Recent Advances in Steroid Discovery: Structural Diversity and Bioactivity of Marine and Terrestrial Steroids
Abstract
1. Introduction
2. Marine Organisms
2.1. Marine Invertebrates
2.2. Soft Corals
2.3. Marine and Mangrove-Associated Fungi
3. Terrestrial Organisms
3.1. Plants
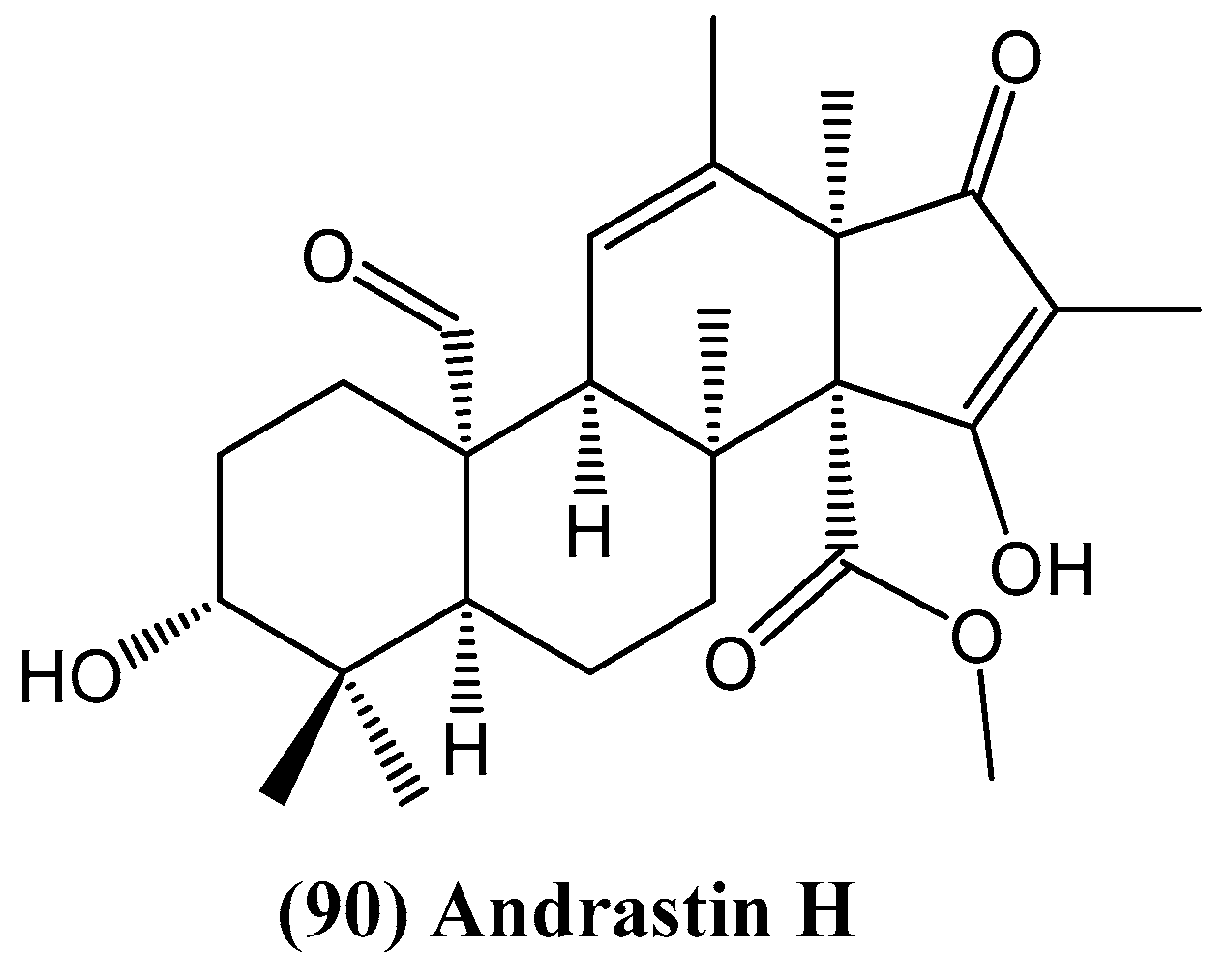
3.2. Fungi
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Theodorakidou, M.; Nikola, O.; Lambrou, G. The multiple roles of steroids and anabolic steroids and its relations to cardiovascular and musculoskeletal pathology: A brief review. J. Res. Prev. Med. Sci. 2018, 2, 22–30. [Google Scholar]
- Herbert, J. Neurosteroids: A new regulatory function in the nervous system. J. Psychiatry Neurosci. 2001, 26, 421–422. [Google Scholar]
- Auchus, R. Steroid assays, and endocrinology: Best practices for basic scientists. Endocrinology 2014, 155, 2049–2051. [Google Scholar]
- Chapman, K.; Odermatt, A. Steroids: Modulators of inflammation and immunity. J. Steroid Biochem. Mol. Biol. 2010, 120, 67–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fenchel, D.; Levkovitz, Y.; Vainer, E.; Kaplan, Z.; Zohar, J.; Cohen, H. Beyond the HPA-axis: The role of the gonadal steroid hormone receptors in modulating stress-related responses in an animal model of PTSD. Eur. Neuropsychopharmacol. 2015, 25, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.; Short, K.; Hooper, S. The science of steroids. Semin. Fetal Neonatal Med. 2019, 24, 170–175. [Google Scholar]
- Pugeat, M.; Dunn, J.; Nisula, B. Transport of steroid hormones: Interaction of 70 drugs with testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J. Clin. Endocrinol. Metab. 1981, 53, 69–75. [Google Scholar] [PubMed]
- Taylor, S.; Ayer, J.; Morris, R. Cortical steroids in treatment of cancer; observations on effects of pituitary adrenocorticotropic hormone (ACTH) and cortisone in far advanced cases. JAMA 1950, 144, 1058–1064. [Google Scholar]
- Siminoski, K.; Murphy, R.; Rennert, P.; Heinrich, G. Cortisone, testosterone, and aldosterone reduce levels of nerve growth factor messenger ribonucleic acid in L-929 fibroblasts. Endocrinology 1987, 121, 1432–1437. [Google Scholar]
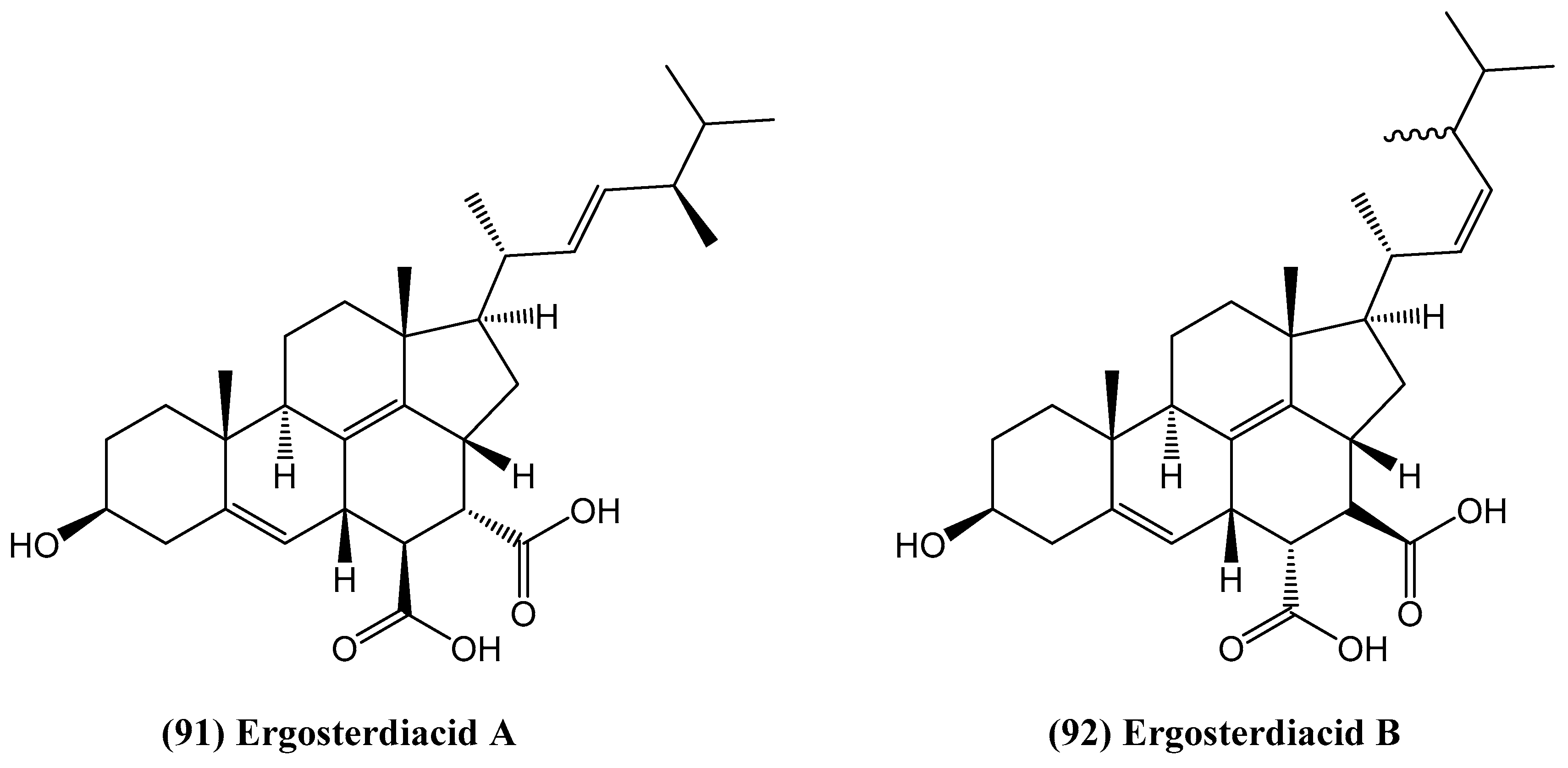
- Pang, C.; Chen, Y.-H.; Bian, H.-H.; Zhang, J.-P.; Su, L.; Han, H.; Zhang, W. Anti-inflammatory ergosteroid derivatives from the coral-associated fungi Penicillium oxalicum HL-44. Molecules 2023, 28, 7784. [Google Scholar] [CrossRef]
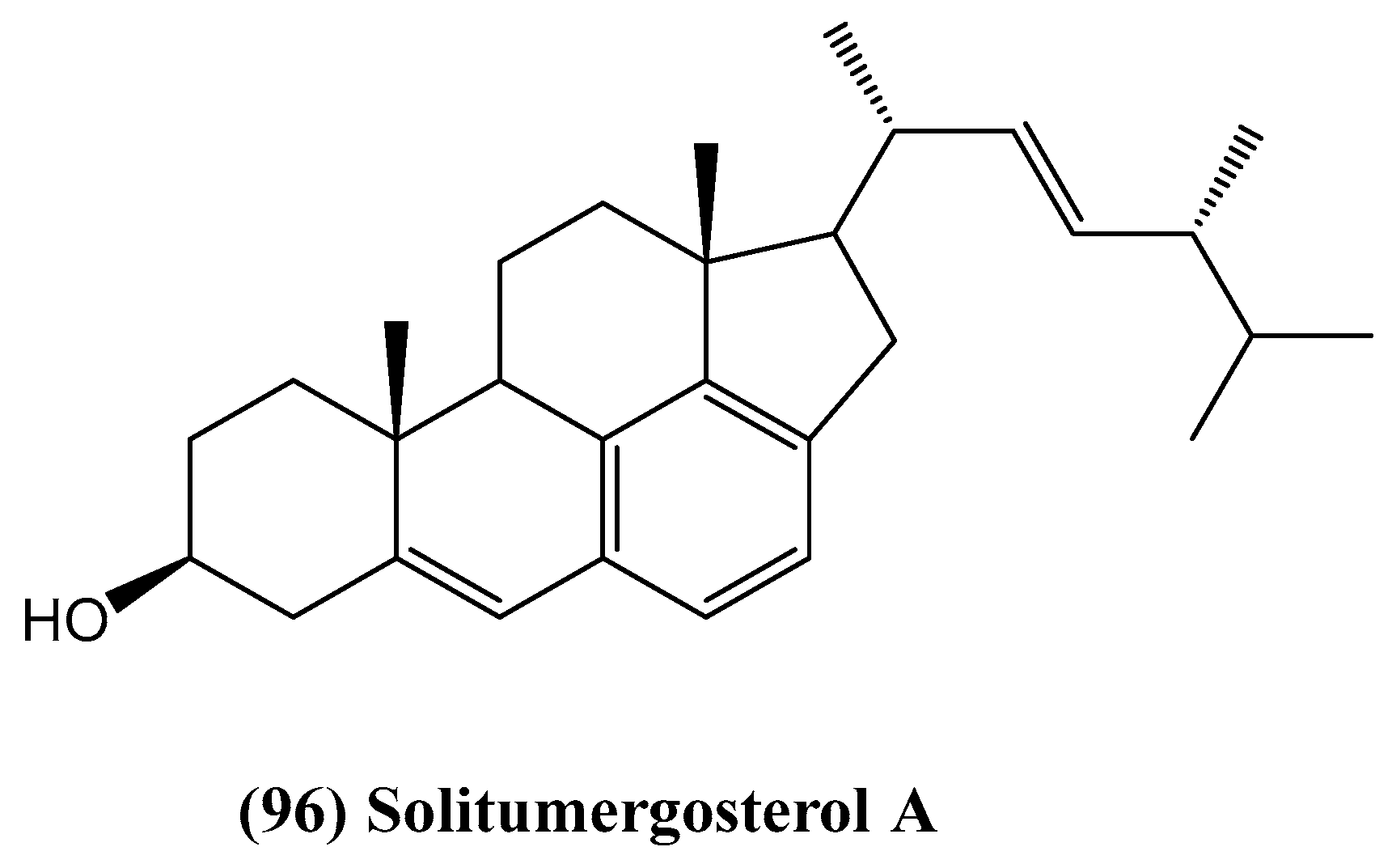
- He, Z.-H.; Xie, C.-L.; Hao, Y.-J.; Xu, L.; Wang, C.-F.; Hu, M.-Y.; Li, S.-J.; Zhong, T.-H.; Yang, X.-W. Solitumergosterol A, a unique 6/6/6/6/5 steroid from the deep-sea-derived Penicillium solitum MCCC 3A00215. Org. Biomol. Chem. 2021, 19, 9369–9372. [Google Scholar] [PubMed]
- Qiu, Y.; Chen, S.; Yu, M.; Shi, J.; Liu, J.; Li, X.; Chen, J.; Sun, X.; Huang, G.; Zheng, C. Natural products from marine-derived fungi with anti-inflammatory activity. Mar. Drugs 2024, 22, 433. [Google Scholar] [CrossRef]
- Xu, M.; Dong, P.; Tian, X.; Wang, C.; Huo, X.; Zhang, B.; Wu, L.; Deng, S.; Ma, X. Drug interaction study of natural steroids from herbs specifically toward human UDP-glucuronosyltransferase (UGT) 1A4 and their quantitative structure activity relationship (QSAR) analysis for prediction. Pharmacol. Res. 2016, 110, 139–150. [Google Scholar] [PubMed]
- Clanton, N.; Hastings, S.; Foultz, G.; Contreras, J.; Yee, S.; Arman, H.; Risinger, A.; Frantz, D. Synthesis and biological evaluations of electrophilic steroids inspired by the taccalonolides. ACS Med. Chem. Lett. 2020, 11, 2534–2543. [Google Scholar] [PubMed]
- Fu, X.; Ferreira, M.; Schmitz, F.; Kelly, M. Tamosterone sulfates: A C-14 epimeric pair of polyhydroxylated sterols from a new Oceanapiid sponge genus. J. Org. Chem. 1999, 64, 6706–6709. [Google Scholar]
- Makarieva, T.; Shubina, L.; Kalinovsky, A.; Stonik, V.; Elyakov, G. Steroids in porifera. II. Steroid derivatives from two sponges of the family Halichondriidae: Sokotrasterol sulfate, a marine steroid with a new pattern of side chain alkylation. Steroids 1983, 42, 267–281. [Google Scholar]
- Moses, T.; Papadopoulou, K.; Osbourn, A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Chakraborty, H.; Kar, B.; Choi, W.; Lee, B.; Bhakta, T.; Atanasov, A.; Kim, H. Therapeutic value of steroidal alkaloids in cancer: Current trends and future perspectives. Int. J. Cancer 2019, 145, 1731–1744. [Google Scholar]
- Jiang, Q.; Chen, M.; Cheng, K.; Yu, P.; Wei, X.; Shi, Z. Therapeutic potential of steroidal alkaloids in cancer and other diseases. Med. Res. Rev. 2016, 36, 119–143. [Google Scholar]
- Lu, Y.; Luo, J.; Kong, L. Steroidal alkaloid saponins and steroidal saponins from Solanum surattense. Phytochemistry 2011, 72, 668–673. [Google Scholar] [CrossRef]
- Zhang, X.; Ito, Y.; Liang, J.; Liu, J.; He, J.; Sun, W. Therapeutic effects of total steroid saponin extracts from the rhizome of Dioscorea zingiberensis C.H. Wright in Freund’s complete adjuvant induced arthritis in rats. Int. Immunopharmacol. 2014, 23, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.; Bachran, D.; Panjideh, H.; Schellmann, N.; Weng, A.; Melzig, M.; Sutherland, M.; Bachran, C. Saponins as tools for improved targeted tumor therapies. Curr. Drug Targets 2009, 10, 140–151. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Gu, Y. Secondary metabolites from the South China Sea invertebrates: Chemistry and biological activity. Curr. Med. Chem. 2006, 13, 2041–2090. [Google Scholar] [CrossRef]
- Proksch, P.; Ebel, R.; Edrada, R.; Wray, V.; Steube, K. Bioactive natural products from marine invertebrates and associated fungi. Prog. Mol. Subcell. Biol. 2003, 37, 117–142. [Google Scholar]
- Kobayashi, J. Search for new bioactive marine natural products and application to drug development. Chem. Pharm. Bull. 2016, 64, 1079–1083. [Google Scholar]
- Gomes, A.; Freitas, A.; Rocha-Santos, T.; Duarte, A. Bioactive compounds derived from echinoderms. RSC Adv. 2014, 4, 29365–29382. [Google Scholar] [CrossRef]
- Ha, D.; Kicha, A.; Kalinovsky, A.; Malyarenko, T.; Popov, R.; Malyarenko, O.; Ermakova, S.; Thủy, T.; Long, P.; Ivanchina, N. Asterosaponins from the tropical starfish Acanthaster planci and their cytotoxic and anticancer activities in vitro. Nat. Prod. Res. 2019, 35, 548–555. [Google Scholar]
- Vien, L.; Hanh, T.; Huong, P.; Tu, V.; Thanh, N.; Lyakhova, E.; Cuong, N.; Nam, N.; Kiem, P.; Minh, C.; et al. New steroidal glycosides from the starfish Acanthaster planci. Chem. Nat. Compd. 2016, 52, 1056–1060. [Google Scholar]
- Itakura, Y.; Komori, T. Biologically active glycosides from Asteroidea, X. steroid oligoglycosides from the starfish Acanthaster planci L., 3. Structures of four new oligoglycoside sulfates. Eur. J. Org. Chem. 1986, 1986, 499–508. [Google Scholar]
- Vien, L.; Hanh, T.; Huong, P.; Dang, N.; Thanh, N.; Lyakhova, E.; Cuong, N.; Nam, N.; Kiem, P.; Kicha, A.; et al. Pyrrole oligoglycosides from the starfish Acanthaster planci suppress lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophages. Chem. Pharm. Bull. 2016, 64, 1654–1657. [Google Scholar]
- Hafez, M.; Okbah, M.; Ibrahim, H.; Hussein, A.; Moneim, N.; Ata, A. First report of steroid derivatives isolated from starfish Acanthaster planci with anti-bacterial, anti-cancer and anti-diabetic activities. Nat. Prod. Res. 2021, 36, 5545–5552. [Google Scholar] [PubMed]
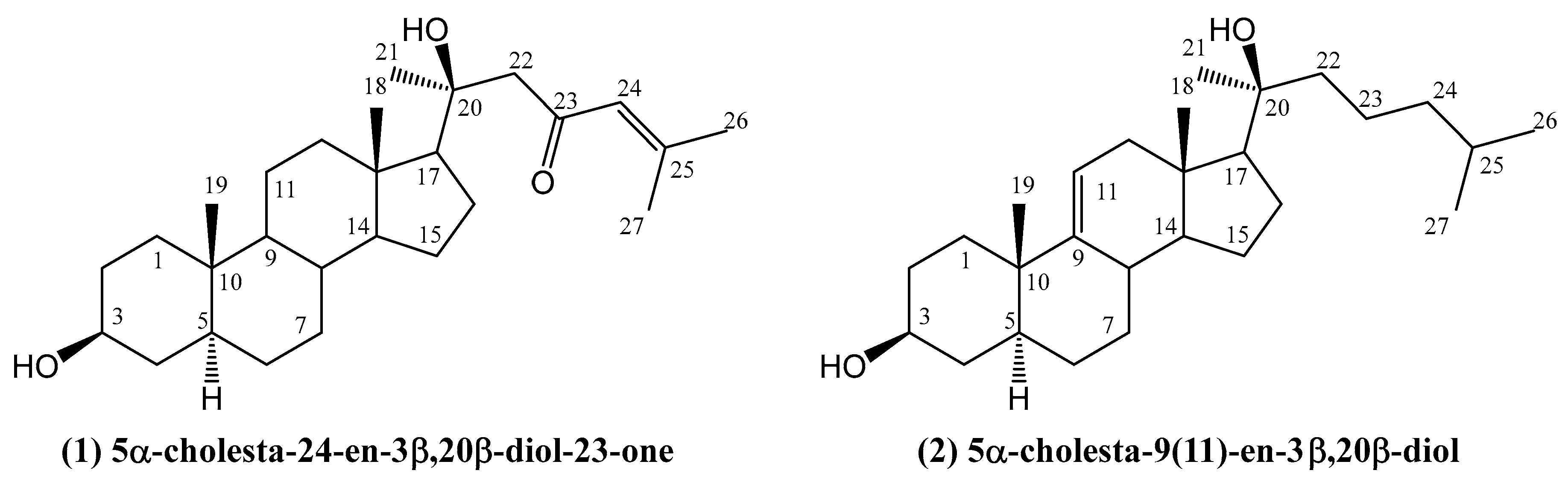
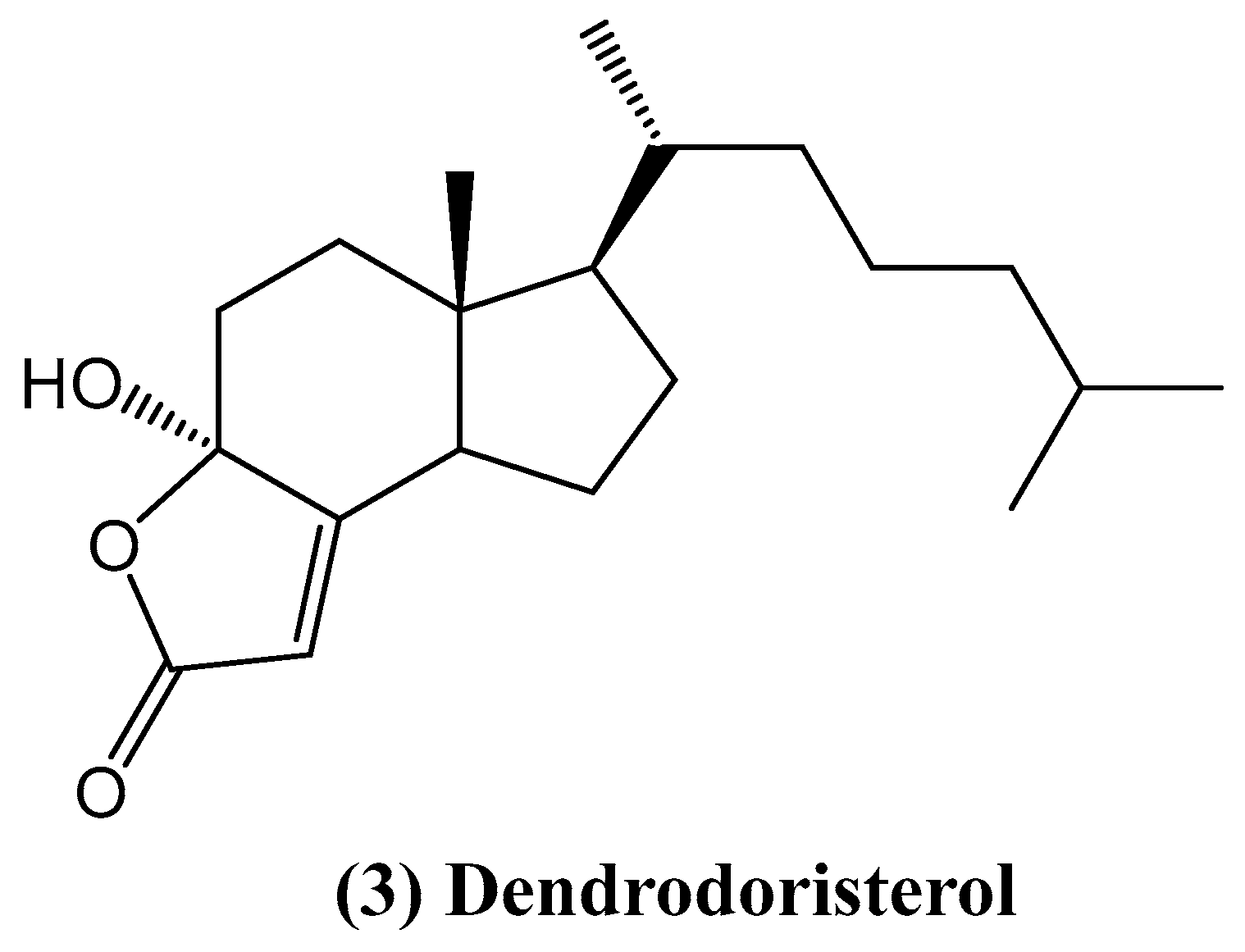
- Huong, P.; Phong, N.; Thao, N.; Binh, P.; Thao, D.; Thanh, N.; Cuong, N.; Nam, N.; Thung, D.; Minh, C. Dendrodoristerol, a cytotoxic C20 steroid from the Vietnamese nudibranch mollusk Dendrodoris fumata. J. Asian Nat. Prod. Res. 2020, 22, 193–200. [Google Scholar] [PubMed]
- Pansini, M.; Ciminiello, P.; Fattorusso, E.; Magno, S.; Mangoni, A. Incisterols, a new class of highly degraded sterols from the marine sponge Dictyonella incisa. J. Am. Chem. Soc. 1990, 112, 3505–3509. [Google Scholar]
- Chianese, G.; Sepe, V.; Limongelli, V.; Renga, B.; D’Amore, C.; Zampella, A.; Taglialatela-Scafati, O.; Fiorucci, S. Incisterols, highly degraded marine sterols, are a new chemotype of PXR agonists. Steroids 2014, 83, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Gavagnin, M.; Carbone, M.; Nappo, M.; Mollo, E.; Roussis, V.; Cimino, G. First chemical study of anaspidean Syphonota geographica: Structure of degraded sterols aplykurodinone-1 and -2. Tetrahedron 2005, 61, 617–621. [Google Scholar]
- Wu, X.; Lin, S.; Zhu, C.; Yue, Z.; Yu, Y.; Zhao, F.; Liu, B.; Dai, J.; Shi, J. Homo- and heptanor-sterols and tremulane sesquiterpenes from cultures of Phellinus igniarius. J. Nat. Prod. 2010, 73, 1294–1300. [Google Scholar]
- Quang, T.H.; Lee, D.-S.; Han, S.J.; Kim, I.C.; Yim, J.H.; Kim, Y.-C.; Oh, H. Steroids from the cold water starfish Ctenodiscus crispatus with cytotoxic and apoptotic effects on human hepatocellular carcinoma and glioblastoma cells. Bull. Korean Chem. Soc. 2014, 35, 2335–2340. [Google Scholar]
- Xiao, D.-J.; Peng, X.-D.; Deng, S.-Z.; Ma, W.-J.; Wu, H.-M. Structure elucidation of (3E)-Cholest-4-en-3,6-dione-3-oxime in marine sponge Cinachyrella australiensis from the South China Sea. Chin. J. Org. Chem. 2005, 25, 1606–1609. [Google Scholar]
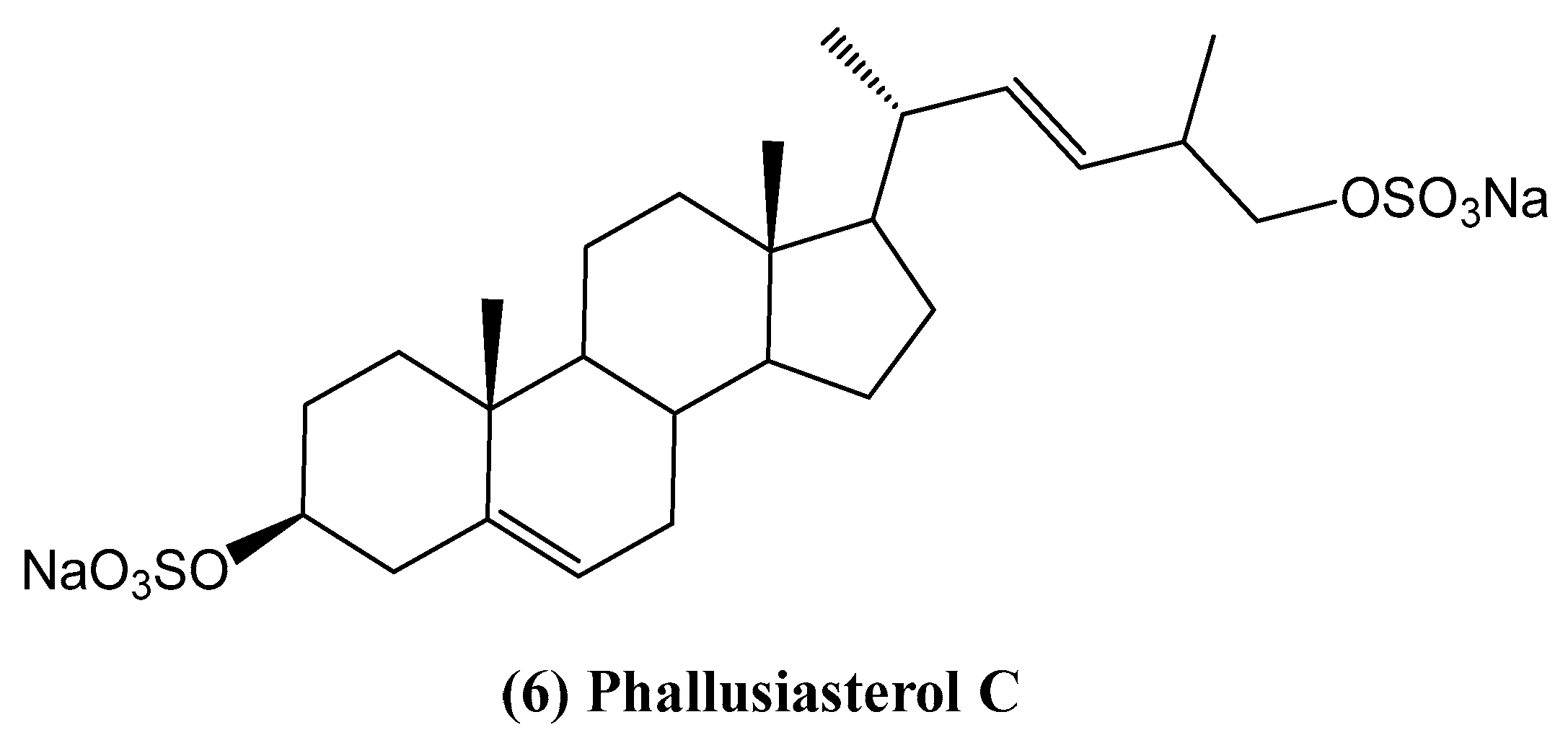
- Imperatore, C.; Senese, M.; Aiello, A.; Luciano, P.; Fiorucci, S.; D’Amore, C.; Carino, A.; Menna, M. Phallusiasterol C, A new disulfated steroid from the mediterranean tunicate Phallusia fumigata. Mar. Drugs 2016, 14, 117. [Google Scholar] [CrossRef]
- Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A. Structures of new polar steroids from the far-eastern starfish Ctenodiscus crispatus. Russ. Chem. Bull. Int. Ed. 2005, 54, 1266–1271. [Google Scholar]
- Festa, C.; de Marino, S.; D’Auria, M.V.; Bifulco, G.; Renga, B.; Fiorucci, S.; Petek, S.; Zampella, A. Solomonsterols A and B from Theonella swinhoei. The first example of C-24 and C-23 sulfated sterols from a marine source endowed with a PXR agonistic activity. J. Med. Chem. 2011, 54, 401–405. [Google Scholar] [CrossRef]
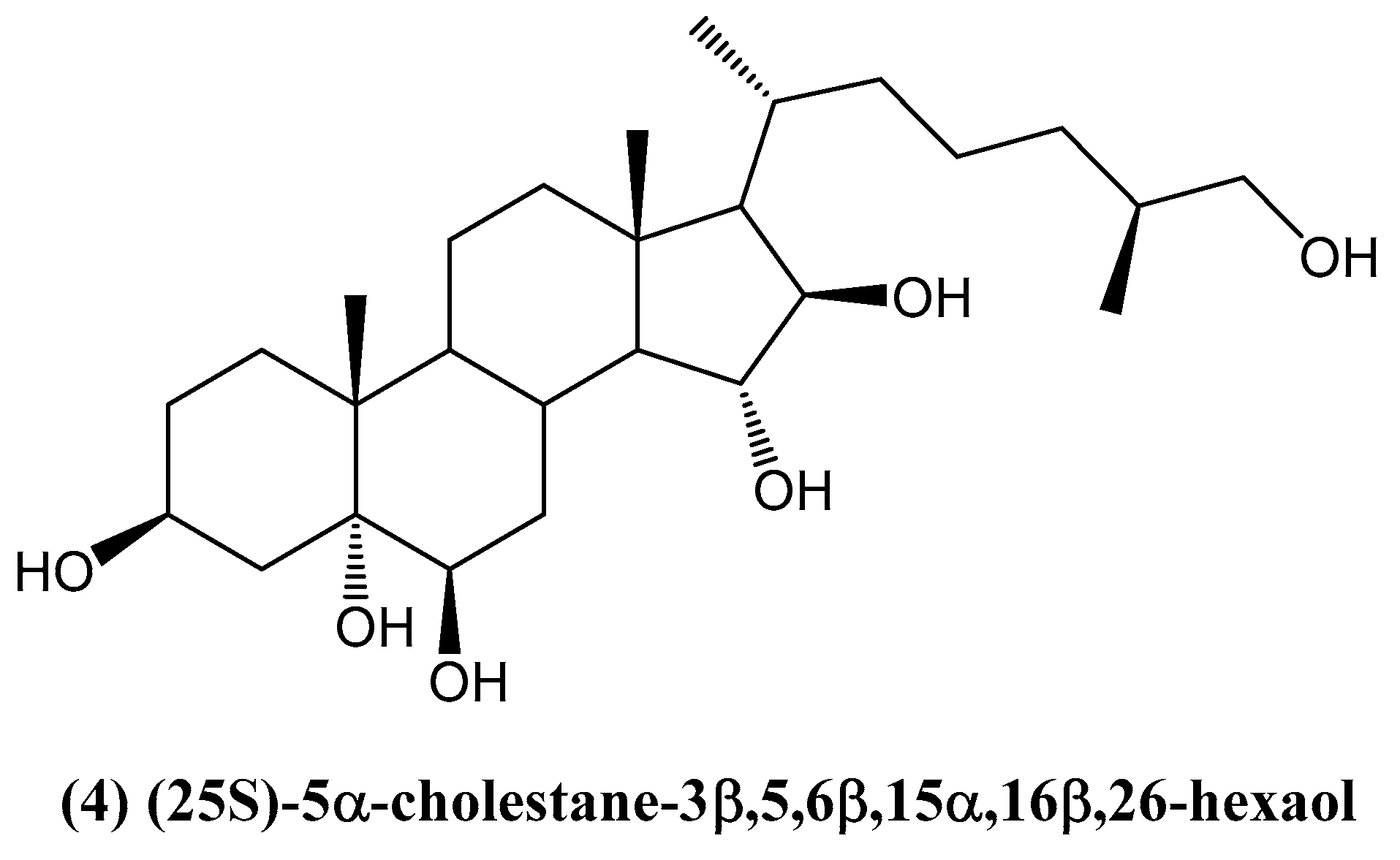
- Tran, T.H.H.; Vien, L.T.; Vinh, L.B.; Thanh, N.V.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Kiem, P.V.; Minh, C.V. Further highly hydroxylated steroids from the Vietnamese starfish Archaster typicus. Chem. Pharm. Bull. 2016, 64, 1523–1527. [Google Scholar]
- Minale, L.; Riccio, R.; Zollo, F. Structural studies on chemical constituents of echinoderms. Stud. Nat. Prod. Chem. 1995, 15, 43–110. [Google Scholar]
- Dong, G.; Xu, T.; Yang, B.; Lin, X.; Zhou, X.; Yang, X.; Liu, Y. Chemical constituents and bioactivities of starfish. Chem. Biodivers. 2011, 8, 740–791. [Google Scholar] [CrossRef]
- Kicha, A.A.; Ivanchina, N.V.; Huong, T.T.T.; Kalinovsky, A.I.; Dmitrenok, P.S.; Long, P.Q. Minor asterosaponin archasteroside C from the starfish Archaster typicus. Russ. Chem. Bull. 2010, 59, 2133–2136. [Google Scholar] [CrossRef]
- Kicha, A.A.; Ivanchina, N.V.; Huong, T.T.T.; Kalinovsky, A.I.; Dmitrenok, P.S.; Fedorov, S.N.; Dyshlovoy, S.A.; Long, P.Q.; Stonik, V.A. Two new asterosaponins, archasterosides A and B, from the vietnamese starfish Archaster typicus and their anticancer properties. Bioorg. Med. Chem. Lett. 2010, 20, 3826–3830. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Chen, X.Q.; Dong, G.; Zhou, X.F.; Chai, X.Y.; Li, Y.Q.; Yang, B.; Zhang, W.D.; Liu, Y. Isolation and structural characterisation of five new and 14 known metabolites from the commercial starfish Archaster typicus. Food Chem. 2011, 124, 1634–1638. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Huong, T.T.; Kalinovsky, A.I.; Dmitrenok, P.S.; Agafonova, I.G.; Long, P.Q.; Stonik, V.A. Highly hydroxylated steroids of the starfish Archaster typicus from the Vietnamese waters. Steroids 2010, 75, 897–904. [Google Scholar]
- Riccio, R.; Santaniello, M.; Greco, O.S.; Minale, L. Structure elucidation of (22E,24R,25R)-24-methyl-5α-cholest-22-ene-3β,4β,5,6α,8,14,15α,25,26-nonaol and (22E,24S)-24-methyl–5α-cholest-22-ene-3β,4β,5,6α,8,14,15α,25,28-nonaol, minor marine polyhydroxysteroids isolated from the starfish Archaster typicus. J. Chem. Soc. Perkin Trans. 1 1989, 4, 823–826. [Google Scholar] [CrossRef]
- Mattia, C.A.; Mazzarella, L.; Puliti, R.; Riccio, R.; Minale, L. Structure and stereochemistry of (24R)-27-nor-5α-cholestane-3β,4β,5,6α,7β,8,14,15α,24-nonaol: A highly hydroxylated marine steroid from the starfish Archaster typicus. Acta Cryst. 1988, C44, 2170–2173. [Google Scholar] [CrossRef]
- Riccio, R.; Squillace Greco, O.; Minale, L.; Laurent, D.; Duhet, D. Highly hydroxylated marine steroids from the starfish Archaster typicus. J. Chem. Soc. Perkin Trans. 1 1986, 1, 665–670. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, Z.; Wang, D.; Yan, Z.; Sun, X.; Li, X.; Yin, X.; Wang, S.; Li, K. Pectiniferosides A–J: Diversified glycosides of polyhydroxy steroids isolated from the sea star Patiria (=Asterina) pectinifera. Mar. Drugs 2024, 22, 545. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A.; Riguera, R.; Jiménez, C. Hemolytic polar steroidal constituents of the starfish Aphelasterias japonica. J. Nat. Prod. 2000, 63, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Ivanchina, N.V.; Kicha, A.A.; Kalinovsky, A.I.; Dmitrenok, P.S.; Dmitrenok, A.S.; Chaikina, E.L.; Stonik, V.A.; Gavagnin, M.; Cimino, G. Polar steroidal compounds from the far eastern starfish Henricia leviuscula. J. Nat. Prod. 2006, 69, 224–228. [Google Scholar] [CrossRef]
- Chludil, H.D.; Muniain, C.C.; Seldes, A.M.; Maier, M.S. Cytotoxic and antifungal triterpene glycosides from the patagonian sea cucumber Hemoiedema spectabilis. J. Nat. Prod. 2002, 65, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Kicha, A.A.; Tolkanov, D.K.; Malyarenko, T.V.; Malyarenko, O.S.; Kuzmich, A.S.; Kalinovsky, A.I.; Popov, R.S.; Stonik, V.A.; Ivanchina, N.V.; Dmitrenok, P.S. Sulfated polyhydroxysteroid glycosides from the Sea of Okhotsk starfish Henricia leviuscula spiculifera and potential mechanisms for their observed anti-cancer activity against several types of human cancer cells. Mar. Drugs 2024, 22, 294. [Google Scholar] [CrossRef]
- Kicha, A.A.; Malyarenko, T.V.; Kuzmich, A.S.; Malyarenko, O.S.; Kalinovsky, A.I.; Popov, R.S.; Tolkanov, D.K.; Ivanchina, N.V. Rare ophiuroid-type steroid 3β,21-, 3β,22-, and 3α,22-disulfates from the slime sea star Pteraster marsippus and their colony-inhibiting effects against human breast cancer cells. Mar. Drugs 2024, 22, 43. [Google Scholar] [CrossRef]
- Kicha, A.A.; Kalinovsky, A.I.; Malyarenko, T.V.; Malyarenko, O.S.; Ermakova, S.P.; Popov, R.S.; Stonik, V.A.; Ivanchina, N.V. Disulfated ophiuroid type steroids from the far eastern starfish Pteraster marsippus and their cytotoxic activity on the models of 2D and 3D cultures. Mar. Drugs 2022, 20, 164. [Google Scholar] [CrossRef] [PubMed]
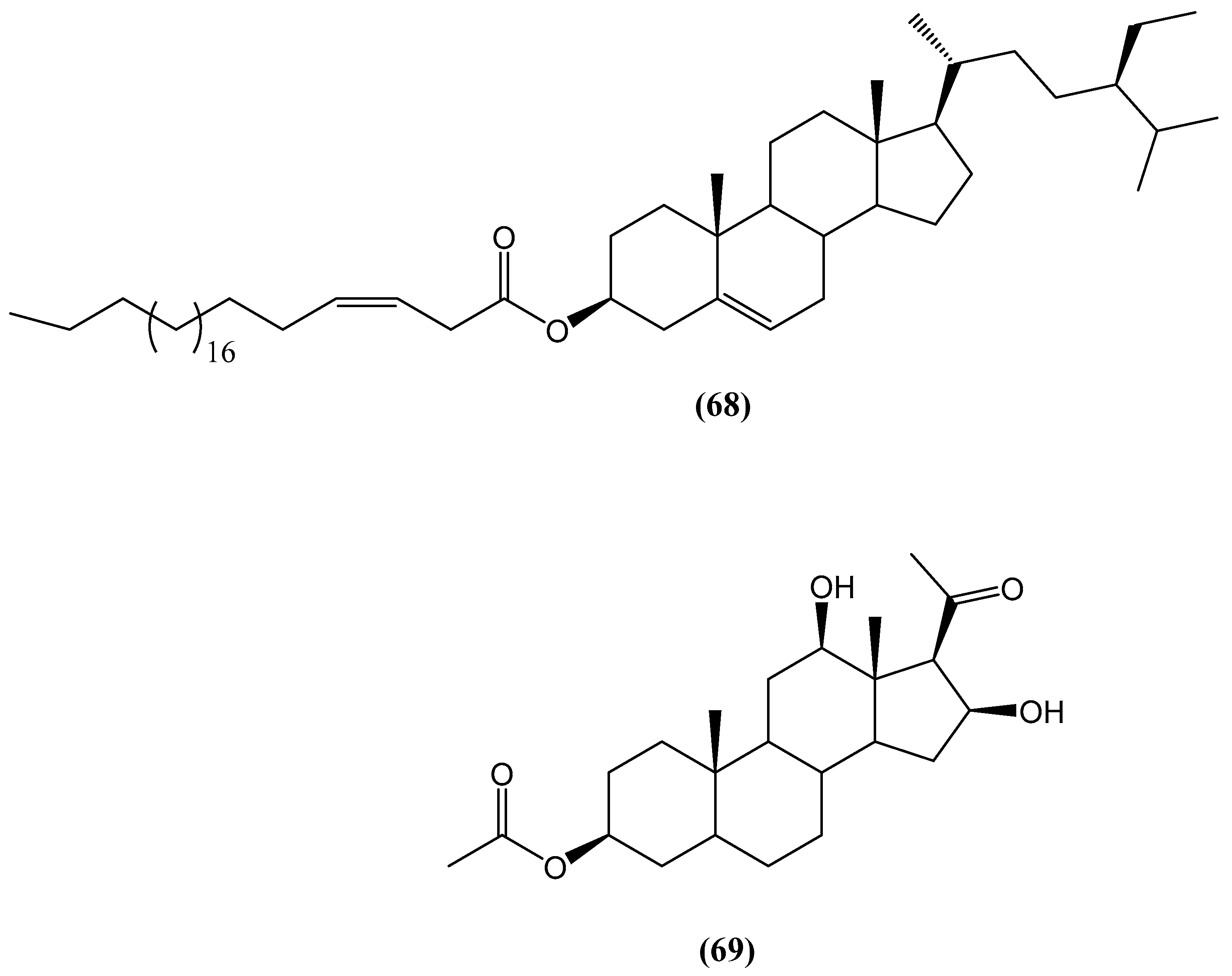
- Malyarenko, T.V.; Kicha, A.A.; Malyarenko, O.S.; Zakharenko, V.M.; Kotlyarov, I.P.; Kalinovsky, A.I.; Popov, R.S.; Svetashev, V.I.; Ivanchina, N.V. New conjugates of polyhydroxysteroids with long-chain fatty acids from the deep-water far eastern starfish Ceramaster patagonicus and their anticancer activity. Mar. Drugs 2020, 18, 260. [Google Scholar] [CrossRef]
- Tian, X.-R.; Gao, Y.-Q.; Tian, X.-L.; Li, J.; Tang, H.-F.; Li, Y.-S.; Lin, H.-W.; Ma, Z.-Q. New cytotoxic secondary metabolites from marine bryozoan Cryptosula pallasiana. Mar. Drugs 2017, 15, 120. [Google Scholar] [CrossRef]
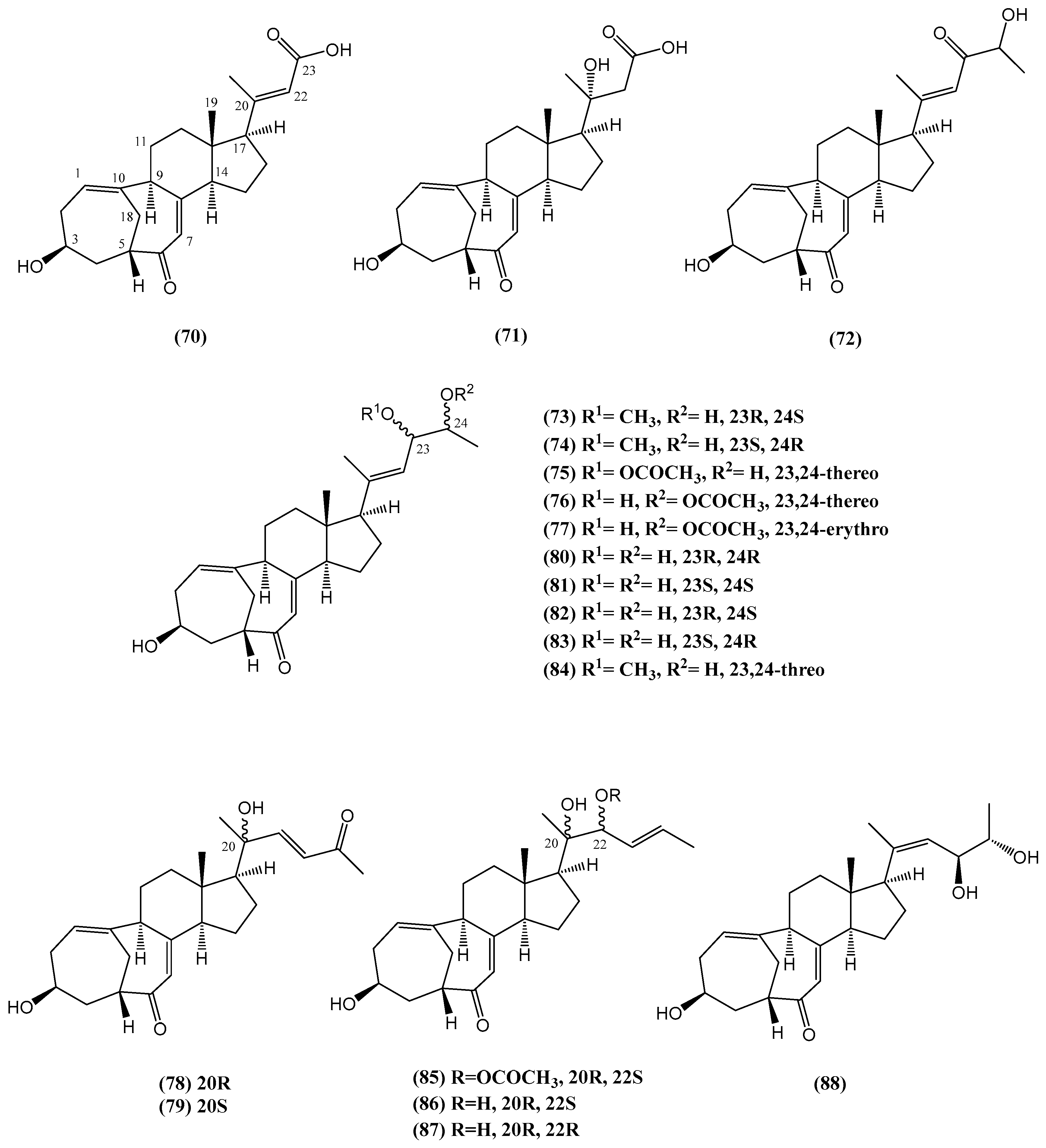
- Cao, F.; Liu, H.-Y.; Zhao, J.; Fang, Y.-C.; Wang, C.-Y. New 19-oxygenated steroid from the South China Sea gorgonian Dichotella gemmacea. Nat. Prod. Res. 2015, 29, 169–173. [Google Scholar]
- Tsai, C.-R.; Huang, C.-Y.; Chen, B.-W.; Tsai, Y.-Y.; Shih, S.-P.; Hwang, T.-L.; Dai, C.-F.; Wang, S.-Y.; Sheu, J.-H. New bioactive steroids from the soft coral Klyxum flaccidum. RSC Adv. 2015, 5, 12546–12554. [Google Scholar]
- Ninh, T.N.; Huong, P.T.M.; Thanh, N.V.; Chi, N.T.P.; Dang, N.H.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Kiem, P.V.; Minh, C.V. Cytotoxic steroids from the Vietnamese soft coral Sinularia conferta. Chem. Pharm. Bull. 2017, 65, 300–305. [Google Scholar]
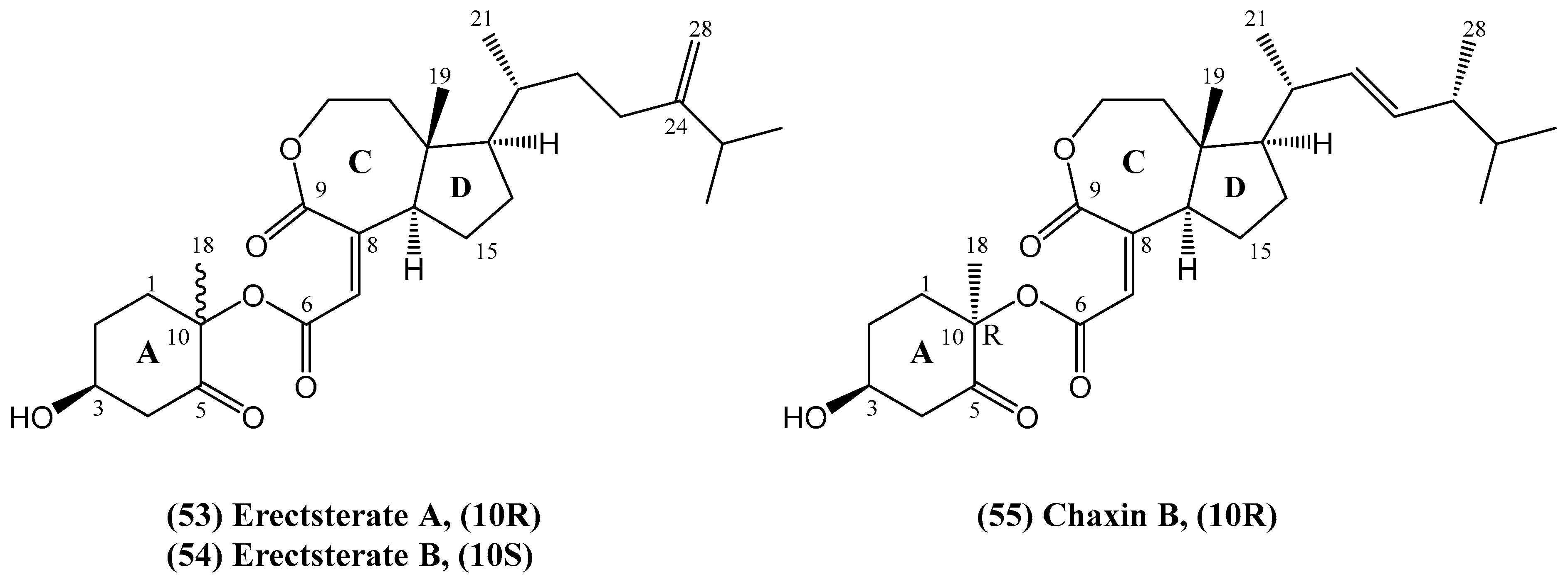
- Liu, J.; Wu, X.; Yang, M.; Gu, Y.-C.; Yao, L.-G.; Huan, X.-J.; Miao, Z.-H.; Luo, H.; Guo, Y.-W. Erectsterates A and B, a pair of novel highly degraded steroid derivatives from the South China Sea soft coral Sinularia erecta. Steroids 2020, 161, 108681. [Google Scholar] [PubMed]
- Choi, J.-H.; Ogawa, A.; Abe, N.; Masuda, K.; Koyama, T.; Yazawa, K.; Kawagishi, H. Chaxines B, C, D, and E from the edible mushroom Agrocybe chaxingu. Tetrahedron 2009, 65, 9850–9853. [Google Scholar]
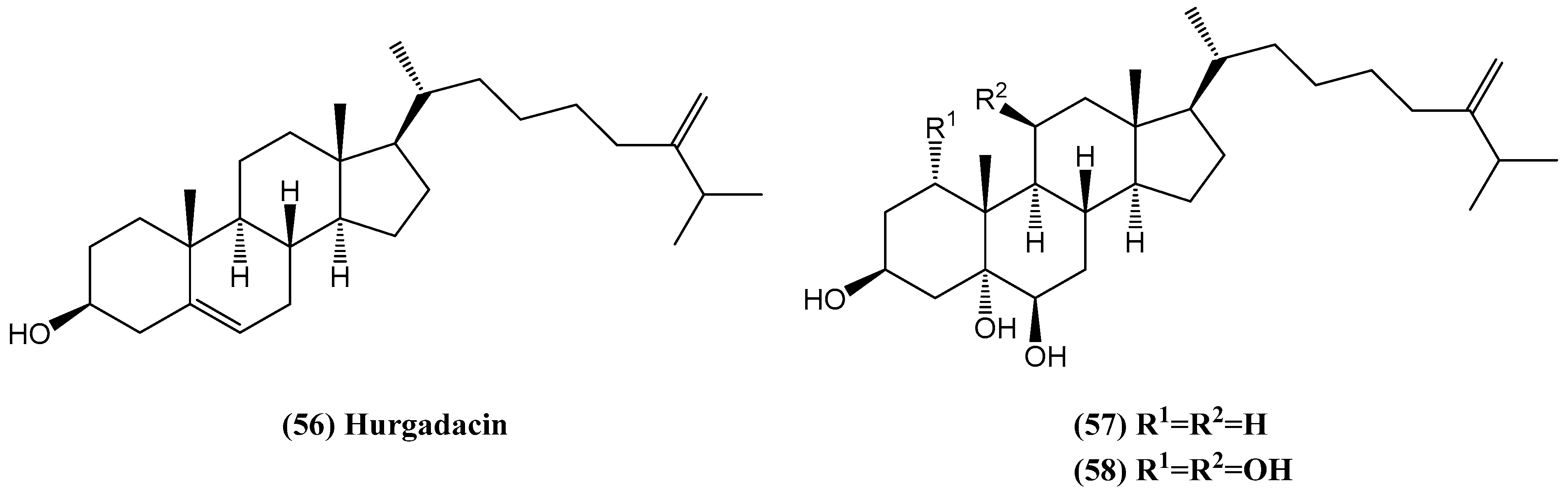
- Shaaban, M.; Shaaban, K.A.; Ghani, M.A. Hurgadacin: A new steroid from Sinularia polydactyla. Steroids 2013, 78, 866–873. [Google Scholar] [CrossRef]
- Mohammed, R.; Seliem, M.A.E.; Mohammed, T.A.A.; Abed-ElFatah, A.; Abo-Youssef, A.M.; Thabet, M.M. Bioactive secondary metabolites from the Red Sea soft coral Heteroxenia fuscescens. Int. J. Appl. Res. Nat. Prod. 2012, 4, 15–27. [Google Scholar]
- Coll, J.C. The chemistry and chemical ecology of octocorals (Coelenterata, Anthozoa, Octocorallia). Chem. Rev. 1992, 92, 613–631. [Google Scholar]
- Shaaban, M.; Shaaban, K.A.; Abd-Alla, H.I.; Hanna, A.G.; Laatsch, H. Dendrophen, a novel glycyrrhetyl amino acid from Dendronephthya hemprichi. Z. Naturforsch. B 2011, 66, 1199–1207. [Google Scholar]
- Ghandourah, M.A. Cytotoxic Ketosteroids from the Red Sea Soft Coral Dendronephthya sp. Open Chem. 2023, 21, 20220327. [Google Scholar]
- Abdel-Lateff, A.; Alarif, W.M.; Asfour, H.Z.; Ayyad, S.-E.N.; Khedr, A.; Badria, F.A.; Al-Lihaibi, S.S. Cytotoxic effects of three new metabolites from Red Sea marine sponge, Petrosia sp. Environ. Toxicol. Pharmacol. 2014, 37, 928–935. [Google Scholar] [PubMed]
- Alarif, W.M.; Abdel-Lateff, A.; Al-Lihaibi, S.S.; Ayyad, S.-E.N.; Badria, F.A.; Alsofyani, A.A.; Abou-Elnaga, Z.S. Marine bioactive steryl esters from the Red Sea black coral Antipathes dichotoma. CLEAN Soil Air Water 2013, 41, 1116–1121. [Google Scholar] [CrossRef]
- Al-Lihaibi, S.S.; Ayyad, S.-E.N.; Shaher, F.; Alarif, W.M. Antibacterial sphingolipid and steroids from the black coral Antipathes dichotoma. Chem. Pharm. Bull. 2010, 58, 1635–1638. [Google Scholar]
- Mohamed, G.A.; Abd-Elrazek, A.E.E.; Hassanean, H.A.; Youssef, D.T.A.; Soest, R. New compounds from the Red Sea marine sponge Echinoclathria gibbosa. Phytochem. Lett. 2014, 9, 51–58. [Google Scholar]
- Jia, T.Z.; Liu, H.B.; Fang, Y.C.; Zhu, T.J.; Gu, Q.Q.; Zhu, W.M. Advances in secondary metabolites of fungi isolated from the marine alga. Chin. J. Antibiot. 2006, 31, 328–334. [Google Scholar]
- König, G.M.; Kehraus, S.; Seibert, S.F.; Abdel-Lateff, A.; Müller, D. Natural products from marine organisms and their associated microbes. ChemBioChem 2006, 7, 229–238. [Google Scholar]
- Bugni, T.S.; Ireland, C.M. Marine-Derived Fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004, 21, 143–163. [Google Scholar] [CrossRef]
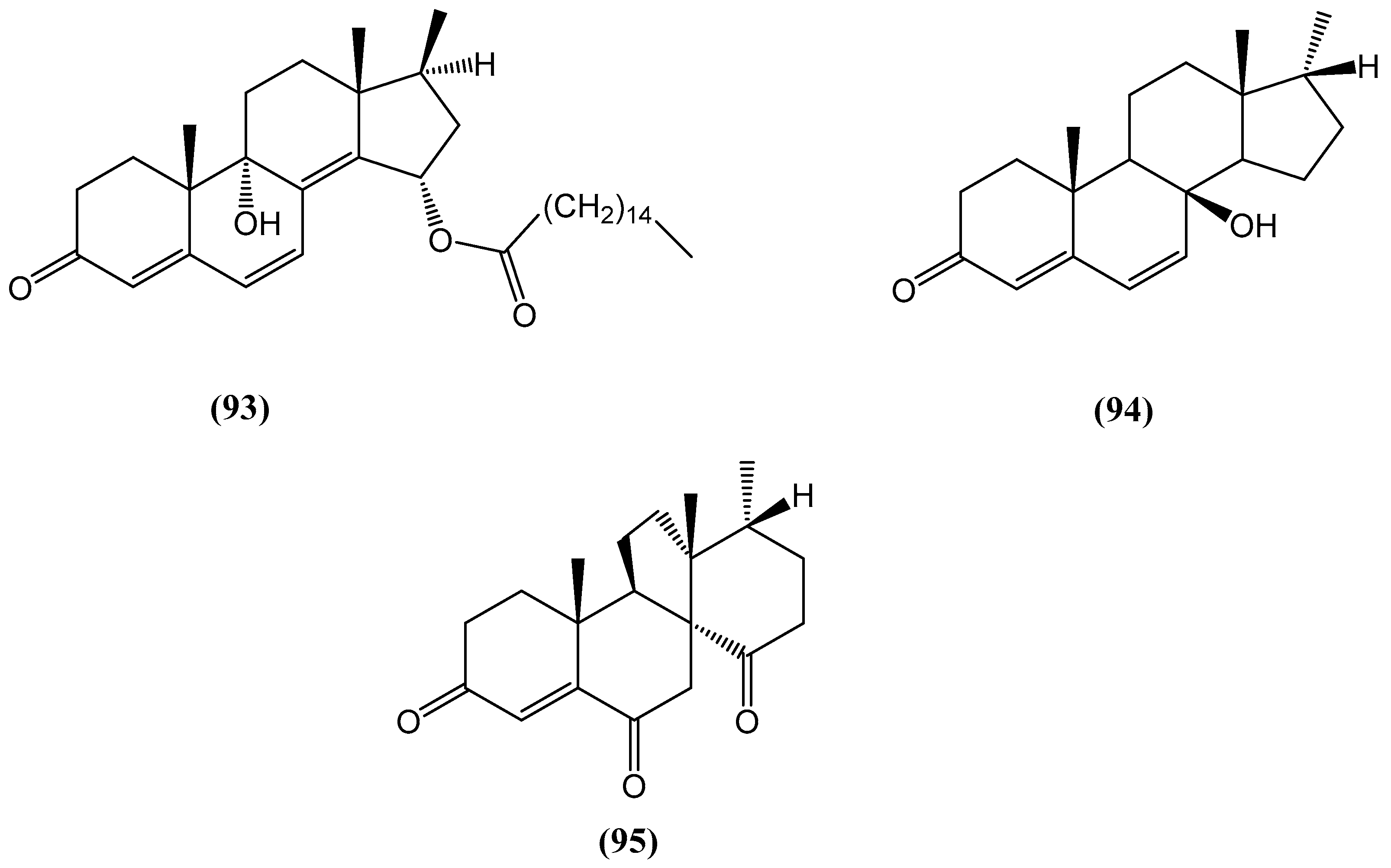
- He, Z.-H.; Xie, C.-L.; Wu, T.; Yue, Y.-T.; Wang, C.-F.; Xu, L.; Xie, M.-M.; Zhang, Y.; Hao, Y.-J.; Xu, R.; et al. Tetracyclic steroids bearing a bicyclo[4.4.1] ring system as potent antiosteoporosis agents from the deep-sea-derived fungus Rhizopus sp. W23. J. Nat. Prod. 2022, 86, 157–165. [Google Scholar] [CrossRef]
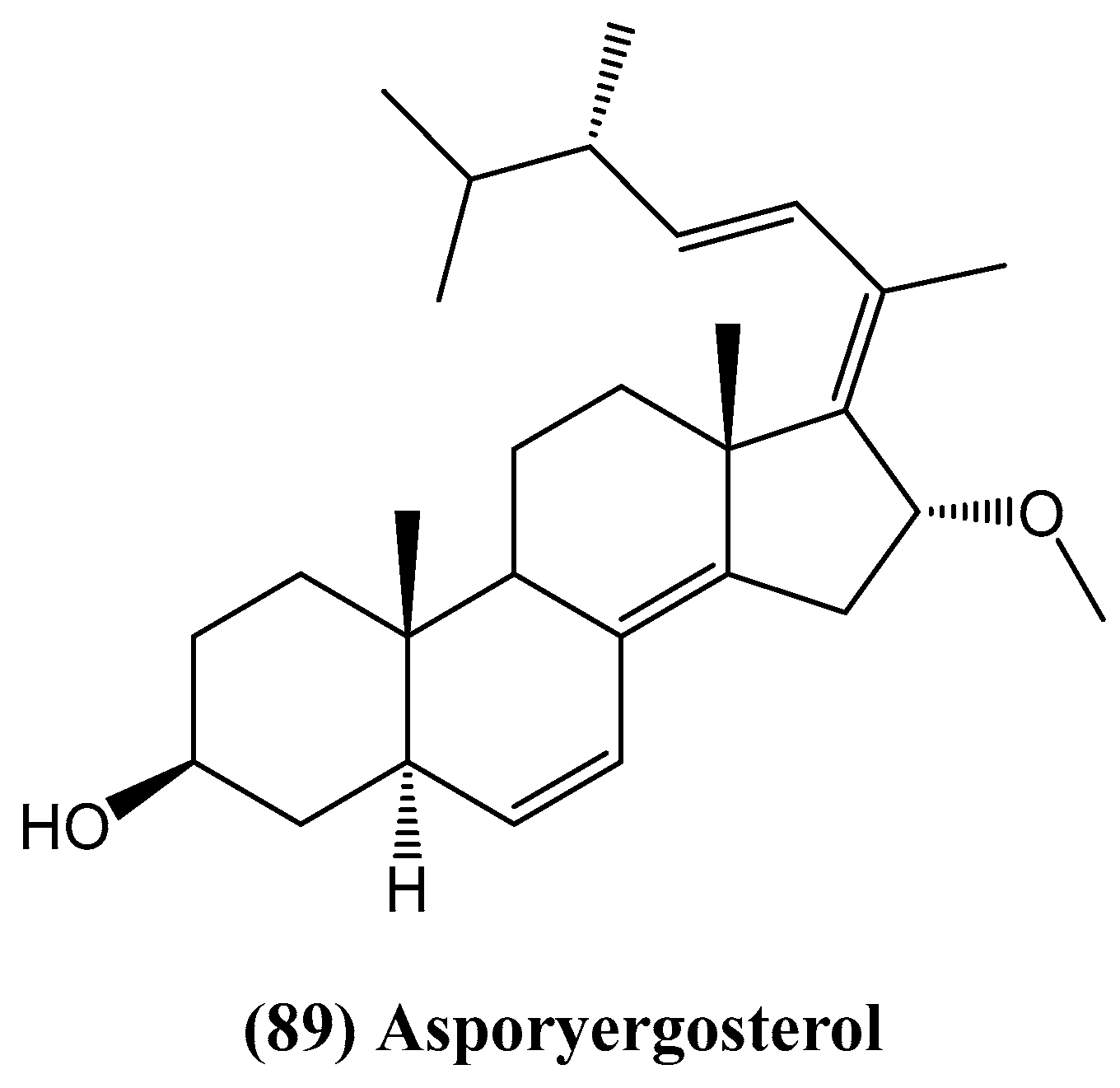
- Qiao, M.-F.; Ji, N.-Y.; Liu, X.-H.; Li, F.; Xue, Q.-Z. Asporyergosterol, a new steroid from an algicolous isolate of Aspergillus oryzae. Nat. Prod. Commun. 2010, 5, 1575–1578. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Xu, Z.; Bai, Q.; Zhou, X.; Zheng, C.; Bai, M.; Chen, G. Biological secondary metabolites from the Lumnitzera littorea-derived fungus Penicillium oxalicum HLLG-13. Mar. Drugs 2023, 21, 22. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, Z.; Qiu, P.; Wang, Q.; Yan, J.; Lu, Y.; Wasu, P.A.; Hong, K.; She, Z. Two new bioactive steroids from a mangrove-derived fungus Aspergillus sp. Steroids 2018, 140, 32–38. [Google Scholar] [CrossRef]
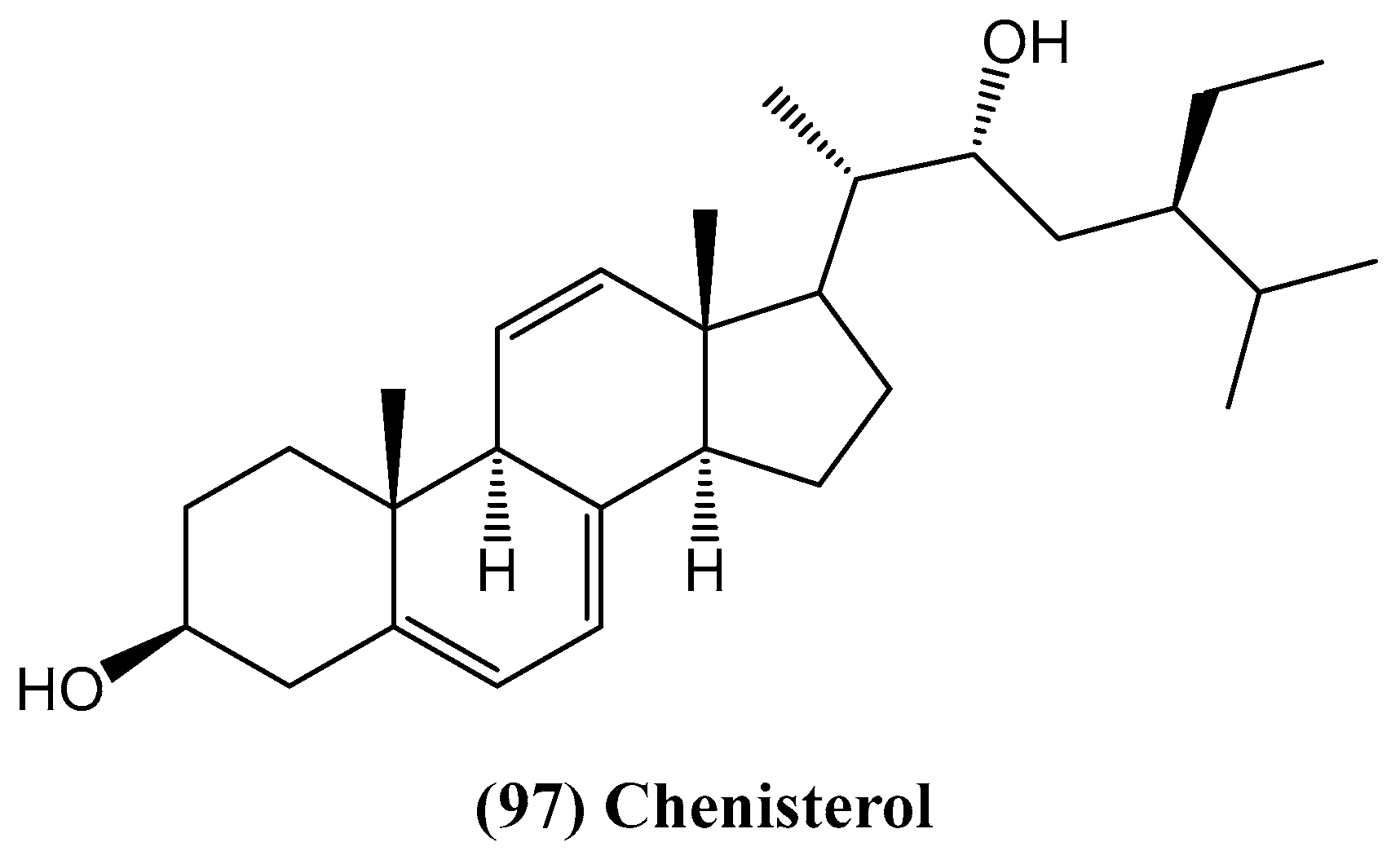
- Afaq, S.; Fatima, I.; Inamullah, F.; Khan, S.; Kazmi, M.H.; Malik, A.; Tareen, R.B.; Ali, M.S.; Farhad, M.Z.; Abbas, T. Chenisterol, a new antimicrobial steroid from Chenopodium badachschanicum. Chem. Nat. Compd. 2018, 54, 710–713. [Google Scholar] [CrossRef]
- Kokanova-Nedialkova, Z.; Nedialkov, P.T.; Nikolov, S.D. The genus Chenopodium: Phytochemistry, ethnopharmacology and pharmacology. Phcog. Rev. 2009, 3, 280–306. [Google Scholar]
- Li, B.; Wang, R.-Y.; Wang, M.-J.; Hu, F.-D.; Fei, D.-Q.; Zhang, Z.-X. Triterpenoids, steroids, and other constituents from the roots of Codonopsis pilosula var. modesta. Chem. Nat. Compd. 2022, 58, 611–615. [Google Scholar] [CrossRef]
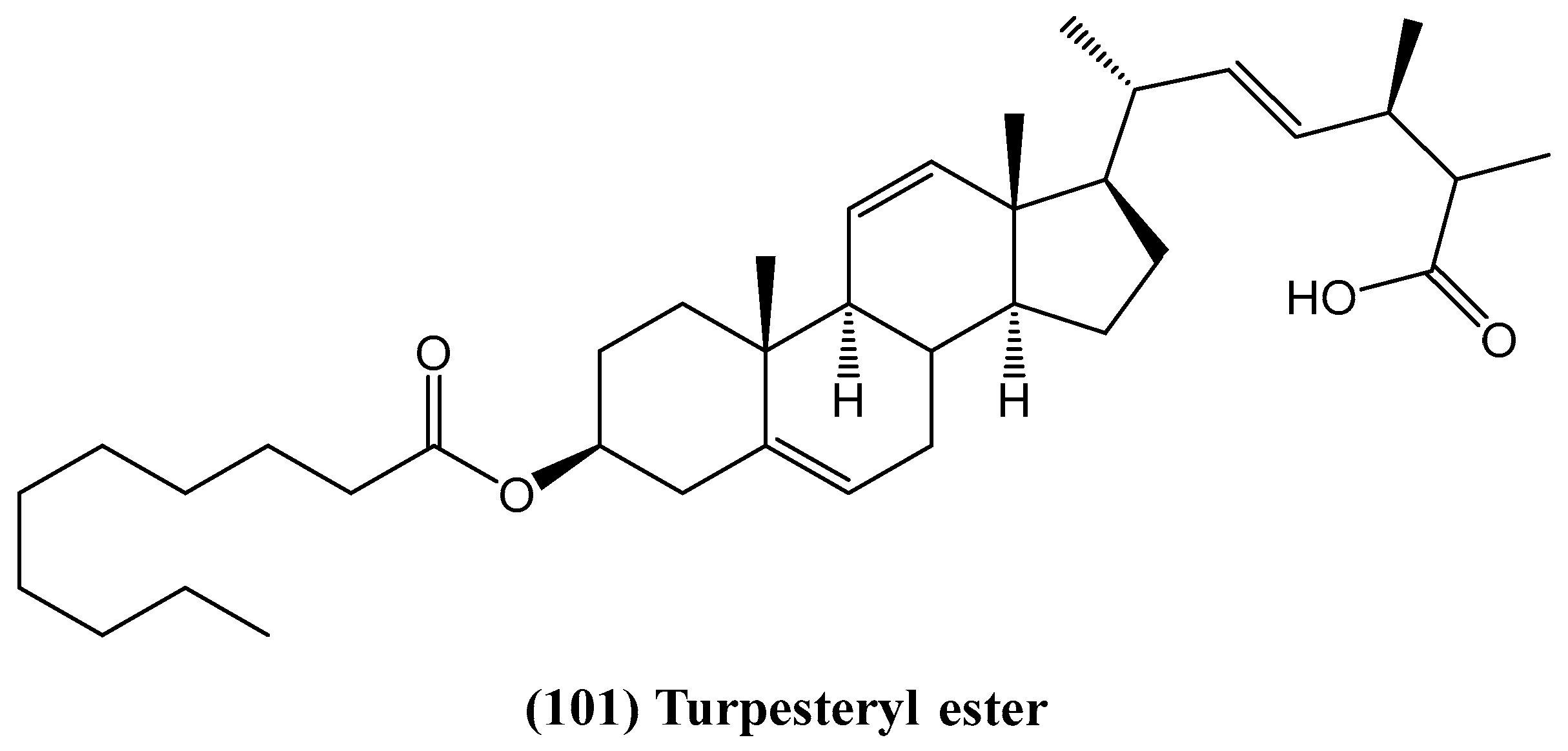
- Arif, Z.; Khan, S.; Farheen, S.; Kazmi, M.H.; Fatima, I.; Malik, A.; Ali, M.S.; Inamullah, F.; Afaq, S.; Shaikh, S.A.; et al. Turpesteryl ester, a new antibacterial steroid from Ipomoea turpethum. Chem. Nat. Compd. 2020, 56, 197–201. [Google Scholar] [CrossRef]
- Austin, D.F.; Huaman, Z. A synopsis of Ipomoea (Convolvulaceae) in the Americas. Taxon 1996, 45, 399–419. [Google Scholar] [CrossRef]
- Harun-Or-Rashid, M.; Gafur, M.A.; Sadik, M.G.; Rahman, M.A.A. Biological activities of a new acrylamide derivative from Ipomoea turpethum. Pak. J. Biol. Sci. 2002, 5, 968–971. [Google Scholar] [CrossRef]
- Sayed, H.M.; Mohamed, M.H.; Farag, S.F.; Mohamed, G.A.; Proksch, P. A new steroid glycoside and furochromones from Cyperus rotundus L. Nat. Prod. Res. 2007, 21, 343–350. [Google Scholar] [CrossRef]
- Suh, W.S.; Lee, S.Y.; Park, J.E.; Kim, D.H.; Kim, S.Y.; Lee, K.R. Two new steroidal alkaloids from the bulbs of Fritillaria thunbergii. Heterocycles 2018, 96, 921–930. [Google Scholar]
- Koduru, S.; Grierson, D.S.; van de Venter, M.; Afolayan, A.J. Anticancer activity of steroid alkaloids isolated from Solanum aculeastrum. Pharm. Biol. 2007, 45, 613–618. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Xu, F.; Morikawa, T.; Pongpiriyadacha, Y.; Nakamura, S.; Asao, Y.; Kumahara, A.; Matsuda, H. Medicinal Flowers. XII.1) New spirostane-type steroid saponins with antidiabetogenic activity from Borassus flabellifer. Chem. Pharm. Bull. 2007, 55, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Jansz, E.; Nikawela, J.K.; Theivendirarajah, K. Studies on the bitter principle and debittering of Palmyrah fruit pulp. J. Sci. Food Agric. 1994, 65, 185–189. [Google Scholar] [CrossRef]
- Ariyasena, D.D.; Jansz, E.R.; Abeysekera, A. Some studies directed at increasing the potential use of palmyrah (Borassus flabellifer L) fruit pulp. J. Sci. Food Agric. 2001, 81, 1347–1352. [Google Scholar] [CrossRef]
- Ahmad, A.; Gupta, G.; Afzal, M.; Kazmi, I.; Anwar, F. Antiulcer and antioxidant activities of a new steroid from Morus alba. Life Sci. 2013, 92, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Singab, A.N.; El-Beshbishy, H.A.; Yonekawa, M.; Nomura, T.; Fukai, T. Hypoglycemic effect of Egyptian Morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005, 100, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Choi, D.H.; Kim, E.J.; Kim, H.Y.; Kwon, T.O.; Kang, D.G.; Lee, H.S. Hypotensive, hypolipidemic, and vascular protective effects of Morus alba L. in rats fed an atherogenic diet. Am. J. Chin. Med. 2011, 39, 39–52. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, N.J.; Choi, J.S.; Park, J.C. Determination of flavonoid by HPLC and biological activities from the leaves of Cudrania tricuspidata Bureau. J. Korean Soc. Food Sci. Nutr. 1993, 22, 68–72. [Google Scholar]
- Arabshahi-Delouee, S.; Urooj, A. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem. 2007, 102, 1233–1240. [Google Scholar] [CrossRef]
- Wattanapitayakul, S.K.; Chularojmontri, L.; Herunsalee, A.; Charuchong-kolwongse, S.; Niumsakul, S.; Bauer, J.A. Screening of antioxidants from medicinal plants for cardioprotective effect against doxorubicin toxicity. Basic Clin. Pharmacol. Toxicol. 2005, 96, 80–87. [Google Scholar] [CrossRef]
- Choi, E.M.; Hwang, J.K. Effects of Morus alba leaf extract on the production of nitric oxide, prostaglandin E2 and cytokines in RAW264.7 macrophages. Fitoterapia 2005, 76, 608–613. [Google Scholar] [CrossRef]
- Niidome, T.; Takahashi, K.; Goto, Y.; Goh, S.M.; Tanaka, N.; Kamei, K. Mulberry leaf extract prevents amyloid beta-peptide fibril formation and neurotoxicity. Neuroreport 2007, 18, 813–816. [Google Scholar] [PubMed]
- Ameen, A.M.; Hapipah, M.A.; Abdul, A.K.; Mohd, N.S.; Salmah, I. Evaluation of the antiulcer activities of Morus alba extracts in experimentally induced gastric ulcer in rats. Biomed. Res. 2009, 20, 35–39. [Google Scholar]
- Wu, S.-B.; Bao, Q.-Y.; Wang, W.-X.; Zhao, Y.; Xia, G.; Zhao, Z.; Zeng, H.; Hu, J.-F. Cytotoxic triterpenoids and steroids from the bark of Melia azedarach. Planta Med. 2011, 77, 922–928. [Google Scholar] [CrossRef]
- Su, L.; Chen, G.; Feng, S.-G.; Wang, W.; Li, Z.-F.; Chen, H.; Pei, Y.-H. Steroidal saponins from Tribulus terrestris. Steroids 2009, 74, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.-P.; Wu, K.-L.; Yu, H.-S.; Pang, X.; Liu, J.; Han, L.-F.; Zhang, J.; Zhao, Y.; Xiong, C.-Q.; Song, X.-B.; et al. Steroidal saponins from Tribulus terrestris. Phytochemistry 2014, 107, 182–189. [Google Scholar] [PubMed]
- Tan, Z.; Zhao, J.-L.; Liu, J.-M.; Zhang, M.; Chen, R.-D.; Xie, K.-B.; Chen, D.-W.; Dai, J.-G. Lanostane triterpenoids and ergostane-type steroids from the cultured mycelia of Ganoderma capense. J. Asian Nat. Prod. Res. 2018, 20, 844–851. [Google Scholar]
- Hen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D.R.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 2012, 3, 913. [Google Scholar]
- Müller, C.I.; Kumagai, T.; O’Kelly, J.; Seeram, N.P.; Heber, D.; Koeffler, H.P. Ganoderma lucidum causes apoptosis in leukemia, lymphoma and multiple myeloma cells. Leuk. Res. 2006, 30, 841–848. [Google Scholar]
- Lu, Q.Y.; Jin, Y.S.; Zhang, Q.; Zhang, Z.; Heber, D.; Go, V.L. Ganoderma lucidum extracts inhibit growth and induce actin polymerization in bladder cancer cells in vitro. Cancer Lett. 2004, 216, 9–20. [Google Scholar]
- Isaka, M.; Chinthanom, P.; Sappan, M.; Danwisetkanjana, K.; Boonpratuang, T.; Choeyklin, R. Antitubercular lanostane triterpenes from cultures of the Basidiomycete Ganoderma sp. BCC 16642. J. Nat. Prod. 2016, 79, 161–169. [Google Scholar] [CrossRef]
- Min, B.S.; Nakamura, N.; Miyashiro, H.; Bae, K.W.; Hattori, M. Triterpenes from the spores of Ganoderma lucidum and their inhibitory activity against HIV-1 Protease. Chem. Pharm. Bull. 1998, 46, 1607–1612. [Google Scholar] [CrossRef]
- Kim, H.W.; Shim, M.J.; Choi, E.C.; Kim, B.K. Inhibition of cytopathic effect of human immunodeficiency virus-1 by water-soluble extract of Ganoderma lucidum. Arch. Pharm. Res. 1997, 20, 425–431. [Google Scholar] [CrossRef]
- Ma, H.-T.; Hsieh, J.-F.; Chen, S.-T. Anti-diabetic effects of Ganoderma lucidum. Phytochemistry 2015, 114, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Christen, M.; Plitzko, I.; Zimmermann, S.; Brun, R.; Kaiser, M.; Hamburger, M. Antiplasmodial lanostanes from the Ganoderma lucidum mushroom. J. Nat. Prod. 2010, 73, 897–900. [Google Scholar] [CrossRef]
- Chepkirui, C.; Sum, W.C.; Cheng, T.; Matasyoh, J.C.; Decock, C.; Stadler, M. Aethiopinolones A–E, new pregnenolone type steroids from the East African Basidiomycete Fomitiporia aethiopica. Molecules 2018, 23, 369. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.E.; Kehraus, S.; König, G.M. Caught between triterpene- and steroid-metabolism: 4a-Carboxylic pregnane-derivative from the marine alga-derived fungus Phaeosphaeria spartinae. Steroids 2013, 78, 880–883. [Google Scholar]
- Yin, R.; Zhao, Z.; Ji, X.; Dong, Z.; Li, Z.; Feng, T.; Liu, J. Steroids and sesquiterpenes from cultures of the fungus Phellinus igniarius. Nat. Prod. Bioprospect. 2015, 5, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Yang, K.; Wu, C.; Gao, S.; Wang, P.; Chen, L.; Li, H. New natural inhibitors of hexokinase 2 (HK2): Steroids from Ganoderma sinense. Fitoterapia 2018, 125, 123–129. [Google Scholar] [CrossRef]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. Hexokinase-2 bound to mitochondria: Cancer’s stygian link to the “Warburg effect” and a pivotal target for effective therapy. Semin. Cancer Biol. 2009, 19, 17–24. [Google Scholar] [CrossRef]
- Tan, V.P.; Miyamoto, S. HK2/hexokinase-II integrates glycolysis and autophagy to confer cellular protection. Autophagy 2015, 11, 963–964. [Google Scholar] [CrossRef]
- Krasnov, G.S.; Dmitriev, A.A.; Lakunina, V.A.; Kirpiy, A.A.; Kudryavtseva, A.V. Targeting VDAC-bound hexokinase II: A promising approach for concomitant anticancer therapy. Expert Opin. Ther. Targets 2013, 17, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Ros, S.; Schulze, A. Glycolysis back in the limelight: Systemic targeting of HK2 blocks tumor growth. Cancer Discov. 2013, 3, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.C.; Wang, Q.; Bhaskar, P.T.; Miller, L.; Wang, Z.; Wheaton, W.; Chandel, N.; Laakso, M.; Muller, W.J.; Allen, E.L. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 2013, 24, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, R.-K.; Sang, P.; Xie, Y.-X.; Zhang, Y.; Zhou, Z.-H.; Wang, K.-K.; Zhou, F.-M.; Ji, X.-B.; Liu, W.-J.; et al. HK2 and LDHA upregulation mediate hexavalent chromium-induced carcinogenesis, cancer development and prognosis through miR-218 inhibition. Ecotoxicol. Environ. Saf. 2024, 279, 116500. [Google Scholar] [CrossRef]
- Wang, X.; Ma, M.; Shao, S.; Xu, X.; Qin, C.; Gao, R.; Zhang, Z. TWIST1 Regulates HK2 ubiquitination degradation to promote pancreatic cancer invasion and metastasis. Cancer Cell Int. 2025, 25, 37. [Google Scholar] [CrossRef]



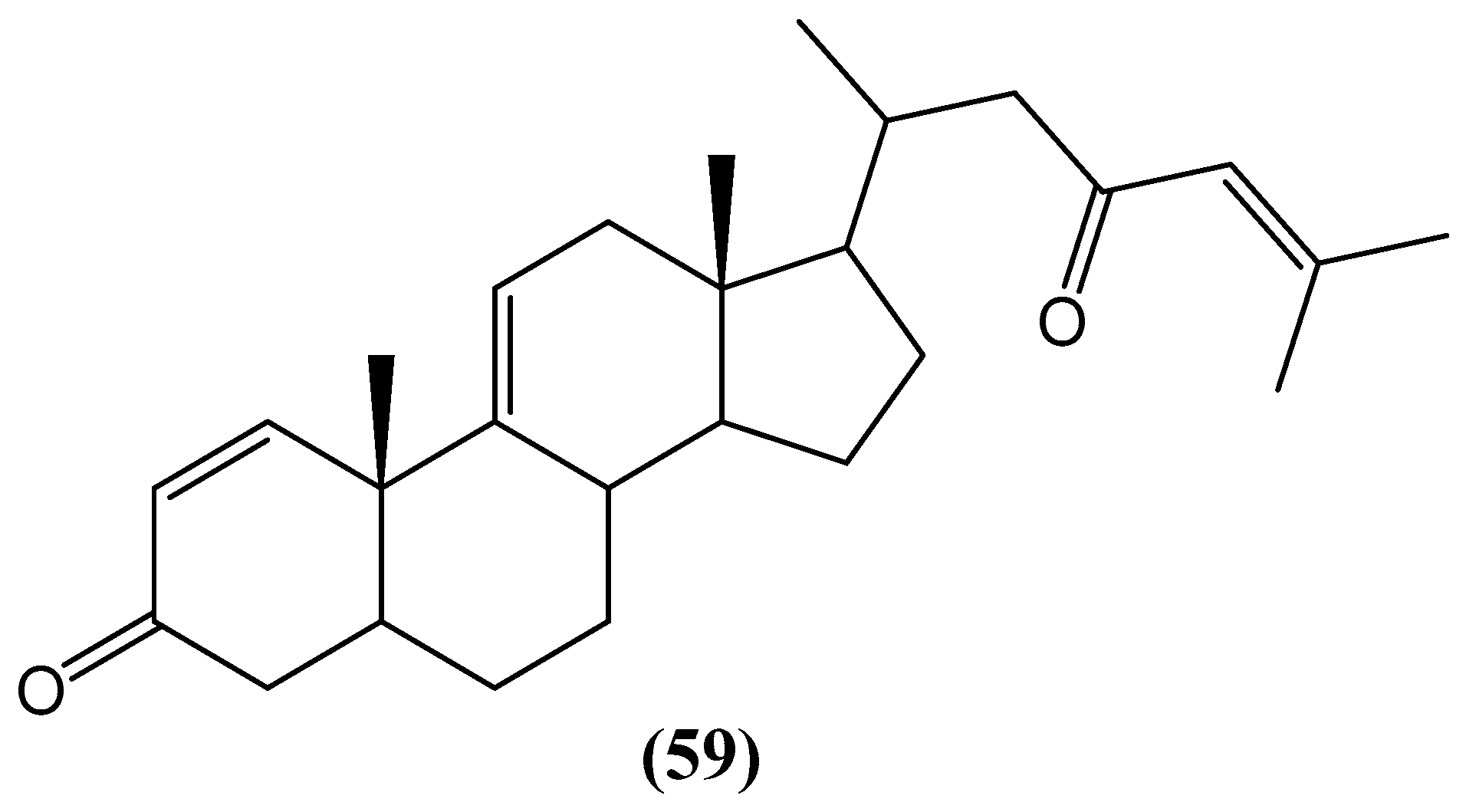
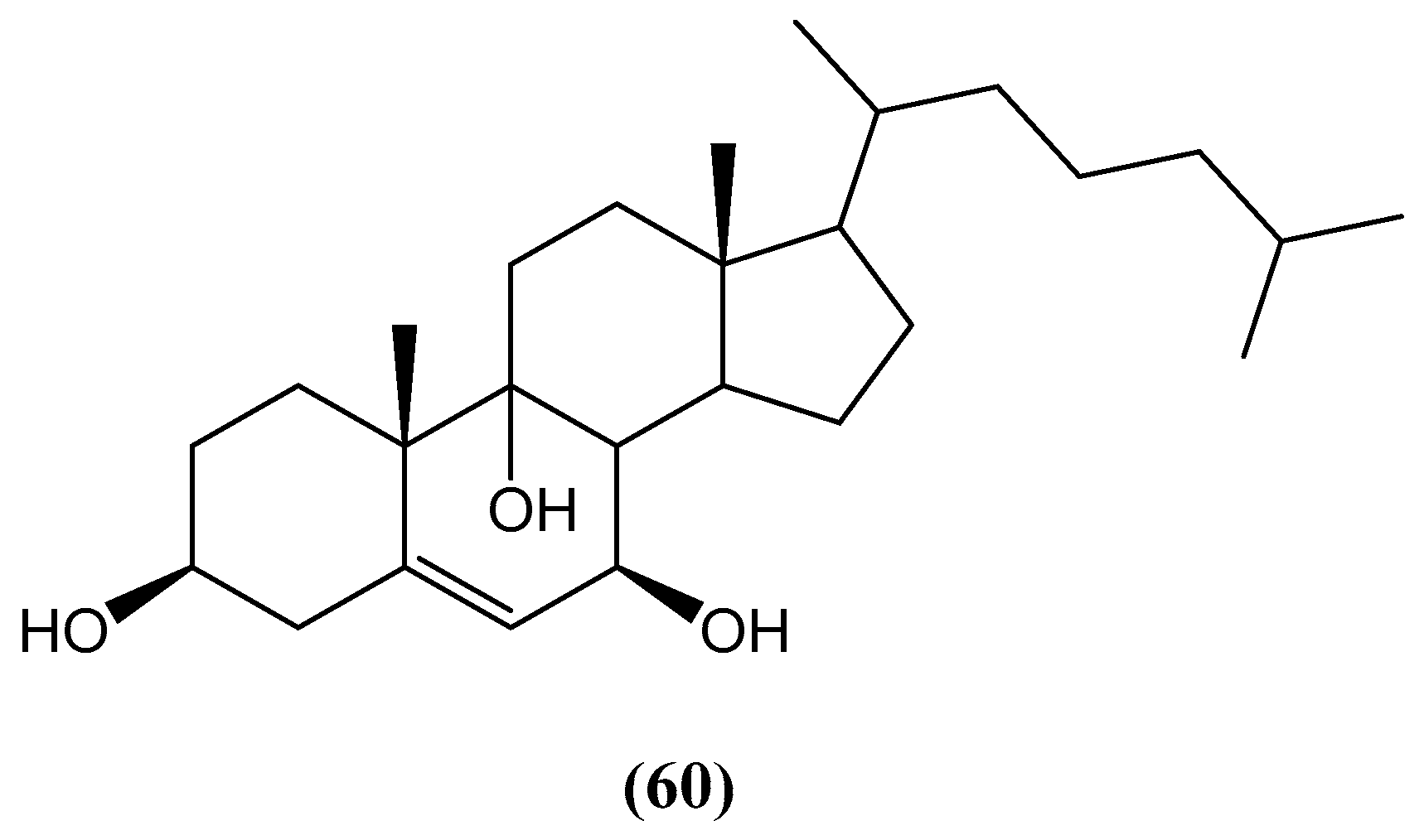
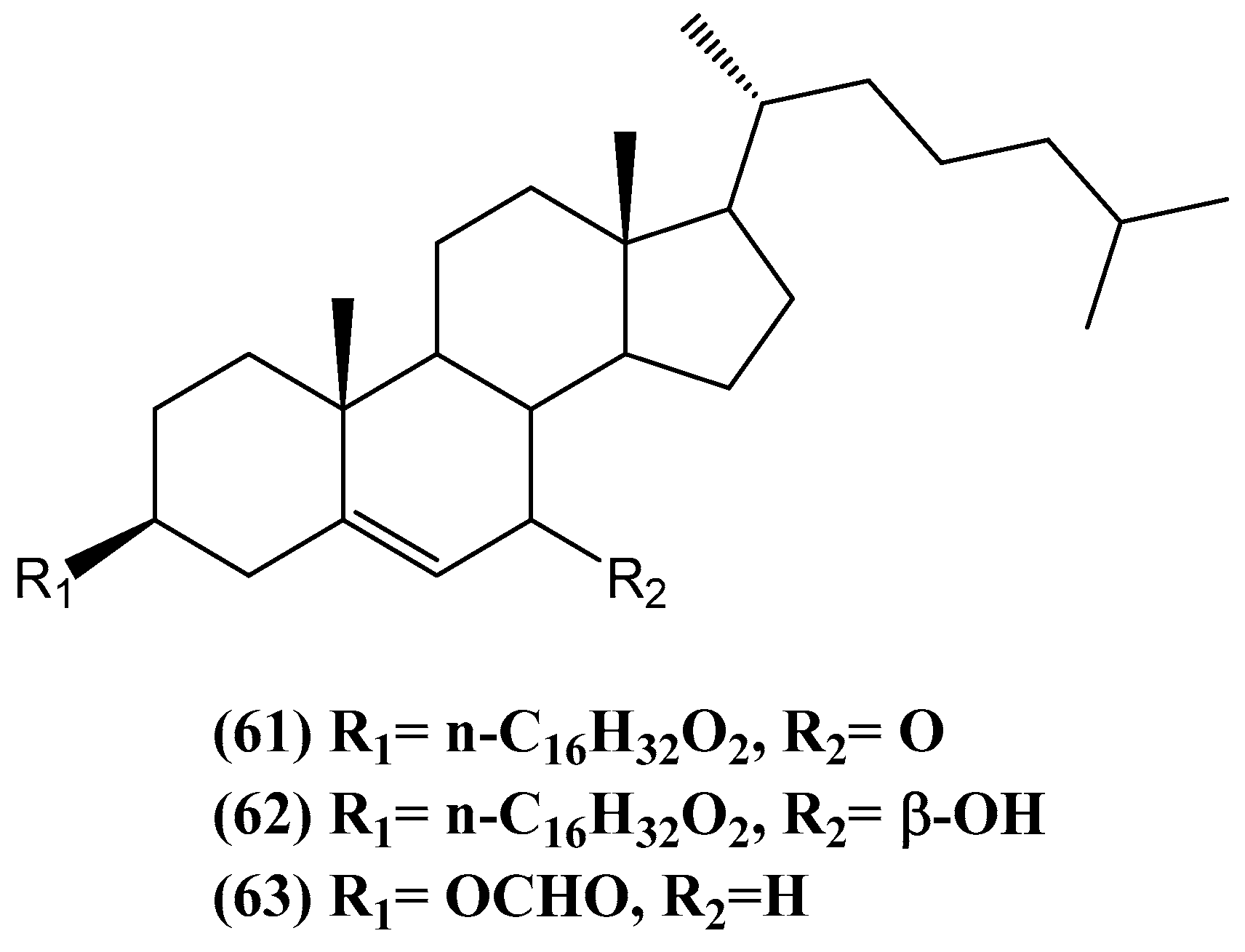
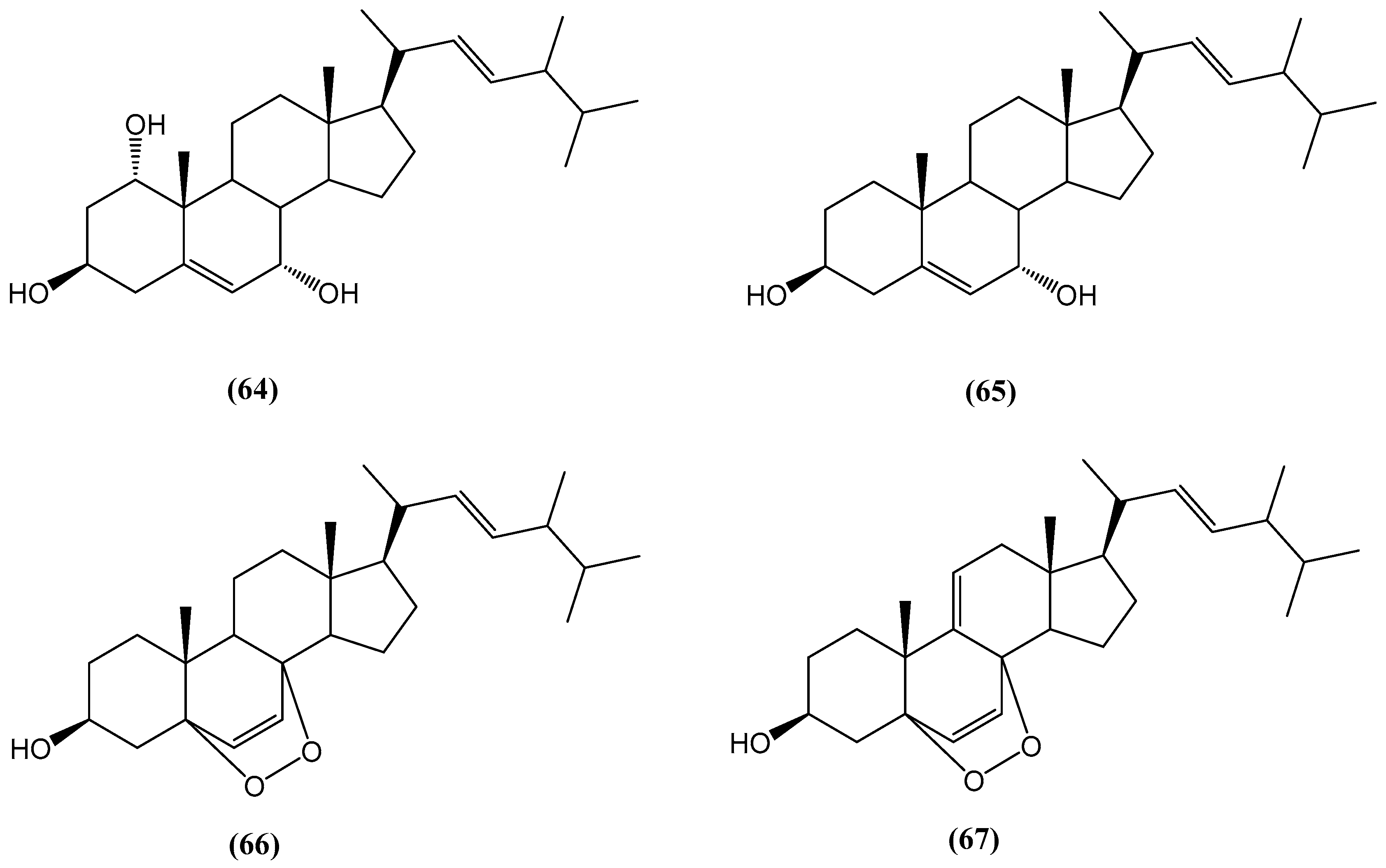

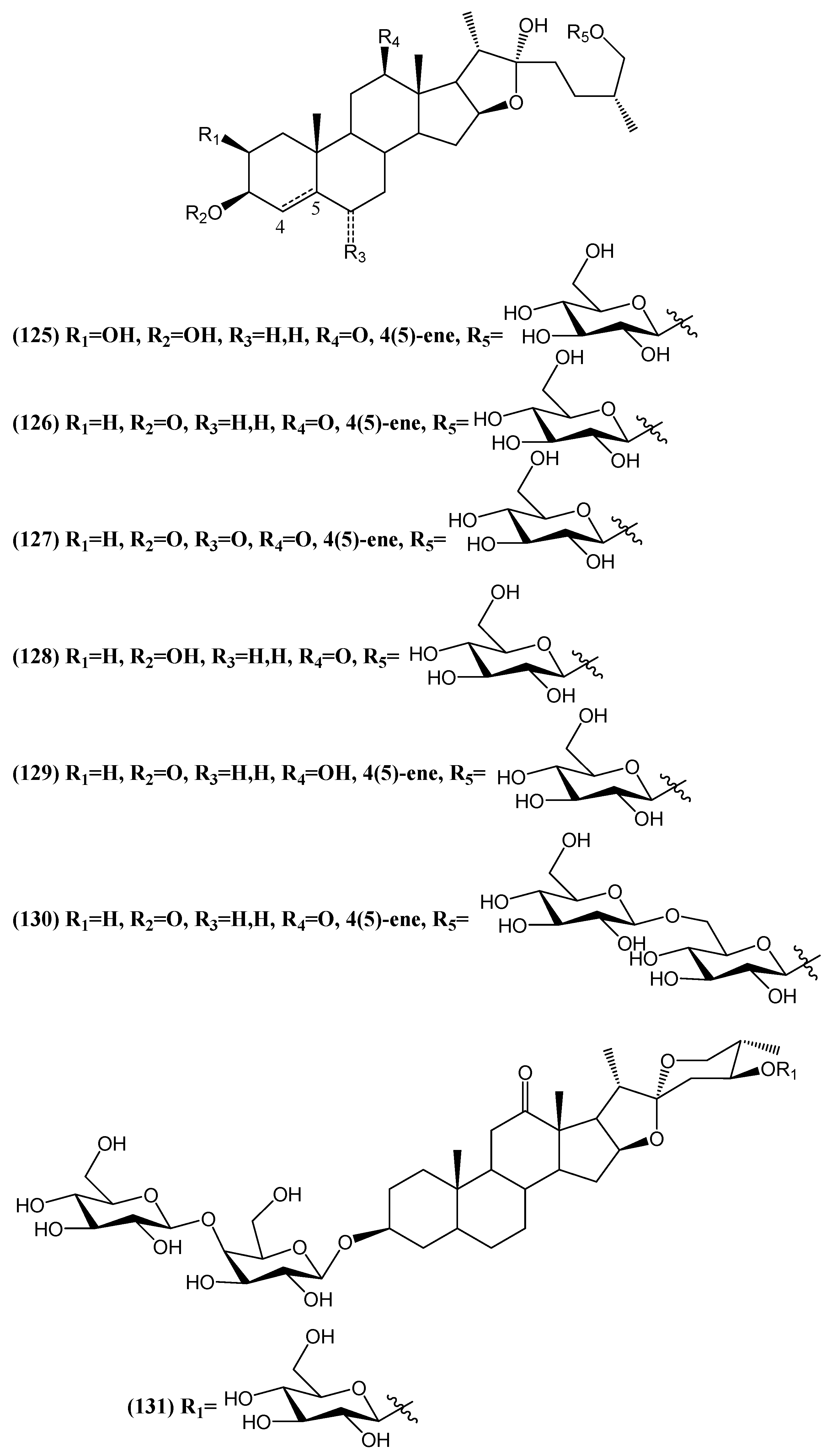

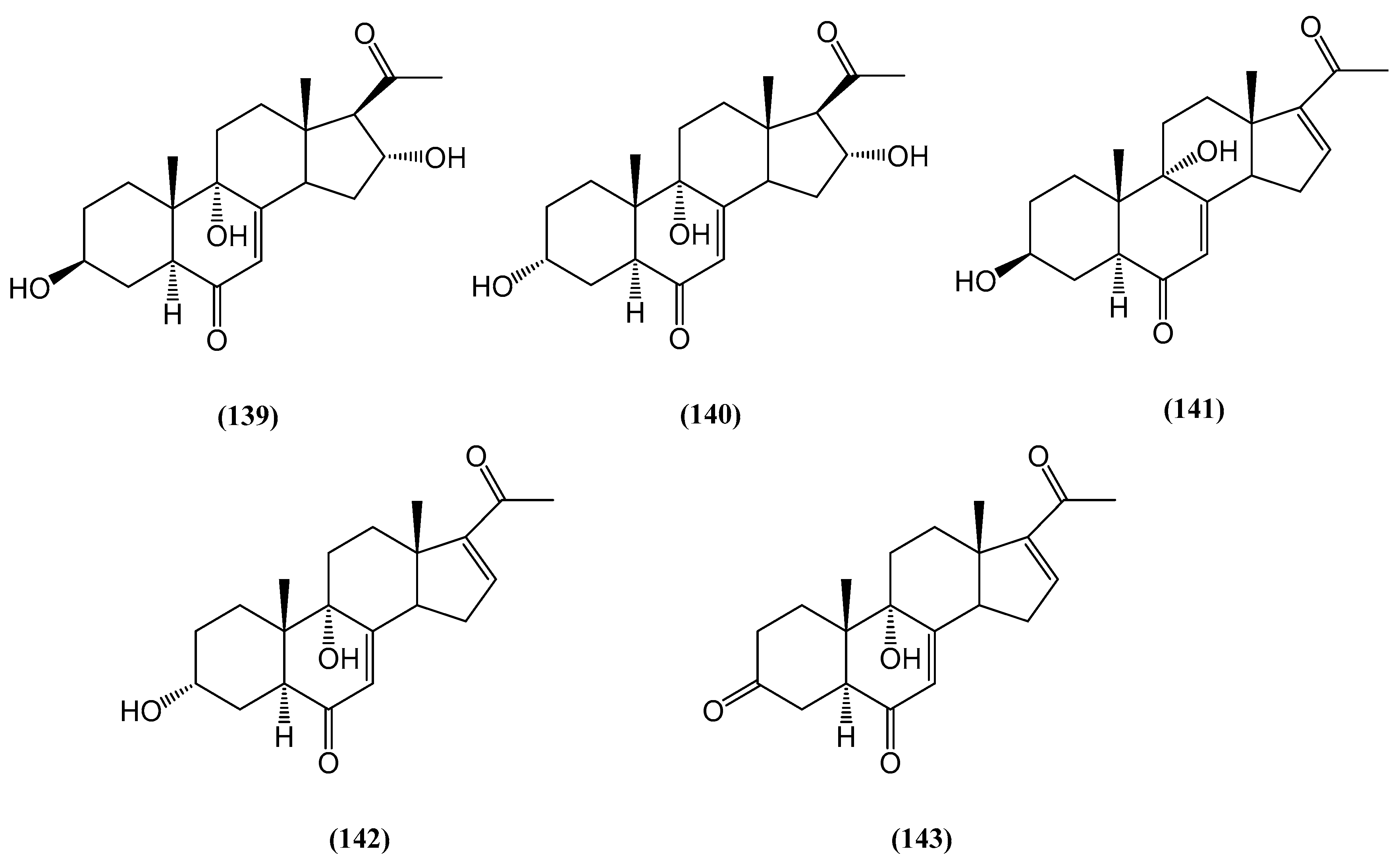
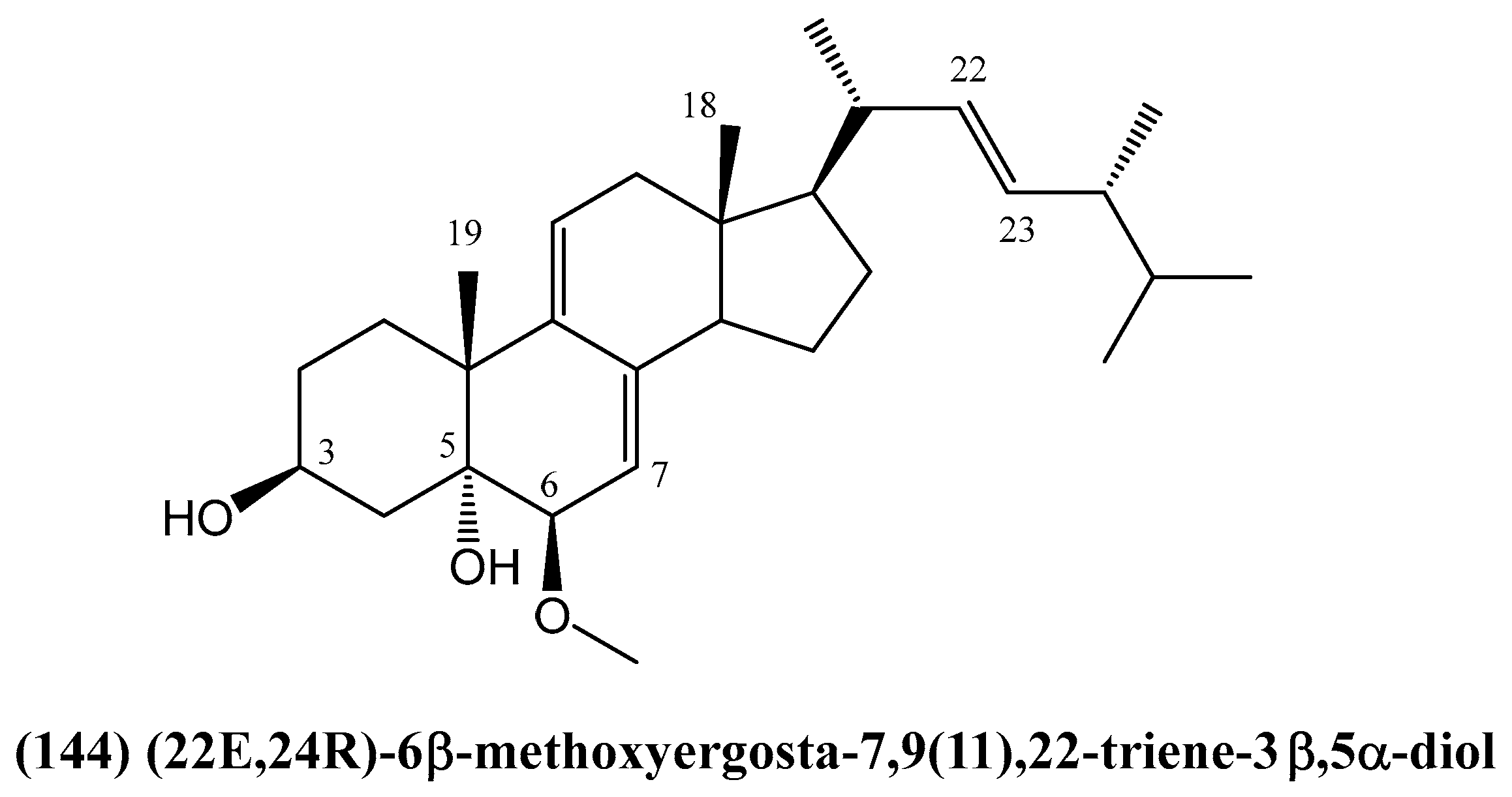
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajdaś, G.; Koenig, H.; Pospieszny, T. Recent Advances in Steroid Discovery: Structural Diversity and Bioactivity of Marine and Terrestrial Steroids. Int. J. Mol. Sci. 2025, 26, 3203. https://doi.org/10.3390/ijms26073203
Hajdaś G, Koenig H, Pospieszny T. Recent Advances in Steroid Discovery: Structural Diversity and Bioactivity of Marine and Terrestrial Steroids. International Journal of Molecular Sciences. 2025; 26(7):3203. https://doi.org/10.3390/ijms26073203
Chicago/Turabian StyleHajdaś, Grzegorz, Hanna Koenig, and Tomasz Pospieszny. 2025. "Recent Advances in Steroid Discovery: Structural Diversity and Bioactivity of Marine and Terrestrial Steroids" International Journal of Molecular Sciences 26, no. 7: 3203. https://doi.org/10.3390/ijms26073203
APA StyleHajdaś, G., Koenig, H., & Pospieszny, T. (2025). Recent Advances in Steroid Discovery: Structural Diversity and Bioactivity of Marine and Terrestrial Steroids. International Journal of Molecular Sciences, 26(7), 3203. https://doi.org/10.3390/ijms26073203





