Coupled Influence of Magnetic Biochar and Solution Chemistries on Retention and Release of Nanoplastics in Porous Media
Abstract
1. Introduction
2. Results and Discussion
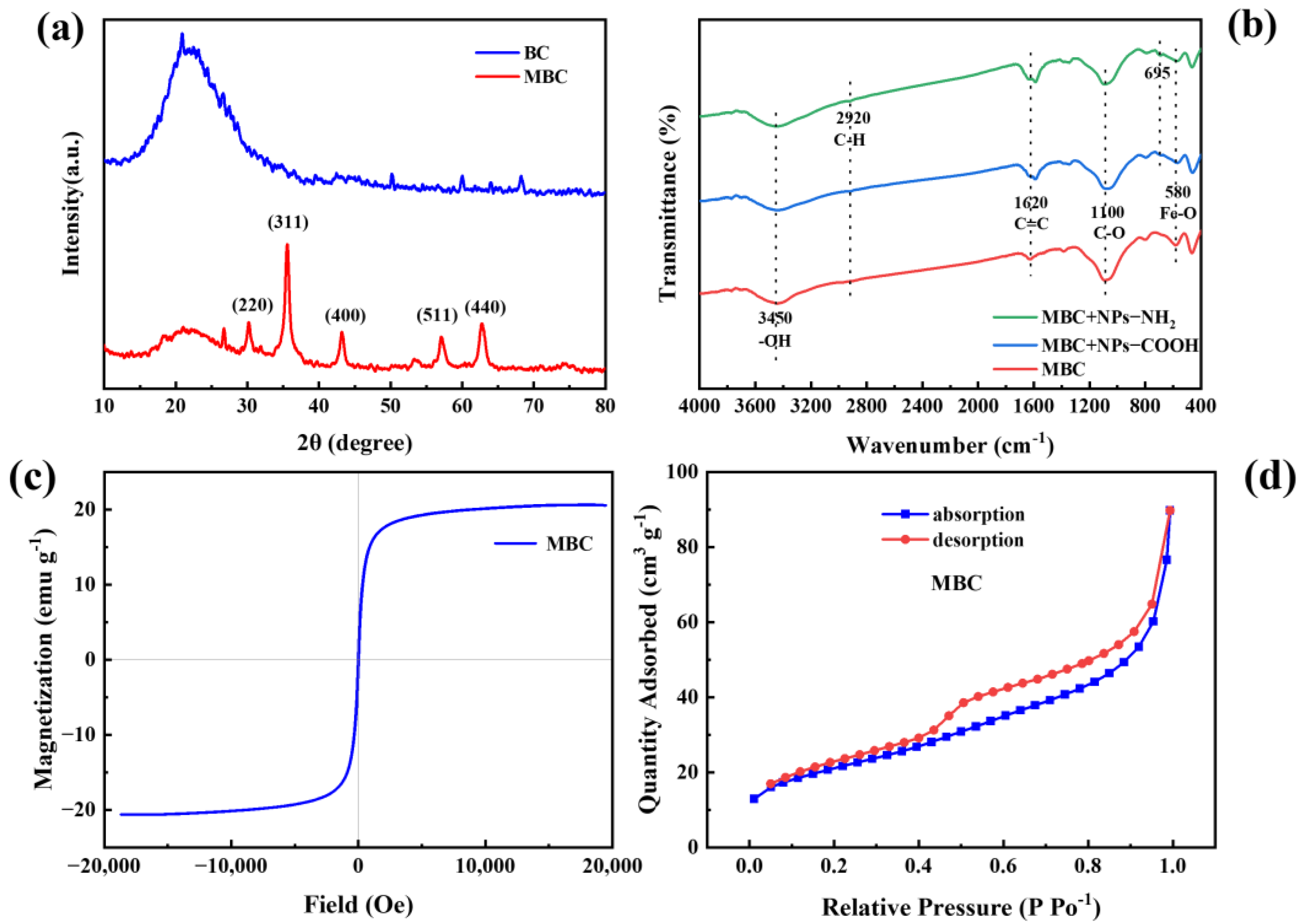
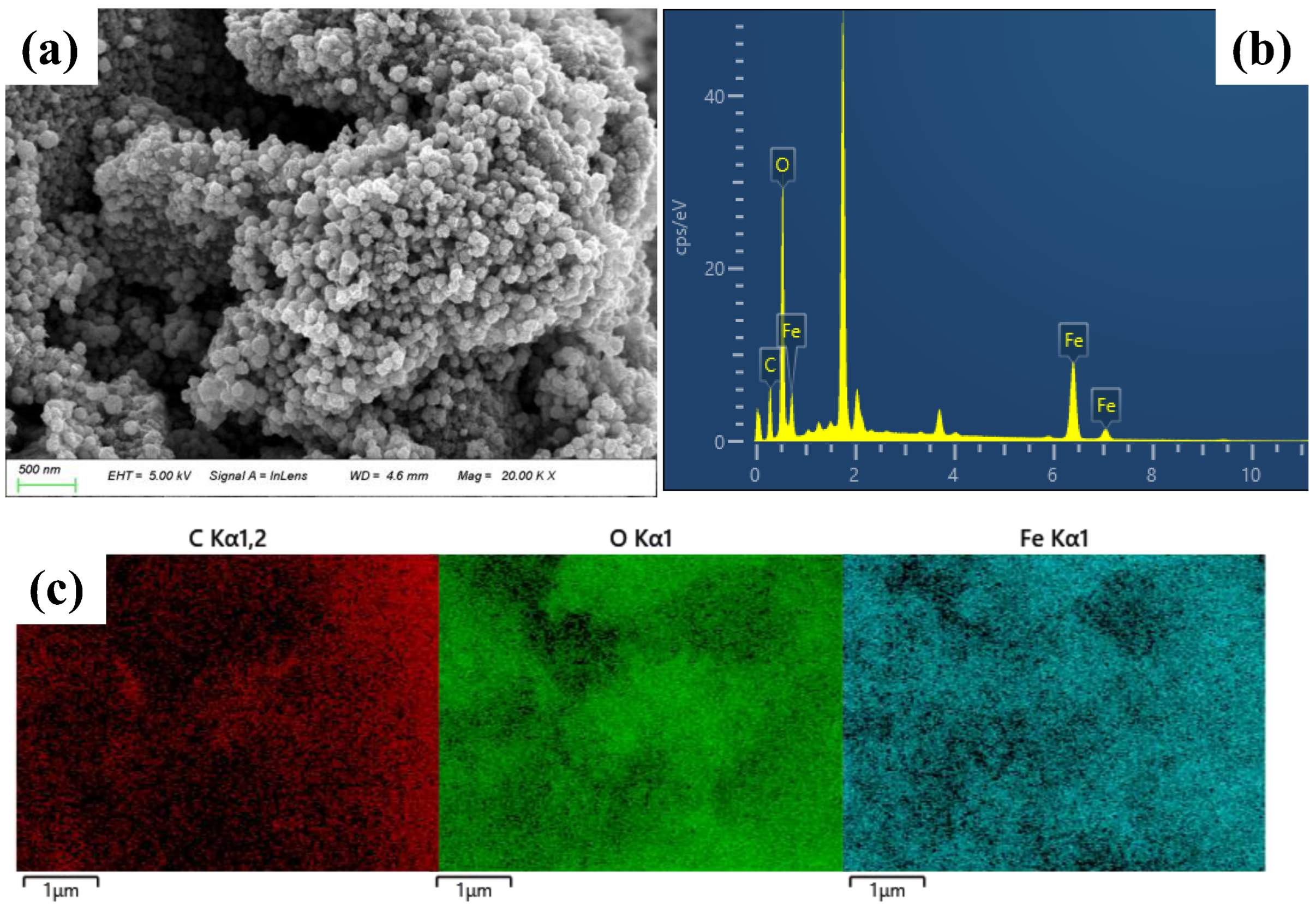
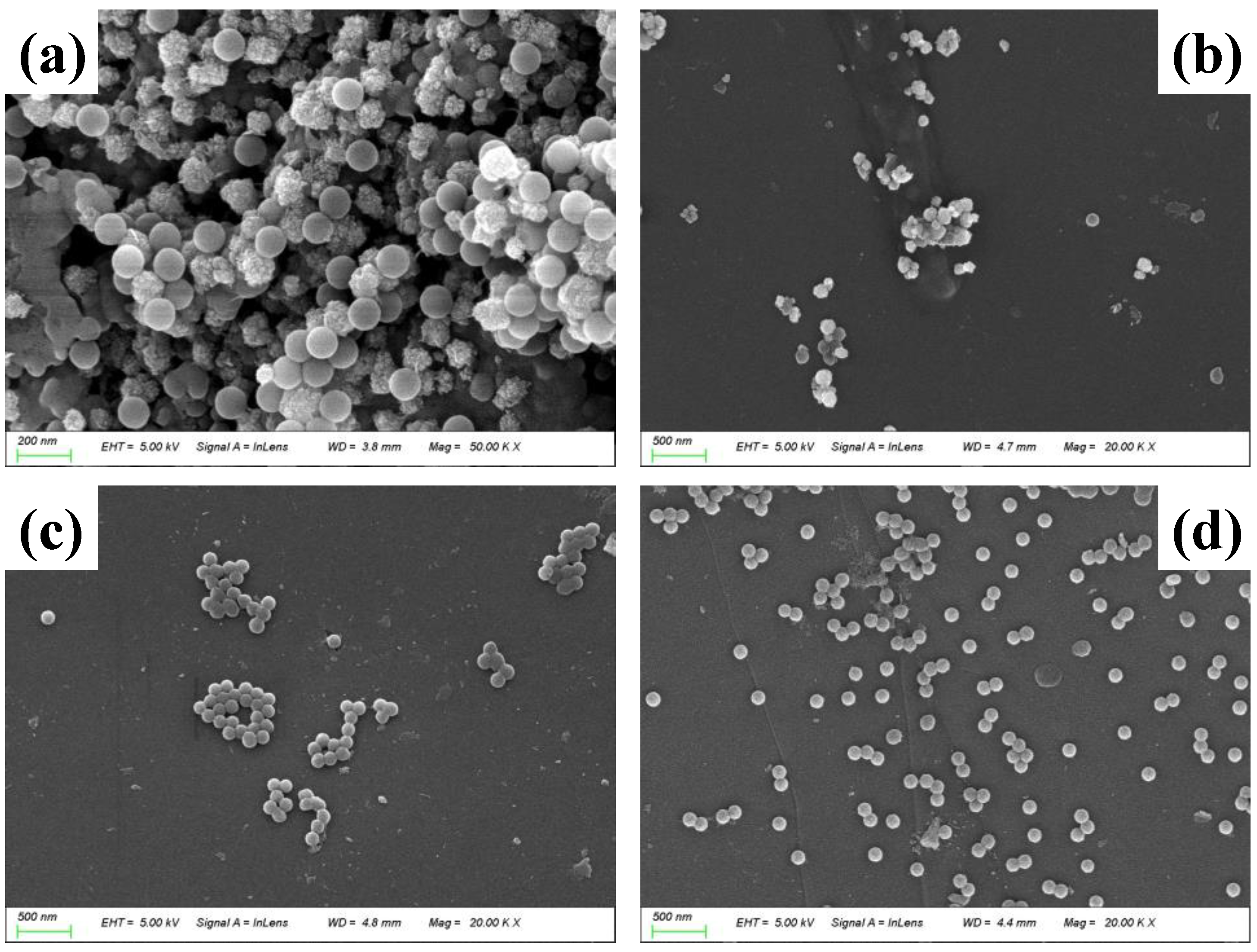
2.1. Characterization of NPs and MBC
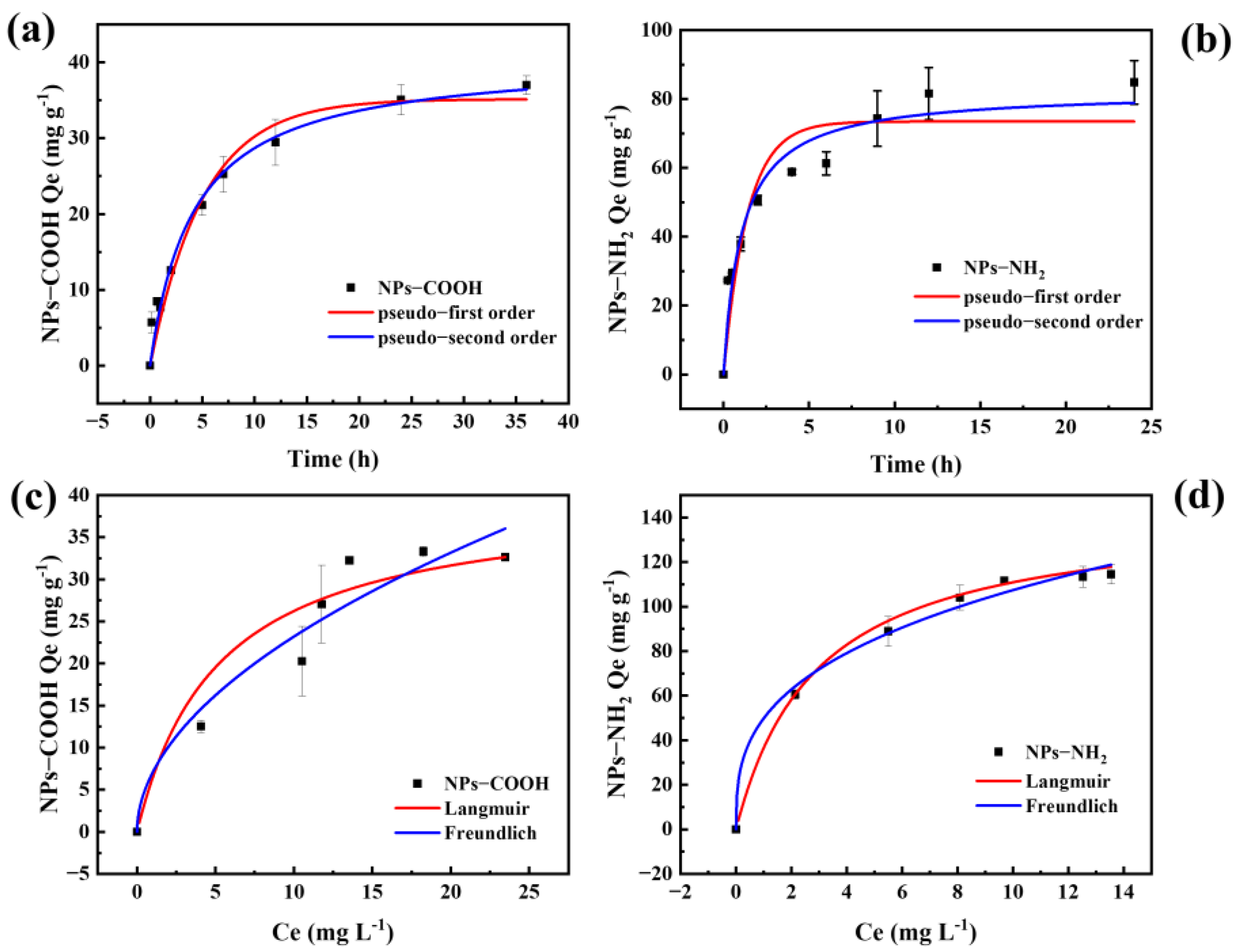
2.2. Adsorption of NPs on MBC
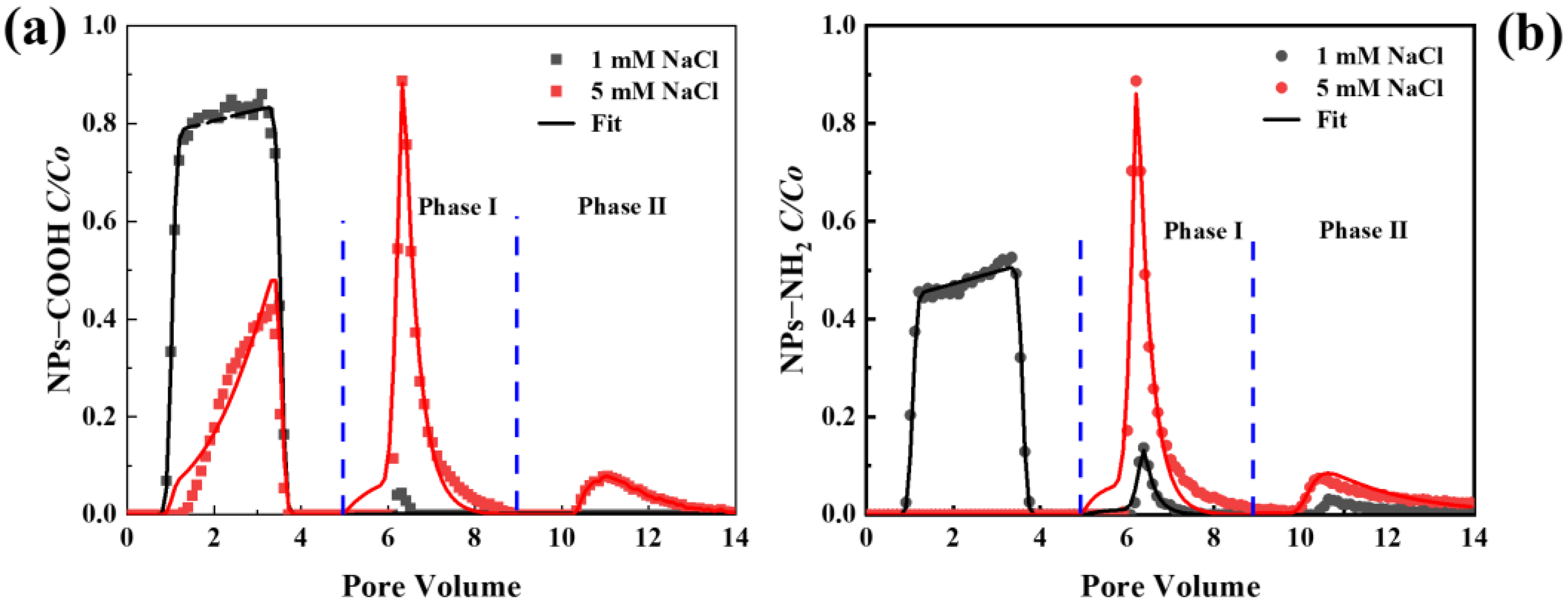
2.3. Transport and Release of NPs in Saturated Porous Media
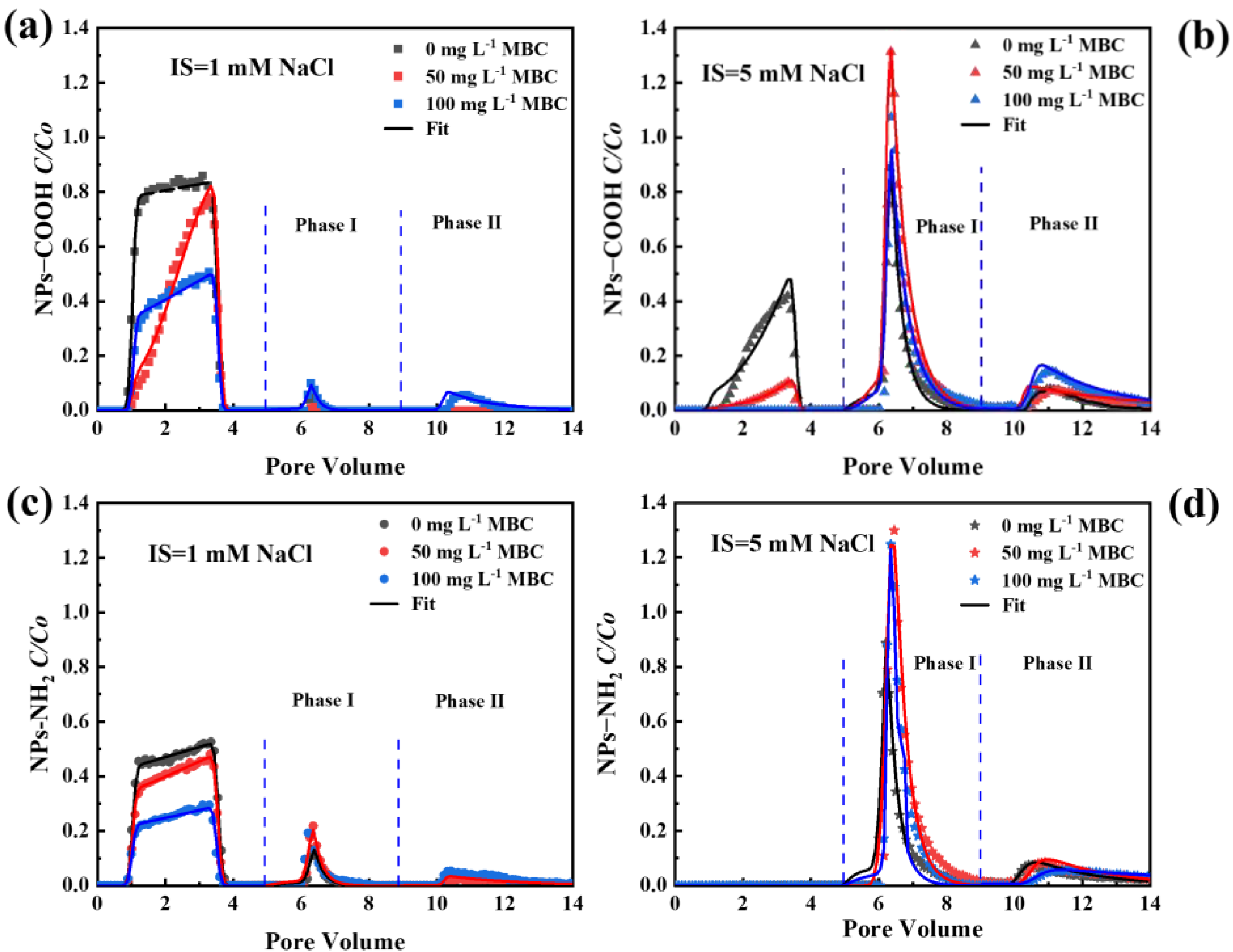
2.4. Effect of MBC on Transport and Release of NPs
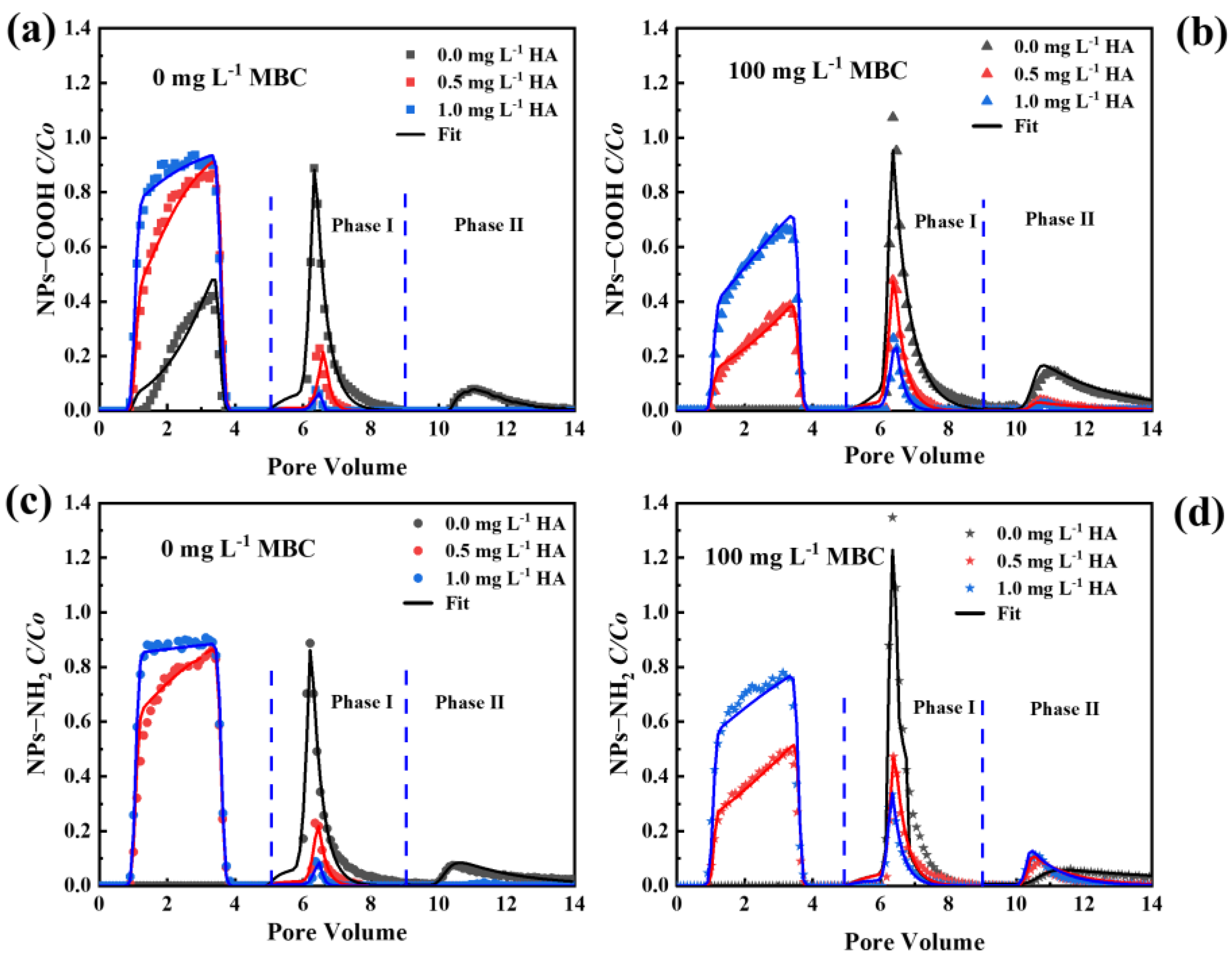
2.5. Effect of HA on Transport and Release of NPs
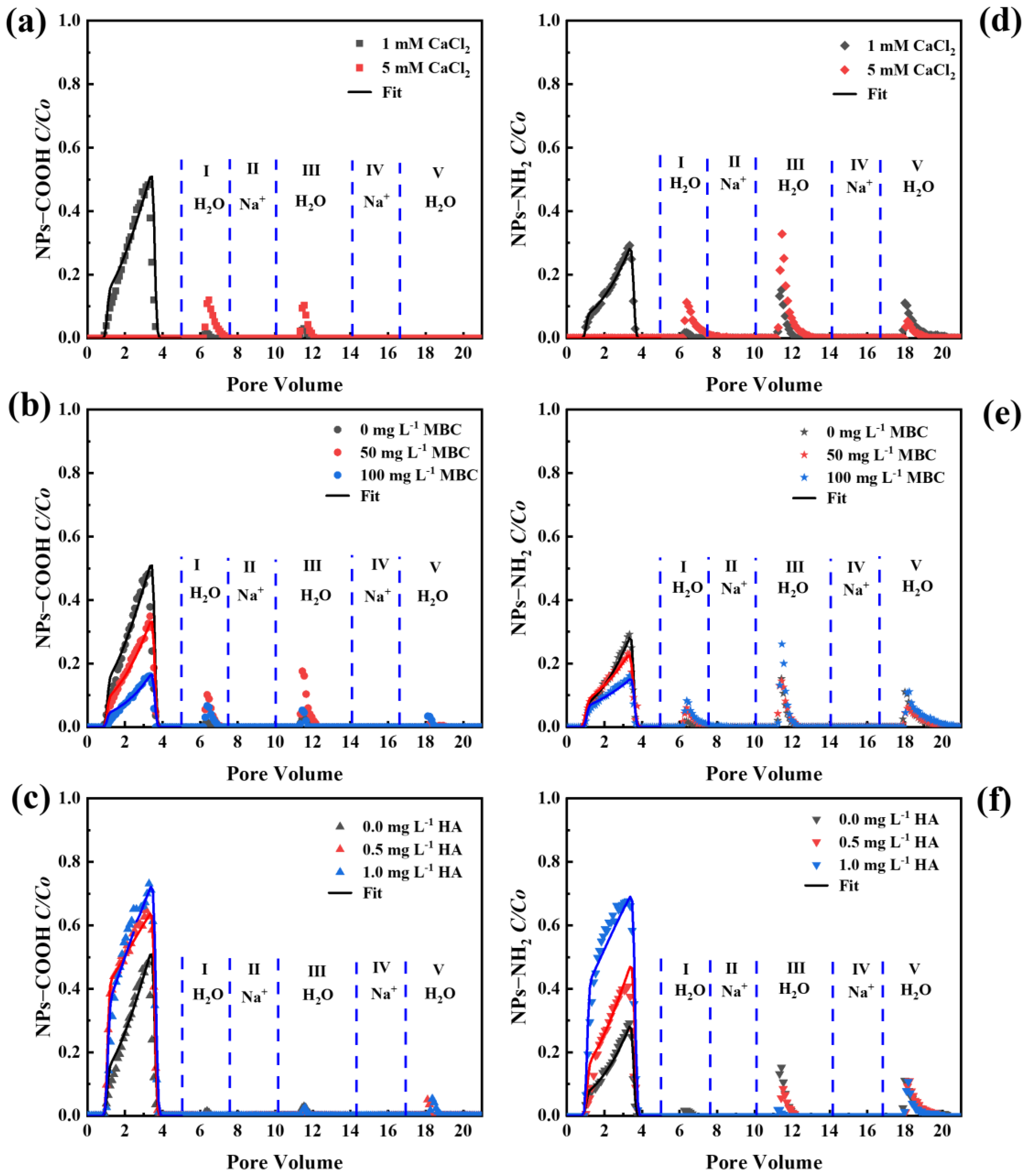
2.6. Effect of Divalent Cations on the Transport and Release of NPs
3. Materials and Methods
3.1. NPs, MBC, Solution Chemistries and Porous Media
3.2. Batch Experiments
3.3. Column Experiments
3.3.1. Transport Experiments
3.3.2. Release Experiments
3.4. Adsorption and Transport Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Ou, Q.; van der Hoek, J.P.; Liu, G.; Lompe, K.M. Photo-oxidation of Micro- and Nanoplastics: Physical, Chemical, and Biological Effects in Environments. Environ. Sci. Technol. 2024, 58, 991–1009. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, L.; Zhuang, Z.; Zhuang, Q.; Dong, S.; Zhao, X.; Mao, L. Nanoplastics in soil plastisphere: Occurrence, bio-interactions and environmental risks. Nano Today 2024, 58, 102409. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Liang, X.; Lu, S.; Ren, J.; Zhang, Y.; Han, Z.; Gao, B.; Sun, K. Effects of microplastics on soil carbon pool and terrestrial plant performance. Carbon Res. 2024, 3, 37. [Google Scholar] [CrossRef]
- Stephanie, C.; Charlotte, H.; Thorsten, H.; Thilo, H. Microplastics and nanoplastics barely enhance contaminant mobility in agricultural soils. Commun. Earth Environ. 2021, 2, 193. [Google Scholar] [CrossRef]
- Bhat, M.A.; Gedik, K.; Gaga, E.O. Atmospheric micro (nano) plastics: Future growing concerns for human health. Air Qual. Atmos. Health 2022, 16, 233–262. [Google Scholar] [CrossRef]
- Maity, S.; Guchhait, R.; Sarkar, M.B.; Pramanick, K. Occurrence and distribution of micro/nanoplastics in soils and their phytotoxic effects: A review. Plant Cell Environ. 2022, 45, 1011–1028. [Google Scholar] [CrossRef]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Bhagat, J.; Nishimura, N.; Shimada, Y. Toxicological interactions of microplastics/nanoplastics and environmental contaminants: Current Knowledge and Future Perspectives. J. Hazard. Mater. 2020, 405, 123913. [Google Scholar] [CrossRef]
- Liu, P.; Dai, J.; Huang, K.; Yang, Z.; Zhang, Z.; Guo, X. Sources of micro(nano)plastics and interaction with co-existing pollutants in wastewater treatment plants. Crit. Rev. Environ. Sci. Technol. 2022, 53, 865–885. [Google Scholar] [CrossRef]
- Okoffo, E.D.; O’Brien, S.; Ribeiro, F.; Burrows, S.D.; Toapanta, T.; Rauert, C.; O’Brien, J.W.; Tscharke, B.J.; Wang, X.; Thomas, K.V. Plastic particles in soil: State of the knowledge on sources, occurrence and distribution, analytical methods and ecological impacts. Environ. Sci. Process. Impacts 2021, 23, 240–274. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef]
- Yasir, A.M.; Ma, J.; Ouyang, X.; Zhao, J.; Zhao, Y.; Weng, L.; Islam, M.S.; Chen, Y.; Li, Y. Effects of selected functional groups on nanoplastics transport in saturated media under diethylhexyl phthalate co-contamination conditions. Chemosphere 2021, 286, 131965. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhu, L.; Zhang, W.; Huang, R.; Lv, X.; Jing, X.; Yang, Z.; Wang, J.; Qiu, Y. Role of surface functionalities of nanoplastics on their transport in seawater-saturated sea sand. Environ. Pollut. 2019, 255, 113177. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Luo, C.; Wang, Y.; Zhao, Y.; Qiu, Y. Nanoplastics dominate the cotransport of small-scale plastics in seawater-saturated porous media. Water Res. 2022, 221, 118773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, B.; Jiang, N.; Pang, K.; Wu, W.; Yin, X. Effect of water-soluble polymers on the transport of functional group-modified polystyrene nanoplastics in goethite-coated saturated porous media. J. Hazard. Mater. 2024, 469, 134044. [Google Scholar] [CrossRef]
- Wu, X.; Lyu, X.; Li, Z.; Gao, B.; Zeng, X.; Wu, J.; Sun, Y. Transport of polystyrene nanoplastics in natural soils: Effect of soil properties, ionic strength and cation type. Sci. Total Environ. 2020, 707, 136065. [Google Scholar] [CrossRef]
- He, L.; Wu, D.; Rong, H.; Li, M.; Tong, M.; Kim, H. Influence of Nano- and Microplastic Particles on the Transport and Deposition Behaviors of Bacteria in Quartz Sand. Environ. Sci. Technol. 2018, 52, 11555–11563. [Google Scholar] [CrossRef]
- Yuan, R.; Zhang, W.; Tao, X.; Wang, S.; Zhang, L. Coupled effects of high pH and chemical heterogeneity on colloid retention and release in saturated porous media. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124285. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Lin, H.; Chang, Z.; Li, Z.; Riaz, A.; Hou, E. Magnesium-doped biochars increase soil phosphorus availability by regulating phosphorus retention, microbial solubilization and mineralization. Biochar 2024, 6, 68. [Google Scholar] [CrossRef]
- Pradhan, S.; Parthasarathy, P.; Mackey, H.R.; Al-Ansari, T.; McKay, G. Food waste biochar: A sustainable solution for agriculture application and soil–water remediation. Carbon Res. 2024, 3, 41. [Google Scholar] [CrossRef]
- Ji, G.; Xing, Y.; You, T. Biochar as adsorbents for environmental microplastics and nanoplastics removal. J. Environ. Chem. Eng. 2024, 12, 113377. [Google Scholar] [CrossRef]
- Gouma, V.; Tziasiou, C.; Pournara, A.D.; Giokas, D.L. A novel approach to sorbent-based remediation of soil impacted by organic micropollutants and heavy metals using granular biochar amendment and magnetic separation. J. Environ. Chem. Eng. 2022, 10, 107316. [Google Scholar] [CrossRef]
- Liang, M.; Ding, Y.; Zhang, Q.; Wang, D.; Li, H.; Lu, L. Removal of aqueous Cr(VI) by magnetic biochar derived from bagasse. Sci. Rep. 2020, 10, 21473. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.; He, L.; Zhang, M.; Lv, W.; Yang, K.; Tong, M. Addition of biochar as thin preamble layer into sand filtration columns could improve the microplastics removal from water. Water Res. 2022, 221, 118783. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dan, Y.; Diao, Y.; Liu, F.; Wang, H.; Sang, W.; Zhang, Y. Transport characteristics of polystyrene microplastics in saturated porous media with biochar/Fe3O4-biochar under various chemical conditions. Sci. Total Environ. 2022, 847, 157576. [Google Scholar] [CrossRef]
- Tong, M.; Li, T.; Li, M.; He, L.; Ma, Z. Cotransport and deposition of biochar with different sized-plastic particles in saturated porous media. Sci. Total Environ. 2020, 713, 136387. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Zhang, J.; Wang, Z.; Guo, Z.; Ali, J.; Wang, L.; Yu, Z.; Zhang, X.; Sun, Y. Effective removal of microplastics by filamentous algae and its magnetic biochar: Performance and mechanism. Chemosphere 2024, 358, 142152. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Zhivulin, V.E. Controlled modification of polyvinylidene fluoride as a way for carbyne synthesis. Polym. Degrad. Stab. 2022, 203, 110054. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Besedina, N.A.; Brzhezinskaya, M.; Malkov, M.N.; Stolyarova, D.Y.; Arutyunyan, A.F.; Struchkov, N.S.; Saveliev, S.D.; Diankin, I.D.; et al. Guiding graphene derivatization for covalent immobilization of aptamers. Carbon 2022, 196, 264–279. [Google Scholar] [CrossRef]
- Lee, E.-J.; Lee, J.-W. Synergistic effect of adsorption and photolysis on methylene blue removal by magnetic biochar derived from lignocellulosic biomass. Bioresour. Technol. 2024, 407, 131124. [Google Scholar] [CrossRef]
- Dong, P.; Liang, Y.; Shen, C.; Jiang, E.; Bradford, S.A. Dual roles of goethite coating on the transport of plastic nanoparticles in heterogeneous porous media: The significance of collector surface roughness. J. Hazard. Mater. 2024, 470, 134153. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wang, S.; Sun, B.; Han, Y.; Zhang, Z.; Shen, X.; Li, Z. Adsorption efficiency and in-situ catalytic thermal degradation behaviour of microplastics from water over Fe-modified lignin-based magnetic biochar. Sep. Purif. Technol. 2025, 353, 128468. [Google Scholar] [CrossRef]
- Peng, X.; Yang, Y.; Wang, J.; Yuan, W.; Guo, Y.; Hu, W.; Yang, X. Cu/Fe co-modified nitrogen self-doped biochar as a heterogeneous Fenton-like catalyst for degradation of organic pollutants: Synthesis, performance, and mechanistic study. J. Environ. Chem. Eng. 2023, 11, 110866. [Google Scholar] [CrossRef]
- Gao, W.; Wang, X.; Diao, Y.; Gong, Y.; Miao, J.; Sang, W.; Yuan, H.; Shen, Z.; El-sayed, M.E.A.; Abdelhafeez, I.A. Co-impacts of cation type and humic acid on migration of polystyrene microplastics in saturated porous media. J. Environ. Manag. 2024, 358, 120918. [Google Scholar] [CrossRef]
- Dong, S.; Cai, W.; Xia, J.; Sheng, L.; Wang, W.; Liu, H. Aggregation kinetics of fragmental PET nanoplastics in aqueous environment: Complex roles of electrolytes, pH and humic acid. Environ. Pollut. 2021, 268, 115828. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Huang, Q.-X.; Chi, Y.; Yan, J.-H. Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J. Hazard. Mater. 2021, 419, 126486. [Google Scholar] [CrossRef]
- Zhu, N.; Yan, Q.; He, Y.; Wang, X.; Wei, Z.; Liang, D.; Yue, H.; Yun, Y.; Li, G.; Sang, N. Insights into the removal of polystyrene nanoplastics using the contaminated corncob-derived mesoporous biochar from mining area. J. Hazard. Mater. 2022, 433, 128756. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, S.; Su, Y.; Wu, D.; Zhao, Y.; Xie, B. Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chem. Eng. J. 2021, 406, 126804. [Google Scholar] [CrossRef]
- Wu, J.; Yang, C.; Zhao, H.; Shi, J.; Liu, Z.; Li, C.; Song, F. Efficient removal of microplastics from aqueous solution by a novel magnetic biochar: Performance, mechanism, and reusability. Environ. Sci. Pollut. Res. 2022, 30, 26914–26928. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, J.; Dong, P.; Qin, Y.; Zhang, R.; Bradford, S.A. Retention and release of black phosphorus nanoparticles in porous media under various physicochemical conditions. Chemosphere 2023, 339, 139604. [Google Scholar] [CrossRef]
- Ye, X.; Cheng, Z.; Wu, M.; Hao, Y.; Hu, B.X.; Mo, C.; Li, Q.; Xiang, L.; Zhao, H.; Wu, J.; et al. Investigating transport kinetics of polystyrene nanoplastics in saturated porous media. Ecotoxicol. Environ. Saf. 2022, 241, 113820. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Bradford, S.A.; Šimůnek, J.; Klumpp, E. Mechanisms of graphene oxide aggregation, retention, and release in quartz sand. Sci. Total Environ. 2019, 656, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, Y.; Peng, Y.; Meng, T.; Xu, L.; Dong, P. Sensitivity of the Transport of Plastic Nanoparticles to Typical Phosphates Associated with Ionic Strength and Solution pH. Int. J. Mol. Sci. 2022, 23, 9860. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, L.; Chen, H.; Zhang, M. Retention and transport behavior of microplastic particles in water-saturated porous media. Sci. Total Environ. 2022, 808, 152154. [Google Scholar] [CrossRef]
- Abdoul Magid, A.S.I.; Islam, M.S.; Chen, Y.; Weng, L.; Li, J.; Ma, J.; Li, Y. Enhanced adsorption of polystyrene nanoplastics (PSNPs) onto oxidized corncob biochar with high pyrolysis temperature. Sci. Total Environ. 2021, 784, 147115. [Google Scholar] [CrossRef]
- Liang, Y.; Luo, Y.; Shen, C.; Bradford, S.A. Micro- and nanoplastics retention in porous media exhibits different dependence on grain surface roughness and clay coating with particle size. Water Res. 2022, 221, 118717. [Google Scholar] [CrossRef]
- Torkzaban, S.; Bradford, S.A. Critical role of surface roughness on colloid retention and release in porous media. Water Res. 2016, 88, 274–284. [Google Scholar] [CrossRef]
- Sun, H.; Jiao, R.; An, G.; Xu, H.; Wang, D. Influence of particle size on the aggregation behavior of nanoparticles: Role of structural hydration layer. J. Environ. Sci. 2021, 103, 33–42. [Google Scholar] [CrossRef]
- Daugherty, E.E.; Gilbert, B.; Nico, P.S.; Borch, T. Complexation and Redox Buffering of Iron(II) by Dissolved Organic Matter. Environ. Sci. Technol. 2017, 51, 11096–11104. [Google Scholar] [CrossRef]
- Wang, M.; Lu, T.; Chen, W.; Zhang, H.; Qi, W.; Song, Y.; Qi, Z. Enhanced role of humic acid on the transport of iron oxide colloids in saturated porous media under various solution chemistry conditions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125486. [Google Scholar] [CrossRef]
- Xu, L.; Liang, Y.; Liao, C.; Xie, T.; Zhang, H.; Liu, X.; Lu, Z.; Wang, D. Cotransport of micro- and nano-plastics with chlortetracycline hydrochloride in saturated porous media: Effects of physicochemical heterogeneities and ionic strength. Water Res. 2022, 209, 117886. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-J.; Li, Q.-C.; Shan, W.-Y.; Hao, Z.-N.; Li, P.; Liu, J.-F. Heteroaggregation of different surface-modified polystyrene nanoparticles with model natural colloids. Sci. Total Environ. 2021, 784, 147190. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Li, X.; Tao, M.; Cao, X.; Wang, Z.; Xing, B. Cation-π mechanism promotes the adsorption of humic acid on polystyrene nanoplastics to differently affect their aggregation: Evidence from experimental characterization and DFT calculation. J. Hazard. Mater. 2023, 459, 132071. [Google Scholar] [CrossRef]
- Ye, X.; Cheng, Z.; Wu, M.; Hu, B.X.; Mo, C.; Li, Q.; Wu, J.; Wu, J.; Hao, Y.; Lu, G. Determining the mobility of polystyrene nano-plastic in saturated quartz Sand-Limestone porous media. Chem. Eng. Sci. 2022, 260, 117949. [Google Scholar] [CrossRef]
- Liang, Y.; Bradford, S.A.; Simunek, J.; Heggen, M.; Vereecken, H.; Klumpp, E. Retention and Remobilization of Stabilized Silver Nanoparticles in an Undisturbed Loamy Sand Soil. Environ. Sci. Technol 2013, 47, 12229–12237. [Google Scholar] [CrossRef]
- Liang, S.; Shi, S.; Zhang, H.; Qiu, J.; Yu, W.; Li, M.; Gan, Q.; Yu, W.; Xiao, K.; Liu, B.; et al. One-pot solvothermal synthesis of magnetic biochar from waste biomass: Formation mechanism and efficient adsorption of Cr(VI) in an aqueous solution. Sci. Total Environ. 2019, 695, 133886. [Google Scholar] [CrossRef]
- Ho, Y. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Siwek, B.; Zembala, M.; Belouschek, P. Kinetics of localized adsorption of colloid particles. Adv. Colloid Interface Sci. 1994, 48, 151–280. [Google Scholar] [CrossRef]
| Sample | Cation Types | IS | HA (mg L−1) | pH | dp, nm | Zeta Potential, mV |
|---|---|---|---|---|---|---|
| NPs-COOH | 0 | 0 | 0 | 7 | 156.9 ± 1.7 | −22.1 ± 0.1 |
| 0 | 0 | 0 | 10 | 187.3 ± 0.9 | −28.2 ± 0.3 | |
| NaCl | 1 | 0 | 7 | 169.9 ± 0.9 | −17.2 ± 1.7 | |
| NaCl | 5 | 0 | 7 | 171.0 ± 1.3 | −9.0 ± 3.2 | |
| NaCl | 5 | 0.5 | 7 | 170.8 ± 5.4 | −14.3 ± 2.1 | |
| NaCl | 5 | 1 | 7 | 170.7 ± 1.6 | −32.4 ± 0.1 | |
| CaCl2 | 1 | 0 | 7 | 171.2 ± 2.7 | −6.0 ± 4.4 | |
| CaCl2 | 1 | 0.5 | 7 | 170.6 ± 1.1 | −4.5 ± 0.9 | |
| CaCl2 | 1 | 1 | 7 | 169.5 ± 5.5 | −6.6 ± 0.9 | |
| CaCl2 | 5 | 0 | 7 | 215.7 ± 3.2 | −3.6 ± 3.9 | |
| NPs-NH2 | 0 | 0 | 0 | 7 | 162.5 ± 1.0 | −11.9 ± 2.3 |
| 0 | 0 | 0 | 10 | 156.8 ± 5.7 | −26.0 ± 0.9 | |
| NaCl | 1 | 0 | 7 | 163.7 ± 1.0 | −16.7 ± 1.5 | |
| NaCl | 5 | 0 | 7 | 178.1 ± 5.3 | −7.5 ± 0.5 | |
| NaCl | 5 | 0.5 | 7 | 166.8 ± 2.1 | −30.7 ± 1.6 | |
| NaCl | 5 | 1 | 7 | 164.8 ± 2.0 | −38.5 ± 1.6 | |
| CaCl2 | 1 | 0 | 7 | 162.2 ± 0.8 | −7.0 ± 3.7 | |
| CaCl2 | 1 | 0.5 | 7 | 161.5 ± 0.1 | −14.1 ± 2.4 | |
| CaCl2 | 1 | 1 | 7 | 161.2 ± 2.4 | −20.2 ± 0.3 | |
| CaCl2 | 5 | 0 | 7 | 178.5 ± 3.0 | −5.2 ± 2.2 | |
| Sand | 0 | 0 | 0 | 7 | - | −26.4 ± 0.3 |
| 0 | 0 | 0 | 10 | - | −46.8 ± 3.7 | |
| NaCl | 1 | 0 | 7 | - | −35.2 ± 0.6 | |
| NaCl | 5 | 0 | 7 | - | −17.4 ± 1.6 | |
| NaCl | 5 | 0.5 | 7 | - | −36.3 ± 5.3 | |
| NaCl | 5 | 1 | 7 | - | −37.1 ± 1.4 | |
| CaCl2 | 1 | 0 | 7 | - | −20.6 ± 0.7 | |
| CaCl2 | 1 | 0.5 | 7 | - | −26.0 ± 0.2 | |
| CaCl2 | 1 | 1 | 7 | - | −29.4 ± 1.5 | |
| CaCl2 | 5 | 0 | 7 | - | −15.9 ± 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Liang, Y.; Peng, Y. Coupled Influence of Magnetic Biochar and Solution Chemistries on Retention and Release of Nanoplastics in Porous Media. Int. J. Mol. Sci. 2025, 26, 2207. https://doi.org/10.3390/ijms26052207
Qin Y, Liang Y, Peng Y. Coupled Influence of Magnetic Biochar and Solution Chemistries on Retention and Release of Nanoplastics in Porous Media. International Journal of Molecular Sciences. 2025; 26(5):2207. https://doi.org/10.3390/ijms26052207
Chicago/Turabian StyleQin, Yan, Yan Liang, and Yongtao Peng. 2025. "Coupled Influence of Magnetic Biochar and Solution Chemistries on Retention and Release of Nanoplastics in Porous Media" International Journal of Molecular Sciences 26, no. 5: 2207. https://doi.org/10.3390/ijms26052207
APA StyleQin, Y., Liang, Y., & Peng, Y. (2025). Coupled Influence of Magnetic Biochar and Solution Chemistries on Retention and Release of Nanoplastics in Porous Media. International Journal of Molecular Sciences, 26(5), 2207. https://doi.org/10.3390/ijms26052207




