Glycyl-tRNA Synthetase as a Target for Antiviral Drug Screening Against Influenza Virus
Abstract
1. Introduction
2. Results
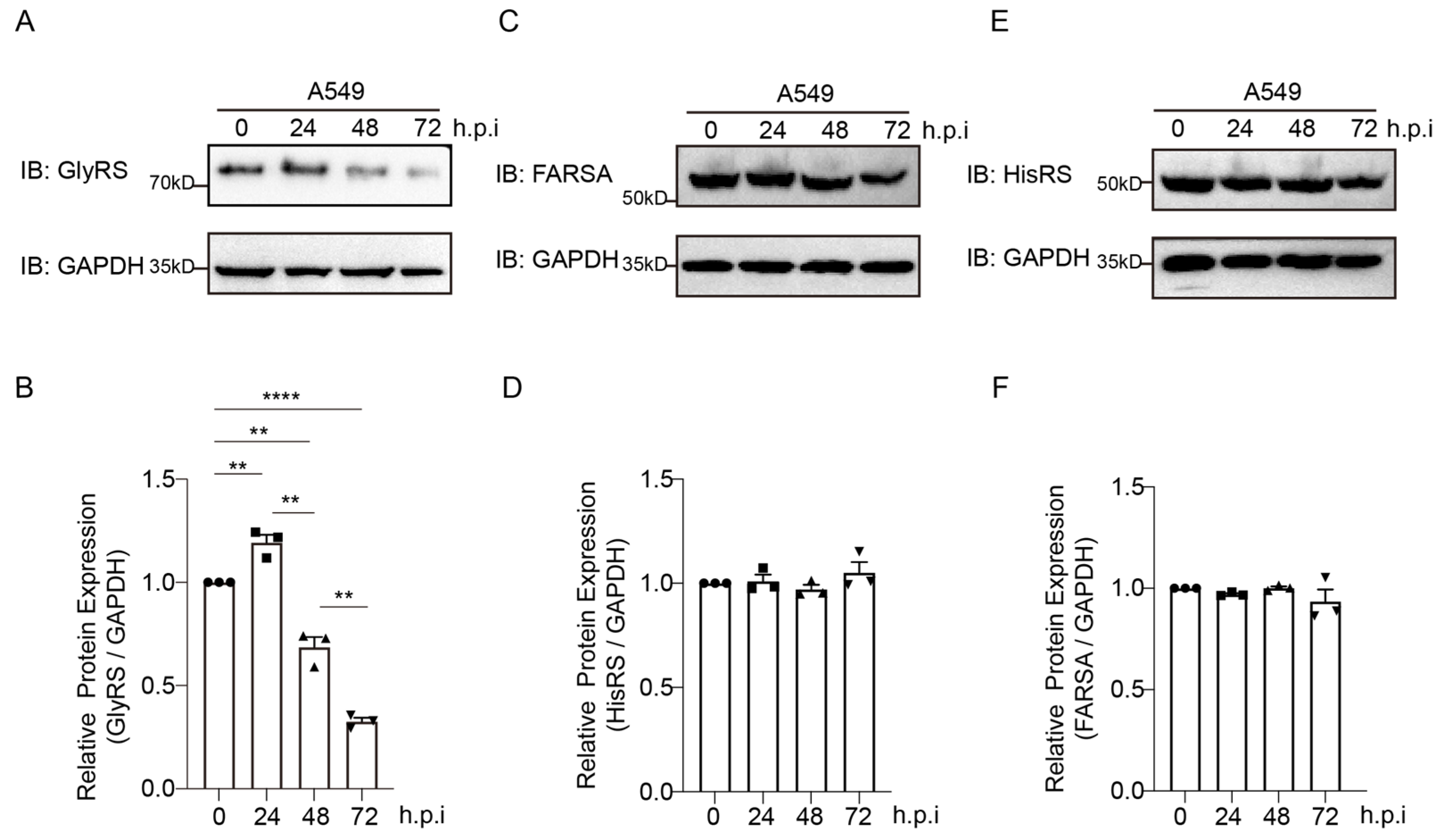
2.1. GlyRS Is Associated with the Proliferation of the PR8 Virus
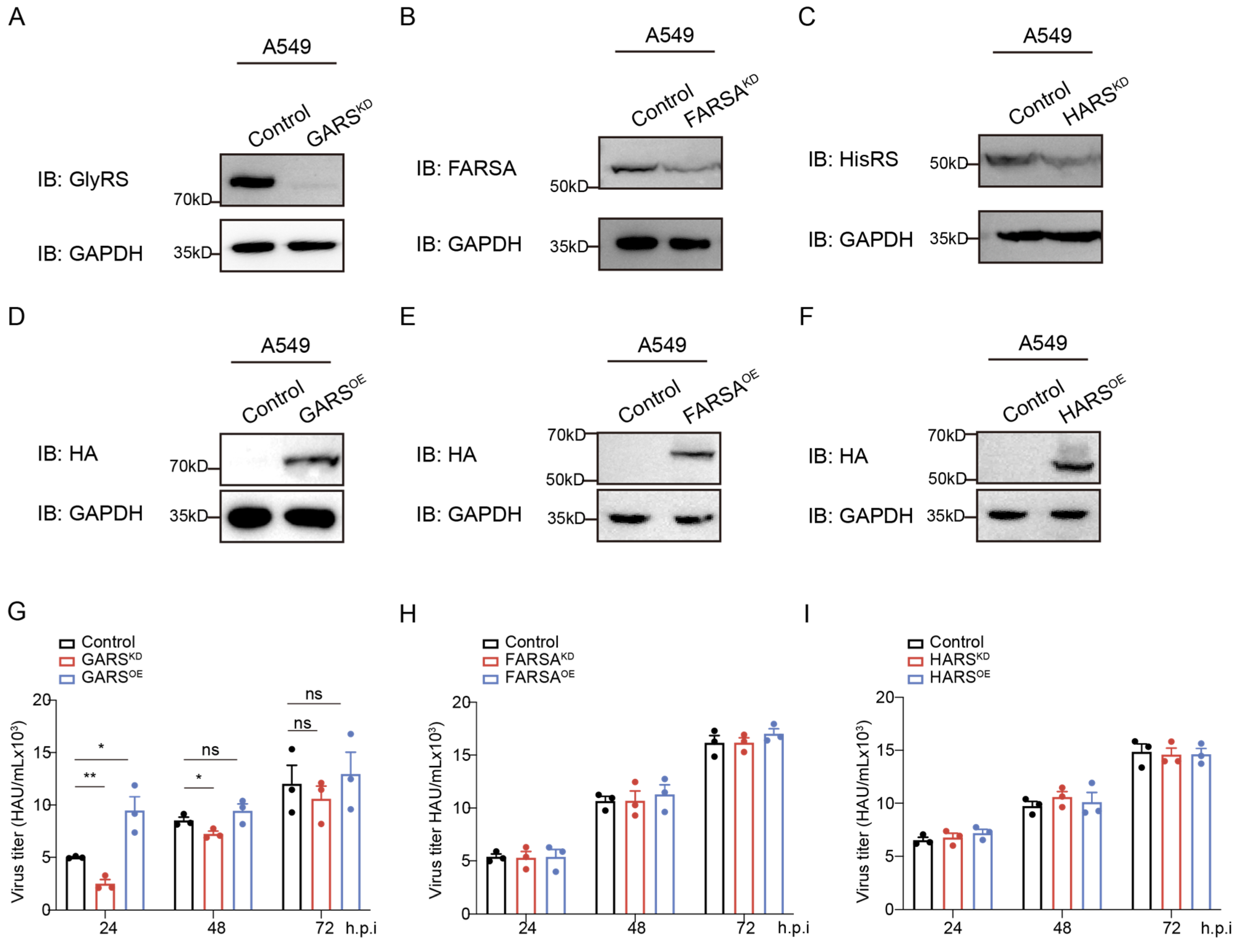
2.2. Knockdown of GlyRS Decreases Infection of Multiple Influenza Virus Strains
2.3. Multi-Approach Drug Screening Based on GlyRS
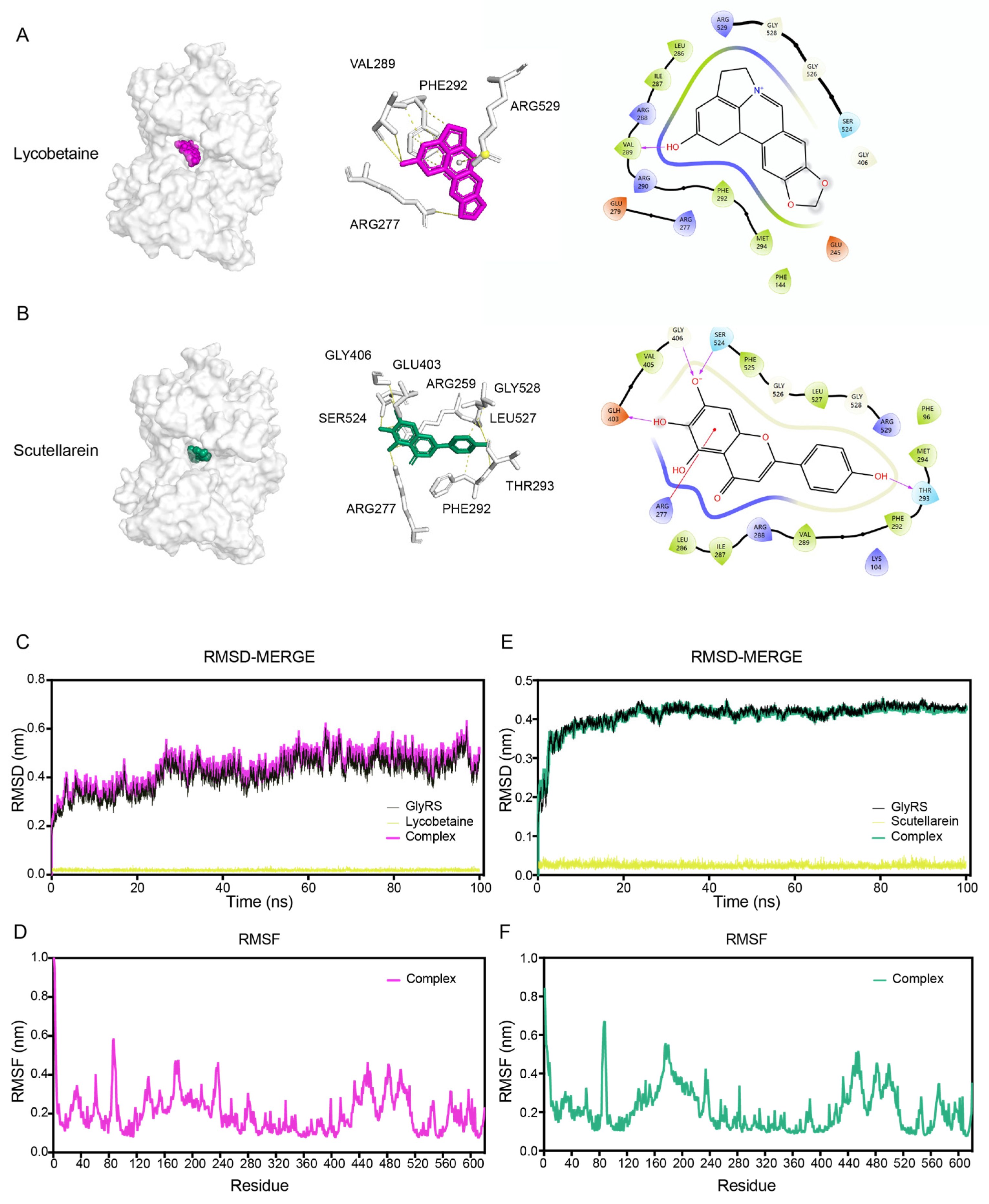
2.4. Binding Mode of Screened Inhibitors with GlyRS
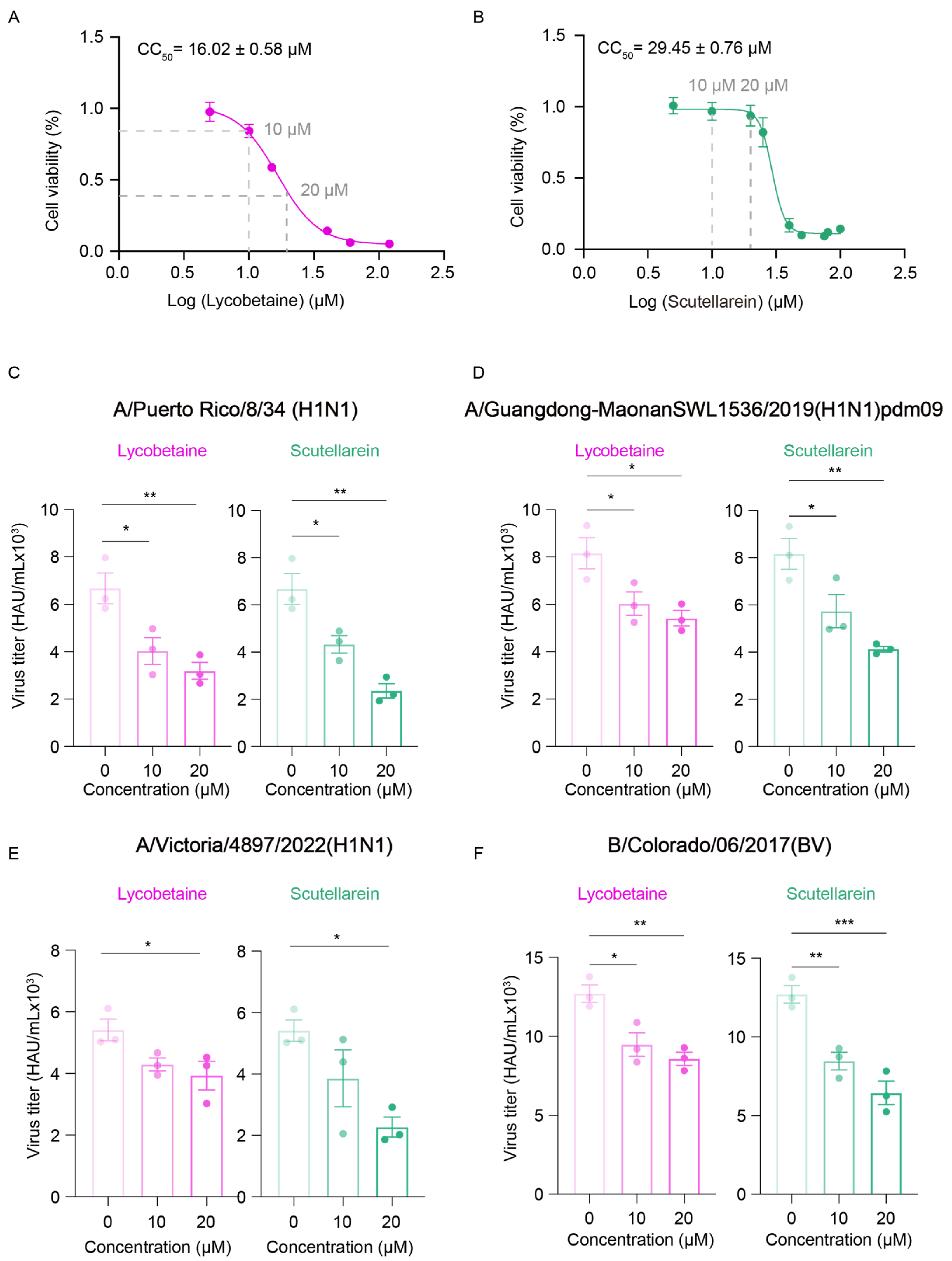
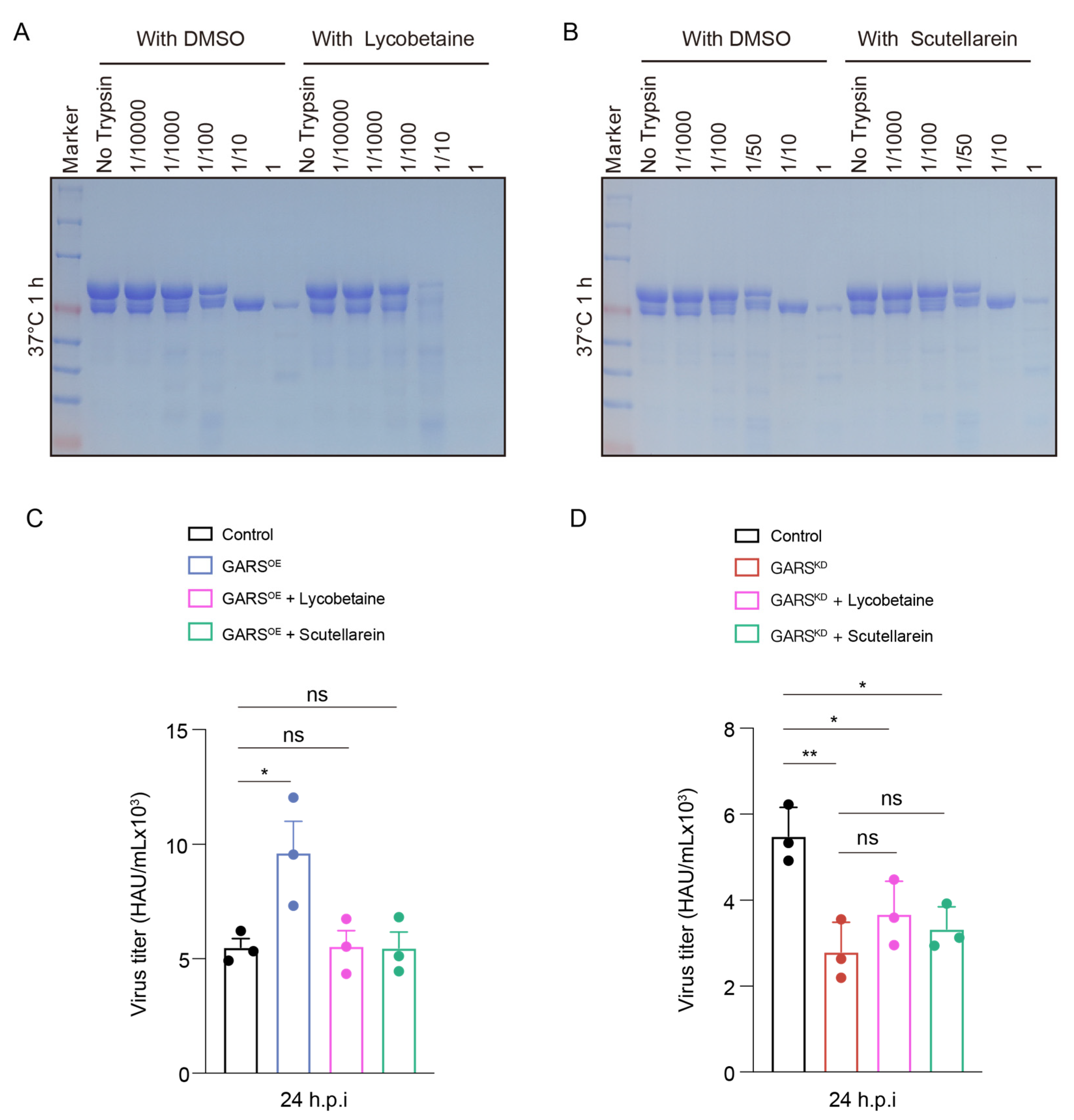
2.5. GlyRS Inhibitors Can Block Influenza Virus
2.6. Lycobetaine Alters Conformation of GlyRS and Blocks Virus by Targeting GlyRS
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Influenza Virus Infection
4.3. Virus Titer Detection
4.4. Construction of shRNA and HA-Tagged Expression Vectors
4.5. Lentivirus Assembly and Infection
4.6. Expression and Purification of GlyRS
4.7. Thermal Shift Assay
4.8. Bio-Layer Interferometry Assay
4.9. Enzyme Inhibition Assay
4.10. Molecular Docking
4.11. Molecular Dynamics Simulation
4.12. Western Blot Assay
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonomini, A.; Felicetti, T.; Pacetti, M.; Bertagnin, C.; Coletti, A.; Giammarino, F.; De Angelis, M.; Poggialini, F.; Macchiarulo, A.; Sabatini, S.; et al. Optimization of Potent, Broad-spectrum, and Specific Anti-influenza Compounds Targeting RNA Polymerase PA-PB1 heterodimerization. Eur. J. Med. Chem. 2024, 277, 116737. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Chokkakula, S.; Song, M.-S. Influenza Treatment: Limitations of Antiviral Therapy and Advantages of Drug Combination Therapy. Microorganisms 2023, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, F.; Wang, R.; Li, F.; Wu, Y.; Kitazato, K.; Wang, Y. HSP90: A promising broad-spectrum antiviral drug target. Arch. Virol. 2017, 162, 3269–3282. [Google Scholar] [CrossRef]
- Mahajan, S.; Choudhary, S.; Kumar, P.; Tomar, S. Antiviral strategies targeting host factors and mechanisms obliging +ssRNA viral pathogens. Bioorg Med. Chem. 2021, 46, 116356. [Google Scholar] [CrossRef]
- Teixeira Alves, L.G.; Baumgardt, M.; Langner, C.; Fischer, M.; Maria Adler, J.; Bushe, J.; Firsching, T.C.; Mastrobuoni, G.; Grobe, J.; Hoenzke, K.; et al. Protective role of the HSP90 inhibitor, STA-9090, in lungs of SARS-CoV-2-infected Syrian golden hamsters. BMJ Open Respir. Res. 2024, 11, e001762. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, J.N.; Zhang, X.M.; Ling, D.D.; Sun, Y.B.; Li, C.Y.; Zhou, Q.Q.; Shi, G.N.; Wang, S.H.; Lin, X.S.; et al. Nanoengineered Red Blood Cells Loaded with TMPRSS2 and Cathepsin L Inhibitors Block SARS-CoV-2 Pseudovirus Entry into Lung ACE2(+) Cells. Adv. Mater. 2024, 36, e2310306. [Google Scholar] [CrossRef]
- Khan, D.; Fox, P.L. Aminoacyl-tRNA synthetase interactions in SARS-CoV-2 infection. Biochem. Soc. Trans. 2023, 51, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Yoon, I.; Han, J.M.; Kim, S. Functional and pathologic association of aminoacyl-tRNA synthetases with cancer. Exp. Mol. Med. 2022, 54, 553–566. [Google Scholar] [CrossRef]
- Kaminska, M.; Shalak, V.; Francin, M.; Mirande, M. Viral hijacking of mitochondrial lysyl-tRNA synthetase. J. Virol. 2007, 81, 68–73. [Google Scholar]
- Li, X.-Q.; Cai, M.-P.; Wang, M.-Y.; Shi, B.-W.; Yang, G.-Y.; Wang, J.; Chu, B.-B.; Ming, S.-L. Pseudorabies virus manipulates mitochondrial tryptophanyl-tRNA synthetase 2 for viral replication. Virol. Sin. 2024, 39, 403–413. [Google Scholar] [CrossRef]
- Oprescu, S.N.; Chepa-Lotrea, X.; Takase, R.; Golas, G.; Markello, T.C.; Adams, D.R.; Toro, C.; Gropman, A.L.; Hou, Y.M.; Malicdan, M.C.V.; et al. Compound heterozygosity for loss-of-function GARS variants results in a multisystem developmental syndrome that includes severe growth retardation. Hum. Mutat. 2017, 38, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Bai, G.; Zhou, H.; Wei, N.; White, N.M.; Lauer, J.; Liu, H.; Shi, Y.; Dumitru, C.D.; Lettieri, K. CMT2D neuropathy is linked to the neomorphic binding activity of glycyl-tRNA synthetase. Nature 2015, 526, 710–714. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, W.; Zhang, J.; Liu, W.; Yin, J.; Shi, D.; Ma, W. A Novel Mitochondrial-Related Nuclear Gene Signature Predicts Overall Survival of Lung Adenocarcinoma Patients. Front. Cell Dev. Biol. 2021, 9, 740487. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Liu, Q.; Liu, Y.; Hou, Y.; Jin, W. Conjoint analysis of circulating tumor cells and solid tumors for exploring potential prognostic markers and constructing a robust novel predictive signature for breast cancer. Cancer Cell Int. 2021, 21, 708. [Google Scholar]
- Khosh Kish, E.; Gamallat, Y.; Choudhry, M.; Ghosh, S.; Seyedi, S.; Bismar, T.A. Glycyl-tRNA Synthetase (GARS) Expression Is Associated with Prostate Cancer Progression and Its Inhibition Decreases Migration, and Invasion In Vitro. Int. J. Mol. Sci. 2023, 24, 4260. [Google Scholar] [CrossRef]
- Feng, M.; Yang, K.; Wang, J.; Li, G.; Zhang, H. First Report of FARSA in the Regulation of Cell Cycle and Survival in Mantle Cell Lymphoma Cells via PI3K-AKT and FOXO1-RAG1 Axes. Int. J. Mol. Sci. 2023, 24, 1608. [Google Scholar] [CrossRef] [PubMed]
- Blocquel, D.; Sun, L.; Matuszek, Z.; Li, S.; Weber, T.; Kuhle, B.; Kooi, G.; Wei, N.; Baets, J.; Pan, T.; et al. CMT disease severity correlates with mutation-induced open conformation of histidyl-tRNA synthetase, not aminoacylation loss, in patient cells. Proc. Natl. Acad. Sci. USA 2019, 116, 19440–19448. [Google Scholar] [CrossRef]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef]
- Goto, T.; Kawai, N.; Bando, T.; Sato, T.; Tani, N.; Chong, Y.; Ikematsu, H. In vitro neuraminidase inhibitory concentrations (IC(50)) of four neuraminidase inhibitors in the Japanese 2023-24 season: Comparison with the 2010-11 to 2022-23 seasons. J. Infect. Chemother. 2024, 31, 102602. [Google Scholar] [CrossRef]
- Ibba, M.; Söll, D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 2000, 69, 617–650. [Google Scholar]
- Mirande, M. Aminoacyl-tRNA synthetase family from prokaryotes and eukaryotes: Structural domains and their implications. Prog. Nucleic Acid Res. Mol. Biol. 1991, 40, 95–142. [Google Scholar] [PubMed]
- de Pouplana, L.s.R.; Schimmel, P. Aminoacyl-tRNA synthetases: Potential markers of genetic code development. Trends Biochem. Sci. 2001, 26, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; You, S.; Hwang, D. Aminoacyl-tRNA synthetases and tumorigenesis: More than housekeeping. Nat. Rev. Cancer 2011, 11, 708–718. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, B.; Nie, A.; Yu, D.; Bian, M. Roles of aminoacyl-tRNA synthetases in cancer. Front. Cell Dev. Biol. 2020, 8, 599765. [Google Scholar] [CrossRef]
- Guo, M.; Yang, X.-L.; Schimmel, P. New functions of aminoacyl-tRNA synthetases beyond translation. Nat. Rev. Mol. Cell Biol. 2010, 11, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Arif, A.; Jia, J.; Halawani, D.; Fox, P.L. Experimental approaches for investigation of aminoacyl tRNA synthetase phosphorylation. Methods 2017, 113, 72–82. [Google Scholar] [CrossRef]
- Yoon, I.; Nam, M.; Kim, H.K.; Moon, H.-S.; Kim, S.; Jang, J.; Song, J.A.; Jeong, S.J.; Kim, S.B.; Cho, S. Glucose-dependent control of leucine metabolism by leucyl-tRNA synthetase 1. Science 2020, 367, 205–210. [Google Scholar] [CrossRef]
- Ognjenović, J.; Simonović, M. Human aminoacyl-tRNA synthetases in diseases of the nervous system. RNA Biol. 2018, 15, 623–634. [Google Scholar] [CrossRef]
- Vondenhoff, G.H.M.; Van Aerschot, A. Aminoacyl-tRNA synthetase inhibitors as potential antibiotics. Eur. J. Med. Chem. 2011, 46, 5227–5236. [Google Scholar] [CrossRef]
- Yang, G.; Liang, Y.; Li, X.; Li, Z.; Qin, Y.; Weng, Q.; Yan, Y.; Cheng, Y.; Qian, Y.; Sun, L. Competitive Inhibition of Okanin against Plasmodium falciparum Tyrosyl-tRNA Synthetase. Int. J. Mol. Sci. 2024, 25, 4751. [Google Scholar] [CrossRef]
- Kurata, K.; James-Bott, A.; Tye, M.A.; Yamamoto, L.; Samur, M.K.; Tai, Y.-T.; Dunford, J.; Johansson, C.; Senbabaoglu, F.; Philpott, M.; et al. Prolyl-tRNA synthetase as a novel therapeutic target in multiple myeloma. Blood Cancer J. 2023, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Lukarska, M.; Palencia, A. Chapter Eleven—Aminoacyl-tRNA synthetases as drug targets. In Enzymes; Ribas de Pouplana, L., Kaguni, L.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 48, pp. 321–350. [Google Scholar]
- Fang, P.; Guo, M. Evolutionary Limitation and Opportunities for Developing tRNA Synthetase Inhibitors with 5-Binding-Mode Classification. Life 2015, 5, 1703–1725. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Hu, M.-H. Preferential stabilization of c-MYC and BCL-2 promoter G-quadruplexes by a natural betaine-type alkaloid. Phytochem. Lett. 2022, 52, 1–6. [Google Scholar] [CrossRef]
- Shen, L.; Zhao, J.; Xia, Y.; Lu, J.; Sun, J.; Tang, J.; Xing, H.; Yin, L.; Yang, Y.; Wang, C. Lycorine derivative effectively inhibits the replication of coronaviruses both in vitro and in vivo. hLife 2024, 2, 75–87. [Google Scholar] [CrossRef]
- Liu, B.s.; Liu, K.; Wang, J.; Shi, Y.m. Anticancer Potential of Nature-Derived Isoquinoline Alkaloids (A Review). Russ. J. Gen. Chem. 2023, 93, 1294–1310. [Google Scholar] [CrossRef]
- Jin, Y.-H.; Min, J.S.; Jeon, S.; Lee, J.; Kim, S.; Park, T.; Park, D.; Jang, M.S.; Park, C.M.; Song, J.H. Lycorine, a non-nucleoside RNA dependent RNA polymerase inhibitor, as potential treatment for emerging coronavirus infections. Phytomedicine 2021, 86, 153440. [Google Scholar]
- Lai, J.; Li, C. Review on the pharmacological effects and pharmacokinetics of scutellarein. Arch. Pharm. 2024, 357, e2400053. [Google Scholar]
- Sun, L.; Zhou, X.-L.; Zhou, Z.-W.; Cui, H. Editorial: Noncanonical functions of Aminoacyl-tRNA synthetases. Front. Physiol. 2023, 14, 1165515. [Google Scholar] [CrossRef]



| Compounds | KD (μM) | Kon (1/MS) × 103 | Kdis (1/s) × 10−2 |
|---|---|---|---|
| Iodoquinol | 7.14 ± 0.41 | 3.10 ± 0.51 | 2.23 ± 0.49 |
| Chloramine-T | 15.09 ± 1.80 | 52.28 ± 13.31 | 78.19 ± 17.95 |
| Lansoprazole | 12.48 ± 1.63 | 7.23 ± 2.14 | 9.25 ± 3.64 |
| Nisoldipine | 47.71 ± 7.18 | 86.16 ± 27.50 | 400 ± 94.49 |
| Velpatasvir | 11.72 ± 1.13 | 15.80 ± 0.71 | 18.46 ± 0.98 |
| Mometasone furoate | 1.82 ± 0.36 | 108.8 ± 67.42 | 31.08 ± 9 03 |
| Carnosic acid | 14.54 ± 0.19 | 1.13 ± 0.26 | 1.12 ± 0.17 |
| Scutellarein | 2 88 ± 0.83 | 3.96 ± 1.05 | 1.09 ± 0.11 |
| N-Acetyl-L-alanine | 24.85 ± 4.33 | 396.20 ± 85.48 | 560 ± 194 |
| Lycobetaine | 43.55 ± 16.37 | 8.48 ± 2 37 | 39.73 ± 11.18 |
| Compounds | Structure | △Tm [°C] a | KD [μM] b | Relative Enzyme Activity [%] c |
|---|---|---|---|---|
| Iodoquinol | 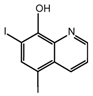 | −11.97 | 7.14 ± 0.41 | 7.0 |
| Chloramine-T | 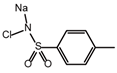 | −5.13 | 15.09 ± 1.90 | 86.9 |
| Lansoprazole | 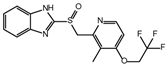 | −5.03 | 12.48 ± 1.63 | 72.3 |
| Nisoldipine |  | −8.96 | 47.71 ± 7.18 | 67.3 |
| Velpatasvir |  | −5.4 | 11.72 ± 1.13 | 78.5 |
| Mometasone furoate |  | −11.58 | 1.82 ± 0.36 | 91.2 |
| Carnosic acid |  | −5.9 | 14.54 ± 0.19 | 75.4 |
| Scutellarein | 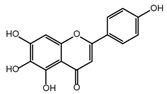 | −5.3 | 2.88 ± 0.83 | 27.3 |
| N-Acetyl-L-alanine | 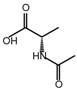 | 5.96 | 24.85 ± 4.33 | 88.1 |
| Lycobetaine |  | −13.01 | 43.55 ± 16.37 | 8.0 |
| Complex | Docking Score | H-Bond | π-Stacking | Hydrophobic Interactions | Salt Bridges | ||
|---|---|---|---|---|---|---|---|
| Residues | Number | Residues | Residues | Number | Residues | ||
| GlyRS- Iodoquinol | −6.88 | VAL289 | 1 | PHE292 | |||
| ARG529 | 2 | ||||||
| GlyRS- Chloramine-T | −6.371 | ARG529 | 1 | PHE292 | VAL289 | 1 | |
| PHE292 | 1 | ||||||
| ARG529 | 1 | ||||||
| GlyRS- Lansoprazole | −7.265 | GLY406 | 1 | PHE292 | GLU403 | ||
| ARG529 | 1 | ||||||
| GlyRS- Nisoldipine | −6.553 | GLU245 | 1 | LYS 143 | 2 | LYS143 | |
| PHE144 | 1 | ARG410 | |||||
| GlyRS-Velpatasvir | −7.77 | HIS162 | 1 | HIS162 | 1 | ||
| ARG288 | 1 | LYS165 | 1 | ||||
| ARG529 | 1 | ARG277 | 1 | ||||
| ILE287 | 1 | ||||||
| PHE292 | 2 | ||||||
| TYR399 | 1 | ||||||
| GlyRS- Mometasone furoate | −7.627 | LYS48 | 1 | ARG49 | ARG376 | ||
| GLU52 | 1 | ||||||
| ILE287 | 1 | ||||||
| TYR399 | 1 | ||||||
| ILE402 | 1 | ||||||
| GLU403 | 1 | ||||||
| GlyRS- Carnosic acid | −7.646 | GLU245 | 1 | PHE144 | 1 | HIS140 | |
| ARG410 | 1 | GLU245 | 1 | LYS143 | |||
| GLU522 | 1 | ARG410 | |||||
| GlyRS- Scutellarein | −8.573 | ARG277 | 1 | PHE292 | 1 | ||
| TH293 | 2 | LEU527 | 1 | ||||
| GLU403 | 2 | ||||||
| GLY406 | 1 | ||||||
| SER524 | 1 | ||||||
| GLY528 | 1 | ||||||
| ARG529 | 2 | ||||||
| GlyRS- N-Acetyl-L-alanine | −4.918 | VAL289 | 2 | ARG288 | 1 | ARG288 | |
| ARG529 | 1 | VAL289 | 1 | ||||
| PHE292 | 1 | ||||||
| GlyRS- Lycobetaine | −8.587 | VAL289 | VAL289 | 1 | |||
| PHE292 | 5 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, X.; Liang, J.; Meng, X.; Zhu, C.; Yang, G.; Liang, Y.; Zhou, Q.; Qin, Q.; Li, Z.; et al. Glycyl-tRNA Synthetase as a Target for Antiviral Drug Screening Against Influenza Virus. Int. J. Mol. Sci. 2025, 26, 2912. https://doi.org/10.3390/ijms26072912
Zhang J, Li X, Liang J, Meng X, Zhu C, Yang G, Liang Y, Zhou Q, Qin Q, Li Z, et al. Glycyl-tRNA Synthetase as a Target for Antiviral Drug Screening Against Influenza Virus. International Journal of Molecular Sciences. 2025; 26(7):2912. https://doi.org/10.3390/ijms26072912
Chicago/Turabian StyleZhang, Jingjing, Xiaorong Li, Jingxian Liang, Xinru Meng, Chenchen Zhu, Guangpu Yang, Yali Liang, Qikai Zhou, Qianni Qin, Zan Li, and et al. 2025. "Glycyl-tRNA Synthetase as a Target for Antiviral Drug Screening Against Influenza Virus" International Journal of Molecular Sciences 26, no. 7: 2912. https://doi.org/10.3390/ijms26072912
APA StyleZhang, J., Li, X., Liang, J., Meng, X., Zhu, C., Yang, G., Liang, Y., Zhou, Q., Qin, Q., Li, Z., Zhang, T., Liu, G., & Sun, L. (2025). Glycyl-tRNA Synthetase as a Target for Antiviral Drug Screening Against Influenza Virus. International Journal of Molecular Sciences, 26(7), 2912. https://doi.org/10.3390/ijms26072912






