Graphitic Carbon Nitride in Oral Health: Emerging Applications, Antimicrobial Potential, and Future Perspectives
Abstract
1. Introduction
- The ultrathin morphology enables effective dispersion in liquid media, allowing g-C3N4 sheets to interact with filler particles before polymerization, thereby promoting well-organized composite microstructures.
- The moderate reactivity of graphite nitride offers greater process control than graphene oxide, which is often excessively reactive.
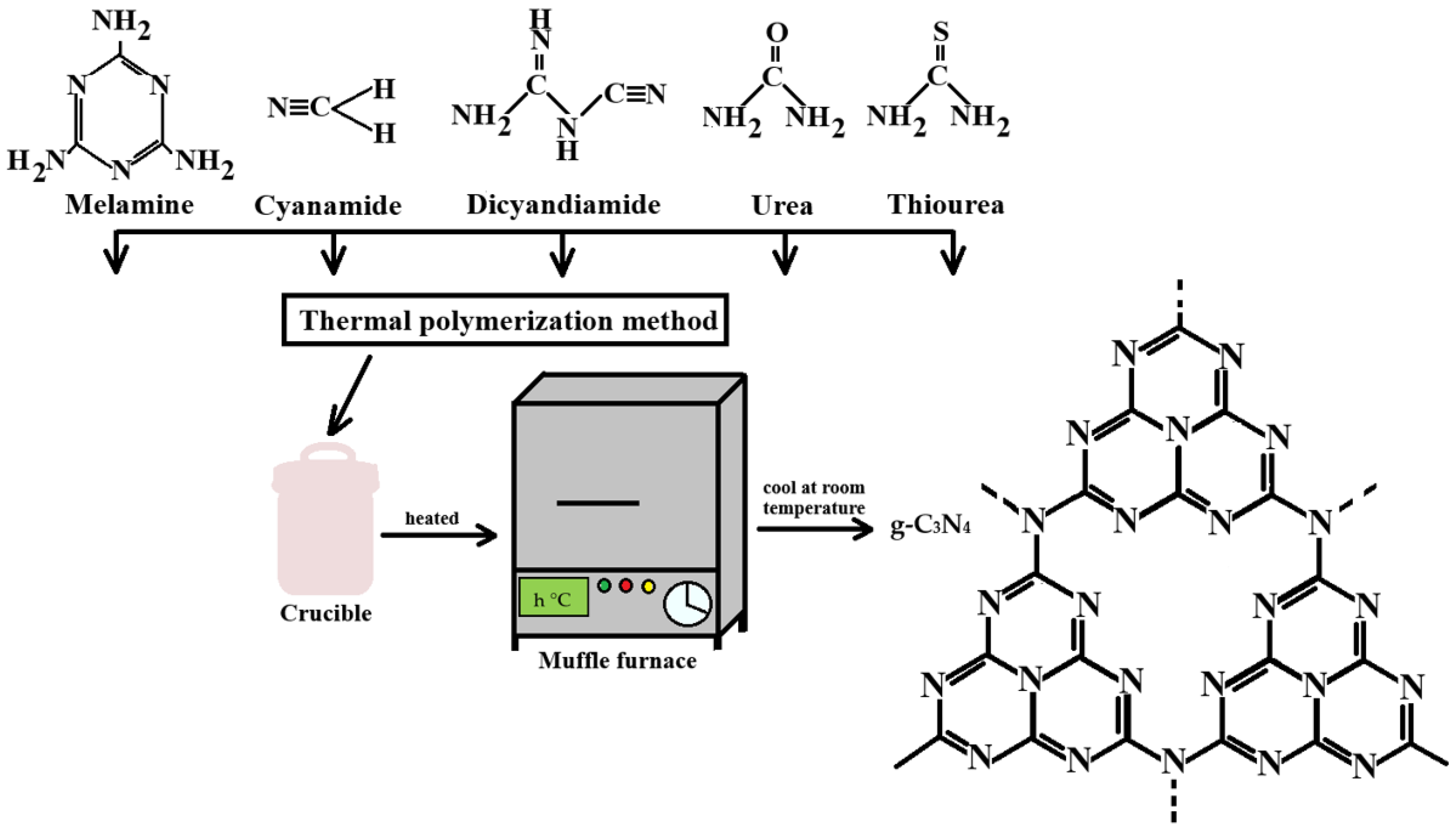
2. Synthesis of Graphitic Carbon Nitride
2.1. Thermal Polymerization
2.2. Solvothermal/Hydrothermal Method
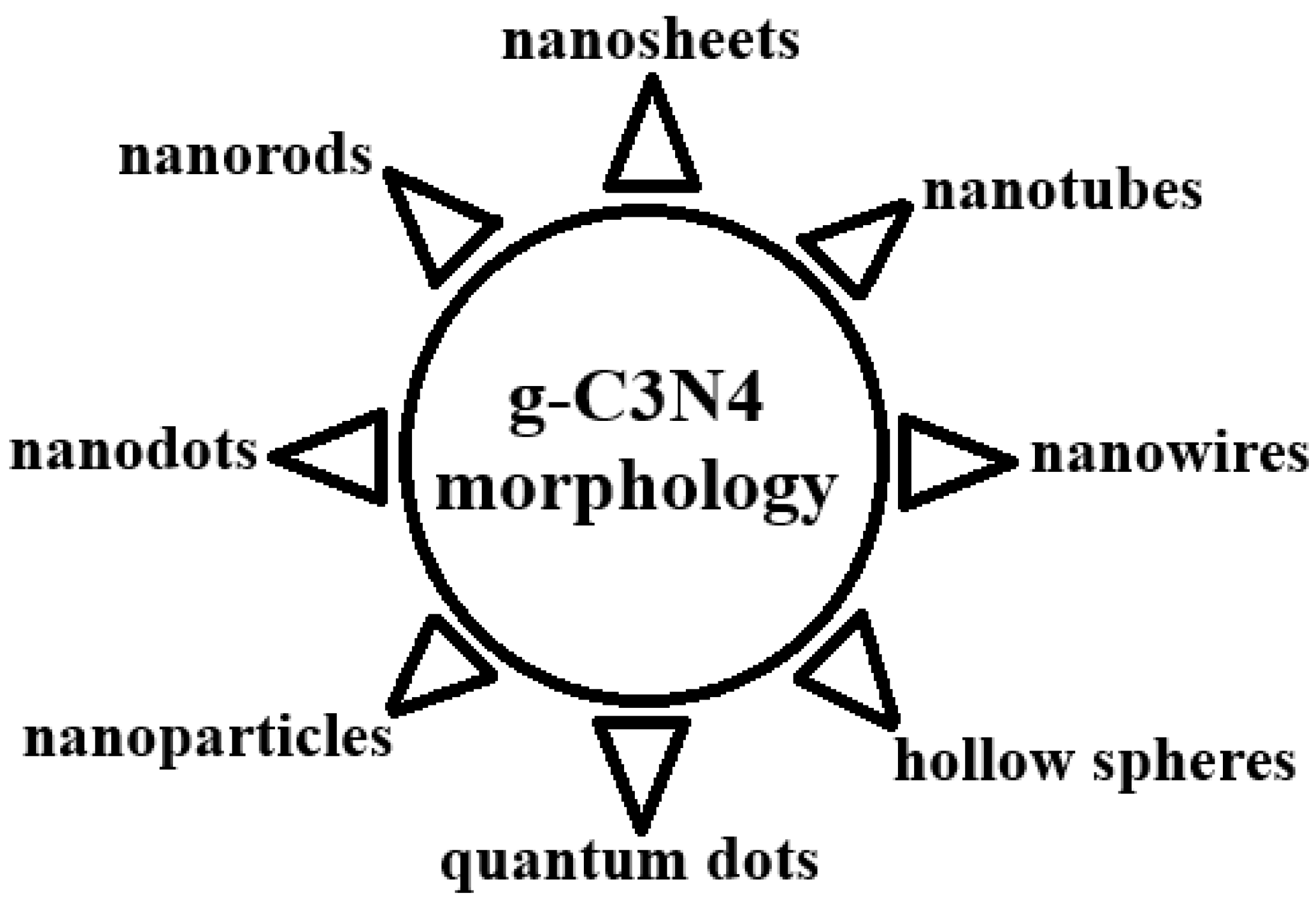
2.3. Morphology of g-C3N4
3. g-C3N4 Activities
4. g-C3N4 in Oral Dentistry
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| STM | Scanning Tunneling Microscopy |
| HOPG | Highly Oriented Pyrolytic Graphite |
| g-C3N4 | Graphitic carbon nitride |
References
- Drdanová, A.P.; Krajčovičová, T.E.; Gál, M.; Nemčeková, K.; Imreová, Z.; Ryba, J.; Naumowicz, M.; Homola, T.; Mackuľak, T.; Svitková, V. Unveiling Versatile Applications and Toxicity Considerations of Graphitic Carbon Nitride. Int. J. Mol. Sci. 2024, 25, 7634. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.; Wu, Y.; Chen, F. Emerging Roles of Graphitic Carbon Nitride-based Materials in Biomedical Applications. ACS Biomater. Sci. Eng. 2024, 10, 4645–4661. [Google Scholar] [CrossRef]
- Liu, M.-X.; Zhang, J.-Y.; Zhang, X.-L. Application of graphite carbon nitride in the field of biomedicine: Latest progress and challenges. Mater. Chem. Phys. 2022, 281, 125925. [Google Scholar] [CrossRef]
- Qi, K.; Liu, S.; Zada, A. Graphitic carbon nitride, a polymer photocatalyst. J. Taiwan Inst. Chem. Eng. 2020, 109, 111–123. [Google Scholar] [CrossRef]
- Ghashghaee, M.; Azizi, Z.; Ghambarian, M. Conductivity tuning of charged triazine and heptazine graphitic carbon nitride (g-C3N4) quantum dots via nonmetal (B, O, S, P) doping: DFT calculations. J. Phys. Chem. Solids 2020, 141, 109422. [Google Scholar] [CrossRef]
- Abdullahi, M.T.; Ali, M.; Farooq, W.; Khan, M.; Younas, M.; Tahir, M.N. Solvothermal synthesis of carbon nitride (g-C3N4): Bandgap engineering for improved photocatalytic performance. Sustain. Energ. Fuels 2025, 9, 1109–1119. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Zhang, J.; Xu, H.; Yu, X. Recent progress in graphitic carbon nitride-based materials for antibacterial applications: Synthesis, mechanistic insights, and utilization. Microstructures 2024, 4, 2024017. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Peera, S.G.; Lee, T.G.; Myla, C.R.; Lee, D.-Y.; Shim, J.; Balasingam, S.K. Recent Trends in Graphitic Carbon Nitride-Based Binary and Ternary Heterostructured Electrodes for Photoelectrochemical Water Splitting. Processes 2021, 9, 1959. [Google Scholar] [CrossRef]
- Kyriakos, P.; Hristoforou, E.; Belessiotis, G.V. Graphitic Carbon Nitride (g-C3N4) in Photocatalytic Hydrogen Production: Critical Overview and Recent Advances. Energies 2024, 17, 3159. [Google Scholar] [CrossRef]
- Antolini, E. The Application of 2D Graphitic Carbon Nitride (g-C3N4) and Hexagonal Boron Nitride (h-BN) in Low-Temperature Fuel Cells: Catalyst Supports, ORR Catalysts, and Membrane Fillers. Molecules 2025, 30, 1852. [Google Scholar] [CrossRef]
- Yan, Y.; Meng, Q.; Tian, L.; Cai, Y.; Zhang, Y.; Chen, Y. Engineering of g-C3N4 for Photocatalytic Hydrogen Production: A Review. Int. J. Mol. Sci. 2024, 25, 8842. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Antonietti, M. Graphitic carbon nitride “reloaded”: Emerging applications beyond (photo)catalysis. Chem. Soc. Rev. 2016, 45, 2308–2326. [Google Scholar] [CrossRef] [PubMed]
- Ozyurt, A.K.; Baykara, M.Z. Inhomogeneous Nanoscale Conductivity and Friction on Graphite Terraces Explored via Atomic Force Microscopy. Lubricants 2024, 12, 462. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Y.; Wu, K. Simultaneous observation of the triangular and honeycomb structures on highly oriented pyrolytic graphite at room temperature: An STM study. Surf. Sci. 2006, 600, 729–734. [Google Scholar] [CrossRef]
- Tyagi, M.; Tiwari, A.A.; Mathew, J.; Sahdev, D.; Dey, S.; Chandran, M. Scanning Tunneling Microscope-Characterization of Chemical Vapor Deposition-Graphene: Ripples and Twisted Bi-layers at Multiple Scales. Phys. Status Solidi B 2025, 262, 2400210. [Google Scholar] [CrossRef]
- Hembacher, S.; Giessibl, F.J.; Mannhart, J.; Quate, C.F. Revealing the hidden atom in graphite by low-temperature atomic force microscopy. Proc. Natl. Acad. Sci. USA 2003, 100, 12539–12542. [Google Scholar] [CrossRef]
- Serfözö, N.E.; Moldovan, M.; Prodan, D.; Ilie, N. Development and Evaluation of a Novel Self-Etch Dental Adhesive Incorporating Graphene Oxide–Zirconia (GO-ZrO2) and Hydroxyapatite–Zinc (HA-Zn) for Enhanced Bond Strength, Biocompatibility, and Long-Term Stability. Nanomaterials 2025, 15, 803. [Google Scholar] [CrossRef]
- Prodan, D.; Moldovan, M.; Cuc, S.; Sarosi, C.; Petean, I.; Filip, M.; Carpa, R.; Doukeh, R.; Mirica, I.-C. Advanced Dentistry Biomaterials Containing Graphene Oxide. Polymers 2024, 16, 1743. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.; Dudea, D.; Cuc, S.; Sarosi, C.; Prodan, D.; Petean, I.; Furtos, G.; Ionescu, A.; Ilie, N. Chemical and Structural Assessment of New Dental Composites with Graphene Exposed to Staining Agents. J. Funct. Biomater. 2023, 14, 163. [Google Scholar] [CrossRef]
- Lopes, P.M.P.; Moldovan, D.; Fechete, R.; Prodan, D.; Pop, C.R.; Rotar, A.M.; Popescu, V. Swelling and Antimicrobial Activity Characterization of a GO-Reinforced Gelatin—Whey Hydrogel. Gels 2023, 9, 18. [Google Scholar] [CrossRef]
- Bacali, C.; Carpa, R.; Buduru, S.; Moldovan, M.L.; Baldea, I.; Constantin, A.; Moldovan, M.; Prodan, D.; Dascalu, L.M.; Lucaciu, O.; et al. Association of Graphene Silver Polymethyl Methacrylate (PMMA) with Photodynamic Therapy for Inactivation of Halitosis Responsible Bacteria in Denture Wearers. Nanomaterials 2021, 11, 1643. [Google Scholar] [CrossRef]
- Bhanderi, D.; Lakhani, P.; Modi, C.K. Graphitic carbon nitride (g-C3N4) as an emerging photocatalyst for sustainable environmental applications: A comprehensive review. RSC Sustain. 2024, 2, 265–287. [Google Scholar] [CrossRef]
- Madankar, R.; Umekar, M.; Bhusari, G.; Mondal, A.; Raish, M.; Afzal, M.; Norek, M.; Chaudhary, R. Rapid synthesis of graphitic carbon nitride nanosheets as an efficient adsorbent for removal of Methylene Blue and Rhodamine B from Aqueous Solutions. Sci. Rep. 2025, 15, 28999. [Google Scholar] [CrossRef]
- Idris, A.O.; Oseghe, E.O.; Msagati, T.A.M.; Kuvarega, A.T.; Feleni, U.; Mamba, B. Graphitic Carbon Nitride: A Highly Electroactive Nanomaterial for Environmental and Clinical Sensing. Sensors 2020, 20, 5743. [Google Scholar] [CrossRef]
- Joy, J.; George, E.; Vijayan, P.P.; Anas, S.; Thomas, S. An overview of synthesis, morphology, and versatile applications of nanostructured graphitic carbon nitride (g-C3N4). J. Ind. Eng. Chem. 2024, 133, 74–89. [Google Scholar] [CrossRef]
- Madkour, L.H. Graphitic Carbon Nitride Quantum Dots (g-C3N4): Fundamentals and Applications. Int. Res. J. Basic Clin. Stu. 2023, 8, 1–14. [Google Scholar] [CrossRef]
- Huang, S.-E.; Tan, K.-H.; Sahu, R.S.; Geleta, T.A.; Miri, A.; Lin, C.-Y.; Shih, Y.-H.; Chen, W.-L. Facile Synthesis and Optimization of Graphitic Carbon Nitride Nanoparticles to Effectively Photodegrade Tetracycline under Visible Light in Water. ACS Agric. Sci. Technol. 2025, 5, 235–245. [Google Scholar] [CrossRef]
- Luo, X.; Dong, Y.; Wang, D.; Duan, Y.; Lei, K.; Mao, L.; Li, Y.; Zhao, Q.; Sun, Y. Facile synthesis of g-C3N4 nanosheets for effective degradation of organic pollutants via ball milling. Rev. Adv. Mater. Sci. 2023, 62, 20230123. [Google Scholar] [CrossRef]
- Molaei, P.; Rahimi-Moghadam, F. Porous g-C3N4 nanosheets through facile thermal polymerization of melamine in the air for photocatalyst application. J. Mater. Sci. Mater. Electron. 2021, 32, 19655–19666. [Google Scholar] [CrossRef]
- Xia, P.; Li, G.; Li, X.; Yuan, S.; Wang, K.; Huang, D.; Ji, Y.; Dong, Y.; Wu, X.; Zhu, L.; et al. Synthesis of g-C3N4 from Various Precursors for Photocatalytic H2 Evolution under the Visible Light. Crystals 2022, 12, 1719. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, G.; Hua, Z.; Chen, X.; Xu, X. Effects of thermal program on physicochemical properties and photocatalytic activity of g-C3N4 prepared by dicyandiamide pyrolysis. Diam. Relat. Mater. 2024, 141, 110614. [Google Scholar] [CrossRef]
- Alwin, E.; Nowicki, W.; Wojcieszak, R.; Zieliński, M.; Pietrowski, M. Elucidating the structure of the graphitic carbon nitride nanomaterials via X-ray photoelectron spectroscopy and X-ray powder diffraction techniques†. Dalton Trans. 2020, 49, 12805. [Google Scholar] [CrossRef]
- Bi, X.; Wang, L.-Z.; Zhai, D.-H.; Wang, L.; Yang, H.; Du, G.-H. In-situ synthesis of g-C3N4 with nitrogen vacancy and cyano group via one-pot method for enhanced photocatalytic activity. Sci. Rep. 2025, 15, 19864. [Google Scholar] [CrossRef] [PubMed]
- Banu, A.; Sinha, B.; Sikdar, S. Synthesis of polymeric 2D-graphitic carbon nitride (g-C3N4) nanosheets for sustainable photodegradation of organic pollutants. Heliyon 2024, 10, e33354. [Google Scholar] [CrossRef]
- Ahmari, A.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Khanbeigi, K.A. A green approach for preparation of chitosan/hydroxyapatite/graphitic carbon nitride hydrogel nanocomposite for improved 5-FU delivery. Int. J. Biol. Macromol. 2024, 258, 128736. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Liu, E.; Shi, J.; Lin, X.; Sheng, L.; Zhang, M.; Wang, L.; Chen, J. A direct one-step synthesis of ultrathin g-C3N4 nanosheets from thiourea for boosting solar photocatalytic H2 evolution. Int. J. Hydrogen Energy 2019, 44, 7194–7204. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Liu, Y.; Alharbi, N.S.; Rabah, S.O.; Wang, S.; Wang, X. Synthesis and fabrication of g-C3N4-based materials and their application in elimination of pollutants. Sci. Total Environ. 2020, 731, 139054. [Google Scholar] [CrossRef]
- Singhal, M.; Jangir, S.; Upadhyay, S.; Rajawat, D.S. Advances in graphitic carbon nitride: A comprehensive review of synthesis, properties, applications and recent developments with photoelectrochemical water splitting. Discov. Chem. 2024, 1, 64. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, M.; Chen, D.; Wu, J.; Gao, S.; Zhao, Y.; Yang, J.; Li, S.; Meng, J. Preparation, Modification, and Application of Graphitic Carbon Nitride in Photocatalytic Degradation of Antibiotics. Processes 2025, 13, 3365. [Google Scholar] [CrossRef]
- Ma, C.; Hu, Z.; Zhao, T.; Gu, Z.; Yuan, Q.; Chen, B. Structural engineering of g-C3N4 for enhanced antibacterial efficacy. J. Mater. Chem. B 2025, 13, 7528–7553. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, X.; Wang, H.; Nie, R. Synthesis and biomedical applications of graphitic carbon nitride quantum dots. J.Mater. Chem. B 2019, 7, 5432–5448. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Xiu, S.; Meng, L.-Y.; Quan, B. Solvothermal synthesis and applications of micro/nano carbons: A review. Chem. Eng. J. 2023, 451, 138572. [Google Scholar] [CrossRef]
- Kunhikrishnan, L.; Vishal, K.; Palaniyappan, S. Mechanical and Thermal Characterization on Synthesized Silane-Treated Graphitic Carbon Nitride (g-C3N4) Reinforced 3D Printed Poly (Lactic Acid) Composite. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1234–1245. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, C.; Meng, X.; Li, Z. Joule heating assisted solvothermal growth of C3N4 on carbon paper for effective photothermal-photocatalytic water splitting for hydrogen evolution. Sep. Purif. Technol. 2025, 354, 129202. [Google Scholar] [CrossRef]
- Montigaud, H.; Tanguy, B.; Demazeau, G.; Alves, I.; Courjault, S. C3N4: Dream or reality? Solvothermal synthesis as macroscopic samples of the C3N4 graphitic form. J. Mater. Sci. 2000, 35, 2547–2552. [Google Scholar] [CrossRef]
- Sasikumar, G.K.; Raja, P.U.M.; Jerome, P.; Shenthilkumar, R.R.; Balla, P. Graphitic Carbon Nitride: A Novel Two-Dimensional Metal-Free Carbon-Based Polymer Material for Electrochemical Detection of Biomarkers. C 2024, 10, 98. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Li, B.; Sun, H.; Wang, S.; Duan, X. Morphology-dependent photocatalysis of graphitic carbon nitride for sustainable remediation of aqueous pollutants: A mini review. J. Environ. Chem. Eng. 2022, 10, 107438. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Huang, F.; Song, S.; Ai, G.; Xin, X.; Zhao, B.; Zheng, Y.; Zhang, Z. Recent Advances in g-C3N4-Based Materials and Their Application in Energy and Environmental Sustainability. Molecules 2023, 28, 432. [Google Scholar] [CrossRef]
- Hayat, A.; Sohail, M.; Jery, A.E.; Al-Zaydi, K.M.; Alshammari, K.F.; Khan, J.; Ali, H.; Ajmal, Z.; Taha, T.A.; Din, I.U.; et al. Different Dimensionalities, Morphological Advancements and Engineering of g-C3N4-Based Nanomaterials for Energy Conversion and Storage. Chem. Rec. 2023, 23, e202200171. [Google Scholar] [CrossRef]
- Liu, M.; Yang, H.; Zhang, D.; Yu, Y.; Yang, X.; Cai, Q. Photodynamic antibacterial property of g-C3N4 formulated dental resin composite. Mater. Today Commun. 2025, 47, 113045. [Google Scholar] [CrossRef]
- Thurston, J.H.; Hunter, N.M.; Wayment, L.J.; Cornell, K.A. Urea-derived graphitic carbon nitride (u-g-C3N4) films with highly enhanced antimicrobial and sporicidal activity. J. Colloid Interface Sci. 2017, 505, 910–918. [Google Scholar] [CrossRef]
- Alonso, A.V.; Murugesan, A.; Gogoi, R.; Chandrabose, S.; Abass, K.S.; Sharma, V.; Kandhavelu, M. Graphitic carbon nitride nanoparticle: g-C3N4 synthesis, characterization, and its biological activity against glioblastoma. Eur. J. Pharmacol. 2025, 1003, 177999. [Google Scholar] [CrossRef]
- Yoshihara, N.; Lopes, M.; Santos, I.; Kopke, B.; Almeida, C.; Araújo, J.; Fechine, P.B.A.; Santos-Oliveira, R.; Sant’Anna, C. Graphitic carbon nitride as a novel anticancer agent: Potential mechanisms and efficacy in prostate cancer and glioblastoma treatment. Biomater. Sci. 2024, 12, 5547–5561. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Zhang, W.; Wang, P.; Wang, C. Metal-free virucidal effects induced by g-C3N4 under visible light irradiation: Statistical analysis and parameter optimization. Chemosphere 2018, 195, 551–558. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Shuai, D.; Naraginti, S.; Wang, D.; Zhang, W. Visible-light-driven photocatalytic inactivation of MS2 by metal-free g-C3N4: Virucidal performance and mechanism. Water Res. 2016, 106, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Dandia, A.; Gupta, S.L.; Saini, P.; Sharma, R.; Meena, S.; Parewa, V. Structure couture and appraisal of catalytic activity of carbon nitride (g-C3N4) based materials towards sustainability. Curr. Res. Green Sustain. Chem. 2020, 3, 100039. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Zong, R.; Zhu, Y. Enhancement of visible light photocatalytic activities via porous structure of g-C3N4. Appl. Catal. B- Environ. 2014, 147, 229–235. [Google Scholar] [CrossRef]
- Zheng, M.; Li, W.; Ma, F.; Liang, M. Graphitic carbon nitride-based materials: Applications in medical diagnostics, therapeutics, and combined cancer therapies. J. Mater. Chem. C 2025, 13, 17483. [Google Scholar] [CrossRef]
- He, J.; Cui, S.; Hou, Y.; Liu, S.; Zhang, Z.; Zhao, M.; He, L.; Wang, R.; Liu, S. Bifunctional defect mediated direct Z-scheme g-C3N4−x/Bi2O3−y heterostructures with enhanced piezo-photocatalytic properties for efficient tooth whitening and biofilm eradication. J. Mater. Chem. B 2023, 11, 7103–7116. [Google Scholar] [CrossRef]
- Jiawen, G.; Xing, W. Carbon Nitride Nanocomposites for Periodontitis Targeted Antibacterial and Osteogenesis. Int. Dent. J. 2025, 75, 104670. [Google Scholar] [CrossRef]



| Precursors | Temperature (°C) | Heating Time (h) | Ramp Rate (°C/min) | Organoleptic Properties | Surface Area | Structural Type | References |
|---|---|---|---|---|---|---|---|
| Melamine | 550 °C 700 °C | 4 | 5 °C/min | yellow powder | 38.38 m2/g at 700 °C 10.38 m2/g at 500 °C | bulk g-C3N4 | [27,28,29,30] |
| Dicyanamide | 500 °C 550 °C | 4 2 | 10 °C/min 15 °C/min | yellow material | 9.82 m2/g 14.87 m2/g | porous structure, more amino functional group smore stable chemical bond structures | [30,31,32] |
| Urea | 550 °C | 2, 3, 4, 5 | 5 °C/min | light-yellow product | 108.83 m2/g | layered nanostructure, laminar morphology -orm stacked CN sheets in a complex reaction enhanced crystallinity | [9,27,31,33,34,35] |
| Thiourea | 580 °C | 4 | 2.3 °C/min | Pale yellow | 92.8 m2/g larger specific surface area | nanosheet structures faster photoinduced electron–hole pair transfer efficiency | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paltinean, G.A.; Moldovan, M.; Sarosi, C.; Silaghi-Dumitrescu, L.; Cuc, S.; Furtos, G.; Petean, I.; Chis, I.C. Graphitic Carbon Nitride in Oral Health: Emerging Applications, Antimicrobial Potential, and Future Perspectives. Int. J. Mol. Sci. 2025, 26, 11860. https://doi.org/10.3390/ijms262411860
Paltinean GA, Moldovan M, Sarosi C, Silaghi-Dumitrescu L, Cuc S, Furtos G, Petean I, Chis IC. Graphitic Carbon Nitride in Oral Health: Emerging Applications, Antimicrobial Potential, and Future Perspectives. International Journal of Molecular Sciences. 2025; 26(24):11860. https://doi.org/10.3390/ijms262411860
Chicago/Turabian StylePaltinean, Gertrud Alexandra, Marioara Moldovan, Codruta Sarosi, Laura Silaghi-Dumitrescu, Stanca Cuc, Gabriel Furtos, Ioan Petean, and Irina Camelia Chis. 2025. "Graphitic Carbon Nitride in Oral Health: Emerging Applications, Antimicrobial Potential, and Future Perspectives" International Journal of Molecular Sciences 26, no. 24: 11860. https://doi.org/10.3390/ijms262411860
APA StylePaltinean, G. A., Moldovan, M., Sarosi, C., Silaghi-Dumitrescu, L., Cuc, S., Furtos, G., Petean, I., & Chis, I. C. (2025). Graphitic Carbon Nitride in Oral Health: Emerging Applications, Antimicrobial Potential, and Future Perspectives. International Journal of Molecular Sciences, 26(24), 11860. https://doi.org/10.3390/ijms262411860