natl. Acad. Sci. USA 1993, 90, 1474–1478. [Google Scholar] [CrossRef]
- Palm, G.J.; Lubkowski, J.; Derst, C.; Schleper, S.; Röhm, K.H.; Wlodawer, A. A Covalently Bound Catalytic Intermediate in Escherichia Coli Asparaginase: Crystal Structure of a Thr-89-Val Mutant. FEBS Lett. 1996, 390, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Maggi, M.; Meli, M.; Colombo, G.; Scotti, C. Revealing Escherichia Coli Type II L-Asparaginase Active Site Flexible Loop in Its Open, Ligand-Free Conformation. Sci. Rep. 2021, 11, 18885. [Google Scholar] [CrossRef] [PubMed]
- Loch, J.I.; Jaskolski, M. Structural and Biophysical Aspects of L-Asparaginases: A Growing Family with Amazing Diversity. IUCrJ 2021, 8, 514–531. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Yasutake, Y.; Morita, H.; Tanaka, I. Structure of the Type I L-Asparaginase from the Hyperthermophilic Archaeon Pyrococcus Horikoshii at 2.16 Å Resolution. Biol. Crystallogr. 2005, 61, 294–301. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Xu, S.; Xu, M.; Yang, T.; Wang, L.; Zhang, H.; Fang, H.; Osire, T.; Rao, Z. Insight into the Thermostability of Thermophilic L-Asparaginase and Non-Thermophilic L-Asparaginase II through Bioinformatics and Structural Analysis. Appl. Microbiol. Biotechnol. 2019, 103, 7055–7070. [Google Scholar] [CrossRef]
- Borek, D.; Kozak, M.; Pei, J.; Jaskolski, M. Crystal Structure of Active Site Mutant of Antileukemic L-Asparaginase Reveals Conserved Zinc-Binding Site. FEBS J. 2014, 281, 4097–4111. [Google Scholar] [CrossRef]
- Howard, J.B.; Carpenter, F.H. L-Asparaginase from Erwinia Carotovora. Substrate Specificity and Enzymatic Properties. J. Biol. Chem. 1972, 247, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Citri, N.; Zvk, N. Stereospecificity of the Catalytic Reaction of L-Asparaginase. Biochemistry 1972, 11, 2103–2109. [Google Scholar] [CrossRef]
- Husain, I.; Sharma, A.; Kumar, S.; Malik, F. Purification and Characterization of Glutaminase Free Asparaginase from Pseudomonas Otitidis: Induce Apoptosis in Human Leukemia MOLT-4 Cells. Biochimie 2016, 121, 38–51. [Google Scholar] [CrossRef]
- Chohan, S.M.; Rashid, N. TK1656, a Thermostable l-Asparaginase from Thermococcus Kodakaraensis, Exhibiting Highest Ever Reported Enzyme Activity. J. Biosci. Bioeng. 2013, 116, 438–443. [Google Scholar] [CrossRef]
- Guo, J.; Coker, A.R.; Wood, S.P.; Cooper, J.B.; Chohan, S.M.; Rashid, N.; Akhtar, M. Structure and Function of the Thermostable l-Asparaginase from Thermococcus Kodakarensis. Acta Crystallogr. Sect. D Struct. Biol. 2017, 73, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Tomar, R.; Sharma, P.; Srivastava, A.; Bansal, S.; Ashish; Kundu, B. Structural and Functional Insights into an Archaeal L-Asparaginase Obtained through the Linker-Less Assembly of Constituent Domains. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014, 70, 3187–3197. [Google Scholar] [CrossRef]
- Yun, M.K.; Nourse, A.; White, S.W.; Rock, C.O.; Heath, R.J. Crystal Structure and Allosteric Regulation of the Cytoplasmic Escherichia Coli L-Asparaginase I. J. Mol. Biol. 2007, 369, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Wu, L.Y.; Lu, C.; Pan, X.M. Determinants of Thermostability in Serine Hydroxymethyltransferase Identified by Principal Component Analysis. Sci. Rep. 2017, 7, 46463. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tomar, R.; Yadav, S.S.; Badmalia, M.D.; Nath, S.K.; Ashish; Kundu, B. Heat Induces End to End Repetitive Association in P. Furiosus l-Asparaginase Which Enables Its Thermophilic Property. Sci. Rep. 2020, 10, 21702. [Google Scholar] [CrossRef]
- Bansal, S.; Srivastava, A.; Mukherjee, G.; Pandey, R.; Verma, A.K.; Mishra, P.; Kundu, B. Hyperthermophilic Asparaginase Mutants with Enhanced Substrate Affinity and Antineoplastic Activity: Structural Insights on Their Mechanism of Action. FASEB J. 2012, 26, 1161–1171. [Google Scholar] [CrossRef]
- Sania, A.; Muhammad, M.A.; Sajed, M.; Ahmad, N.; Aslam, M.; Tang, X.-F.; Rashid, N. Engineering Tk1656, a Highly Active l-Asparaginase from Thermococcus Kodakarensis, for Enhanced Activity and Stability. Int. J. Biol. Macromol. 2024, 281, 136442. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Xu, S.; Zhang, H.; Xu, M.; Yang, T.; Wang, L.; Qian, H.; Zhang, H.; Fang, H.; et al. Simultaneous Cell Disruption and Semi-Quantitative Activity Assays for High-Throughput Screening of Thermostable L-Asparaginases. Sci. Rep. 2018, 8, 7915. [Google Scholar] [CrossRef]
- Long, S.; Zhang, X.; Rao, Z.; Chen, K.; Xu, M.; Yang, T.; Yang, S. Amino Acid Residues Adjacent to the Catalytic Cavity of Tetramer L-Asparaginase II Contribute Significantly to Its Catalytic Efficiency and Thermostability. Enzym. Microb. Technol. 2016, 82, 15–22. [Google Scholar] [CrossRef]
- Ran, T.; Jiao, L.; Wang, W.; Chen, J.; Chi, H.; Lu, Z.; Zhang, C.; Xu, D.; Lu, F. Structures of l-Asparaginase from Bacillus Licheniformis Reveal an Essential Residue for Its Substrate Stereoselectivity. J. Agric. Food Chem. 2021, 69, 223–231. [Google Scholar] [CrossRef]
- Novak, E.K.; Phillips, A.W. L Glutamine as a Substrate for L Asparaginase from Serratia Marcescens. J. Bacteriol. 1974, 117, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Morikawa, Y.; Tanaka, M. L-Asparaginase from Escherichia Coli: Part II. Substrate Specificity Studies. Agric. Biol. Chem. 1971, 35, 743–751. [Google Scholar] [CrossRef]
- Anishkin, A.; Vanegas, J.M.; Rogers, D.M.; Lorenzi, P.L.; Chan, W.K.; Purwaha, P.; Weinstein, J.N.; Sukharev, S.; Rempe, S.B. Catalytic Role of the Substrate Defines Specificity of Therapeutic L-Asparaginase. J. Mol. Biol. 2015, 427, 2867–2885. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, N.; Sharma, M.; Kumar, P.; Singh, R.P. Biochemical and Molecular Insights on the Bioactivity and Binding Interactions of Bacillus Australimaris NJB19 L-Asparaginase. Int. J. Biol. Macromol. 2022, 215, 1–11. [Google Scholar] [CrossRef]
- Aghaiypour, K.; Wlodawer, A.; Lubkowski, J. Structural Basis for the Activity and Substrate Specificity of Erwinia Chrysanthemi L-Asparaginase. Biochemistry 2001, 40, 5655–5664. [Google Scholar] [CrossRef]
- Chi, H.; Chen, M.; Jiao, L.; Lu, Z.; Bie, X.; Zhao, H.; Lu, F. Characterization of a Novel L-Asparaginase from Mycobacterium Gordonae with Acrylamide Mitigation Potential. Foods 2021, 10, 2819. [Google Scholar] [CrossRef]
- Citri, N.; Kitron, N.; Zyk, N. Stereospecific Features of the Conformative Response of L-Asparaginase. Biochemistry 1972, 11, 2110–2116. [Google Scholar] [CrossRef]
- Dobryakova, N.; Zhdanov, D.; Dumina, M.; Aleksandrova, S.; Pokrovskaya, M.; Genin, A.; Shishparenok, A.; Zhgun, A.; Kudryashova, E.V. Thermal Inactivation Mechanism and Structural Features Providing Enhanced Thermal Stability of Hyperthermophilic Thermococcus Sibiricus L-Asparaginase in Comparison with Mesophilic and Thermophilic L-Asparaginases. Catalysts 2023, 13, 832. [Google Scholar] [CrossRef]
- Matsumoto, M.; Homma, H.; Long, Z.; Imai, K.; Iida, T.; Maruyama, T.; Aikawa, Y.; Endo, I.; Yohda, M. Occurrence of Free D-Amino Acids and Aspartate Racemases in Hyperthermophilic Archaea. J. Bacteriol. 1999, 181, 6560–6563. [Google Scholar] [CrossRef]
- Goodfriend, G.A. Rapid Racemization of Aspartic Acid in Mollusc Shells and Potential for Dating over Recent Centuries. Nature 1992, 357, 399–401. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, N.-H.; Teng, Z.-J.; Chen, Y.; Wang, P.; Zhang, Y.-Z.; Fu, H.-H.; Chen, X.-L.; Zhang, Y.-Q. Evidence for Archaeal Metabolism of D-Amino Acids in the Deep Marine Sediments. Sci. Total Environ. 2024, 948, 174723. [Google Scholar] [CrossRef] [PubMed]
- Sasabe, J.; Suzuki, M. Emerging Role of D-Amino Acid Metabolism in the Innate Defense. Front. Microbiol. 2018, 9, 933. [Google Scholar] [CrossRef] [PubMed]
- Warrell, R.P.; Arlin, Z.A.; Gee, T.S.; Chou, T.C.; Roberts, J.; Young, C.W. Clinical Evaluation of Succinylated Acinetobacter Glutaminase-Asparaginase in Adult Leukemia. Cancer Treat. Rep. 1982, 66, 1479–1485. [Google Scholar]
- Kafkewitz, D.; Bendich, A. Enzyme-Induced Asparagine and Glutamine Depletion and Immune System Function. Am. J. Clin. Nutr. 1983, 37, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.H.G.; da Silva Fiúza, T.; de Morais, S.B.; Trevizani, R. Circumventing the Side Effects of L-Asparaginase. Biomed. Pharmacother. 2021, 139, 111616. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Su, Y.; Zhang, J.Y.; Antanasijevic, A.; Caffrey, M.; Schalk, A.M.; Liu, L.; Rondelli, D.; Oh, A.; Mahmud, D.L.; et al. A Novel L-Asparaginase with Low L-Glutaminase Coactivity Is Highly Efficacious against Both T- and B-Cell Acute Lymphoblastic Leukemias In Vivo. Cancer Res. 2018, 78, 1549–1560. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Su, Y.; Lavie, A. Design and Characterization of Erwinia Chrysanthemi L-Asparaginase Variants with Diminished L-Glutaminase Activity. J. Biol. Chem. 2016, 291, 17664–17676. [Google Scholar] [CrossRef]
- Safary, A.; Moniri, R.; Hamzeh-Mivehroud, M.; Dastmalchi, S. Highly Efficient Novel Recombinant L-Asparaginase with No Glutaminase Activity from a New Halo-Thermotolerant Bacillus Strain. BioImpacts 2019, 9, 15. [Google Scholar] [CrossRef]
- Brumano, L.P.; da Silva, F.V.S.; Costa-Silva, T.A.; Apolinario, A.C.; Santos, J.H.P.M.; Kleingesinds, E.K.; Monteiro, G.; de Oliveira Rangel-Yagui, C.; Benyahia, B.; Junior, A.P. Development of L-Asparaginase Biobetters: Current Research Status and Review of the Desirable Quality Profiles. Front. Bioeng. Biotechnol. 2018, 6, 212. [Google Scholar] [CrossRef]
- Dumina, M.; Kalinin, S.; Zhdanov, D. Potential of Hyperthermophilic L-Asparaginase from Thermococcus Sibiricus to Mitigate Dietary Acrylamide Assessed Using a Simplified Food System. Foods 2025, 14, 1720. [Google Scholar] [CrossRef]
- Kalinin, S.G.; Zhdanov, D.D.; Dumina, M.V. Comparative Activity of L-Asparaginases from Thermophiles Thermococcus Sibiricus and Melioribacter Roseus in Acrylamide Reduction. Microbiology 2024, 93, S78–S81. [Google Scholar] [CrossRef]
- Wriston, J.C.; Yellin, T.O. L-Asparaginase: A Review. In Advances in Enzymology and Related Areas of Molecular Biology; Wiley: Hoboken, NJ, USA, 2006; Volume 39. [Google Scholar]
- Wade, H.E.; Robinson, H.K.; Phillips, B.W. Asparaginase and Glutaminase Activities of Bacteria. Microbiology 1971, 69, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Pokrovskaya, M.V.; Aleksandrova, S.S.; Pokrovsky, V.S.; Veselovsky, A.V.; Grishin, D.V.; Abakumova, O.Y.; Podobed, O.V.; Mishin, A.A.; Zhdanov, D.D.; Sokolov, N.N. Identification of Functional Regions in the Rhodospirillum Rubrum L-Asparaginase by Site-Directed Mutagenesis. Mol. Biotechnol. 2015, 57, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully Automated Protein-Ligand Interaction Profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef]



| Substrate | Km, mM | Vmax, mM/min | ||
|---|---|---|---|---|
| TsAI | TsAID54G/T56Q | TsAI | TsAID54G/T56Q | |
| L-asparagine | 3.10 ± 0.15 | 6.03 ± 0.15 | 4.02 ± 0.38 | 8.17 ± 0.24 |
| D-asparagine | 8.63 ± 0.48 | 9.18 ± 0.86 | 0.66 ± 0.15 | 0.59 ± 0.12 |
| L-glutamine | 11.09 ± 0.25 | 11.41 ± 0.27 | 0.28 ± 0.09 | 0.25 ± 0.06 |
| D-glutamine | 13.86 ± 0.86 | 14.40 ± 1.60 | 0.18 ± 0.03 | 0.18 ± 0.04 |
| Enzyme | Organism | PDB ID | Sequence Length | Type /Localization | Oligomeric State | Specific Activity, U/mg/Topt, °C | Refs. |
|---|---|---|---|---|---|---|---|
| Hyperthermophilic L-asparaginases | |||||||
| TsAI | Thermococcus sibiricus | 9UFQ | 331 | I-like/cytosolic | Homodimer | 2066/90 | [10] |
| TsAID54G/T56Q | Thermococcus sibiricus | 9UFR | 331 | I-like/cytosolic | Homodimer | 5038/90 | [10] |
| TkA | Thermococcus kodakarensis | 5OT0 | 328 | I-like/cytosolic | Homodimer | 2350/85 | [21,22] |
| PhA | Pyrococcus horikoshii | 1WLS | 328 | I-like/cytosolic | Homodimer | No data | [15] |
| PfA | Pyrococcus furiosus | 4Q0M | 327 | I-like/cytosolic | Homodimer | 330/80 | [23] |
| Mesophilic L-asparaginases | |||||||
| EcAI | Escherichia coli | 2P2D | 358 | I type/cytosolic | Homotetramer | [24] | |
| EcAII | Escherichia coli | 6UOG | 326 | II type/periplasmic | Homotetramer | 91/37 | |
| TsAI | TsAID54G/T56Q | |
|---|---|---|
| Data collection | ||
| Diffraction source | Institute of Organic Chemistry RAS (Rigaku OD XtaLAB Synergy-S) | |
| Wavelength (Å) | 1.54 | |
| Temperature (K) | 100 | |
| Detector | HyPix-6000HE | |
| Crystal-to-detector distance (mm) | 37 | 38 |
| Rotation range per image (°) | 0.15 | 0.4 |
| Total rotation range (°) | 300 | 360 |
| Space group | P22121 | |
| a, b, c (Å) | 45.19, 57.79, 131.16 | 45.01, 57.45, 130.65 |
| α, β, γ (°) | 90.00, 90.00, 90.00 | 90.00, 90.00, 90.00 |
| Average mosaicity (°) | 0.89 | 1.25 |
| Resolution range (Å) | 24.11–1.90 (1.94–1.90) | 22.70–1.90 (1.94–1.90) |
| Completeness (%) | 95.3 (92.8) | 99.4 (96.3) |
| Average multiplicity | 11.2 (13.2) | 12.0 (8.2) |
| 〈I/σ(I)〉 | 21.7 (7.8) | 31.5 (5.0) |
| Rmeas (%) | 8.4 (32.1) | 8.7 (42.5) |
| CC1/2 | 99.9 (97.5) | 99.9 (93.7) |
| Refinement | ||
| Rfact (%) | 16.7 | 17.5 |
| Rfree (%) | 21.4 | 22.5 |
| RMSD Bonds (Å) | 0.01 | 0.02 |
| RMSD Angles (°) | 1.80 | 2.15 |
| Ramachandran plot | ||
| Ramachandran favoured (%) | 97.0 | 96.9 |
| Ramachandran allowed (%) | 2.4 | 2.5 |
| PDB ID | 9UFQ | 9UFR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumina, M.V.; Zhdanov, D.D.; Veselovsky, A.V.; Pokrovskaya, M.V.; Aleksandrova, S.S.; Minyaev, M.E.; Varfolomeeva, L.A.; Matyuta, I.O.; Boyko, K.M.; Zhgun, A.A. Hyperthermophilic L-Asparaginase from Thermococcus sibiricus and Its Double Mutant with Increased Activity: Insights into Substrate Specificity and Structure. Int. J. Mol. Sci. 2025, 26, 5437. https://doi.org/10.3390/ijms26125437
Dumina MV, Zhdanov DD, Veselovsky AV, Pokrovskaya MV, Aleksandrova SS, Minyaev ME, Varfolomeeva LA, Matyuta IO, Boyko KM, Zhgun AA. Hyperthermophilic L-Asparaginase from Thermococcus sibiricus and Its Double Mutant with Increased Activity: Insights into Substrate Specificity and Structure. International Journal of Molecular Sciences. 2025; 26(12):5437. https://doi.org/10.3390/ijms26125437
Chicago/Turabian StyleDumina, Maria V., Dmitry D. Zhdanov, Alexander V. Veselovsky, Marina V. Pokrovskaya, Svetlana S. Aleksandrova, Mikhail E. Minyaev, Larisa A. Varfolomeeva, Ilya O. Matyuta, Konstantin M. Boyko, and Alexander A. Zhgun. 2025. "Hyperthermophilic L-Asparaginase from Thermococcus sibiricus and Its Double Mutant with Increased Activity: Insights into Substrate Specificity and Structure" International Journal of Molecular Sciences 26, no. 12: 5437. https://doi.org/10.3390/ijms26125437
APA StyleDumina, M. V., Zhdanov, D. D., Veselovsky, A. V., Pokrovskaya, M. V., Aleksandrova, S. S., Minyaev, M. E., Varfolomeeva, L. A., Matyuta, I. O., Boyko, K. M., & Zhgun, A. A. (2025). Hyperthermophilic L-Asparaginase from Thermococcus sibiricus and Its Double Mutant with Increased Activity: Insights into Substrate Specificity and Structure. International Journal of Molecular Sciences, 26(12), 5437. https://doi.org/10.3390/ijms26125437







