Involvement of miR775 in the Post-Transcriptional Regulation of Fructose-1,6-Bisphosphate Aldolase in Maize (Zea mays L.) Leaves Under Hypoxia
Abstract
1. Introduction
2. Results
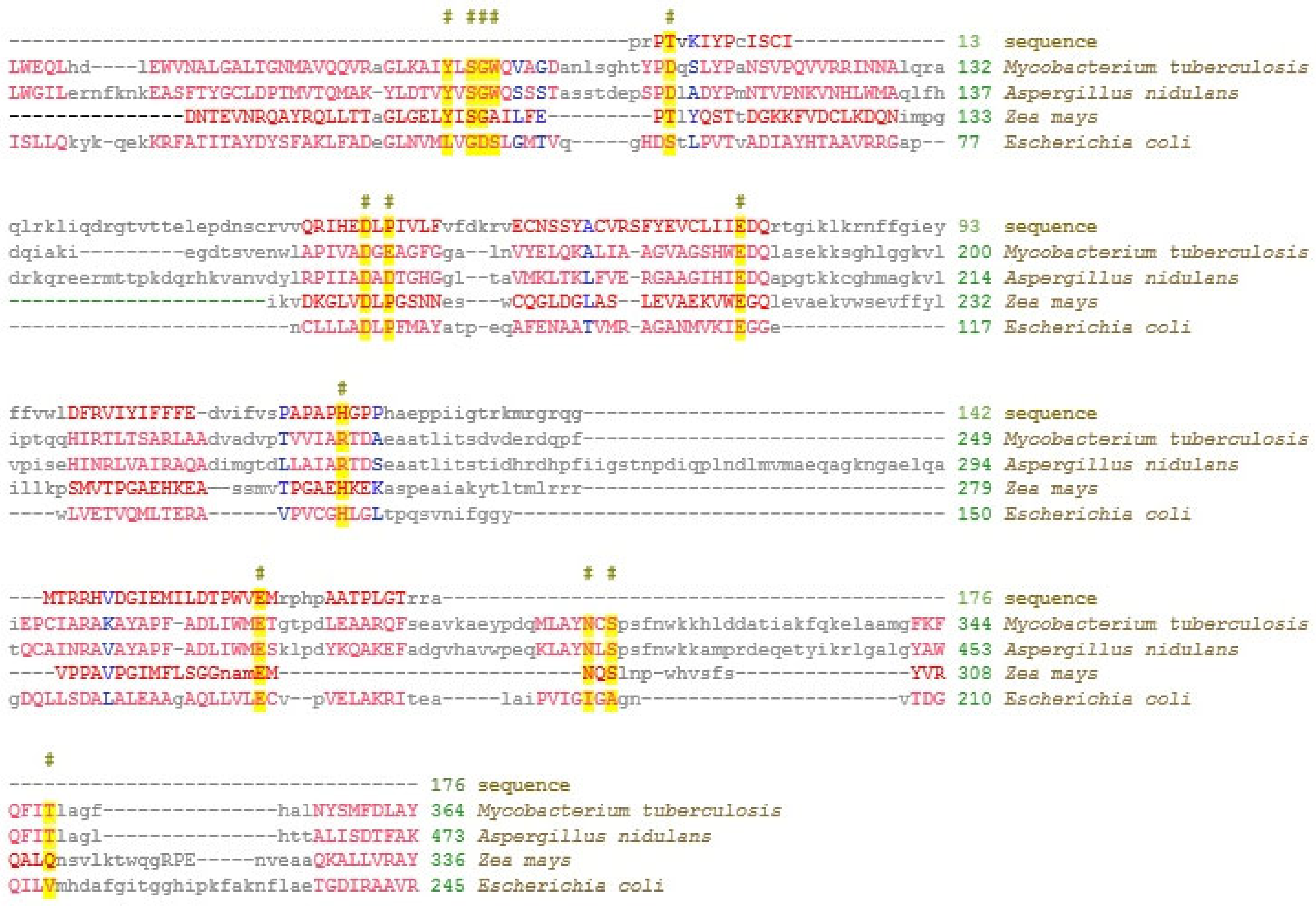
2.1. Identification of miR775 Targets
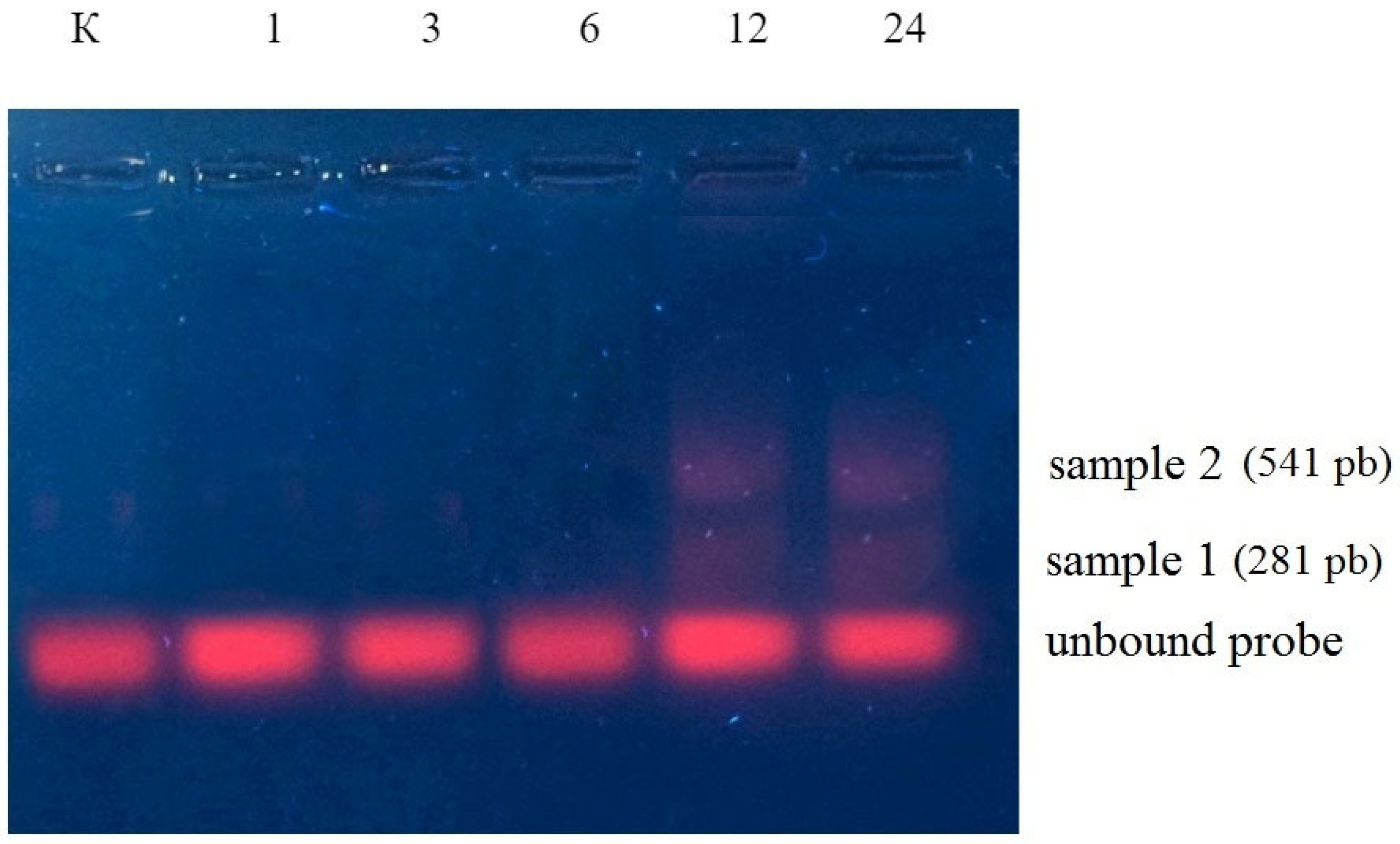
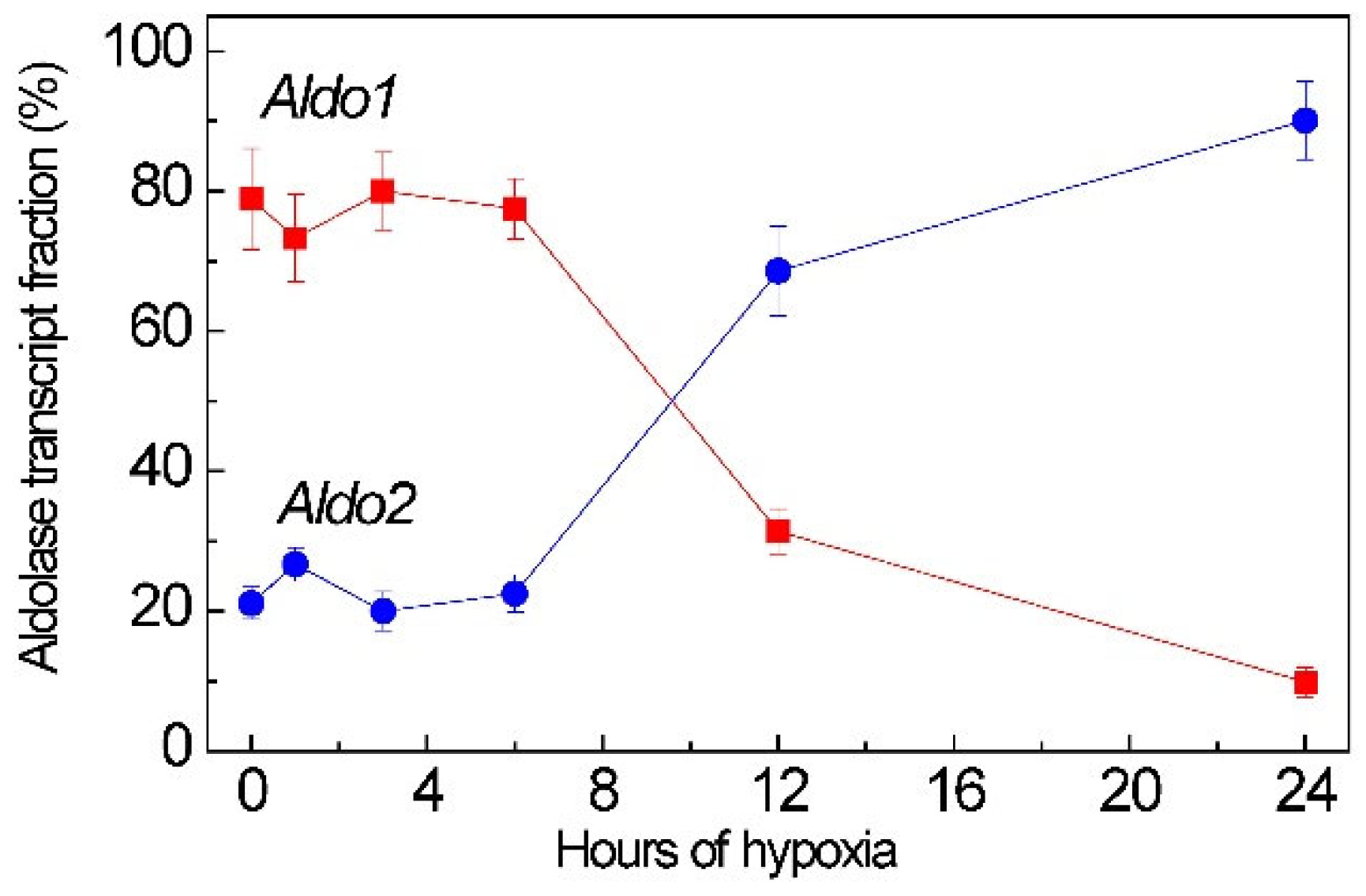
2.2. Aldolase Genes Aldo1 and Aldo2 Transcript Is the Target of miR775A
2.3. Aldolase and Glucose-6-Phosphate Dehydrogenase Activities Under Hypoxic Conditions
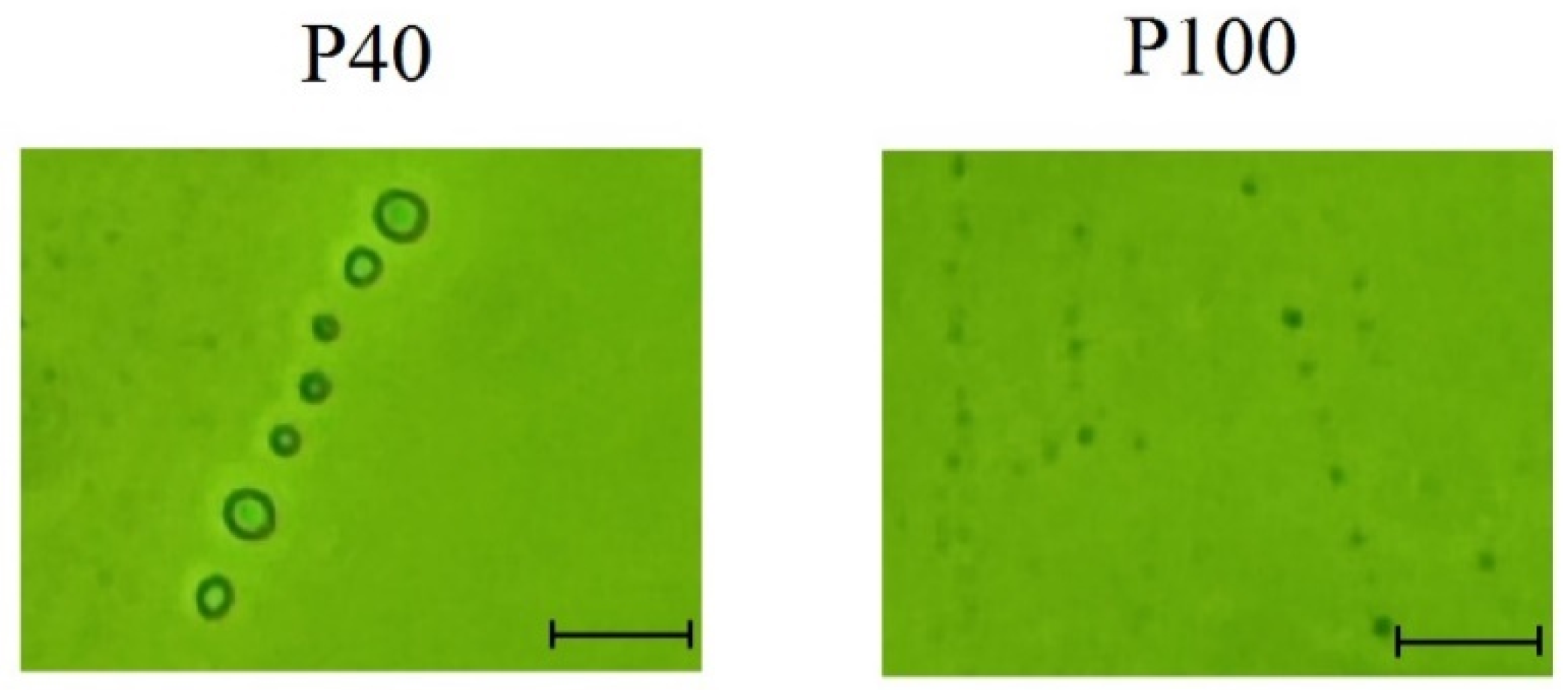
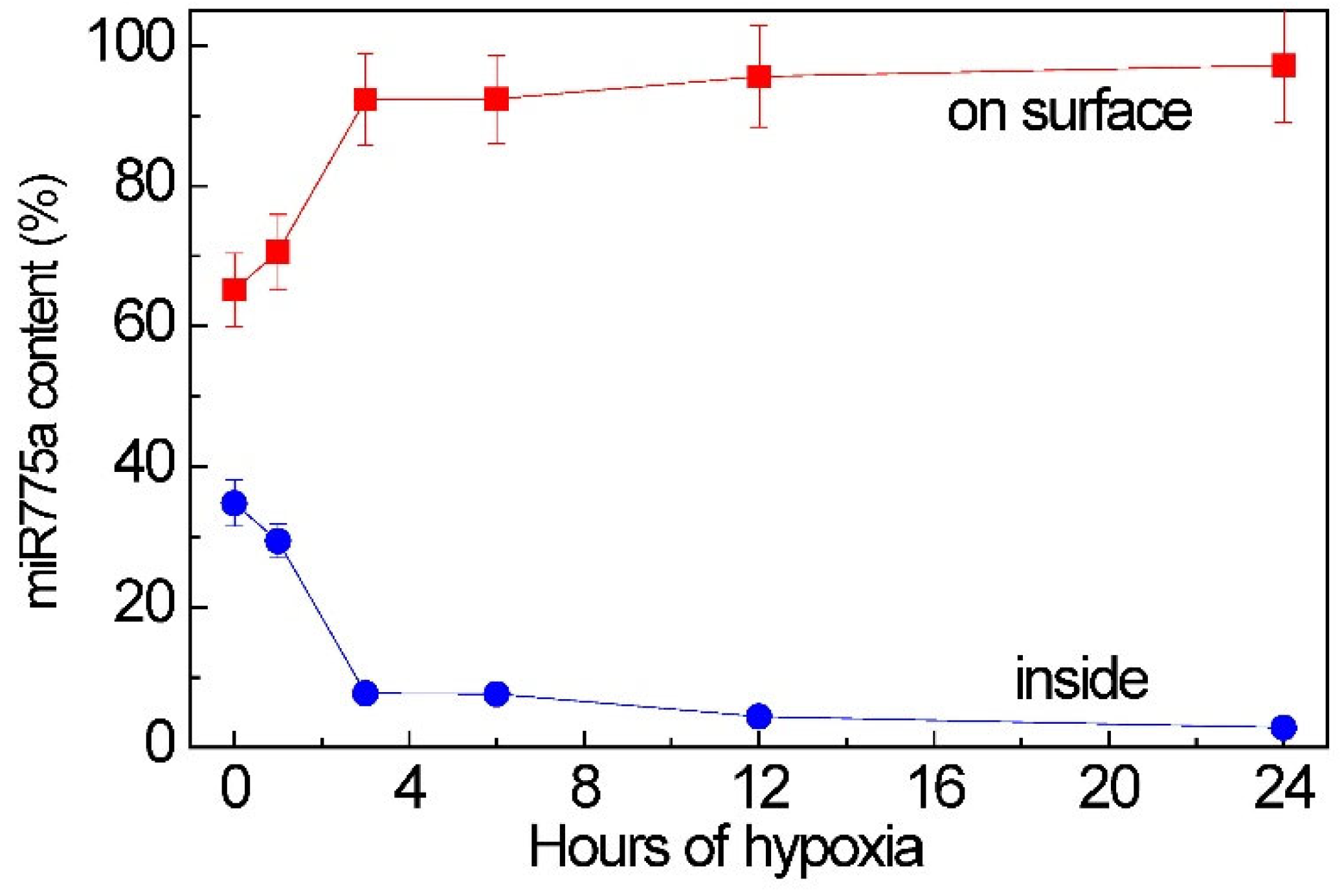
2.4. Participation of Extracellular Vesicles in miR775A Transport
3. Discussion
4. Material and Methods
4.1. Object of Investigation
4.2. Creating Hypoxic Conditions
4.3. Isolation of Total mRNA
4.4. Analytical Electrophoresis of Nucleic Acids
4.5. Reverse Transcription
4.6. Real-Time Polymerase Chain Reaction
- –
- for Aldo1 (LOC100286050): forward—5′-AAGCCCGAAGACACCGATCT-3′; reverse—5′-AAGCAACAGATTTCGCGGTG-3′;
- –
- for Aldo2 (LOC100272913): forward—5′-GTGCCAACAACCTCTACGT-3′; reverse—5′-TCTGTTGTGTTGGCACAGG-3′.
4.7. Hybridization of Cellular RNA with the Fluorescent Probe ROX-miR775A
4.8. Measurement of Total Enzymatic Activity
4.9. Isolation of Extracellular Plant Vesicles
4.10. Inhibitory Assay
4.11. Statistical Data Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGO1 | ARGONAUTE1 |
| EV | extracellular vesicle |
| miRNA | microRNA |
| miR775A | microRNA 775A |
| OPPP | oxidative pentose phosphate pathway |
| pri-miR | initially transcribed long primary transcripts from the precursor gene of the corresponding microRNA |
| pre-miR | hairpin-shaped precursor processed into microRNA |
| TF | transcription factor |
| TCA cycle | tricarboxylic acid cycle |
References
- Bailey-Serres, J.; Chang, R. Sensing and signalling in response to oxygen deprivation in plants and other organisms. Ann. Bot. 2005, 96, 507–518. [Google Scholar] [CrossRef]
- León, J.; Castillo, M.C.; Gayubas, B. The hypoxia-reoxygenation stress in plants. J. Exp. Bot. 2021, 72, 5841–5856. [Google Scholar] [CrossRef]
- Ullah, N.; Tan, D.K.Y.; Ahmad, W.; Pampana, S. Editorial: Adaptation of plants to waterlogging and hypoxia. Front. Plant Sci. 2024, 15, 1425012. [Google Scholar] [CrossRef] [PubMed]
- Šečić, E.; Kogel, K.H.; Ladera-Carmona, M.J. Biotic stress-associated microRNA families in plants. J. Plant Physiol. 2021, 263, 153451. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Domingues, M.N.; Sforça, M.L.; Soprano, A.S.; Lee, J.; de Souza, T.A.C.B.; Cassago, A.; Portugal, R.V.; de Mattos Zeri, A.C.; Murakami, M.T.; Sadanandom, A.; et al. Structure and Mechanism of Dimer-Monomer Transition of a Plant Poly(A)-Binding Protein upon RNA Interaction: Insights into Its Poly(A) Tail Assembly. J. Mol. Biol. 2015, 427, 2491–2506. [Google Scholar] [CrossRef]
- Mayberry, L.K.; Allen, M.L.; Nitka, K.R.; Campbell, L.; Murphy, P.A.; Browning, K.S. Plant cap-binding complexes eukaryotic initiation factors eIF4F and eIFISO4F: Molecular specificity of subunit binding. J. Biol. Chem. 2011, 286, 42566–42574. [Google Scholar] [CrossRef] [PubMed]
- Keiper, B.D. Cap-Independent mRNA Translation in Germ Cells. Int. J. Mol. Sci. 2019, 20, 173. [Google Scholar] [CrossRef] [PubMed]
- Ghram, M.; Morris, G.; Culjkovic-Kraljacic, B.; Mars, J.C.; Gendron, P.; Skrabanek, L.; Revuelta, M.V.; Cerchietti, L.; Guzman, M.L.; Borden, K.L.B. The eukaryotic translation initiation factor eIF4E reprograms alternative splicing. EMBO J. 2023, 42, e110496. [Google Scholar] [CrossRef]
- Iwakawa, H.O.; Tomari, Y. Life of RISC: Formation, action, and degradation of RNA-induced silencing complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Skopelitis, D.S.; Hill, K.; Klesen, S.; Marco, C.F.; von Born, P.; Chitwood, D.H.; Timmermans, M.C.P. Gating of miRNA movement at defined cell-cell interfaces governs their impact as positional signals. Nat. Commun. 2018, 9, 3107. [Google Scholar] [CrossRef]
- Levine, A. Regulation of stress responses by intracellular vesicle trafficking? Plant Physiol. Biochem. 2002, 40, 531–535. [Google Scholar] [CrossRef]
- López de Las Hazas, M.C.; Tomé-Carneiro, J.; Del Pozo-Acebo, L.; Del Saz-Lara, A.; Chapado, L.A.; Balaguer, L.; Rojo, E.; Espín, J.C.; Crespo, C.; Moreno, D.A.; et al. Therapeutic potential of plant-derived extracellular vesicles as nanocarriers for exogenous miRNAs. Pharmacol. Res. 2023, 198, 106999. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Bailey-Serres, J. Plant responses to hypoxia—Is survival a balancing act? Trends Plant Sci. 2004, 9, 449–456. [Google Scholar] [CrossRef]
- Loreti, E.; van Veen, H.; Perata, P. Plant responses to flooding stress. Curr. Opin. Plant Biol. 2016, 33, 64–71. [Google Scholar] [CrossRef]
- Perata, P.; Pozueta-Romero, J.; Akazawa, T.; Yamaguchi, J. Effect of anoxia on starch breakdown in rice and wheat seeds. Planta 1992, 188, 611–618. [Google Scholar] [CrossRef]
- Guglielminetti, L.; Yamaguchi, J.; Perata, P.; Alpi, A. Amylolytic Activities in Cereal Seeds under Aerobic and Anaerobic Conditions. Plant Physiol. 1995, 109, 1069–1076. [Google Scholar] [CrossRef]
- Singh, P.K.; Mehla, K.; Hollingsworth, M.A.; Johnson, K.R. Regulation of Aerobic Glycolysis by microRNAs in Cancer. Mol. Cell. Pharmacol. 2011, 3, 125–134. [Google Scholar] [PubMed]
- Suriya Muthukumaran, N.; Velusamy, P.; Akino Mercy, C.S.; Langford, D.; Natarajaseenivasan, K.; Shanmughapriya, S. MicroRNAs as Regulators of Cancer Cell Energy Metabolism. J. Pers. Med. 2022, 12, 1329. [Google Scholar] [CrossRef]
- Ye, Z.; Zeng, J.; Long, L.; Ye, L.; Zhang, G. Identification of microRNAs in response to low potassium stress in the shoots of Tibetan wild barley and cultivated. Curr. Plant Biol. 2021, 25, 100193. [Google Scholar] [CrossRef]
- Chatterjee, Y.; Tomar, S.; Mishra, M.; Pareek, A.; Singla-Pareek, S.L. OsLdh7 Overexpression in Rice Confers Submergence Tolerance by Regulating Key Metabolic Pathways: Anaerobic Glycolysis, Ethanolic Fermentation and Amino Acid Metabolism. Plant Cell Environ. 2025, in press. [Google Scholar] [CrossRef]
- Botha, F.C.; O’Kennedy, M.M. Characterization of the Cytosolic Aldolase from Germinating Phaseolus vulgaris Seeds. J. Plant Physiol. 1989, 135, 433–438. [Google Scholar] [CrossRef]
- Yemelyanov, V.V.; Puzanskiy, R.K.; Shishova, M.F. Plant Life with and without Oxygen: A Metabolomics Approach. Int. J. Mol. Sci. 2023, 24, 16222. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Vaucheret, H.; Trejo, J.; Bartel, D.P. A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes Dev. 2006, 20, 3407–3425. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, D.; Spriggs, A.; Yang, J.; Pogson, B.J.; Dennis, E.S.; Wilson, I.W. Hypoxia-responsive microRNAs and trans-acting small interfering RNAs in Arabidopsis. J. Exp. Bot. 2010, 61, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Xu, Y.; Mattson, N.; Li, X.; Wang, B.; Zhang, X.; Jiang, H.; Liu, X.; Wang, Y.; Yao, D. Identification of Submergence-Responsive MicroRNAs and Their Targets Reveals Complex MiRNA-Mediated Regulatory Networks in Lotus (Nelumbo nucifera Gaertn). Front. Plant Sci. 2017, 8, 6. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Z.; Zhuang, Y.; Suo, Y.; Du, J.; Gao, Z.; Pan, J.; Li, L.; Wang, T.; Xiao, L.; et al. MicroRNA775 regulates intrinsic leaf size and reduces cell wall pectin levels by targeting a galactosyltransferase gene in Arabidopsis. Plant Cell 2021, 33, 581–602. [Google Scholar] [CrossRef] [PubMed]
- Fedorin, D.N.; Eprintsev, A.T.; Chuykova, V.O.; Igamberdiev, A.U. Participation of miR165a in the Phytochrome Signal Transduction in Maize (Zea mays L.) Leaves under Changing Light Conditions. Int. J. Mol. Sci. 2024, 25, 5733. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.N.; Srivastava, D. MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell 2010, 7, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.C.; Singh, J.; Barik, S. MicroRNAs: Processing, Maturation, Target Recognition and Regulatory Functions. Mol. Cell. Pharmacol. 2011, 3, 83–92. [Google Scholar] [PubMed]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef] [PubMed]
- Fedorin, D.N.; Khomutova, A.E.; Eprintsev, A.T. Participation of MiR775A in Post-transcriptional Regulation of Glycerol-3-phosphate acyltransferases in Corn Leaves under Hypoxia. J. Stress Physiol. Biochem. 2024, 20, 117–122. [Google Scholar]
- Baumberger, N.; Baulcombe, D.C. Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc. Natl. Acad. Sci. USA 2005, 102, 11928–11933. [Google Scholar] [CrossRef] [PubMed]
- Fedorin, D.N.; Khomutova, A.E.; Eprintsev, A.T. Changes in the content of microRNA775A and its role in post-transcriptional regulation of targeted genes in corn leaves under hypoxia. Biol. Bull. Rev. 2024, 14, 879–885. [Google Scholar] [CrossRef]
- Graciet, E.; Lebreton, S.; Gontero, B. Emergence of new regulatory mechanisms in the Benson-Calvin pathway via protein-protein interactions: A glyceraldehyde-3-phosphate dehydrogenase/CP12/phosphoribulokinase complex. J. Exp. Bot. 2004, 55, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Xue, Z.; Li, Q.; Cai, B. Structures, characteristics and functions of fructose-1,6-bisphosphate aldolase in various tissues. Acta Soc. Bot. Pol. 2023, 92, 1–15. [Google Scholar] [CrossRef]
- Kelley, P.M.; Freeling, M. Anaerobic expression of maize fructose-1,6-diphosphate aldolase. J. Biol. Chem. 1984, 259, 14180–14183. [Google Scholar] [CrossRef] [PubMed]
- Zabalza, A.; van Dongen, J.T.; Froehlich, A.; Oliver, S.N.; Faix, B.; Gupta, K.J.; Schmälzlin, E.; Igal, M.; Orcaray, L.; Royuela, M.; et al. Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiol. 2009, 149, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- António, C.; Päpke, C.; Rocha, M.; Diab, H.; Limami, A.M.; Obata, T.; Fernie, A.R.; van Dongen, J.T. Regulation of primary metabolism in response to low oxygen availability as revealed by carbon and nitrogen isotope redistribution. Plant Physiol. 2016, 170, 43–56. [Google Scholar] [CrossRef]
- Cho, H.Y.; Loreti, E.; Shih, M.C.; Perata, P. Energy and sugar signaling during hypoxia. New Phytol. 2021, 229, 57–63. [Google Scholar] [CrossRef] [PubMed]
- van Veen, H.; Triozzi, P.M.; Loreti, E. Metabolic strategies in hypoxic plants. Plant Physiol. 2024, 197, kiae564. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Kleczkowski, L.A. Pyrophosphate as an alternative energy currency in plants. Biochem. J. 2021, 478, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Kleczkowski, L.A. Magnesium and cell energetics in plants under anoxia. Biochem. J. 2011, 437, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.H.; Bednarz, H.; Gödde, V.; Niehaus, K.; Zörb, C. Metabolic responses of sugar beet to the combined effect of root hypoxia and NaCl-salinity. J. Plant Physiol. 2021, 267, 153545. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Chen, X. Small RNA metabolism in Arabidopsis. Trends Plant Sci. 2008, 13, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Rego, E.C.S.; Pinheiro, T.D.M.; Fonseca, F.C.A.; Gomes, T.G.; Costa, E.C.; Bastos, L.S.; Alves, G.S.C.; Cotta, M.G.; Amorim, E.P.; Ferreira, C.F.; et al. Characterization of microRNAs and Target Genes in Musa acuminata subsp. burmannicoides, var. Calcutta 4 during Interaction with Pseudocercospora musae. Plants 2023, 12, 1473. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Gilroy, S. Analysis of plant flooding response. Methods Enzymol. 2022, 680, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Renziehausen, T.; Frings, S.; Schmidt-Schippers, R. ‘Against all floods’: Plant adaptation to flooding stress and combined abiotic stresses. Plant J. 2024, 117, 1836–1855. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Verwoerd, T.C.; Dekker, B.M.; Hoekema, A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989, 17, 2362. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.F. Stem-loop RT-qPCR for miRNAs. Curr. Protoc. Mol. Biol. 2011, 95, Unit 15.10. [Google Scholar] [CrossRef] [PubMed]
- Nicot, N.; Hausman, J.F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Párraga Solórzano, P.K.; Bastille, T.S.; Radin, J.N.; Kehl-Fie, T.E. A Manganese-independent Aldolase Enables Staphylococcus aureus to Resist Host-imposed Metal Starvation. mBio 2023, 14, e0322322. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, X.; Chang, N.; Nan, W.; Wang, S.; Ruan, M.; Sun, L.; Li, S.; Bi, Y. Cytosolic Glucose-6-Phosphate Dehydrogenase is Involved in Seed Germination and Root Growth under Salinity in Arabidopsis. Front. Plant Sci. 2019, 10, 182. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Cai, Q.; Jin, H. Effective methods for isolation and purification of extracellular vesicles from plants. J. Integr. Plant Biol. 2021, 63, 2020–2030. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Pearson: Hoboken, NJ, USA, 1999; ISBN 978-0130815422. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorin, D.N.; Khomutova, A.E.; Eprintsev, A.T.; Igamberdiev, A.U. Involvement of miR775 in the Post-Transcriptional Regulation of Fructose-1,6-Bisphosphate Aldolase in Maize (Zea mays L.) Leaves Under Hypoxia. Int. J. Mol. Sci. 2025, 26, 865. https://doi.org/10.3390/ijms26030865
Fedorin DN, Khomutova AE, Eprintsev AT, Igamberdiev AU. Involvement of miR775 in the Post-Transcriptional Regulation of Fructose-1,6-Bisphosphate Aldolase in Maize (Zea mays L.) Leaves Under Hypoxia. International Journal of Molecular Sciences. 2025; 26(3):865. https://doi.org/10.3390/ijms26030865
Chicago/Turabian StyleFedorin, Dmitry N., Anna E. Khomutova, Alexander T. Eprintsev, and Abir U. Igamberdiev. 2025. "Involvement of miR775 in the Post-Transcriptional Regulation of Fructose-1,6-Bisphosphate Aldolase in Maize (Zea mays L.) Leaves Under Hypoxia" International Journal of Molecular Sciences 26, no. 3: 865. https://doi.org/10.3390/ijms26030865
APA StyleFedorin, D. N., Khomutova, A. E., Eprintsev, A. T., & Igamberdiev, A. U. (2025). Involvement of miR775 in the Post-Transcriptional Regulation of Fructose-1,6-Bisphosphate Aldolase in Maize (Zea mays L.) Leaves Under Hypoxia. International Journal of Molecular Sciences, 26(3), 865. https://doi.org/10.3390/ijms26030865






