Endothelial-Protective Actions of Diethylether Extract from Gentiana kochiana and Xanthone Gentiacaulein Against Oxidized LDL-Induced Injury—In Vitro Evaluation
Abstract
1. Introduction
2. Results
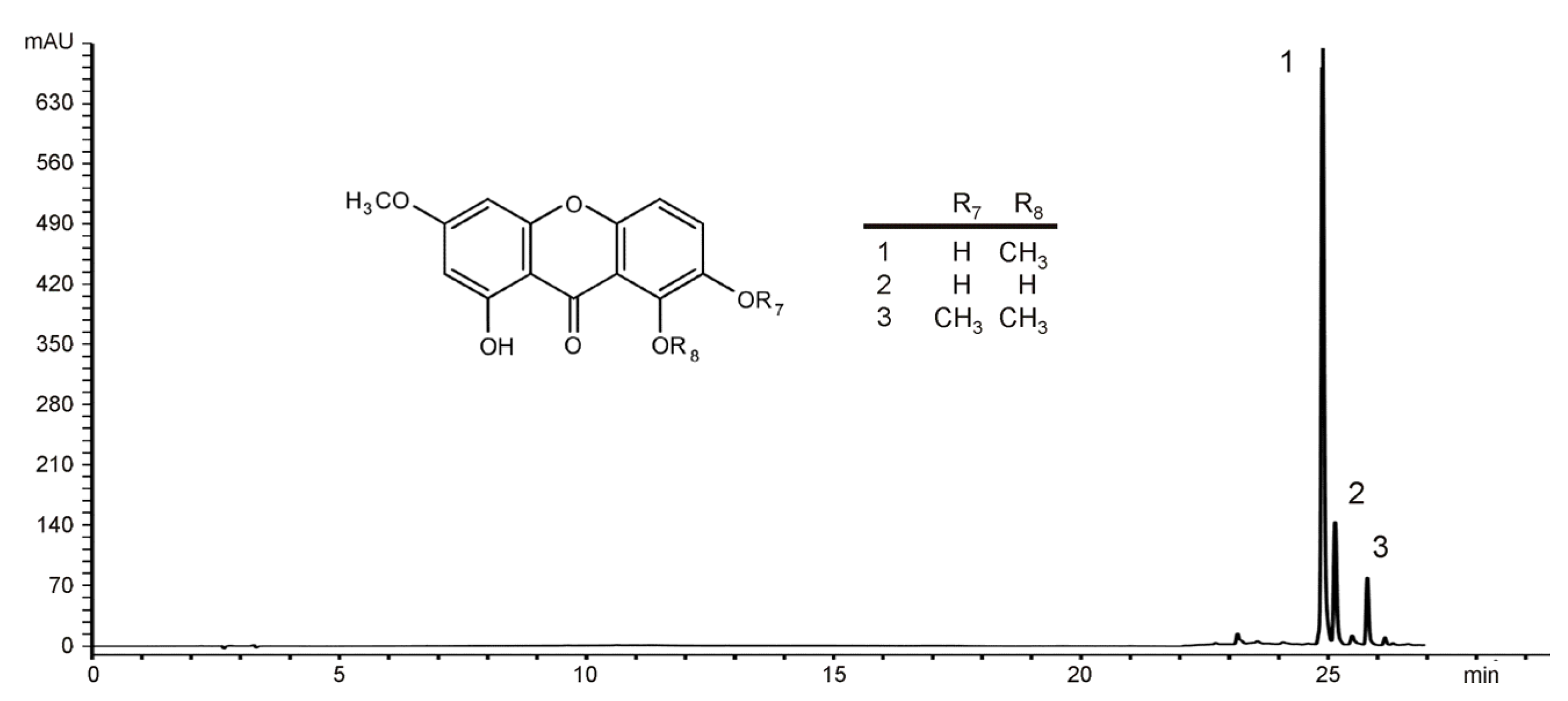
2.1. Identification of the Main Constituents from EE of G. kochiana
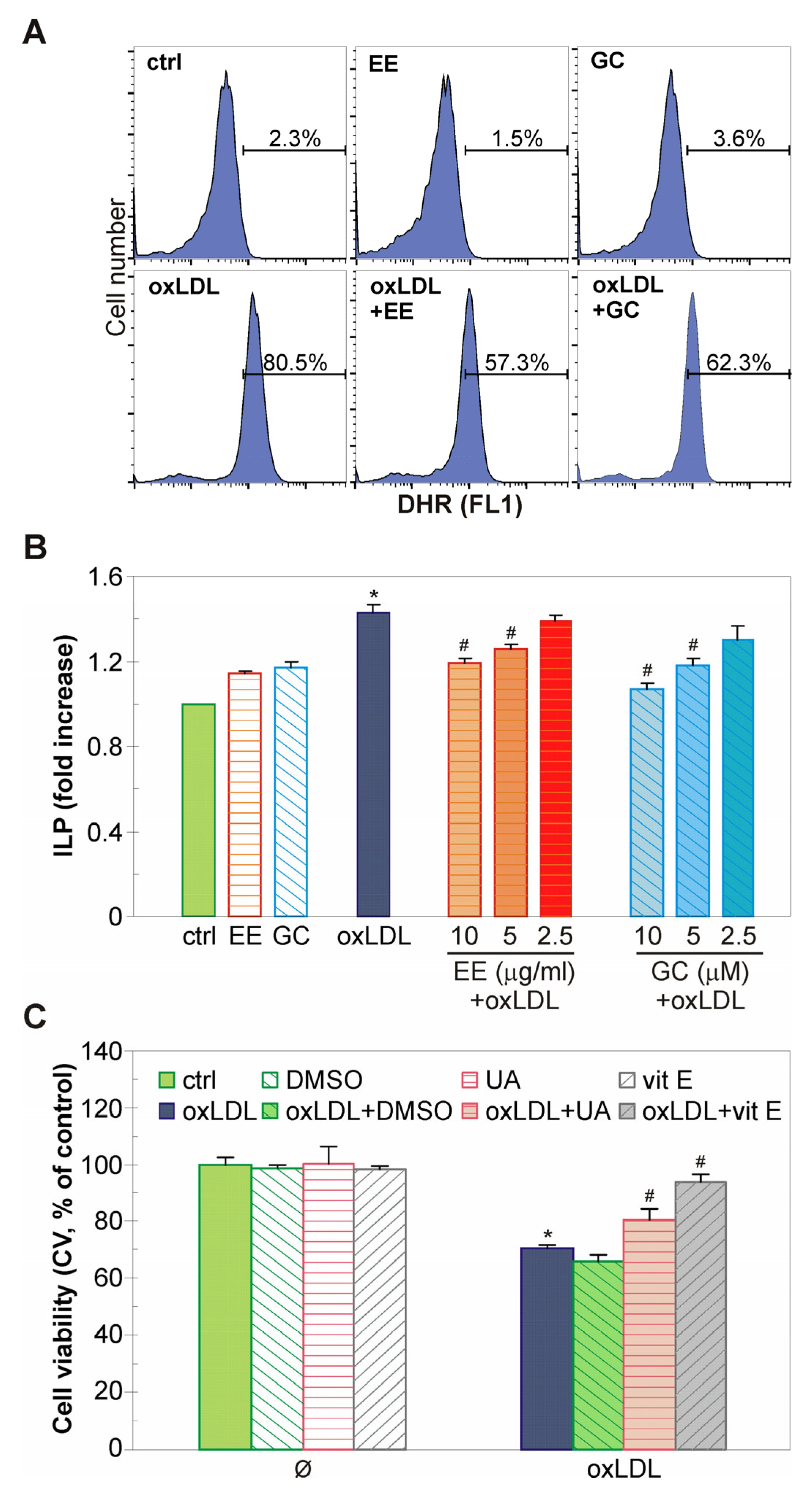
2.2. The EE of G. kochiana and GC Protect Endothelial EA.hy926 Cells from oxLDL-Induced Death
2.3. The Antiapoptotic Action of EE from G. kochiana and GC Is Associated with Mitochondrial Stabilization and Caspase Inhibition
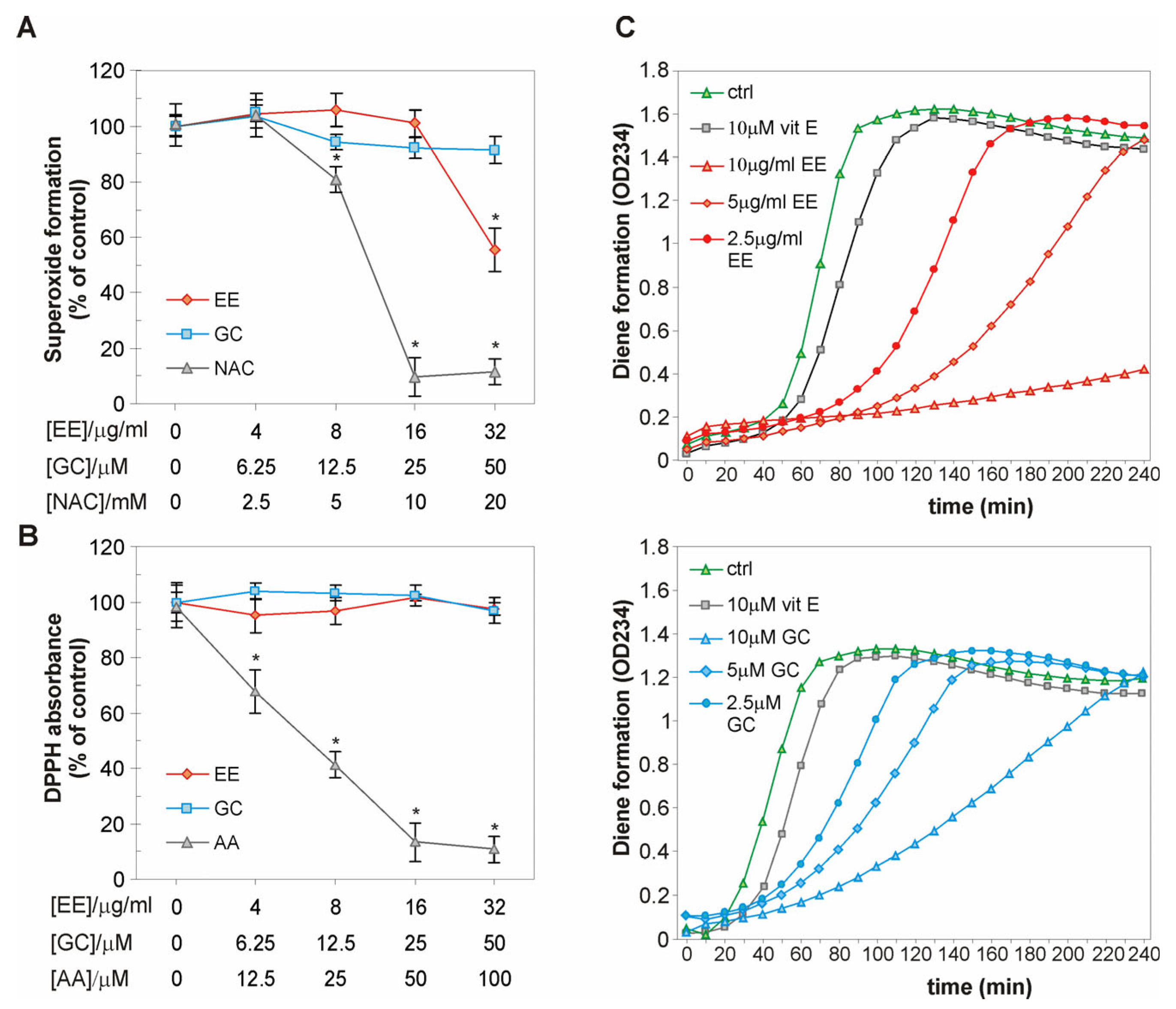
2.4. The EE of G. kochiana and GC Decrease Intracellular ROS Accumulation and Inhibit Lipid Peroxidation in oxLDL-Treated Endothelial Cells
2.5. The EE of G. kochiana and GC Do Not Exert Antiradical Activity, but Delay Cu2+-Induced Oxidation of LDL Particles
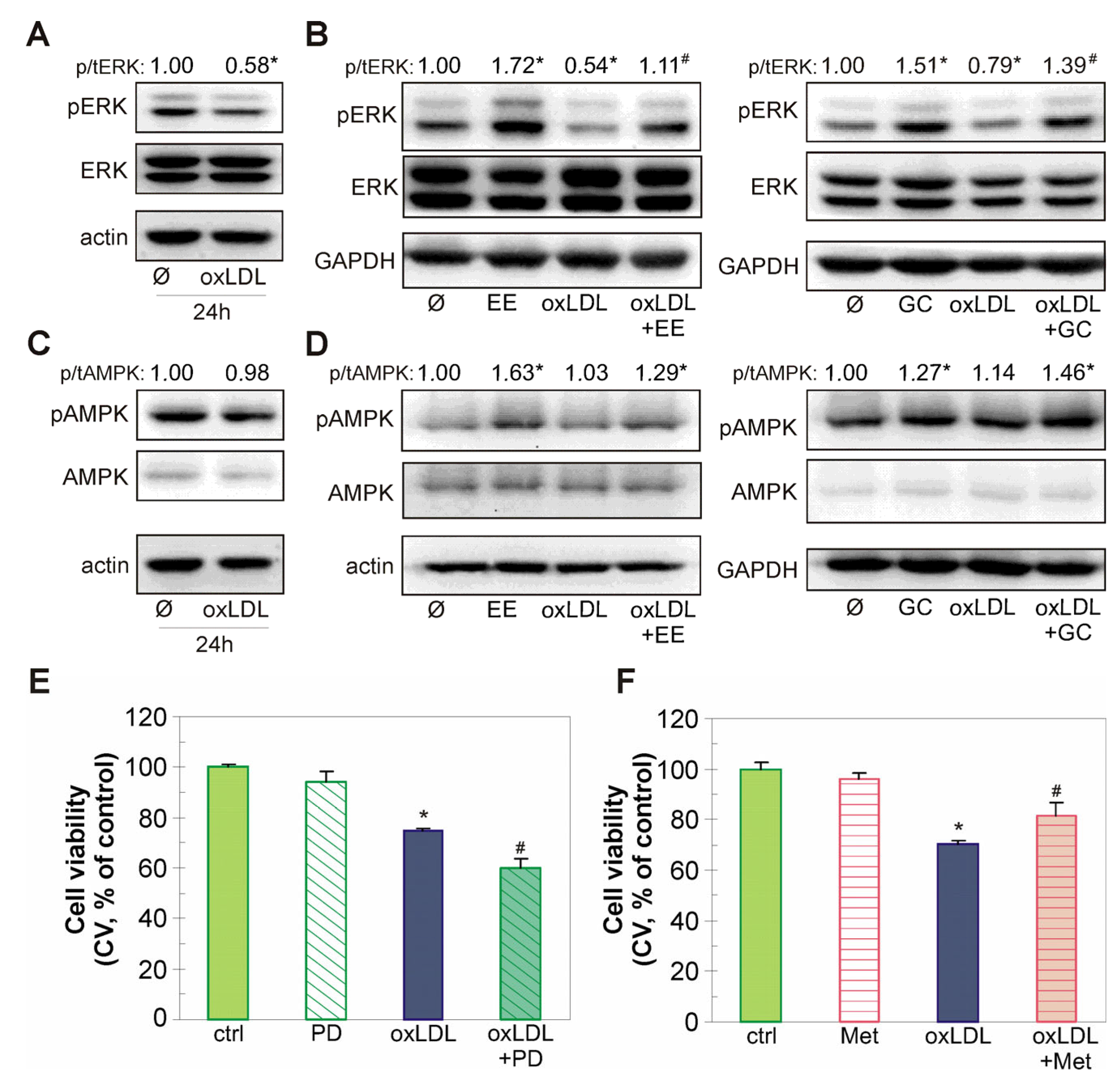
2.6. The Endothelial-Protective Action of EE and GC Is Associated with Reactivation of the Akt/CREB/eNOS Axis, ERK Kinase, and Restoration of NO Levels in oxLDL-Treated Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. Plant Material and HPLC Analysis
4.2. Cell Culture and Treatments
4.3. Oxidation of Low-Density Lipoprotein
4.4. Cell Viability
4.5. Light Microscopy
4.6. Detection of Apoptosis by Annexin V-FITC/PI Double Staining
4.7. Cell Cycle Analysis
4.8. Caspase Activation
4.9. Mitochondrial Membrane Potential Assessment
4.10. Measurement of ROS Accumulation
4.11. TBARS Assay
4.12. Antiradical Activity Assessment
4.13. Conjugated Diene Formation
4.14. Measurement of NO Production
4.15. Immunoblot
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 27 November 2023).
- Di Pietro, N.; Baldassarre, M.P.A.; Cichelli, A.; Pandolfi, A.; Formoso, G.; Pipino, C. Role of Polyphenols and Carotenoids in Endothelial Dysfunction: An Overview from Classic to Innovative Biomarkers. Oxidative Med. Cell. Longev. 2020, 2020, 6381380. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yan, X.; Tang, Z.; Feng, B. Association between circulating oxidized OxLDL/LDL-C ratio and the severity of coronary atherosclerosis, along with other emerging biomarkers of cardiovascular disease in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2022, 191, 110040. [Google Scholar] [CrossRef] [PubMed]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic Insights into the Oxidized Low-Density Lipoprotein-Induced Atherosclerosis. Oxid. Med. Cell. Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhou, Y.; Nabavi, S.M.; Sahebkar, A.; Little, P.J.; Xu, S.; Weng, J.; Ge, J. Mechanisms of Oxidized LDL-Mediated Endothelial Dysfunction and Its Consequences for the Development of Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 925923. [Google Scholar] [CrossRef]
- Hong, C.G.; Florida, E.; Li, H.; Parel, P.M.; Mehta, N.N.; Sorokin, A.V. Oxidized low-density lipoprotein associates with cardiovascular disease by a vicious cycle of atherosclerosis and inflammation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 1023651. [Google Scholar] [CrossRef]
- Ahmadi, A.; Jamialahmadi, T.; Sahebkar, A. Polyphenols and atherosclerosis: A critical review of clinical effects on LDL oxidation. Pharmacol. Res. 2022, 184, 106414. [Google Scholar] [CrossRef]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Lanuza, F.; Zamora-Ros, R.; Bondonno, N.P.; Meroño, T.; Rostgaard-Hansen, A.L.; Riccardi, G.; Tjønneland, A.; Landberg, R.; Halkjær, J.; Andres-Lacueva, C. Dietary polyphenols, metabolic syndrome and cardiometabolic risk factors: An observational study based on the DCH-NG subcohort. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1167–1178. [Google Scholar] [CrossRef]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef]
- Jiang, D.; Dai, Z.; Li, Y. Pharmacological Effects of Xanthones as Cardiovascular Protective Agents. Cardiovasc. Drug Rev. 2004, 22, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-R.; Wang, Y.-H.; Zhong, Y.-N.; Che, T.-T.; Hu, Y.; Bao, J.; Meng, N. Protective Effect of Flavonoids from a Deep-Sea-Derived Arthrinium sp. against ox-LDL-Induced Oxidative Injury through Activating the AKT/Nrf2/HO-1 Pathway in Vascular Endothelial Cells. Mar. Drugs 2021, 19, 712. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.-J.; Jiang, J.-L.; Zhu, H.-Q.; Tan, G.-S.; Liu, S.-Q.; Xu, K.-P.; Li, Y.-J. Demethylbellidifolin preserves endothelial function by reduction of the endogenous nitric oxide synthase inhibitor level. J. Ethnopharmacol. 2004, 93, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.-J.; Jiang, J.-L.; Tan, G.-S.; Huang, Z.-Z.; Deng, H.-W.; Li, Y.-J. Demethylbellidifolin Inhibits Adhesion of Monocytes to Endothelial Cells via Reduction of Tumor Necrosis Factor alpha and Endogenous Nitric Oxide Synthase Inhibitor Level. Planta Medica 2003, 69, 1150–1152. [Google Scholar] [CrossRef] [PubMed]
- John, O.D.; Mushunje, A.T.; Surugau, N.; Guad, R. The metabolic and molecular mechanisms of α-mangostin in cardiometabolic disorders (Review). Int. J. Mol. Med. 2022, 50, 120. [Google Scholar] [CrossRef]
- Ren, K.; Li, H.; Zhou, H.-F.; Liang, Y.; Tong, M.; Chen, L.; Zheng, X.-L.; Zhao, G.-J. Mangiferin promotes macrophage cholesterol efflux and protects against atherosclerosis by augmenting the expression of ABCA1 and ABCG1. Aging 2019, 11, 10992–11009. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, G.; Liu, H.; Xing, N.; Sun, Y.; Zhai, Y.; Yang, B.; Kong, A.-N.T.; Kuang, H.; Wang, Q. Cardioprotective effect of the xanthones from Gentianella acuta against myocardial ischemia/reperfusion injury in isolated rat heart. Biomed. Pharmacother. 2017, 93, 626–635. [Google Scholar] [CrossRef]
- Manganelli, R.E.U.; Chericoni, S.; Baragatti, B. Ethnopharmacobotany in Tuscany: Plants used as antihypertensives. Fitoterapia 2000, 71, S95–S100. [Google Scholar] [CrossRef]
- Wunir, C. Khasbagan Ewenki folk medicinal plants and its comparison with Mongolian medicine. Chin. J. Ethnomed. Ethnopharm. 2009, 18, 156–158. [Google Scholar]
- Krstić-Milošević, D. Chemical Investigation of Pharmacologically Active Secondary Metabolites of Some Species from Genus Gentiana. Ph.D. Thesis, Faculty of Chemistry, University of Belgrade, Belgrade, Serbia, 2008. [Google Scholar]
- Baragatti, B.; Calderone, V.; Testai, L.; Martinotti, E.; Chericoni, S.; Morelli, I. Vasodilator activity of crude methanolic extract of Gentiana kokiana Perr. et Song. (Gentianaceae). J. Ethnopharmacol. 2001, 79, 369–372. [Google Scholar] [CrossRef]
- Chericoni, S.; Testai, L.; Calderone, V.; Flamini, G.; Nieri, P.; Morelli, I.; Martinotti, E. The Xanthones Gentiacaulein and Gentiakochianin are Responsible for the Vasodilator Action of the Roots of Gentiana kochiana. Planta Medica 2003, 69, 770–772. [Google Scholar] [CrossRef] [PubMed]
- Isakovic, A.; Jankovic, T.; Harhaji, L.; Kostic-Rajacic, S.; Nikolic, Z.; Vajs, V.; Trajkovic, V. Antiglioma action of xanthones from Gentiana kochiana: Mechanistic and structure–activity requirements. Bioorganic Med. Chem. 2008, 16, 5683–5694. [Google Scholar] [CrossRef] [PubMed]
- Tomić, M.; Tovilović, G.; Butorović, B.; Krstić, D.; Janković, T.; Aljančić, I.; Menković, N. Neuropharmacological evaluation of diethylether extract and xanthones of Gentiana kochiana. Pharmacol. Biochem. Behav. 2005, 81, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Nauman, M.C.; Johnson, J.J. The purple mangosteen (Garcinia mangostana): Defining the anticancer potential of selected xanthones. Pharmacol. Res. 2021, 175, 106032. [Google Scholar] [CrossRef]
- Hang, L.; Peng, Y.; Xiang, R.; Li, X.; Li, Z. Ox-LDL Causes Endothelial Cell Injury Through ASK1/NLRP3-Mediated Inflammasome Activation via Endoplasmic Reticulum Stress. Drug Des. Devel. Ther. 2020, 14, 731–744. [Google Scholar] [CrossRef]
- Lara-Guzman, O.J.; Gil-Izquierdo, Á.; Medina, S.; Osorio, E.; Álvarez-Quintero, R.; Zuluaga, N.; Oger, C.; Galano, J.-M.; Durand, T.; Muñoz-Durango, K. Oxidized LDL triggers changes in oxidative stress and inflammatory biomarkers in human macrophages. Redox Biol. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef]
- Baiseitova, A.; Shah, A.B.; Khan, A.M.; Idrees, M.; Kim, J.H.; Lee, Y.H.; Kong, I.-K.; Park, K.H. Antioxidant potentials of furanodihydrobenzoxanthones from Artocarpus elasticus and their protection against oxLDL induced injury in SH-SY5Y cells. Biomed. Pharmacother. 2023, 165, 115278. [Google Scholar] [CrossRef]
- Luo, Y.; Lu, S.; Dong, X.; Xu, L.; Sun, G.; Sun, X. Dihydromyricetin protects human umbilical vein endothelial cells from injury through ERK and Akt mediated Nrf2/HO-1 signaling pathway. Apoptosis 2017, 22, 1013–1024. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, D.-L.; Jia, M.; Hao, W.-H.; Li, Y.-J. Mangiferin inhibits high-fat diet induced vascular injury via regulation of PTEN/AKT/eNOS pathway. J. Pharmacol. Sci. 2018, 137, 265–273. [Google Scholar] [CrossRef]
- Liu, S.; Yan, W.; Hu, Y.; Wu, H. Shikonin Alleviates Endothelial Cell Injury Induced by ox-LDL via AMPK/Nrf2/HO-1 Signaling Pathway. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, J.; Rondeau, P.; Veeren, B.; Faccini, J.; Gonthier, M.-P.; Meilhac, O.; Vindis, C. Antioxidant and Cytoprotective Properties of Polyphenol-Rich Extracts from Antirhea borbonica and Doratoxylon apetalum against Atherogenic Lipids in Human Endothelial Cells. Antioxidants 2021, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Kanoi, R.; Loachan, P.; Das, S.; Rao, B.S.S. Mangiferin, a naturally occurring polyphenol, mitigates oxidative stress induced premature senescence in human dermal fibroblast cells. Mol. Biol. Rep. 2021, 48, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Y.; Song, J.; Hou, F.; Liu, B.; Li, A. Mangiferin protects mitochondrial function by preserving mitochondrial hexokinase-II in vessel endothelial cells. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1829–1839. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Chung, P.-C.; Owaga, E.E.; Tsai, I.-J.; Wang, P.-Y.; Tsai, J.-I.; Yeh, T.-S.; Hsieh, R.-H. Alpha-mangostin from mangosteen (Garcinia mangostana Linn.) pericarp extract reduces high fat-diet induced hepatic steatosis in rats by regulating mitochondria function and apoptosis. Nutr. Metab. 2016, 13, 1–10. [Google Scholar] [CrossRef]
- Pardo-Andreu, G.L.; Paim, B.A.; Castilho, R.F.; Velho, J.A.; Delgado, R.; Vercesi, A.E.; Oliveira, H.C. Mangifera indica L. extract (Vimang®) and its main polyphenol mangiferin prevent mitochondrial oxidative stress in atherosclerosis-prone hypercholesterolemic mouse. Pharmacol. Res. 2008, 57, 332–338. [Google Scholar] [CrossRef]
- Qu, K.; Yan, F.; Qin, X.; Zhang, K.; He, W.; Dong, M.; Wu, G. Mitochondrial dysfunction in vascular endothelial cells and its role in atherosclerosis. Front. Physiol. 2022, 13, 1084604. [Google Scholar] [CrossRef]
- Kou, X.; Song, L.; Wang, Y.; Yu, Q.; Ju, H.; Yang, A.; Shen, R. Design, synthesis and anti-Alzheimer’s disease activity study of xanthone derivatives based on multi-target strategy. Bioorganic Med. Chem. Lett. 2019, 30, 126927. [Google Scholar] [CrossRef]
- Pinto, M.M.M.; Palmeira, A.; Fernandes, C.; Resende, D.I.S.P.; Sousa, E.; Cidade, H.; Tiritan, M.E.; Correia-da-Silva, M.; Cravo, S. From Natural Products to New Synthetic Small Molecules: A Journey through the World of Xanthones. Molecules 2021, 26, 431. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Q.; Chen, B.; Luo, X.; Chen, D. Structure–Activity Relationship and Prediction of the Electron-Transfer Potential of the Xanthones Series. ChemistryOpen 2018, 7, 730–736. [Google Scholar] [CrossRef]
- Félix, R.; Valentão, P.; Andrade, P.B.; Félix, C.; Novais, S.C.; Lemos, M.F.L. Evaluating the In Vitro Potential of Natural Extracts to Protect Lipids from Oxidative Damage. Antioxidants 2020, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Uvarani, C.; Chandraprakash, K.; Sankaran, M.; Ata, A.; Mohan, P.S. Antioxidant and structure–activity relationships of five tetraoxygenated xanthones from Swertia minor (Griscb.) Knobl. Nat. Prod. Res. 2012, 26, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Cidade, H.; Rocha, V.; Palmeira, A.; Marques, C.; Tiritan, M.E.; Ferreira, H.; Lobo, J.S.; Almeida, I.F.; Sousa, M.E.; Pinto, M. In silico and in vitro antioxidant and cytotoxicity evaluation of oxygenated xanthone derivatives. Arab. J. Chem. 2020, 13, 17–26. [Google Scholar] [CrossRef]
- Zhao, Y.; Qian, Y.; Sun, Z.; Shen, X.; Cai, Y.; Li, L.; Wang, Z. Role of PI3K in the Progression and Regression of Atherosclerosis. Front. Pharmacol. 2021, 12, 632378. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Su, B.; Qin, G.; Zhao, L. HMGB1 promotes Ox-LDL-induced endothelial cell damage by inhibiting PI3K/Akt signaling pathway. BMC Cardiovasc. Disord. 2022, 22, 555. [Google Scholar] [CrossRef]
- Mohammed, K.A.K.; Madeddu, P.; Avolio, E. MEK inhibitors: A promising targeted therapy for cardiovascular disease. Front. Cardiovasc. Med. 2024, 11, 1404253. [Google Scholar] [CrossRef]
- Ou, H.; Chou, W.; Chu, P.; Hsieh, P.; Hung, C.; Tsai, K. Fucoxanthin Protects against oxLDL-Induced Endothelial Damage via Activating the AMPK-Akt-CREB-PGC1α Pathway. Mol. Nutr. Food Res. 2019, 63, e1801353. [Google Scholar] [CrossRef]
- Wang, Z.; Bao, Z.; Ding, Y.; Xu, S.; Du, R.; Yan, J.; Li, L.; Sun, Z.; Shao, C.; Gu, W. Nε-carboxymethyl-lysine-induced PI3K/Akt signaling inhibition promotes foam cell apoptosis and atherosclerosis progression. Biomed. Pharmacother. 2019, 115, 108880. [Google Scholar] [CrossRef]
- Berthier, A.; Lemaire-Ewing, S.; Prunet, C.; Montange, T.; Vejux, A.; de Barros, J.P.P.; Monier, S.; Gambert, P.; Lizard, G.; Néel, D. 7-Ketocholesterol-induced apoptosis. FEBS J. 2005, 272, 3093–3104. [Google Scholar] [CrossRef]
- Nam, M.-H.; Son, W.-R.; Yang, S.-Y.; Lee, Y.-S.; Lee, K.-W. Chebulic acid inhibits advanced glycation end products-mediated vascular dysfunction by suppressing ROS via the ERK/Nrf2 pathway. J. Funct. Foods 2017, 36, 150–161. [Google Scholar] [CrossRef]
- Song, W.; Yuan, Y.; Tan, X.; Gu, Y.; Zeng, J.; Song, W.; Xin, Z.; Fang, D.; Guan, R. Icariside II induces rapid phosphorylation of endothelial nitric oxide synthase via multiple signaling pathways. PeerJ 2022, 10, e14192. [Google Scholar] [CrossRef]



| Concentration | Lag Phase Delay (%) | |
|---|---|---|
| 10 μg/mL | n.d. | |
| EE | 5 μg/mL | 136.4 |
| 2.5 μg/mL | 81.8 | |
| 10 μM | 163.3 | |
| GC | 5 μM | 133.3 |
| 2.5 μM | 83.3 | |
| Vit E | 10 μM | 21.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tovilović-Kovačević, G.; Zogović, N.; Ignjatović, Đ.; Tomić, M.; Penjišević, J.; Kukić-Marković, J.; Krstić-Milošević, D. Endothelial-Protective Actions of Diethylether Extract from Gentiana kochiana and Xanthone Gentiacaulein Against Oxidized LDL-Induced Injury—In Vitro Evaluation. Int. J. Mol. Sci. 2025, 26, 1351. https://doi.org/10.3390/ijms26031351
Tovilović-Kovačević G, Zogović N, Ignjatović Đ, Tomić M, Penjišević J, Kukić-Marković J, Krstić-Milošević D. Endothelial-Protective Actions of Diethylether Extract from Gentiana kochiana and Xanthone Gentiacaulein Against Oxidized LDL-Induced Injury—In Vitro Evaluation. International Journal of Molecular Sciences. 2025; 26(3):1351. https://doi.org/10.3390/ijms26031351
Chicago/Turabian StyleTovilović-Kovačević, Gordana, Nevena Zogović, Đurđica Ignjatović, Mirko Tomić, Jelena Penjišević, Jelena Kukić-Marković, and Dijana Krstić-Milošević. 2025. "Endothelial-Protective Actions of Diethylether Extract from Gentiana kochiana and Xanthone Gentiacaulein Against Oxidized LDL-Induced Injury—In Vitro Evaluation" International Journal of Molecular Sciences 26, no. 3: 1351. https://doi.org/10.3390/ijms26031351
APA StyleTovilović-Kovačević, G., Zogović, N., Ignjatović, Đ., Tomić, M., Penjišević, J., Kukić-Marković, J., & Krstić-Milošević, D. (2025). Endothelial-Protective Actions of Diethylether Extract from Gentiana kochiana and Xanthone Gentiacaulein Against Oxidized LDL-Induced Injury—In Vitro Evaluation. International Journal of Molecular Sciences, 26(3), 1351. https://doi.org/10.3390/ijms26031351






