Functional Differentiation and Regulatory Mechanisms of Ferrochelatases HemH1 and HemH2 in Bacillus thuringiensis Under Iron and Oxidative Stress
Abstract
1. Introduction
2. Results
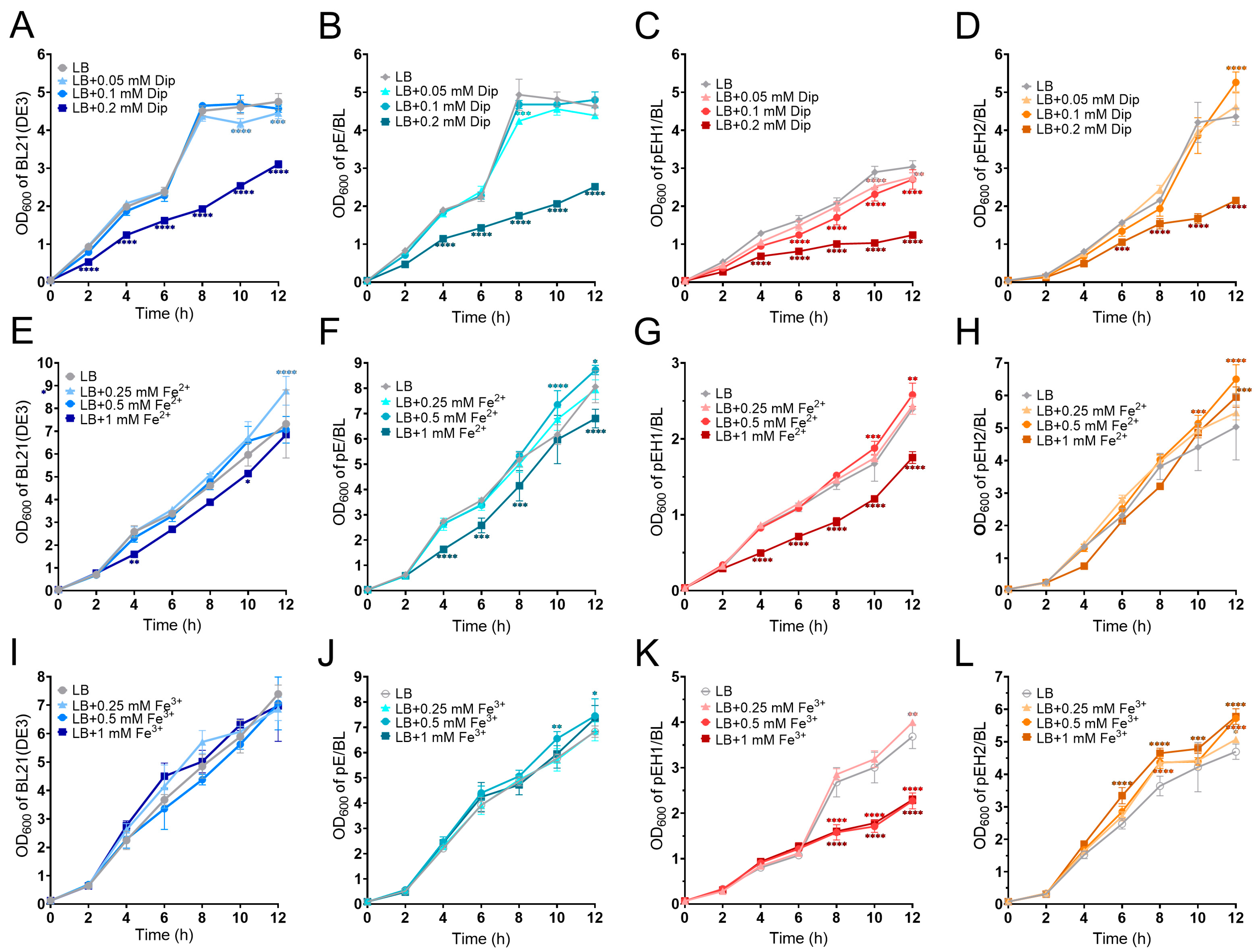
2.1. The Effect of Different Concentrations of Dip, Fe2+, and Fe3+ on Strain Growth
2.2. The Effect of Different Concentrations of H2O2 on Bacterial Growth
2.3. The Effect of Iron-Limited Conditions on the Production of 5-ALA by E. coli Strains
2.4. The Effect of Dip, Fe2+, Fe3+ on the Production of Heme by E. coli Strains
2.5. Metal Ion-Dependent Fur Binding to PhemH1 or PhemH2 Promoters and Binding Region Verification
2.6. Verification of the Binding Regions of Fur with PhemH1 and PhemH2 with Fur by DNase I Footprinting
2.7. The Regulation Mode of Fur on hemH1 and hemH2 and Its Influence by Fe3+
2.8. qRT-PCR Results
3. Discussion
3.1. The Sequence Analysis of Ferrochelatases from Different Strains
3.2. The Effect of Different Concentrations of Dip, Fe2+, and Fe3+ on the Function of Ferrochelatases HemH1 and HemH2
3.3. The Role of hemH1 and hemH2 in Antioxidant Stress
3.4. Differential Regulatory Mechanisms of Fur on hemH1 and hemH2
4. Materials and Methods
4.1. Strains, Plasmids, and Culture Conditions
4.2. Construction of E. coli Recombinant Strains Expressing fur and hemH
4.3. Construction of hemH Knockout, Overexpression, and Complementation Strains
4.4. Determination of the Growth Curve of the Strain
4.5. H2O2 Tolerance Experiment
4.6. Determination of Heme and 5-Aminolevulinic Acid Concentrations
4.7. Gel Mobility Shift Assay (EMSA) and DNase I Footprinting Assay
4.8. Determination of β-Galactosidase Activity
4.9. RNA Extraction and qRT-PCR Analysis
4.10. Bioinformatics and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Layer, G. Heme biosynthesis in prokaryotes. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118861. [Google Scholar] [CrossRef] [PubMed]
- Hamza, I. Intracellular trafficking of porphyrins. ACS Chem. Biol. 2006, 1, 627–629. [Google Scholar] [CrossRef][Green Version]
- Möbius, K.; Arias-Cartin, R.; Breckau, D.; Hännig, A.-L.; Riedmann, K.; Biedendieck, R.; Schröder, S.; Becher, D.; Magalon, A.; Moser, J. Heme biosynthesis is coupled to electron transport chains for energy generation. Proc. Natl. Acad. Sci. USA 2010, 107, 10436–10441. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Z.H.; Shu, J.X.; Jiang, X.H.; Wang, W.; Shi, Z.H.; Lin, Y.W. Mimicking a natural enzyme system: Cytochrome c oxidase-like activity of Cu2O nanoparticles by receiving electrons from cytochrome c. Inorg. Chem. 2017, 56, 9400–9403. [Google Scholar] [CrossRef]
- Richtová, J.; Sheiner, L.; Gruber, A.; Yang, S.M.; Kořený, L.; Striepen, B.; Oborník, M. Using diatom and apicomplexan models to study the heme pathway of Chromera velia. Int. J. Mol. Sci. 2021, 22, 6495. [Google Scholar] [CrossRef] [PubMed]
- Su, H.F.; Chen, X.L.; Chen, S.J.; Guo, M.Z.; Liu, H.L. Applications of the whole-cell system in the efficient biosynthesis of heme. Int. J. Mol. Sci. 2023, 24, 8384. [Google Scholar] [CrossRef]
- Revel, M.; Dimitrov, J.D. Methods for Assessment of Interactions of Proteins with Heme: Application for Complement Proteins and Immunoglobulins. Methods Mol. Biol. 2021, 2227, 227–236. [Google Scholar] [CrossRef]
- Jarzębski, M.; Wieruszewski, M.; Kościński, M.; Rogoziński, T.; Kobus-Cisowska, J.; Szablewski, T.; Perła-Kaján, J.; Waszkowiak, K.; Jakubowicz, J. Heme iron as potential iron fortifier for food application—Characterization by material techniques. Rev. Adv. Mater. Sci. 2023, 62, 20230128. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Z.W.; Zhang, Z.H.; Zhou, J.W.; Li, J.H.; Chen, J.; Du, G.C.; Zhao, X.R. Biosynthesis, acquisition, regulation, and upcycling of heme: Recent advances. Crit. Rev. Biotechnol. 2024, 44, 1422–1438. [Google Scholar] [CrossRef]
- Dailey, H.A.; Medlock, A.E. A primer on heme biosynthesis. Biol. Chem. 2022, 403, 985–1003. [Google Scholar] [CrossRef]
- Videira, M.A.M.; Lobo, S.A.L.; Silva, L.S.O.; Palmer, D.; Warren, M.J.; Prieto, M.; Coutinho, A.; Sousa, F.L.; Fernandes, F.; Saraiva, L.M. Staphylococcus aureus haem biosynthesis and acquisition pathways are linked through haem monooxygenase IsdG. Mol. Microbiol. 2018, 109, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.A.; López-Gomollón, S.; Bes, M.T.; Fillat, M.F.; Pelrato, M.L. Three fur homologues from Anabaena sp. PCC7120: Exploring reciprocal protein-promoter recognition. FEMS Microbiol. Lett. 2004, 236, 275–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qiu, D.; Xie, M.; Dai, J.; An, W.; Wei, H.; Tian, C.; Kempher, M.L.; Zhou, A.; He, Z.; Gu, B.; et al. Differential regulation of the two ferrochelatase paralogues in Shewanella loihica PV-4 in response to environmental stresses. Appl. Environ. Microbiol. 2016, 82, 5077–5088. [Google Scholar] [CrossRef]
- Dai, J.; Liu, Y.; Liu, S.; Li, S.; Gao, N.; Wang, J.; Zhou, J.; Qiu, D. Differential gene content and gene expression for bacterial evolution and speciation of Shewanella in terms of biosynthesis of heme and heme-requiring proteins. BMC Microbiol. 2019, 19, 173. [Google Scholar] [CrossRef]
- Schauder, A.; Avital, A.; Malik, Z. Regulation and gene expression of heme synthesis under heavy metal exposure- review. J. Environ. Pathol. Tox. 2010, 29, 137–158. [Google Scholar] [CrossRef]
- Dutt, S.; Hamza, I.; Bartnikas, T.B. Molecular mechanisms of iron and heme metabolism. Annu. Rev. Nutr. 2022, 42, 311–335. [Google Scholar] [CrossRef]
- Fillat, M.F. The FUR (ferric uptake regulator) superfamily: Diversity and versatility of key transcriptional regulators. Arch. Biochem. Biophys. 2014, 546, 41–52. [Google Scholar] [CrossRef]
- Sarvan, S.; Butcher, J.; Stintzi, A.; Couture, J.F. Variation on a theme: Investigating the structural repertoires used by ferric uptake regulators to control gene expression. Biometals 2018, 31, 681–704. Available online: https://link.springer.com/article/10.1007/s10534-018-0120-8 (accessed on 16 July 2018).
- Troxell, B.; Hassan, H.M. Transcriptional regulation by ferric uptake regulator (Fur) in pathogenic bacteria. Front. Cell. Infect. Microbiol. 2013, 3, 59. [Google Scholar] [CrossRef]
- Troxell, B.; Sikes, M.L.; Fink, R.C.; Vazquez-Torres, A.; Jones-Carson, J.; Hassan, H.M. Fur negatively regulates hns and is required for the expression of hilA and virulence in Salmonella enterica serovar typhimurium. J. Bacteriol. 2011, 193, 497–505. [Google Scholar] [CrossRef]
- Dutter, B.F.; Mike, L.A.; Reid, P.R.; Chong, K.M.; Ramos-Hunter, S.J.; Skaar, E.P.; Sulikowski, G.A. Decoupling Activation of Heme Biosynthesis from Anaerobic Toxicity in a Molecule Active in Staphylococcus aureus. ACS Chem. Biol. 2016, 11, 1354–1361. [Google Scholar] [CrossRef]
- Hansson, M.D.; Karlberg, T.; Soderberg, C.A.G.; Rajan, S.; Warren, M.J.; Al-Karadaghi, S.; Rigby, S.E.J.; Hansson, M. Bacterial ferrochelatase turns human: Tyr13 determines the apparent metal specificity of Bacillus subtilis ferrochelatase. J. Biol. Inorg. Chem. 2011, 16, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lecerof, D.; Fodje, M.N.; Alvarez Leon, R.; Olsson, U.; Hansson, A.; Sigfridsson, E.; Ryde, U.; Hansson, M.; Al-Karadaghi, S. Metal binding to Bacillus subtilis ferrochelatase and interaction between metal sites. J. Biol. Inorg. Chem. 2003, 8, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Nihei, C.; Nakayashiki, T.; Nakamura, K.; Inokuchi, H.; Gennis, R.B.; Kojima, S.; Kita, K. Abortive assembly of succinate-ubiquinone reductase (complex II) in a ferrochelatase-deficient mutant of Escherichia coli. Mol. Genet. Genom. 2001, 265, 394–404. [Google Scholar] [CrossRef]
- Glanville, D.G.; Mullineaux-Sanders, C.; Corcoran, C.J.; Burger, B.T.; Imam, S.; Donohue, T.J.; Ulijasz, A.T. A high-throughput method for identifying novel genes that influence metabolic pathways reveals new iron and heme regulation in Pseudomonas aeruginosa. mSystems 2021, 6, e00933-20. [Google Scholar] [CrossRef]
- Wu, C.K.; Dailey, H.A.; Rose, J.P.; Burden, A.; Sellers, V.M.; Wang, B.C. The 2.0 angstrom structure of human ferrochelatase, the terminal enzyme of heme biosynthesis. Nat. Struct. Biol. 2001, 8, 156–160. [Google Scholar] [CrossRef]
- Hobbs, C.; Reid, J.D.; Shepherd, M. The coproporphyrin ferrochelatase of Staphylococcus aureus: Mechanistic insights into a regulatory iron-binding site. Biochem. J. 2017, 474, 3513–3522. [Google Scholar] [CrossRef] [PubMed]
- Lobo, S.A.L.; Scott, A.; Videira, M.A.M.; Winpenny, D.; Gardner, M.; Palmer, M.J.; Schroeder, S.; Lawrence, A.D.; Parkinson, T.; Warren, M. Staphylococcus Aureus haem biosynthesis: Characterisation of the enzymes involved in final steps of the pathway. Mol. Microbiol. 2015, 97, 472–487. [Google Scholar] [CrossRef]
- Dailey, H.A.; Gerdes, S.; Dailey, T.A.; Burch, J.S.; Phillips, J.D. Noncanonical Coproporphyrin-Dependent Bacterial Heme Biosynthesis Pathway That Does Not Use Protoporphyrin. Proc. Natl. Acad. Sci. USA 2015, 112, 2210–2215. [Google Scholar] [CrossRef]
- Schaible, U.E.; Kaufmann, S.H.E. Iron and Microbial Infection. Nat. Rev. Microbiol. 2004, 2, 946–953. [Google Scholar] [CrossRef]
- Meng, L.; Lei, T. The effect of Fur overexpression on heme synthesis in Escherichia coli. Food Ferment. Ind. 2019, 45, 18–25. [Google Scholar] [CrossRef]
- Porcheron, G.; Dozois, C.M. Interplay between Iron Homeostasis and Virulence: Fur and ryhB as major regulators of bacterial pathogenicity. Vet. Microbiol. 2015, 179, 2–14. [Google Scholar] [CrossRef]
- Llamas, M.A.; Sánchez-Jiménez, A. Iron homeostasis in Pseudomonas Aeruginosa: Targeting iron acquisition and storage as an antimicrobial strategy. In Pseudomonas aeruginosa: Biology, Pathogenesis and Control Strategies; Filloux, A., Ramos, J.-L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 29–68. ISBN 978-3-031-08491-1. [Google Scholar]
- Kwon, S.J.; de Boer, A.L.; Petri, R.; Schmidt-Dannert, C. High-level production of porphyrins in metabolically engineered Escherichia Coli: Systematic extension of a pathway assembled from overexpressed genes involved in heme biosynthesis. Appl. Environ. Microbiol. 2003, 69, 4875–4883. [Google Scholar] [CrossRef] [PubMed]
- Franken, C.; Werner, R.; Haas, H.; Lokman, C.; Hondel, A.; Ram, F.; Weert, S.; Punt, J. The role of coproporphyrinogen III oxidase and ferrochelatase genes in heme biosynthesis and regulation in Aspergillus niger. Appl. Microbiol. Biotechnol. 2013, 97, 9773–9785. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Y.; Gong, K.; Wang, Q.; Liang, Q.; Qi, Q. Constitutive expression of RyhB regulates the heme biosynthesis pathway and increases the 5-aminolevulinic acid accumulation in Escherichia coli. FEMS Microbiol. Lett. 2014, 350, 209–215. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Liu, Q.; Tang, L. Effects of genetic recombination and exogenous iron on heme synthesis in Escherichia coli. Microbiol. China 2023, 50, 3382–3391. [Google Scholar] [CrossRef]
- Wang, M.; Liu, M.; Cheng, A. Functional identification of hemH and transcriptomic analysis of hemH mutant of Riemerella anatipestifer. Acta Microbiol. Sin. 2023, 63, 3083–3095. [Google Scholar] [CrossRef]
- Yu, C.; Wang, N.; Wu, M.; Tian, F.; Chen, H.; Yang, F.; Yuan, X.; Yang, C.H.; He, C. OxyR-regulated catalase CatB promotes the virulence in rice via detoxifying hydrogen peroxide in Xanthomonas oryzae pv. oryzae. BMC Microbiol. 2016, 16, 269. [Google Scholar] [CrossRef]
- Chung, W.-H. Unraveling new functions of superoxide dismutase using yeast model system: Beyond its conventional role in superoxide radical scavenging. J. Microbiol. 2017, 55, 409–416. [Google Scholar] [CrossRef]
- Miller, A.-F. Superoxide dismutases: Active sites that save, but a protein that kills. Curr. Opin. Chem. Biol. 2004, 8, 162–168. [Google Scholar] [CrossRef]
- Zhou, Y.; Lv, H.; Li, H.; Li, J.; Yan, Y.; Liu, F.; Hao, W.; Zhou, Z.; Wang, P.; Zhou, S. Nitroreductase increases menadione-mediated oxidative stress in Aspergillus nidulans. Appl. Environ. Microbiol. 2021, 87, e01758-21. [Google Scholar] [CrossRef]
- Ma, Y.; Song, N. Advances in research on a bacterial ferric uptake regulator. J. Pathog. Biol. 2021, 16, 117–121. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20230424000 (accessed on 1 January 2021).
- Michener, J.K.; Nielsen, J.; Smolke, C.D. Identification and Treatment of Heme Depletion Attributed to Overexpression of a Lineage of Evolved P450 Monooxygenases. Proc. Natl. Acad. Sci. USA 2012, 109, 19504–19509. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Lopez, C.A.; Hu, H.; Xia, Y.; Freedman, D.S.; Reddington, A.P.; Daaboul, G.G.; Unlü, M.S.; Genco, C.A. A high-throughput method to examine protein-nucleotide interactions identifies targets of the bacterial transcriptional regulatory protein Fur. PLoS ONE 2014, 9, e96832. [Google Scholar] [CrossRef]
- Baichoo, N.; Helmann, J.D. Recognition of DNA by Fur: A reinterpretation of the Fur box consensus sequence. J. Bacteriol. 2002, 184, 5826–5832. [Google Scholar] [CrossRef]
- Seo, S.W.; Kim, D.; Latif, H.; O’Brien, E.J.; Szubin, R.; Palsson, B.O. Deciphering Fur transcriptional regulatory network highlights its complex role beyond iron metabolism in Escherichia coli. Nat. Commun. 2014, 5, 4910. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, Q.; Liu, Z.; Zhang, M.; Machado, A.C.D.; Chiu, T.-P.; Feng, C.; Zhang, Q.; Yu, L.; Qi, L.; et al. Mechanistic insights into metal ion activation and operator recognition by the ferric uptake regulator. Nat. Commun. 2015, 6, 7642. [Google Scholar] [CrossRef]
- Su, J.; Li, Z.; Liao, B. Microcalorimetric study of the effect of manganese on the growth and metabolism in a heterogeneously expressing manganese-dependent superoxide dismutase (Mn-SOD) strain. Therm. Anal. Calorim. 2017, 130, 1407–1416. [Google Scholar] [CrossRef]
- Su, J.; Bao, P.; Bai, T.; Deng, L.; Wu, H.; Liu, F.; He, J. CotA, a multicopper oxidase from Bacillus pumilus WH4, exhibits manganese-oxidase activity. PLoS ONE 2013, 8, e60573. [Google Scholar] [CrossRef]
- Su, J.; Deng, L.; Huang, L.; Guo, S.; Liu, F.; He, J. Catalytic oxidation of manganese(II) by multicopper oxidase CueO and characterization of the biogenic Mn oxide. Water Res. 2014, 56, 304–313. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, Y.; Wang, X.; Xie, Y.; Ali, M.K.; He, J. Functional analysis of the sporulation-specific diadenylate cyclase cdaS in Bacillus thuringiensis. Front. Microbiol. 2015, 6, 908. [Google Scholar] [CrossRef] [PubMed]
- Janes, B.K.; Stibitz, S. Routine markerless gene replacement in Bacillus anthracis. Infect. Immun. 2006, 74, 1949–1953. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Li, X.; Qian, H.; Cai, X.; Li, X.; He, J. Poly-β-hydroxybutyrate metabolism is unrelated to the sporulation and parasporal crystal protein formation in Bacillus thuringiensis. Front. Microbiol. 2016, 7, 836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kang, Z.; Ding, W.; Chen, J.; Du, G. Integrated optimization of the in vivo heme biosynthesis pathway and the in vitro iron concentration for 5-Aminolevulinate production. Appl. Biochem. Biotechnol. 2016, 178, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Cao, Z.; Cai, X.; Fang, Y.; An, B.; Li, X.; Zhang, Y.; Tian, H.; Hu, W.; Yan, B.; et al. NupR responding to multiple signals is a nucleoside permease regulator in Bacillus thuringiensis BMB171. Microbiol. Spectr. 2022, 10, e0154322. [Google Scholar] [CrossRef]
- Tang, Q.; Li, X.; Zou, T.; Zhang, H.; Wang, Y.; Gao, R.; Li, Z.; He, J.; Feng, Y. Functional analysis of a c-di-AMP-specific phosphodiesterase mspde from Mycobacterium smegmatis. Int. J. Biol. Sci. 2015, 11, 813–824. [Google Scholar] [CrossRef]
- Peng, Q.; Kao, G.; Qu, N.; Zhang, J.; Li, J.; Song, F. The regulation of exosporium-related genes in Bacillus thuringiensis. Sci. Rep. 2016, 6, 19005. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Luo, Y.; Jiao, T.; Liu, S.; Liang, T.; Mei, H.; Cheng, S.; Yang, Q.; He, J.; Su, J. Functional Differentiation and Regulatory Mechanisms of Ferrochelatases HemH1 and HemH2 in Bacillus thuringiensis Under Iron and Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 2911. https://doi.org/10.3390/ijms26072911
Wang J, Luo Y, Jiao T, Liu S, Liang T, Mei H, Cheng S, Yang Q, He J, Su J. Functional Differentiation and Regulatory Mechanisms of Ferrochelatases HemH1 and HemH2 in Bacillus thuringiensis Under Iron and Oxidative Stress. International Journal of Molecular Sciences. 2025; 26(7):2911. https://doi.org/10.3390/ijms26072911
Chicago/Turabian StyleWang, Jianghan, Yi Luo, Tian Jiao, Shizhen Liu, Ting Liang, Huiting Mei, Shuang Cheng, Qian Yang, Jin He, and Jianmei Su. 2025. "Functional Differentiation and Regulatory Mechanisms of Ferrochelatases HemH1 and HemH2 in Bacillus thuringiensis Under Iron and Oxidative Stress" International Journal of Molecular Sciences 26, no. 7: 2911. https://doi.org/10.3390/ijms26072911
APA StyleWang, J., Luo, Y., Jiao, T., Liu, S., Liang, T., Mei, H., Cheng, S., Yang, Q., He, J., & Su, J. (2025). Functional Differentiation and Regulatory Mechanisms of Ferrochelatases HemH1 and HemH2 in Bacillus thuringiensis Under Iron and Oxidative Stress. International Journal of Molecular Sciences, 26(7), 2911. https://doi.org/10.3390/ijms26072911





