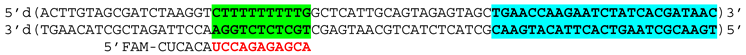2.2. RNA Extension by RNAP in the TRC Substrate
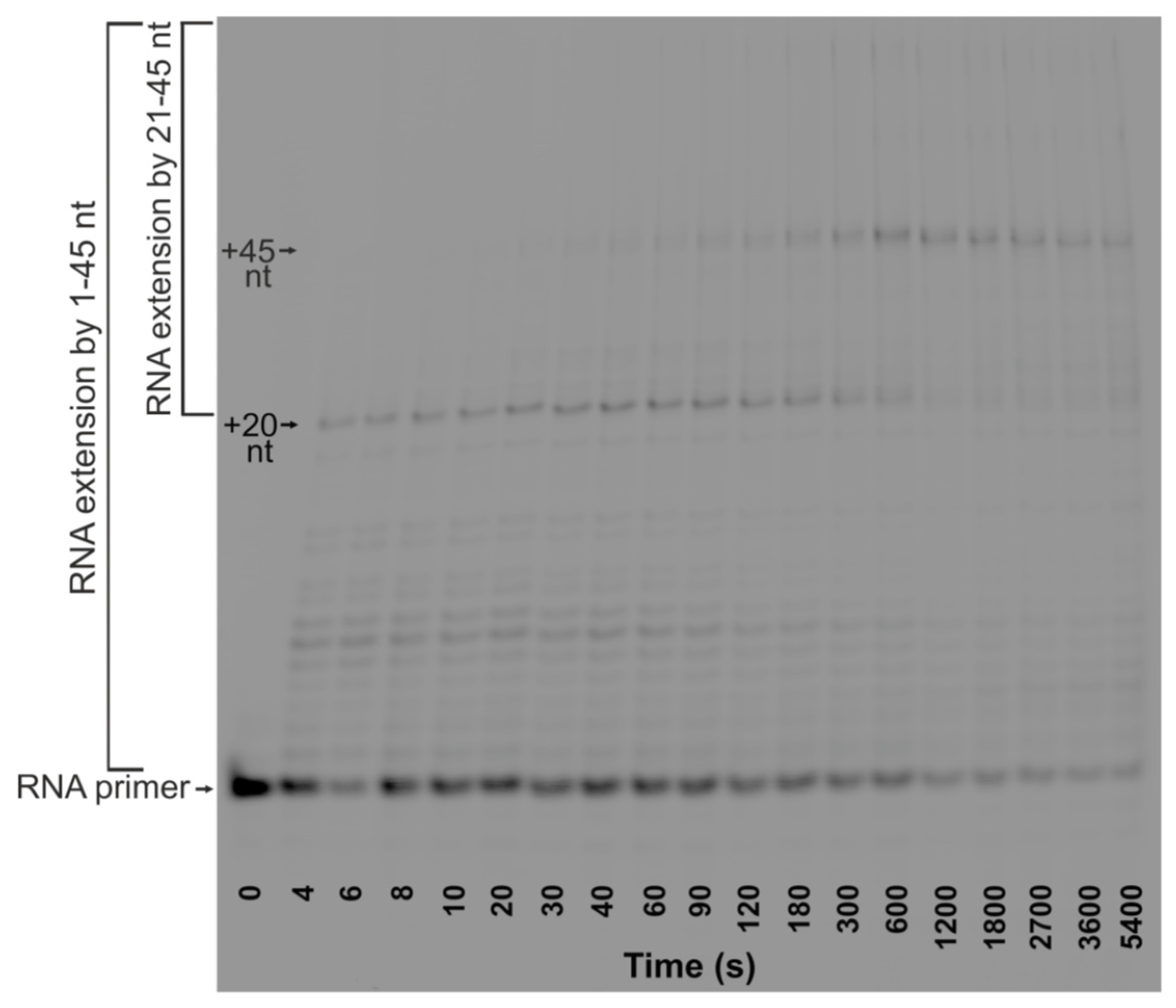
To determine the efficiency of TEC formation and the rate of RNA extension by RNAP in the TRC complexes, kinetic traces of the accumulation of total RNA products from +1 to +45 nt in the presence of Klenow fragment or DNA Pol I were obtained (
Figure 2 and
Figure 3A). The reaction mixtures either contained the dNTPs or did not. In addition, to establish the basis for comparison of RNAP action in the presence and absence of DNA polymerase, we obtained kinetic traces of accumulation of RNA products in the absence of DNA polymerase and DNA primer (in R-loop-substrate-11 which contains the R-loop but lacks the DNA polymerase primer) or in the absence of DNA polymerase alone (in TRC-substrate-11) (see
Table 1).
We have determined the dissociation constant characterizing the stability of the entire RNAP complex with the R-loop containing the 9 nt bubble (R-loop-9) to be 0.3 μM using a microscale thermophoresis assay (see ref. [
23]). This dissociation constant characterizes the entire RNAP complex with R-loop-9 irrespective of whether it is catalytically competent or not. Furthermore, we have determined the dissociation constant characterizing the stability of only the catalytically competent RNAP complex with R-loop-9 to be 0.8 μM from the dependence of the catalytically competent TEC proportion on RNAP concentration (see
Supplementary Materials). The dissociation constant obtained for the catalytically competent TEC was 2.7-fold higher than the dissociation constant of the overall RNAP complex with the R-loop. Based on these data, we have concluded that the concentration of the catalytically competent TEC is lower than that of the entire RNAP complex with the R-loop at the initial moment. We have proposed that some proportion of the RNAP complexes with an R-loop are initially in an active conformation, forming the catalytically competent TEC, while the remainder are initially in a catalytically inactive conformation (probably a backtracked conformation). Through mutual conformational changes (induced fit), the inactive complex can transform into a catalytically competent TEC (see ref. [
23]).
Given the defined dissociation constants, in the present study, we used micromolar concentrations of RNAP to obtain detectable binding of RNAP to the substrate.
The time courses of the accumulation of total RNA products in the absence of DNA polymerase or in the presence of Klenow fragments were fitted to Equation (2): burst exponential growth was followed by linear growth (see
Section 3.4,
Figure 3A). It was found that the presence of a DNA primer alone, or a DNA primer together with the Klenow fragment, does not affect significantly the initial concentration of the catalytically competent TEC ([TEC]
Sum) (corresponding to TEC formation efficiency), the rate of the burst phase (
) (corresponding to RNA synthesis in a processive mode within the catalytically competent TEC), or the rate of the slow linear phase (
) (corresponding to induced fit within the inactive complex of RNAP with an R-loop, leading to the formation of catalytically competent TEC, as was suggested in [
23]), since these parameters were very similar among all kinetic traces obtained in the absence of dNTPs (
Table 2). However, the addition of dNTPs to the reaction mixture with the Klenow fragment increases the rate of induced fit within the inactive complex of RNAP with an R-loop (
) almost two-fold. This result is presumably associated with the DNA primer extension by DNA polymerase leading to the separation of DNA strands in TRC-substrate-11 during the movement of RNAP and DNA polymerase towards one another. The kinetic trace of the accumulation of total RNA products in the presence of DNA Pol I also showed an increase in the product fraction within the time range from 0 to 300 s (
Figure 3A). Moreover, a kink in the kinetic trace was observed at ~300 s, followed by a slow linear decrease in the total product fraction. Therefore, in the presence of DNA Pol I, the time course was fitted to Equation (2) (single-exponential growth followed by linear growth) within the time range from 0 to 300 s. The further linear decrease was fitted to linear Equation (5). It could be suggested that head-on collision of RNAP with full-length DNA Pol I stimulates endonuclease degradation of RNA, which results in a decrease in the total amount of RNA products. This RNA degradation is characterized by the observed rate constant
(
Table 2).
Since the replication fork within the TRC-substrate-11 is located at a distance of 20 nt from the 3′ end of the RNA primer, we analyzed the accumulation of RNA products from +21 to +45 nt (
Figure 3B). The time courses of accumulation of RNA products from +21 to +45 nt were fitted to Equation (2) (single-exponential growth followed by linear growth) in the presence of the Klenow fragment or to single-exponential Equation (1) in the presence of the Klenow fragment together with dNTPs. The amplitude of the burst phase ([TEC]
21÷45) represented the initial proportion of TEC, where the RNA primer was elongated in the region of the replication fork. As can be seen from
Table 2, the presence of the Klenow fragment barely altered this amplitude as well as the observed burst rate constant
(characterizing the pre-steady-state accumulation of long RNA products) and the observed rate constant of the slow phase
(characterizing the steady-state accumulation of long RNA products). These data indicate that Klenow fragment, stalled in the complex with the replication fork, does not stop transcription in the model system of head-on transcription–replication conflict.
In the presence of the Klenow fragment together with dNTPs, the time course of the accumulation of RNA products from +21 to +45 nt was fitted to single-exponential Equation (1). From the time courses presented in
Figure 3B, it is evident that RNA products from +21 to +45 nt accumulate at a similar rate during the first 120 s in the presence and absence of dNTPs. Consequently, the rate constants of the pre-steady-state accumulation of long RNA products (
) in the presence and absence of dNTPs should be similar. At the same time, in the presence of dNTPs, the time course was fitted to a single-exponential equation and yielded the observed rate constant that was 2.7-fold lower than
in the absence of dNTPs. Therefore, the observed rate constant of the accumulation of RNA products from +21 to +45 nt in the presence of the Klenow fragment, together with dNTPs, characterizes the steady-state accumulation of long RNA products
(
Table 2). The rate constant
cannot be determined in the latter case, since
and
are of the same order of magnitude. Thus, the addition of dNTPs to the reaction mixture with the Klenow fragment increased [TEC]
21÷45 nearly two-fold and
by more than two orders of magnitude. It can be concluded that the movement of DNA polymerase towards RNAP (in the presence of dNTPs) increases the rate of steady-state accumulation of long RNA products and the TEC proportion where the RNA primer is elongated up to the edge of the DNA template, probably due to a decrease in paused TECs proportion caused by the separation of DNA strands during the movement of RNAP and DNA polymerase towards one another.
The kinetic trace of the accumulation of RNA products in TRC-substrate-11 from +21 to +45 nt in the presence of full-length DNA Pol I showed an increase in the product fraction within the time range from 0 to 180 s with further kinks, followed by a slow linear decrease in RNA product fraction (
Figure 3B). The time course was fitted to Equation (2) (single-exponential growth followed by linear growth) within the time range from 0 to 180 s and yielded the observed rate constants
and
. The further linear decrease was fitted to linear Equation (5) from 300 s and yielded the observed rate constant
, characterizing the endonuclease degradation of long RNA products (
Table 2). Consequently, in the presence of DNA Pol I, the equilibrium accumulation of RNA products from +21 to +45 nt shifted backwards, as did the equilibrium accumulation of total RNA products. Thus, stalled DNA Pol I seems to stimulate the endonuclease activity of RNAP in the complex with TRC-substrate-11. These results correlate with data described in [
17], demonstrating that the probability of RNAP backtracking increases if there is an obstacle such as the DNA-bound protein. On the other hand, our data revealed that an obstacle such as DNA Pol I (or its Klenow fragment) is not itself a permanent block to transcription, since RNAP transcripts reach +45 nt in the presence of stalled DNA polymerase I. During head-on TRC in vivo, DNA between the TEC and the replication fork is supercoiled. This supercoiling can cause RNAP backtracking and stalling. In the model system used in this study, the supercoiling is absent. Thus, when RNA-elongating TEC collides with DNA polymerase, it remains competent and can displace the DNA polymerase.
Thus, the obtained results reveal that the transcription is not interfered with by the Klenow fragment, which is stalled in the complex with the replication fork in our model system of head-on TRC. The Klenow fragment, which moves along the TRC substrate containing the 11-nt bubble (TRC-substrate-11) towards RNAP, elongating the DNA, accelerates the induced fit within the inactive complex of RNAP with an R-loop leading to the formation of the catalytically competent TEC. In addition, it increases the TEC proportion where the RNA primer is elongated up to the edge of the DNA template and the rate of steady-state accumulation of long RNA products, probably due to a decrease in paused TECs proportion. On the other hand, head-on collision of RNAP with full-length DNA Pol I does not stop RNAP but stimulates its endonuclease activity.
2.3. DNA Extension by DNA Polymerase in the TRC-Substrate
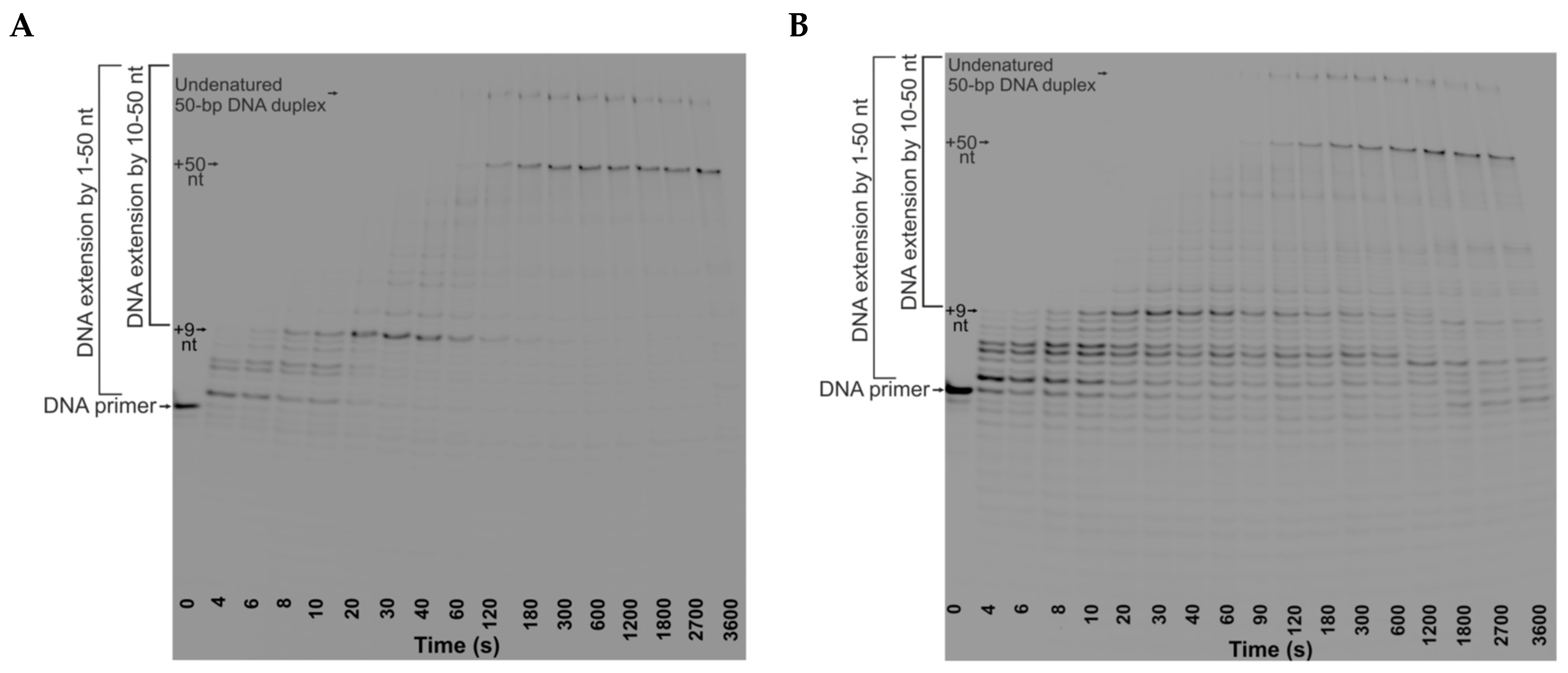
The efficiency and the rate of DNA extension by DNA polymerase in the TRC complexes were also determined from kinetic traces of the accumulation of DNA products by the Klenow fragment or DNA Pol I in the presence of RNAP (
Figure 4 and
Figure 5). The reaction mixtures either contained the NTPs or did not. In addition, to establish the basis for comparison of DNA polymerase action in the presence and absence of RNAP, we obtained time courses of DNA extension in the absence of RNAP. We used TRC-substrate-11, RF-substrate-11 (which contains the replication fork but lacks the RNA primer) as well as Control-substrate-11 (which consists of a nontemplate DNA strand and a DNA primer) (see
Table 1).
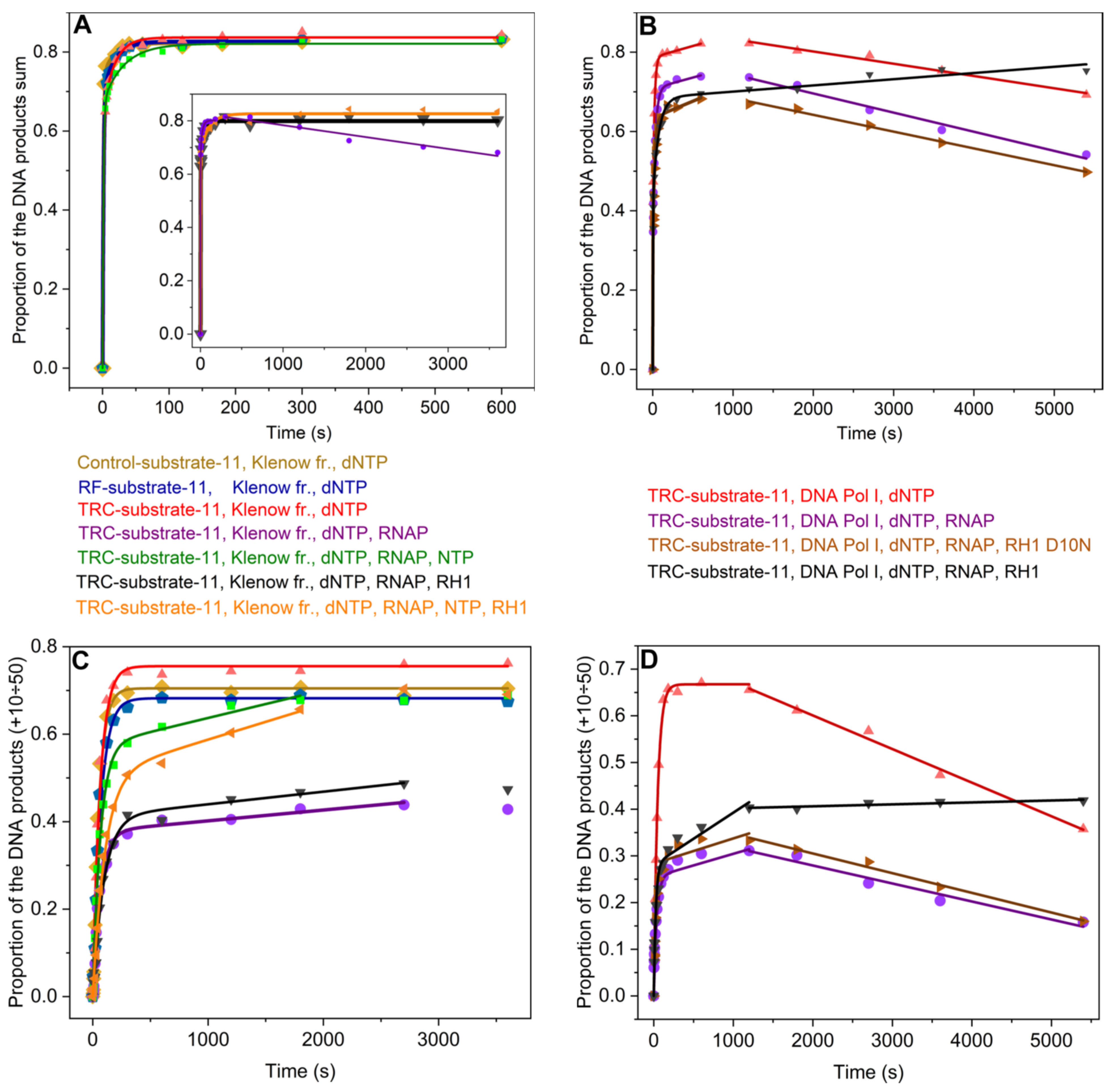
The kinetic traces of accumulation of total products by Klenow fragment in the absence of RNAP or in the presence of RNAP elongating the RNA (in the presence of NTPs) showed the product fraction increase reaching a plateau. These kinetic traces were fitted to biexponential Equation (3) and yielded the observed rate constants
,
and the formation efficiency of the enzyme–substrate complexes [ES]
DNA Sum (
Figure 5A,
Table 3). At the same time, the kinetic traces of accumulation of total products by Klenow fragment in the presence of stalled RNAP (without NTPs) showed an increase in the product fraction within the time range from 0 to 300 s with a kink at ~300 s, followed by a slow linear decrease in product fraction. Thus, the head-on collision of the Klenow fragment with RNAP stalled in the complex with the R-loop shifted the equilibrium accumulation of total DNA products towards the products of the 3′⟶5′ exonuclease activity of DNA polymerase at times exceeding 300 s. Within the time range from 0 to 300 s, the kinetic traces were also fitted to biexponential Equation (3). The further linear decrease in product fraction was fitted to linear Equation (5) and yielded the observed rate constant
. This constant characterizes the 3′⟶5′ exonuclease activity of DNA polymerase, which leads to a decrease in the total amount of DNA products (
Table 3). We did not observe a similar decrease in total amount of DNA products during head-on collision between the Klenow fragment and an elongating RNAP. This is likely because the template and non-template DNA strands separate during the movement of RNAP and DNA polymerase toward each other.
The kinetic traces of accumulation of total products by full-length DNA Pol I in TRC-substrate-11 both in the presence and absence of RNAP showed an increase in the product fraction within the time range from 0 to 600 s with a kink at ~600 s, followed by a slow linear decrease in product fraction (
Figure 5B). Consequently, in the case of DNA Pol I, the equilibrium accumulation of DNA products in TRC-substrate-11 was shifted towards the products of the exonuclease activity of DNA polymerase at times exceeding 600 s irrespective of whether RNAP was present or not. The kinetic traces were fitted to Equation (4) within the time range from 0 to 600 s: biexponential growth was followed by linear growth (
Table 3). The latter slow linear growth corresponded to the steady-state accumulation of total DNA products and was characterized by the observed rate constant
. The further linear decrease was fitted to linear Equation (5) and yielded the observed rate constant
characterizing the 3′⟶5′ exonuclease activity of DNA polymerase.
Figure 4 clearly shows that the first ~5 nucleotides are incorporated much faster than the following ones. Moreover, this is true for TRC-substrate-11, RF-substrate-11, and Control-substrate-11 in the absence and presence of RNAP. Thus, the lower rate of accumulation of total DNA products compared to initial short products is not associated with the presence of the R-loop or RNAP. This is probably caused by DNA polymerase pausing depending on the sequence of the nontemplate DNA strand. Most of the pauses were observed prior to the incorporation of purine deoxynucleosides (
Figure 4,
Table 1). The rate constant
characterizes the pre-steady-state accumulation of initial short DNA products up to ~+5 nt while the rate constant
characterizes the pre-steady-state accumulation of total DNA products up to +50 nt. Total products accumulate more than an order of magnitude slower than the initial short ones.
The presence of RNAP (both in the absence and presence of NTPs) did not affect the accumulation of initial short DNA products by Klenow fragment or the formation efficiency of the complex of Klenow fragment with TRC-substrate-11, since the values of and [ES]DNA Sum were the same in the presence and absence of RNAP. At the same time, the observed rate constant of the accumulation of total DNA products was found to decrease 1.3-fold in the presence of stalled RNAP (in the absence of NTPs) and 1.8-fold in the presence of RNAP together with NTPs. It can be suggested that the stalled RNAP stimulates Klenow fragment pausing in the pre-steady-state phase of head-on TRC. Addition of NTPs to the reaction mixture promotes the movement of RNAP along TRC-substrate-11 towards DNA polymerase, which enhances Klenow fragment pausing in the pre-steady-state phase.
In the case of full-length DNA Pol I, the presence of RNAP did not affect the formation efficiency of the complex of the enzyme and substrate, since the value of [ES]
DNA Sum was the same in the presence and absence of RNAP. On the other hand, the head-on collision of DNA Pol I with stalled RNAP probably stimulated DNA polymerase pausing during the pre-steady-state accumulation of DNA products, since the values of
and
decreased 1.4-fold and 1.6-fold, respectively, upon addition of RNAP to the reaction mixture. The head-on collision of DNA Pol I (but not the Klenow fragment) with stalled RNAP influenced the pre-steady-state accumulation of initial short DNA products up to ~+5 nt, probably due to its 5′⟶3′ exonuclease domain, since this domain causes DNA Pol I to encounter RNAP faster than the Klenow fragment. At the same time, addition of RNAP did not influence the subsequent steady-state extension of total DNA products by DNA Pol I in TRC-substrate-11 (see
in
Table 3). Moreover, our data suggested that stalled RNAP enhanced the exonuclease activity of DNA Pol I in the complex with TRC-substrate-11, since the observed rate constant of the accumulation of exonuclease products (
) was found to increase 1.5-fold when RNAP was added to the reaction mixture.
The replication fork within our TRC substrate is located at a distance of 20 nt from the 3′ end of RNA primer (
Table 1). It is known that the DNA-binding site of RNAP interacts with 2 unpaired nt and ~9 bp of downstream DNA [
14]. Thus, in our model system, +10-th deoxynucleoside can attach to nascent DNA only if DNA polymerase does not collide with RNAP bound in the competent TEC. Therefore, we also analyzed the accumulation of DNA products from +10 to +50 nt to monitor the collision of DNA polymerase with RNAP (
Figure 5C,D).
It turned out that the time courses of the accumulation of DNA products from +10 to +50 nt by Klenow fragment were monophasic in the absence of RNAP (
Figure 5C). Thus, these time courses were fitted to single-exponential Equation (1) and yielded the proportion of the initial catalytically competent enzyme–substrate complex, where the DNA primer was extended by 10–50 nt ([ES]
DNA [10÷50]), and the observed rate constant characterizing the pre-steady-state accumulation of DNA products from +10 to +50 nt (
) (
Table 4). In the case of DNA Pol I, the appropriate time course was also monophasic within the time range from 0 to 1200 s (
Figure 5D). The time course then showed a kink, followed by a slow linear decrease in the product proportion. Thus, the increase in the product proportion in the latter kinetic trace was fitted to single-exponential Equation (1). The further linear decrease was fitted to linear Equation (5) and yielded the observed rate constant characterizing the 3′⟶5′ exonuclease degradation of DNA products from +10 to +50 nt by DNA polymerase (
) (
Table 4). The time courses of the accumulation of DNA products by Klenow fragment from +10 to +50 nt became diphasic when RNAP was added and had burst-like traces. Therefore, the kinetic traces were fitted to Equation (2) (single-exponential growth followed by linear growth) within the time range from 0 to 2700 s (
Figure 5C) and yielded the observed rate constants
(characterizing the pre-steady-state accumulation of DNA products from +10 to +50 nt) and
(characterizing the steady-state accumulation of DNA products from +10 to +50 nt) (
Table 4). At the same time, in the case of DNA Pol I, in the presence of RNAP, the time course showed a diphasic increase in the product proportion within the time range from 0 to 1200 s with a further kink, followed by a slow linear decrease in product proportion (
Figure 5D). Thus, the latter kinetic trace was fitted to Equation (2) (single-exponential growth followed by linear growth) within the time range from 0 to 1200 s. The further linear decrease was fitted to linear Equation (5) (
Table 4). Consequently, in the case of DNA Pol I, the equilibrium accumulation of products from +10 to +50 nt was shifted towards the products of the 3′⟶5′ exonuclease activity of DNA polymerase over long times both in the presence and absence of RNAP.
On the other hand, in the case of the Klenow fragment, we did not observe a backward shift in its equilibrium accumulation of products from +10 to +50 nt (
Figure 5C), whereas its equilibrium accumulation of total DNA products was shifted backwards in the presence of stalled RNAP (without NTPs) (
Figure 5A). Taken in tandem, these data suggest that a stalled TEC shifts the equilibrium accumulation of DNA products towards the products of the 3′⟶5′ exonuclease activity of the Klenow fragment when the Klenow fragment replicates DNA downstream of the stalled TEC.
The proportion of the initial catalytically competent DNA polymerase complex with TRC-substrate-11, where the DNA primer was extended by 10–50 nt ([ES]
DNA [10÷50]) decreased 2.1-fold in the case of the Klenow fragment and 2.6-fold in the case of DNA Pol I when RNAP was added (
Table 4). During RNAP interaction with the TRC-substrate-11, the proportion of the initial catalytically competent TEC ([TEC]
Sum) did not exceed 62% in the absence of DNA polymerase or in the presence of Klenow fragment and did not exceed 72% in the presence of DNA Pol I (
Table 2). At the same time, in the presence of RNAP, the proportion of the initial catalytically competent DNA polymerase complex with TRC-substrate-11 ([ES]
DNA [10÷50]), where the DNA primer was extended by more than 9 nt, was 37% in the case of the Klenow fragment and 25.5% in the case of DNA Pol I (
Table 4). Hence, the DNA extension in the TRC-substrate-11 by more than 9 nt presumably occurred in the proportion of the substrate that was not bound in the catalytically competent TEC. The burst phase in the kinetic traces of accumulation of DNA products from +10 to +50 nt probably corresponded to the DNA extension within the complex of DNA polymerase with the substrate initially unbound in the catalytically competent TEC. Consequently, the head-on conflict of replication with transcription occurs in our model system: RNAP stalled in the catalytically competent TEC interferes with replication.
Consequently, our data reveal that while stalled RNAP poses a strong roadblock for DNA polymerase, stalled Pol I does not obstruct RNAP. RNAP can displace a stalled DNA polymerase during elongation, but DNA Pol I lacks the ability to displace a stalled RNAP. This can probably be explained by the fact that the stability of the catalytically competent RNAP complex with an R-loop is higher than the stability of the DNA polymerase complex with its substrate. This assumption is consistent with data showing that the processivity of RNAP (10
3–10
5 nucleotides [
24]) is significantly higher than that of DNA Pol I (no more than 188 nucleotides [
25]).
On the other hand, the proportion of the initial catalytically competent complex of Klenow fragment with TRC-substrate-11 ([ES]
DNA [10÷50]), where the DNA primer was extended by more than 9 nt, decreased only 1.3-fold in the presence of RNAP together with NTPs (
Table 4). Moreover, in the latter case, the proportion of DNA products extended by more than 9 nt reached the level seen without RNAP within 1800 s (
Figure 5C). It can be proposed that after RNAP displaces the DNA polymerase and reaches the end of the DNA template strand, the DNA polymerase can reassociate with the nontemplate DNA strand (in a complex with the DNA primer) upstream of RNAP and resume DNA elongation, thus overcoming the head-on conflict between replication and transcription. This overcoming was characterized by the observed rate constant of steady-state accumulation of DNA products from +10 to +50 nt
, which was more than three orders of magnitude lower than the pre-steady-state accumulation of these DNA products (compare
in
Table 4). In addition, in ~5% of TRC complexes, the head-on collision of the Klenow fragment or full-length DNA Pol I with stalled RNAP was overcome (
Figure 5C,D,
in
Table 4). In these ~5% of the TRC complexes, RNAP probably formed an incompetent complex with the R-loop, which did not stall replication but stimulated DNA polymerase pausing, resulting in a decrease by more than three orders of magnitude in the rate of accumulation of DNA products from +10 to +50 nt (compare
in
Table 4). The collision between the Klenow fragment and stalled RNAP (occurring in ~5% of TRC complexes) was resolved at a rate two times lower than its collision with an actively elongating RNAP (
in
Table 4).
At the same time, the proportion of the catalytically competent complex of Klenow fragment [ES]
DNA [10÷50] with the R-loop-containing substrate (TRC-substrate-11) and the rate constant of DNA extension by 10–50 nt (
) in this substrate were the same as in the case of complexes with substrates lacking an R-loop (RF-substrate-11 and Control-substrate-11) (
Table 4). Analysis of the accumulation of DNA products from +21 to +50 nt gave the same result (Data not shown). Hence, an R-loop containing an 11-nt bubble in the absence of RNAP did not interfere with replication by Klenow fragment. On the other hand, in the case of full-length DNA Pol I, an R-loop containing an 11-nt bubble in the absence of RNAP also did not stall the replication but shifted the equilibrium accumulation of total DNA products and products from +10 to +50 nt towards the products of the 3′⟶5′ exonuclease activity of DNA polymerase. There is a broad understanding of R-loops as a replication fork barrier. However, RNAP was present in the experimental systems used in all studies where the R-loop was shown to be a replication obstacle [
20,
26]. The results obtained in this study reveal that the isolated R-loop itself does not stall replication by DNA Pol I. The polymerase resolves the R-loop, probably due to its strand displacement activity. Thus, it is the presence of RNAP forming the catalytically competent TEC with the R-loop that interferes with replication by DNA Pol I.
Therefore, our data reveal that the head-on conflict of replication with transcription has been registered in our model system: RNAP stalled in the catalytically competent TEC with an R-loop containing an 11-nt bubble (R-loop-11) interferes with the action of DNA polymerases both lacking and possessing 5′⟶3′ exonuclease activity.
What is more, the stalled RNAP stimulates a pause of the Klenow fragment in the pre-steady-state phase of head-on TRC and the 3′⟶5′ exonuclease activity of the Klenow fragment in the steady-state phase when the Klenow fragment replicates DNA downstream of the stalled RNAP. It was shown that it is the incompetent complex of RNAP with a TRC substrate that stimulates the 3′⟶5′ exonuclease activity of the Klenow fragment during a head-on TRC. Furthermore, when RNAP moves along TRC-substrate-11 towards the Klenow fragment, elongating the RNA, the pausing of the Klenow fragment is enhanced in the pre-steady-state phase; then, in the steady-state phase, the head-on conflict of replication with transcription is slowly overcome in our model system, likely through the reassociation of the displaced DNA polymerase with the nontemplate DNA strand upstream of RNAP. At the same time, our data reveal that free R-loop-11 does not interfere with DNA replication by the Klenow fragment.
In the case of full-length DNA Pol I, the steady-state accumulation of DNA products in TRC-substrate-11 is shifted towards the products of the exonuclease activity of DNA polymerase at times exceeding 600 s irrespectively of whether RNAP was present or not. Thus, R-loop-11 in the absence of RNAP does not stall replication but shifts the equilibrium accumulation of DNA products towards the products of the 3′⟶5′ exonuclease activity of DNA polymerase. The head-on collision of DNA Pol I with stalled RNAP stimulates DNA polymerase pausing during pre-steady-state accumulation of DNA products and enhanced 3′⟶5′ exonuclease activity of DNA Pol I in the steady-state phase. It was shown that the incompetent complex of RNAP with TRC-substrate enhanced the 3′⟶5′ exonuclease activity of DNA Pol I.
Both DNA polymerases used in the study slowly overcame a head-on conflict with stalled RNAP in only ~5% of TRC complexes in our model system. In these ~5% of the TRC complexes, RNAP probably formed an incompetent complex with the R-loop, which does not stall replication but rather stimulates DNA polymerase pausing in the steady-state phase, resulting in a decrease by more than three orders of magnitude in the rate of accumulation of DNA products from +10 to +50 nt.
2.5. DNA Extension by DNA Polymerase in the TRC Substrate in the Presence of RH1
To analyze the ability of RH1 to overcome a head-on conflict of replication and transcription, kinetic traces of accumulation of DNA products by DNA polymerase in the presence of RNAP and wild-type (wt) RH1 or its catalytically inactive mutant form RH1 D10N were obtained (
Figure 5).
The kinetic traces of accumulation of total products by Klenow fragment or DNA Pol I in the presence of stalled RNAP (without NTPs) and wt RH1 did not show a kink followed by a slow linear decrease in product proportion, which was observed in the absence of RH1. The kinetic traces showed an increase in the product fraction. In the case of Klenow fragment, the kinetic trace was fitted to the biexponential Equation (3) and yielded the observed rate constants
,
and the formation efficiency of enzyme–substrate complexes [ES]
DNA Sum (
Figure 5A,
Table 3). In the case of full-length DNA Pol I, the kinetic trace in the presence of RH1 was fitted to Equation (4): biexponential growth was followed by linear growth (
Figure 5B,
Table 3). The latter slow linear growth was characterized by the observed rate constant
. Thus, the addition of wt RH1 to the reaction mixture eliminated the shift in the equilibrium accumulation of total DNA products towards the products of 3′⟶5′ exonuclease activity of DNA polymerase in the case of head-on TRC. On the other hand, RH1 D10N did not affect significantly the kinetic traces of accumulation of DNA products by DNA Pol I in TRC-substrate-11 in the presence of stalled RNAP (
Figure 5B,D,
Table 3 and
Table 4). The appropriate kinetic traces in the presence of RH1 D10N were fitted to the same equations as in its absence. Consequently, the effect of wt RH1 is due to its catalytic activity, not to substrate binding. At the same time, wt RH1 did not affect significantly the kinetic trace of accumulation of DNA products by the Klenow fragment in TRC-substrate-11 in the presence of RNAP and NTPs, probably due to the separation of DNA strands (
Figure 5A,C).
In addition, wt RH1 barely influenced the time course of the accumulation of DNA products from +10 to +50 nt by Klenow fragment in TRC-substrate-11 in the presence of RNAP (
Figure 5C,
Table 4). The kinetic trace in the presence of RH1 was fitted to the same equation as in its absence (Equation (2)—single-exponential growth followed by linear growth). Since the accumulation of DNA products from +10 to +50 nt most probably occurred in the proportion of the substrate that was not bound in the catalytically competent TEC, the latter data indicated that a free R-loop containing an 11-nt bubble (R-loop-11) did not interfere with DNA replication by the DNA polymerase lacking a 5′⟶3′ exonuclease domain. On the contrary, in the cases of DNA Pol I, the addition of wt RH1 to the reaction mixture eliminated the shift in the steady-state accumulation of DNA products from +10 to +50 nt towards the products of the 3′⟶5′ exonuclease activity of DNA polymerase (
Figure 5D). Thus, this kinetic trace was fitted to Equation (2) (single-exponential growth followed by linear growth) within the time range from 0 to 1200 s. The further slow linear increase was fitted to linear Equation (5) and yielded the observed rate constant
. These data indicated that free R-loop shifted the steady-state accumulation of DNA products towards the products of the 3′⟶5′ exonuclease activity of full-length DNA polymerase.
In the case of TRC-substrate-11, the addition of wt RH1 did not increase the values of [ES]
DNA [10÷50] in the presence of RNAP to the level of those observed in the absence of RNAP either for the Klenow fragment or for DNA Pol I (
Table 4). Consequently, wt RH1 alone did not provide a resolution of the head-on conflict of replication and transcription.
Our data revealed that RH1 did not displace stalled RNAP from the competent TEC. On the other hand, RH1 eliminated the shift in the steady-state accumulation of total DNA products towards the products of the 3′⟶5′ exonuclease activity of DNA polymerase during head-on TRC. The R-loop-11 in the absence of RNAP did not interfere with replication by the Klenow fragment. Thus, the cleavage of the free R-loop cannot explain the effect of RH1 on the 3′⟶5′ exonuclease activity of the Klenow fragment. Therefore, the only possible explanation of this effect is that RH1 displaces RNAP from the incompetent complex with the TRC substrate. Consequently, it is the incompetent complex of RNAP with the TRC substrate that stimulates the 3′⟶5′ exonuclease activity of the Klenow fragment (lacking 5′⟶3′ exonuclease activity) during head-on TRC. At the same time, in the case of full-length DNA Pol I (possessing 5′⟶3′ exonuclease activity), both a free R-loop and an incompetent complex of RNAP with TRC substrate stimulate 3′⟶5′ exonuclease activity of DNA polymerase. In addition, the observed rate constant of the pre-steady-state accumulation of total DNA products
by the Klenow fragment was found to decrease 1.5-fold upon the addition of RH1 to the TRC containing stalled RNAP (
Table 3). In TRC containing stalled RNAP and full-length DNA Pol I extending DNA, the observed rate constants of pre-steady-state DNA products accumulation
and
and steady-state DNA products accumulation
decreased 1.3-fold, 2.4-fold, and 4-fold, respectively, upon the addition of RH1. It is likely that RH1 promotes the movement of RNAP towards DNA polymerase in the incompetent complex of RNAP with TRC substrate prior to the dissociation of RNAP from this complex, which enhances DNA polymerase pausing in the pre-steady-state phase. The co-addition of RH1 and NTPs to the TRC containing stalled RNAP and the Klenow fragment extending DNA had a more pronounced effect on DNA polymerase pausing in the pre-steady-state phase. The values of
and
decreased 1.3-fold and 4.5-fold, respectively. Presumably, RH1 promotes the movement of RNAP towards DNA polymerase in the incompetent complex of RNAP with TRC-substrate, whereas NTPs promote such movement in the competent TEC.
Therefore, the results displayed in this work clearly reveal that RH1 alone does not provide a resolution of a head-on conflict of replication and transcription in a complex containing a TRC-substrate with an 11-nt heteroduplex. Our data showed that RH1 does not displace stalled RNAP from a competent TEC containing an R-loop-11. Moreover, RH1 also does not displace stalled RNAP during head-on TRC, when DNA polymerase extending DNA collides with competent TEC. On the other hand, RH1 eliminates the shift in the steady-state accumulation of total DNA products towards the products of 3′⟶5′ exonuclease activity of DNA polymerase during head-on TRC, since RH1 probably displaces RNAP from the incompetent complex with the TRC substrate. The effect of wt RH1 was shown to be due to its catalytic activity rather than substrate binding. Furthermore, RH1 probably promotes the movement of RNAP towards DNA polymerase in the incompetent complex of RNAP with the TRC substrate prior to the dissociation of RNAP from this complex, which enhances DNA polymerase pausing in the pre-steady-state phase. The co-addition of RH1 and NTPs to the TRC containing stalled RNAP and the Klenow fragment, extending DNA, has a more pronounced effect on DNA polymerase pausing in the pre-steady-state phase. Presumably, RH1 promotes the movement of RNAP towards DNA polymerase in the incompetent complex of RNAP with TRC substrate, whereas NTPs promote such movement in a competent TEC.