Cytochalasin B Mitigates the Inflammatory Response in Lipopolysaccharide-Induced Mastitis by Suppressing Both the ARPC3/ARPC4-Dependent Cytoskeletal Changes and the Association Between HSP70 and the NLRP3 Inflammasome
Abstract
1. Introduction
2. Results
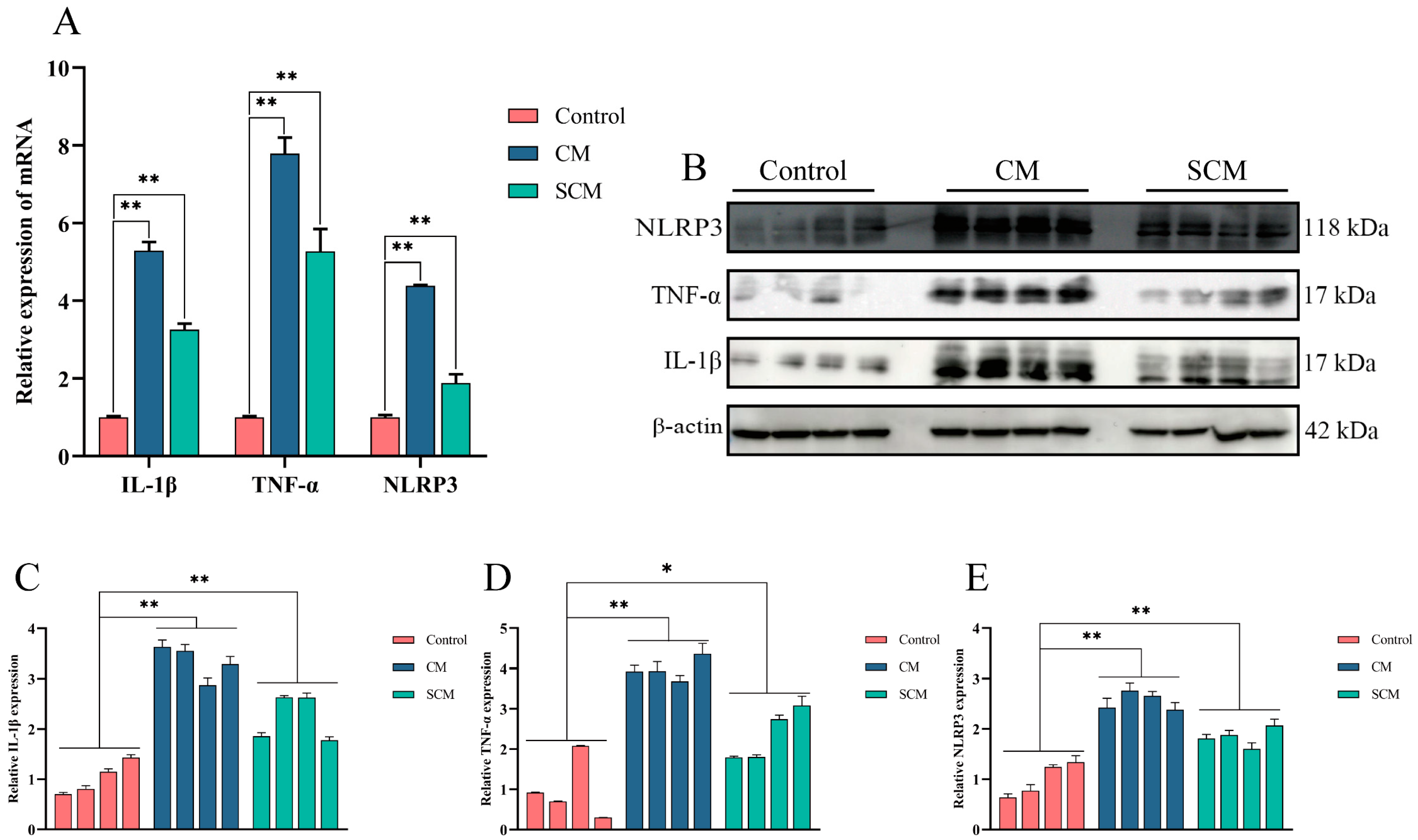
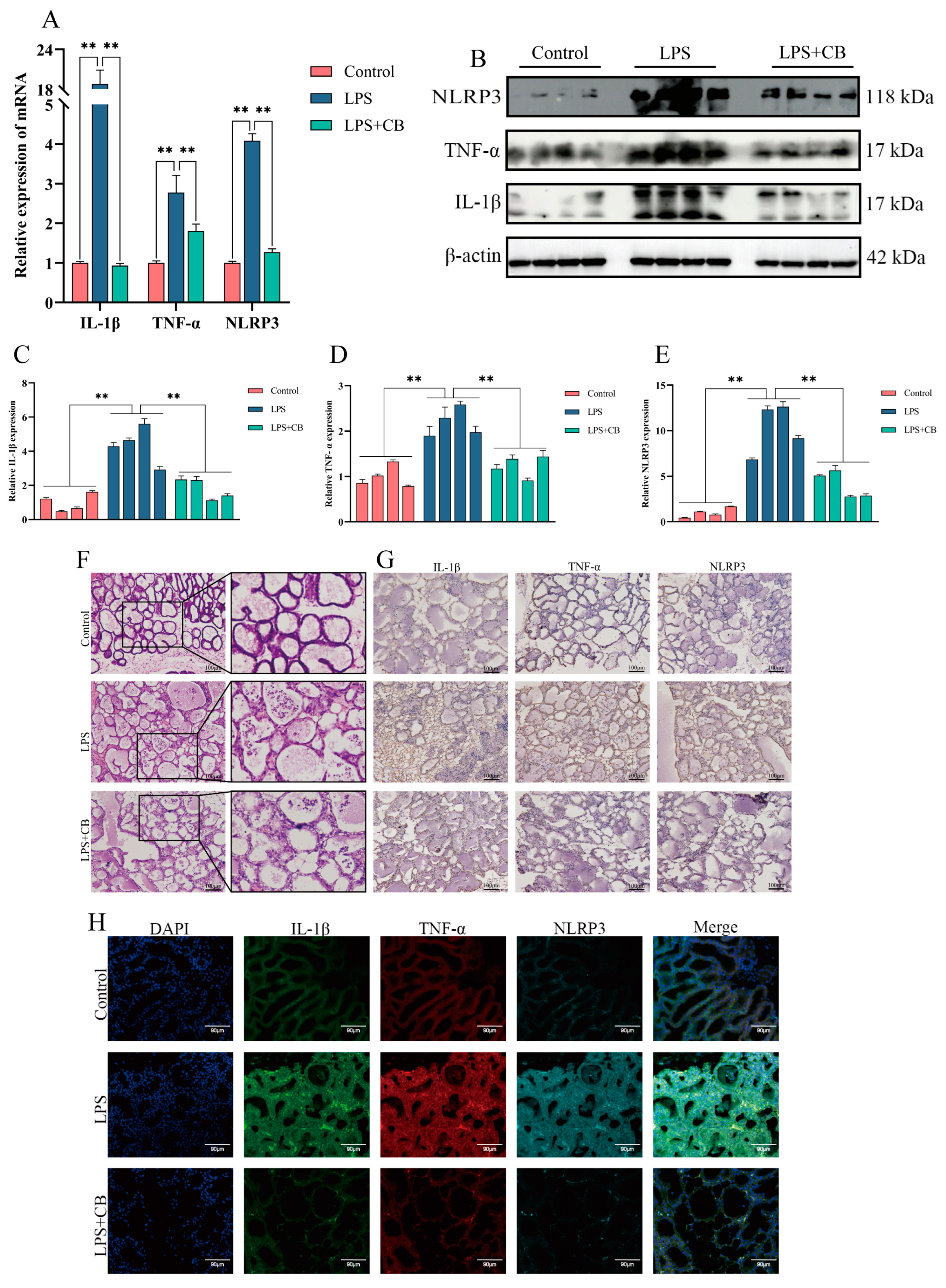
2.1. Expression of Inflammatory Cytokines and Activation of the NLRP3 Inflammasome in Bovine Mastitis Tissue
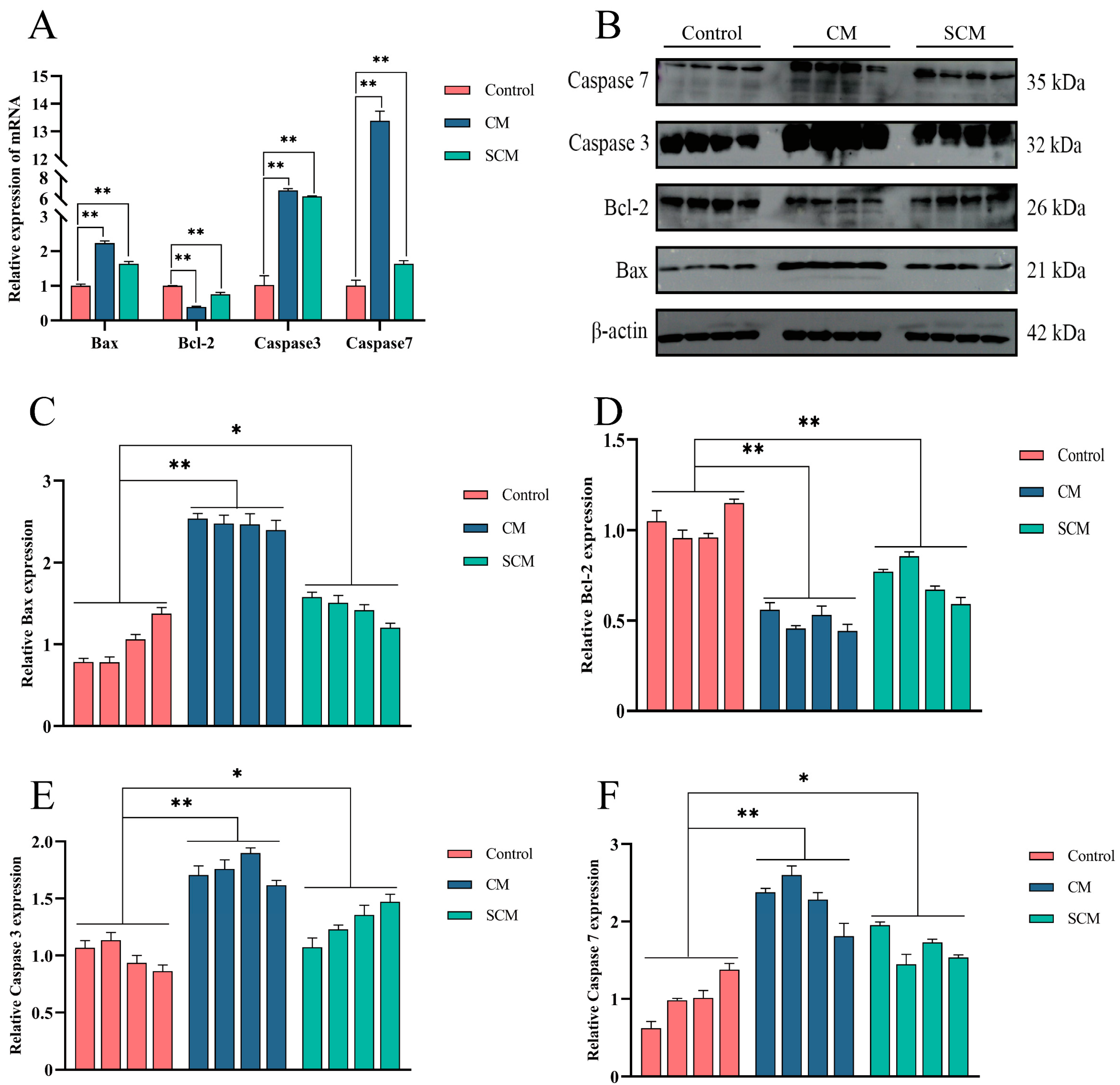
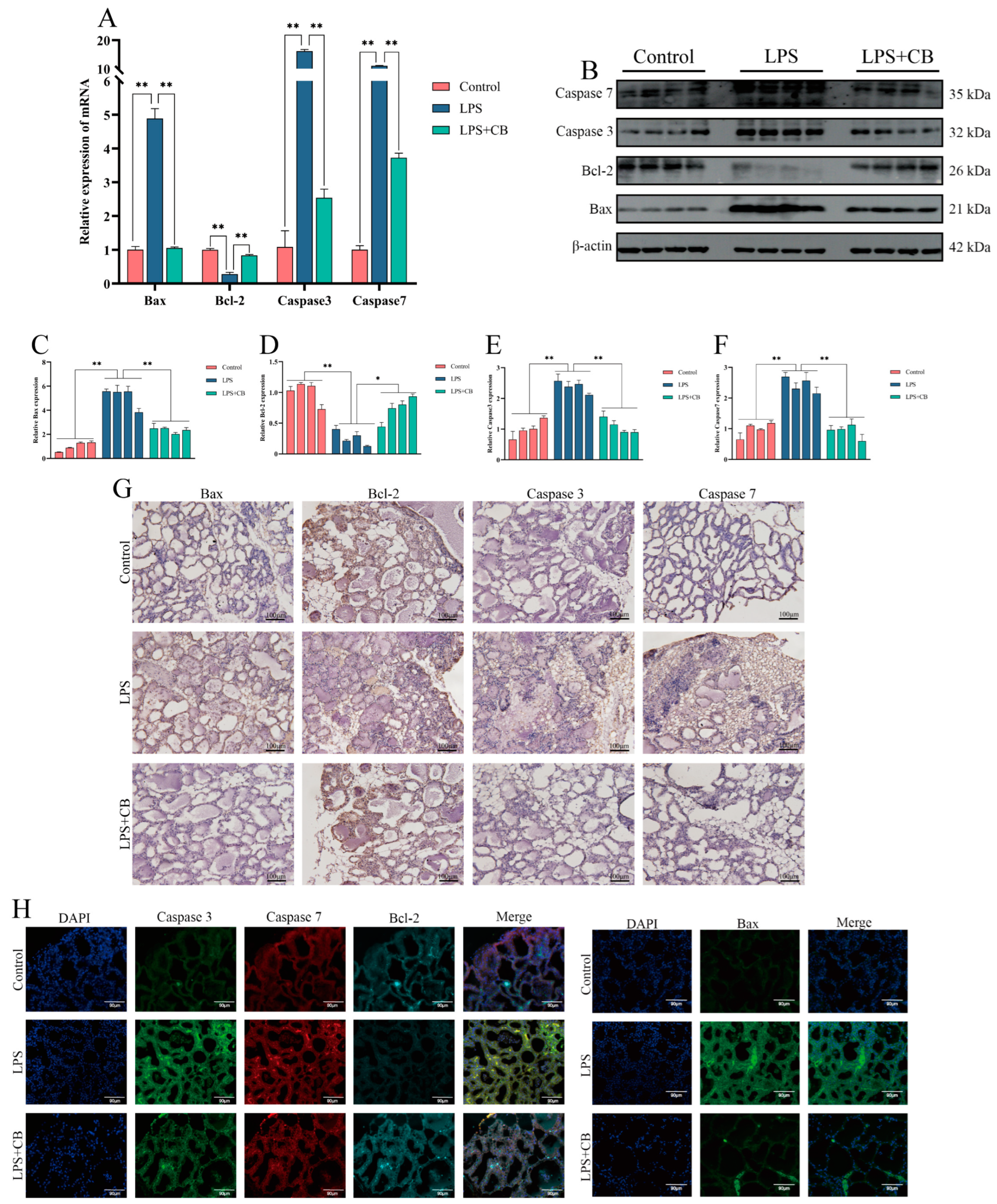
2.2. Increased Apoptosis in Bovine Mastitis Tissue
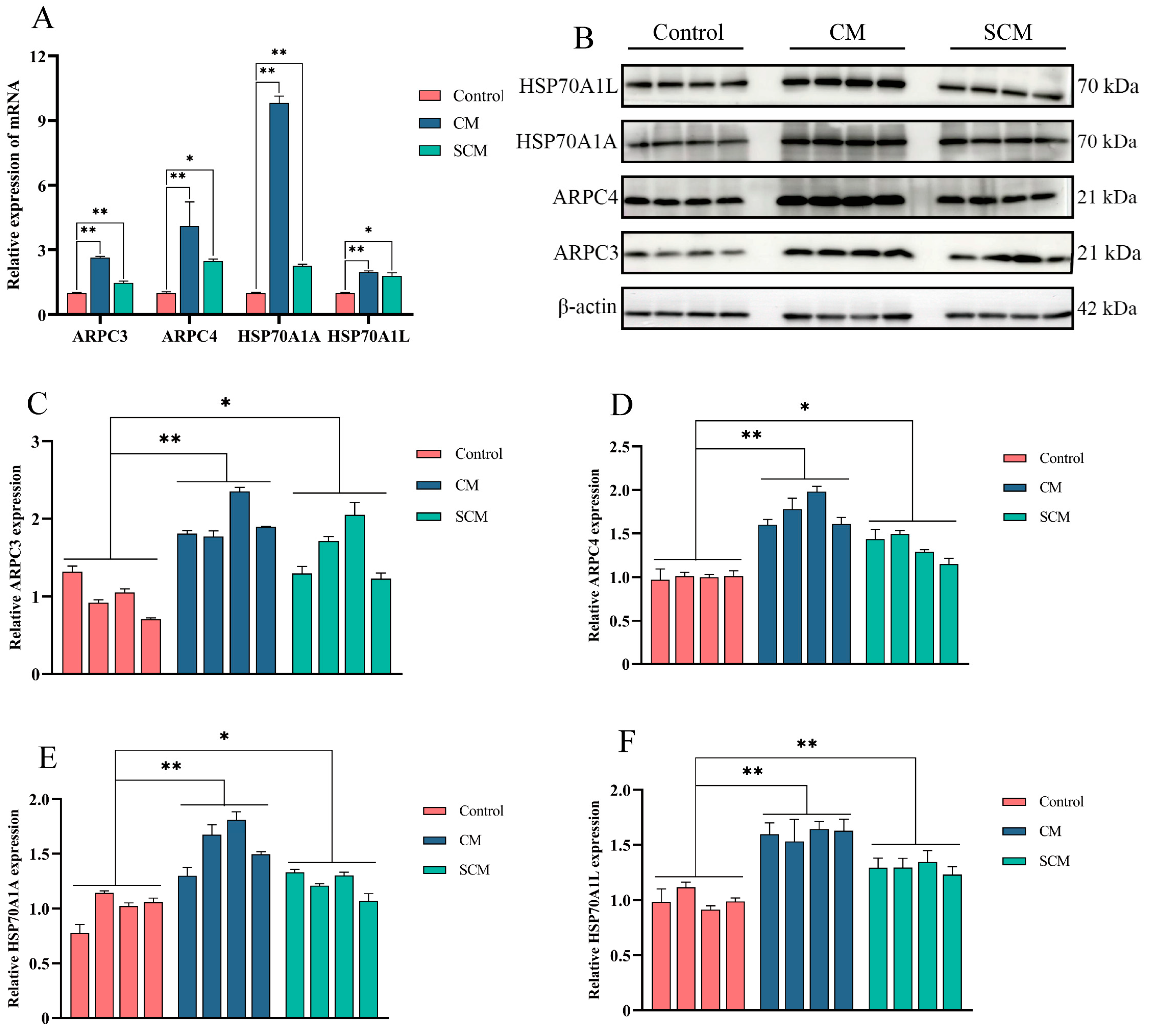
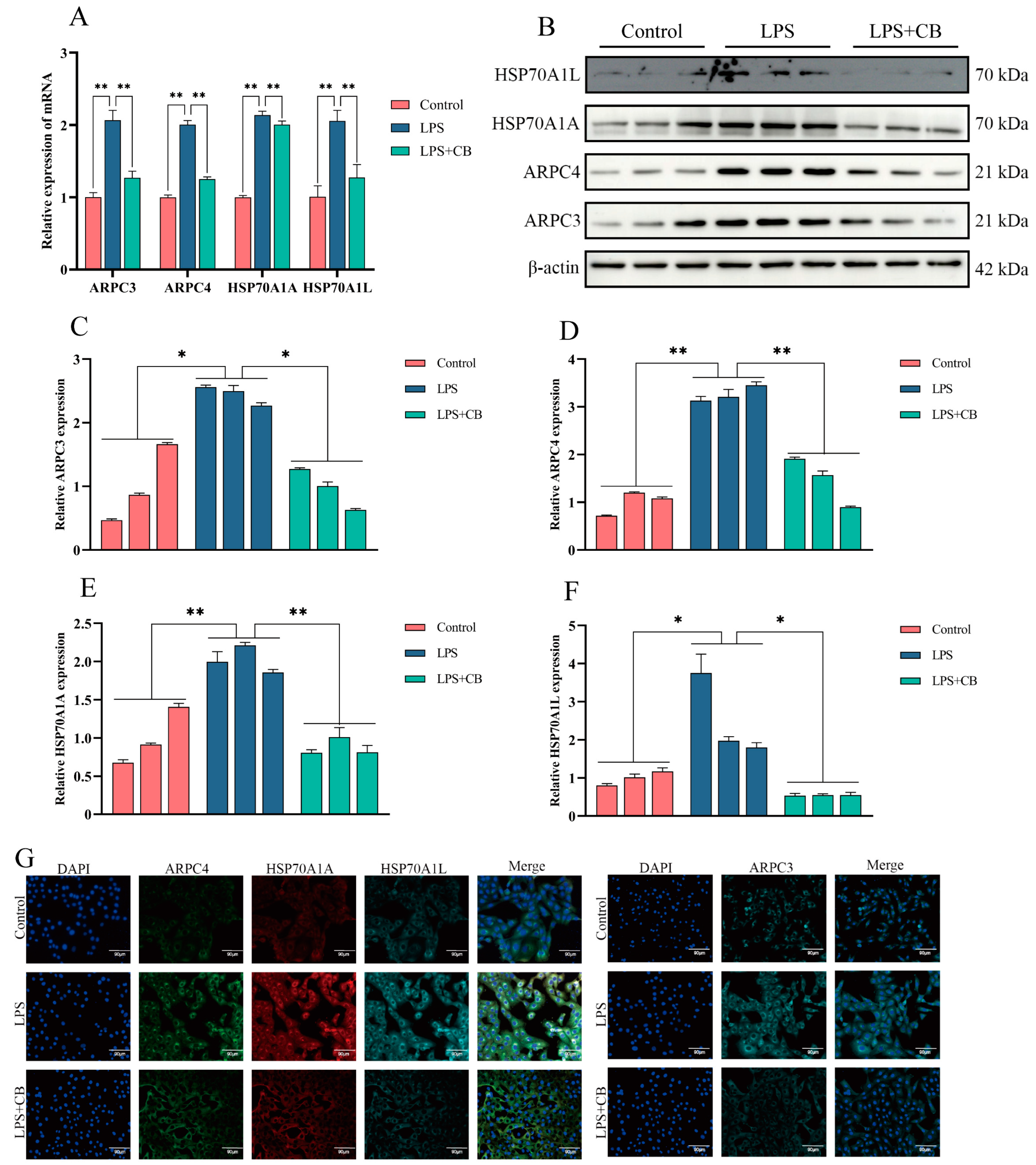
2.3. Enhanced Expression of ARPC3, ARPC4, and HSP70 in Bovine Mastitis Tissue
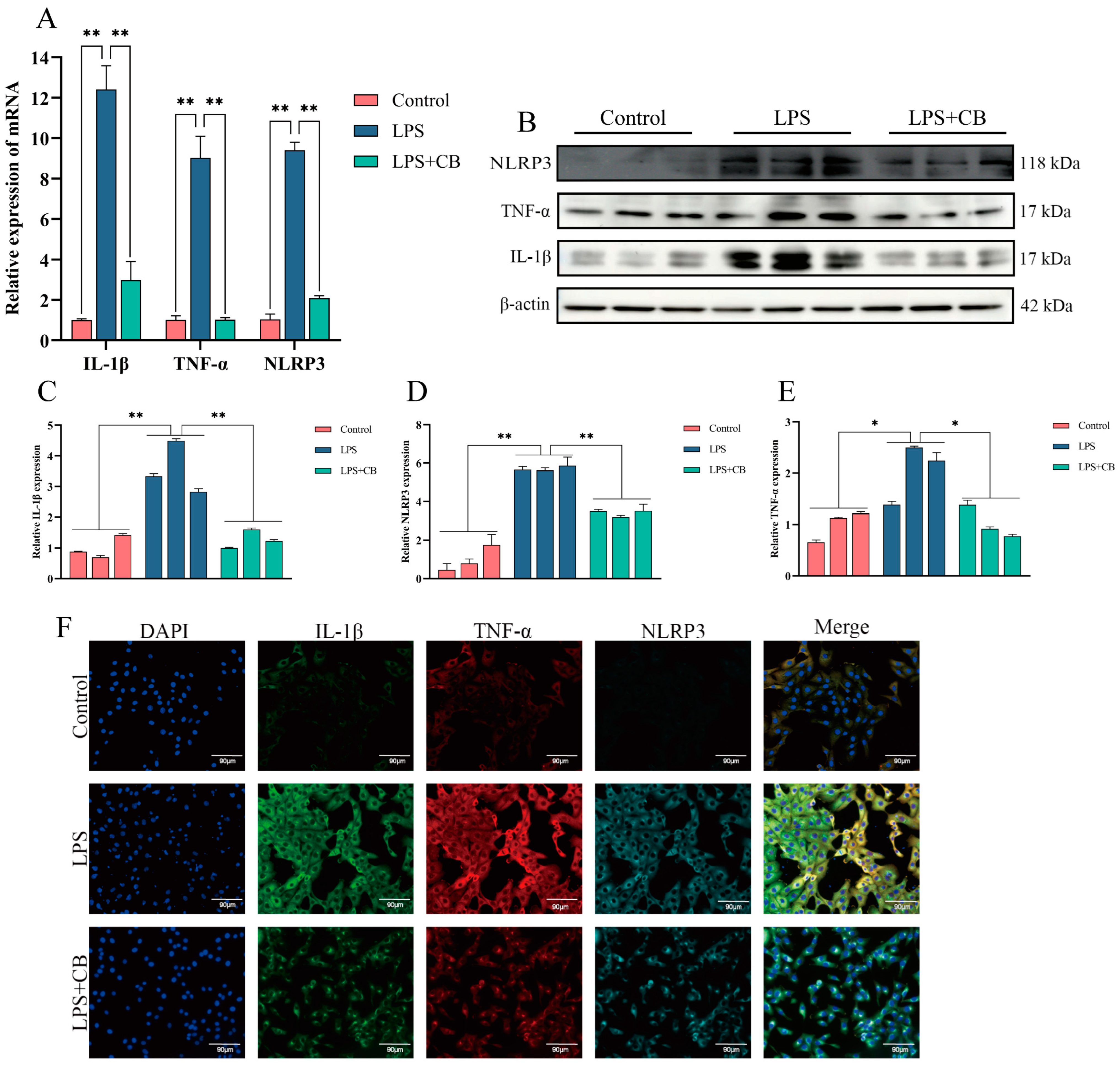
2.4. CB Inhibits LPS-Induced Expression of Inflammatory Cytokines and Activation of the NLRP3 Inflammasome in MAC-T Cells
2.5. CB Inhibits LPS-Induced Apoptosis in MAC-T Cells
2.6. CB Inhibits LPS-Induced Expression of ARPC3, ARPC4, and HSP70 in MAC-T Cells
2.7. CB’s Effects on LPS-Induced Pathological Damage and Inflammatory Responses in Mouse Mastitis Tissue
2.8. CB Inhibits LPS-Induced Apoptosis in Mouse Mammary Cells
2.9. CB Inhibits LPS-Induced Expression of ARPC3, ARPC4, and HSP70 in Mouse Mammary Tissue
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Tissue Sample Preparation
4.3. Animal Housing and Treatment
4.4. RNA Extraction, cDNA Synthesis, and qPCR
4.5. Western Blot
4.6. Immunohistochemistry and Immunofluorescence Staining
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ragul, P.; Gopal Dhinakar, R.; Ranjithkumar, D.; Subbarayalu, R.; Mahesh Prabu, E.; Bhavadharani, P.; Veeramani, V.; Arunkumar, N.; Siddarth, P.; Muthupandian, S. Is AMR in Dairy Products a Threat to Human Health? An Updated Review on the Origin, Prevention, Treatment, and Economic Impacts of Subclinical Mastitis. Infect. Drug Resist. 2023, 16, 155–178. [Google Scholar]
- Ké, L.; Mingyuan, H.; Lin, Z.; Mengyue, T.; Ming, Y.; Jia, L.; Yeru, L.; Dongmin, Z.; Ruonan, L.; Yuzhong, M. Analysis of antimicrobial resistance and genetic correlations of Escherichia coli in dairy cow mastitis. J. Vet. Res. 2022, 66, 571. [Google Scholar]
- Johnzon, C.-F.; Dahlberg, J.; Gustafson, A.-M.; Waern, I.; Moazzami, A.A.; Östensson, K.; Pejler, G. The Effect of Lipopolysaccharide-Induced Experimental Bovine Mastitis on Clinical Parameters, Inflammatory Markers, and the Metabolome: A Kinetic Approach. Front. Immunol. 2018, 9, 1487. [Google Scholar] [CrossRef] [PubMed]
- Luca, P.; Provvidenza Maria, A.; Riccardo, T.; Andrea, A.; Giovannamaria, P.; Gregorio, R.; Claudia, C.; Valeria, P.; Nicoletta, C.; Silvia, C.; et al. Cytochalasin B Influences Cytoskeletal Organization and Osteogenic Potential of Human Wharton’s Jelly Mesenchymal Stem Cells. Pharmaceuticals 2023, 16, 289. [Google Scholar] [CrossRef]
- Mi-Yeon, K.; Jong-Hoon, K.; Jae Youl, C. Cytochalasin B Modulates Macrophage-Mediated Inflammatory Responses. Biomol. Ther. 2014, 22, 295. [Google Scholar]
- Tong, Z.; Liu, N.; Song, H.; Li, J.Q.; Jiang, J.; Zhu, J.; Qi, J. Cytochalasin B inhibits the proliferation of human glioma U251 cells through cell cycle arrest and apoptosis. Genet. Mol. Res. 2014, 13, 10811–10822. [Google Scholar] [CrossRef] [PubMed]
- Fábio, V.; Madalena, R.; Ana, B.; Ana, M.A.; José Guilherme, T.; Maria Filomena, B. P-0080 New Options in Hepatocellular Carcinoma Therapy: The Role of Quercetin and Cytochalasin-B. Ann. Oncol. 2012, 23, 54. [Google Scholar]
- Serge, M.; Avinash, R.S. The cytoskeleton in cell-autonomous immunity: Structural determinants of host defence. Nat. Rev. Immunol. 2015, 15, 559–573. [Google Scholar]
- Richard, J.S.; Virginie, P.H.; Marco, A.P.; Melanie, M.; Rebecca, A.; Marcus, H.; Susanne, M.B.; Jürgen, H.; Robin, A.; Michael, P.W.; et al. HCMV pUL135 Remodels the Actin Cytoskeleton to Impair Immune Recognition of Infected Cells. Cell Host Microbe 2014, 16, 201–214. [Google Scholar]
- Jörg, R.; Mary, S.S.; Dan, W.; Thomas, R.; Klaus, L. Phosphoinositide 3-kinase required for lipopolysaccharide-induced transepithelial neutrophil trafficking in the lung. Eur. Respir. J. 2009, 35, 1137–1147. [Google Scholar]
- Tao, L.; Liu, Y.; Fan, G.; Zhang, H.; Zong, Y.; Yang, X. GRK6 palmitoylation increasing its membrance translocation promotes LPS-induced inflammation by PI3K/AKT pathway in kuppfer cells. Int. Immunopharmacol. 2023, 117, 109933. [Google Scholar] [PubMed]
- Punsiri, M.C.; Caylin, G.W.; Daniel, E.V. Hijacking Host Cell Highways: Manipulation of the Host Actin Cytoskeleton by Obligate Intracellular Bacterial Pathogens. Front. Cell. Infect. Microbiol. 2016, 6, 107. [Google Scholar]
- Christopher, T.J.; Thomas, D.P. Crystals of the Arp2/3 complex in two new space groups with structural information about actin-related protein 2 and potential WASP binding sites. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71, 1161–1168. [Google Scholar]
- Xiong, H.; Han, X.; Cai, L.; Zheng, H. Natural polysaccharides exert anti-tumor effects as dendritic cell immune enhancers. Front. Oncol. 2023, 13, 1274048. [Google Scholar]
- Pan, M.H.; Xu, R.; Zhang, Y.; Yin, L.; Li, R.; Wen, D.; Lu, S.; Gao, Y.; Zhao, X.; Wei, Q.; et al. The Impact of Arp2/3 Complex Inhibition on Cytoskeleton Dynamics and Mitochondrial Function during Goat Oocyte Meiosis. Animals 2023, 13, 263. [Google Scholar] [CrossRef]
- Susannah, B.; Lori, B.; Matthew, D.M.; Carla, A.P.; James, L.M.; Hal, M.H. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J. Clin. Investig. 2013, 123, 4695–4705. [Google Scholar]
- Paul, K.; Kieran, G.M.; Cliona, O.F. Non-canonical Inflammasome-Mediated IL-1β Production by Primary Endometrial Epithelial and Stromal Fibroblast Cells Is NLRP3 and Caspase-4 Dependent. Front. Immunol. 2019, 10, 102. [Google Scholar]
- Hu, X.; Li, D.; Wang, J.; Guo, J.; Li, Y.; Cao, Y.; Zhang, N.; Fu, Y. Melatonin inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in lipopolysaccharide-induced endometritis in mice. Int. Immunopharmacol. 2018, 64, 101–109. [Google Scholar]
- Pierre, M.; Angélique, C.; Valentin, D.; Lionel, A.; Carmen, G.; François, G.; Cédric, R. HSP70 is a negative regulator of NLRP3 inflammasome activation. Cell Death Dis. 2019, 10, 256. [Google Scholar]
- Dong, W.; Chen, Y.; Zhang, Q.; Zhao, X.; Liu, P.; He, H.; Lu, T.; He, Y.; Du, X.; Hu, J.; et al. Effects of lipoteichoic and arachidonic acids on the immune-regulatory mechanism of bovine mammary epithelial cells using multi-omics analysis. Front. Vet. Sci. 2022, 9, 984607. [Google Scholar]
- Yang, H.; Zhang, X.; Li, H.; Ye, Y.; Li, Z.; Han, X.; Hu, Y.; Zhang, C.; Jiang, Y. Heat Shock 70 kDa Protein Cognate 3 of Brown Planthopper Is Required for Survival and Suppresses Immune Response in Plants. Insects 2022, 13, 299. [Google Scholar] [CrossRef]
- Yu, C.; Tracy, S.V.; Peter, P.L.; Earl, G.N.; Currie, R.W. Heat Shock Paradox and a New Role of Heat Shock Proteins and their Receptors as Anti-Inflammation Targets. Inflamm. Allergy Drug Targets 2007, 6, 91–100. [Google Scholar]
- Fang, B.; Yang, T.; Chen, Y.; Duan, Z.; Hu, J.; Wang, Q.; He, Y.; Zhang, Y.; Dong, W.; Zhang, Q.; et al. Activation of ARP2/3 and HSP70 Expression by Lipoteichoic Acid: Potential Bidirectional Regulation of Apoptosis in a Mastitis Inflammation Model. Biomolecules 2024, 14, 901. [Google Scholar] [CrossRef]
- Lara, T.-O.; Mariana, L.; Patricia, D.R. Fighting antibiotic resistance in the local management of bovine mastitis. Biomed. Pharmacother. 2024, 170, 115967. [Google Scholar]
- Winnie, M.; Abdullah Ibn, M.; Bharat, P.; Tobenna, A.; Agnes, K.N. Antibiotic Resistant Enterobacteriaceae in Milk Alternatives. Foods 2021, 10, 3070. [Google Scholar] [CrossRef] [PubMed]
- Meysam, N.; Yongkang, G.; Gerhard, A.; Humblot, P.; Erik, B.R. Gene Networks and Pathways Involved in LPS-Induced Proliferative Response of Bovine Endometrial Epithelial Cells. Genes 2022, 13, 2342. [Google Scholar] [CrossRef] [PubMed]
- Omar, B.; Dong, X.; Alfred, L.R.; Anna, C.; Juan, J.L. Innate immune responses induced by lipopolysaccharide and lipoteichoic acid in primary goat mammary epithelial cells. J. Anim. Sci. Biotechnol. 2017, 8, 842–851. [Google Scholar]
- François, G.; Lionel, A.; Antoine, T.; Laetitia, A.; Yuting, M.; Carla, O.; Karim, V.; Theocharis, P.; Grégoire, M.; Evelyn, U.; et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β–dependent adaptive immunity against tumors. Nat. Med. 2009, 15, 1170–1178. [Google Scholar]
- Che, H.Y.; Zhou, C.H.; Lyu, C.C.; Meng, Y.; He, Y.T.; Wang, H.Q.; Wu, H.Y.; Zhang, J.B.; Yuan, B. Allicin Alleviated LPS-Induced Mastitis via the TLR4/NF-KB Signaling Pathway in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 3805. [Google Scholar]
- Yin, H.; Xue, G.; Dai, A.; Wu, H. Protective Effects of Lentinan Against Lipopolysaccharide-Induced Mastitis in Mice. Front. Pharmacol. 2021, 12, 755768. [Google Scholar]
- Li, Y.; Zhu, Y.; Chu, B.; Liu, N.; Chen, S.; Wang, J. Lactobacillus rhamnosus GR-1 Prevents Escherichia coli-Induced Apoptosis Through PINK1/Parkin-Mediated Mitophagy in Bovine Mastitis. Front. Immunol. 2021, 12, 715098. [Google Scholar]
- Chen, W.; Liu, Y.; Zhang, L.; Gu, X.; Liu, G.; Shahid, M.; Gao, J.; Ali, T.; Han, B. Nocardia cyriacigeogica from Bovine Mastitis Induced In vitro Apoptosis of Bovine Mammary Epithelial Cells via Activation of Mitochondrial-Caspase Pathway. Front. Cell. Infect. Microbiol. 2017, 7, 194. [Google Scholar]
- Campbell, W.G.; Kathryn, R.A. The actin cytoskeleton: A key regulator of apoptosis and ageing? Nat. Rev. Mol. Cell Biol. 2005, 6, 583–589. [Google Scholar]
- Yadaiah, M.; Chao, Y.; Małgorzata, B.; Kelley, A.B.; Adam, Z.; Grzegorz, R.; Tatyana, S.; Roberto, D. PICK1 is implicated in organelle motility in an Arp2/3 complex–independent manner. Mol. Biol. Cell 2015, 26, 1308–1322. [Google Scholar]
- Katrin, G.; Pathmanaban, R.; Azim Dheghani, A.; Marcel, H.S.; Gilles, G.; Martín, S.; Martina, S. More than the “Killer Trait”: Infection with the Bacterial Endosymbiont Caedibacter taeniospiralis Causes Transcriptomic Modulation in Paramecium Host. Genome Biol. Evol. 2018, 10, 646–656. [Google Scholar]
- Susan, L.D.; Timothy, G.B.; Cobb, J.P. The heat shock paradox: Does NF-KB determine cell fate? FASEB J. 2001, 15, 270–274. [Google Scholar]
- Timothy, G.B.; Patricia, A.A.; Elise, H.S.; Gregory, B.B. Induction of heat shock response leads to apoptosis in endothelial cells previously exposed to endotoxin. Am. J. Physiol. Heart Circ. Physiol. 1993, 265, H165–H170. [Google Scholar]


| Species | Gene | GenBank NO. | Sequence (5′–3′) | Length |
|---|---|---|---|---|
| Mouse | IL-1β | NM_008361.4 | GCCACCTTTTGACAGTGATGAG | 135 |
| ATGTGCTGCTGCGAGATTTG | ||||
| TNF-α | NM_013693.3 | AAACCACCAAGTGGAGGAGC | 120 | |
| ACAAGGTACAACCCATCGGC | ||||
| NLRP3 | NM_145827.4 | ATTACCCGCCCGAGAAAGG | 83 | |
| CATGAGTGTGGCTAGATCCAAG | ||||
| BAX | NM_007527.4 | CACTAAAGTGCCCGAGCTGA | 96 | |
| CAGCCACCCTGGTCTTGG | ||||
| Bcl-2 | NM_009741.5 | GAACTGGGGGAGGATTGTGG | 194 | |
| GCATGCTGGGGCCATATAGT | ||||
| Caspase 3 | NM_009810.3 | GGAGCAGCTTTGTGTGTGTG | 242 | |
| AGCCTCCACCGGTATCTTCT | ||||
| Caspase 7 | NM_007611.3 | GAGGAGGACCACAGCAACTC | 238 | |
| CGTCAATGTCGTTGATGGGC | ||||
| ARPC3 | NM_019824.4 | GCCATTTATGCCAAGCCTGC | 151 | |
| TCACAAAGCAAGTCCACCACT | ||||
| ARPC4 | NM_026552.3 | CTGCCACTCTCCGCCCCTAC | 126 | |
| TGCTACTCCTGACTTCGACCTCTG | ||||
| HSP70A1A | NM_005345.6 | GACAAGTGCCAGGAGGTCAT | 155 | |
| CCGAAGCCCCCAGCC | ||||
| HSP70A1L | NM_013558.2 | GACGCCAACGGTATCCTGAA | 176 | |
| TTGGCAGCGATTTTCTCCCT | ||||
| β-actin | NM_007393.5 | GGCTGTATTCCCCTCCATCG | 154 | |
| CCAGTTGGTAACAATGCCATGT | ||||
| Bovine | IL-1β | NM_174093.1 | TCCGACGAGTTTCTGTGTGA | 206 |
| ATACCCAAGGCCACAGGAAT | ||||
| TNF-α | NM_173966.3 | TTGTTCCTCACCCACACCAT | 239 | |
| CCAAAGTAGACCTGCCCAGA | ||||
| NLRP3 | NM_001102219.1 | GGCACCTTTCTTCCATGGCT | 219 | |
| ACCCGGTCAGAGTCCAGAAA | ||||
| BAX | NM_173894.1 | GAGATGAATTGGACAGTAACA | 118 | |
| TTGAAGTTGCCGTCAGAA | ||||
| Bcl-2 | NM_001166486.1 | ATGACCGAGTACCTGAAC | 79 | |
| CATACAGCTCCACAAAGG | ||||
| Caspase 3 | NM_001077840.1 | AGTGGTGCTGAGGATGAC | 135 | |
| ACAAAGAGCCTGGATGAA | ||||
| Caspase 7 | XM_002698509.6 | GAAATTCAGCCTGCTTCGCC | 110 | |
| CCCCCTAAAATGGGCTGTCA | ||||
| ARPC3 | NM_001034271.2 | TTTTCGTTGGGGTGGAGACO | 138 | |
| TCTCTAGGGGCAGGTCCTTI | ||||
| ARPC4 | NM_001076163.1 | CCGTGTCTCTGTGAAGTCGT | 103 | |
| ATCTAATGCCCACCCTGACC | ||||
| HSP70A1A | NM_203322.3 | AGTGCCAGGAGGTGATTTCC | 100 | |
| ATGGGGTTACACACCTGCTC | ||||
| HSP70A1L | NM_001167895.1 | GCCAAGAACCAGGTAGCCAT | 149 | |
| ATTACCTTGGGCTTGCCTCC | ||||
| β-actin | AY141970.1 | CAACCGTGAGAAGATGACCCA | 293 | |
| TGTCACGGACGATTTCCGCTC |
| Name | Manufacturer | Cat.NO. |
|---|---|---|
| IL-1β | Affinity | AF4006 |
| TNF-α | Affinity | AF7014 |
| NLRP3 | Proteintech | 27458-1-AP |
| BAX | Proteintech | 50599-2-IG |
| Bcl-2 | Proteintech | 26593-1-AP |
| Caspase 3 | Bioss | bs-0081R |
| Caspase 7 | Bioss | bsm-60304R |
| ARPC3 | Proteintech | 14652-1-AP |
| ARPC4 | Proteintech | 10930-1-AP |
| HSP70A1A | Proteintech | 10995-1-AP |
| HSP70A1L | Proteintech | 13970-1-AP |
| β-actin | Proteintech | 66009-1-IG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Chen, Y.; Fang, B.; Zhang, J.; Bai, W.; Yang, T.; Zhang, Q.; Liu, P.; Duan, Z.; Lu, T.; et al. Cytochalasin B Mitigates the Inflammatory Response in Lipopolysaccharide-Induced Mastitis by Suppressing Both the ARPC3/ARPC4-Dependent Cytoskeletal Changes and the Association Between HSP70 and the NLRP3 Inflammasome. Int. J. Mol. Sci. 2025, 26, 3029. https://doi.org/10.3390/ijms26073029
Wang A, Chen Y, Fang B, Zhang J, Bai W, Yang T, Zhang Q, Liu P, Duan Z, Lu T, et al. Cytochalasin B Mitigates the Inflammatory Response in Lipopolysaccharide-Induced Mastitis by Suppressing Both the ARPC3/ARPC4-Dependent Cytoskeletal Changes and the Association Between HSP70 and the NLRP3 Inflammasome. International Journal of Molecular Sciences. 2025; 26(7):3029. https://doi.org/10.3390/ijms26073029
Chicago/Turabian StyleWang, An, Yan Chen, Bo Fang, Jiang Zhang, Wenkai Bai, Tingji Yang, Quanwei Zhang, Peiwen Liu, Zhiwei Duan, Ting Lu, and et al. 2025. "Cytochalasin B Mitigates the Inflammatory Response in Lipopolysaccharide-Induced Mastitis by Suppressing Both the ARPC3/ARPC4-Dependent Cytoskeletal Changes and the Association Between HSP70 and the NLRP3 Inflammasome" International Journal of Molecular Sciences 26, no. 7: 3029. https://doi.org/10.3390/ijms26073029
APA StyleWang, A., Chen, Y., Fang, B., Zhang, J., Bai, W., Yang, T., Zhang, Q., Liu, P., Duan, Z., Lu, T., He, Y., Zhang, Y., Zhao, X., & Dong, W. (2025). Cytochalasin B Mitigates the Inflammatory Response in Lipopolysaccharide-Induced Mastitis by Suppressing Both the ARPC3/ARPC4-Dependent Cytoskeletal Changes and the Association Between HSP70 and the NLRP3 Inflammasome. International Journal of Molecular Sciences, 26(7), 3029. https://doi.org/10.3390/ijms26073029





