Physicochemical Characterization and Structural Tuning of PVA/CMC Composites Modified with Montmorillonite and Glycerol: Toward Sustainable Wound Dressings
Abstract
1. Introduction
- carboxymethyl cellulose because it provides excellent swelling capacity, pH sensitivity, biocompatibility, and biodegradability;
- poly(vinyl alcohol) because it enhances the hydration of composite materials and improves their mechanical strength and thermal stability. It also promotes cell adhesion and migration, making it a valuable component in hydrogel composites for biomedical applications;
- montmorillonite because it is used to modify the mechanical and structural properties of the composite. Due to its layered silicate structure and high aspect ratio, MMT restricts polymer chain mobility and increases structural rigidity. It can also increase pore volume and surface area. MMT contributes to enhanced molecular adhesion through multiple interfacial interactions. Its surface contains Si-OH and Al-OH groups and negatively charged silicate layers that can establish hydrogen bonding, electrostatic interactions, and van der Waals forces with the functional groups of the polymer chains (e.g., hydroxyl or carboxyl groups in PVA and CMC). Additionally, exfoliated MMT exposes an additional active surface area. This facilitates stronger adhesion not only between polymer chains but also between the composite and active molecules, improving the potential for molecular entrapment/adsorption or drug-loading efficiency in biomedical systems. Due to its layered structure—composed of negatively charged packets balanced by cations between the packets—it enables further modifications such as cation exchange and interlayer intercalation [14,15,16,17];
- glycerin as a plasticizer to reduce polymer rigidity, decrease brittleness, and enhance ductility;
- citric acid because hydrogels are completely safe during synthesis and have good swelling and biodegradation properties, while also reducing resistance and improving the material’s adhesiveness.
2. Results and Discussion
2.1. Preparation of Composite Materials
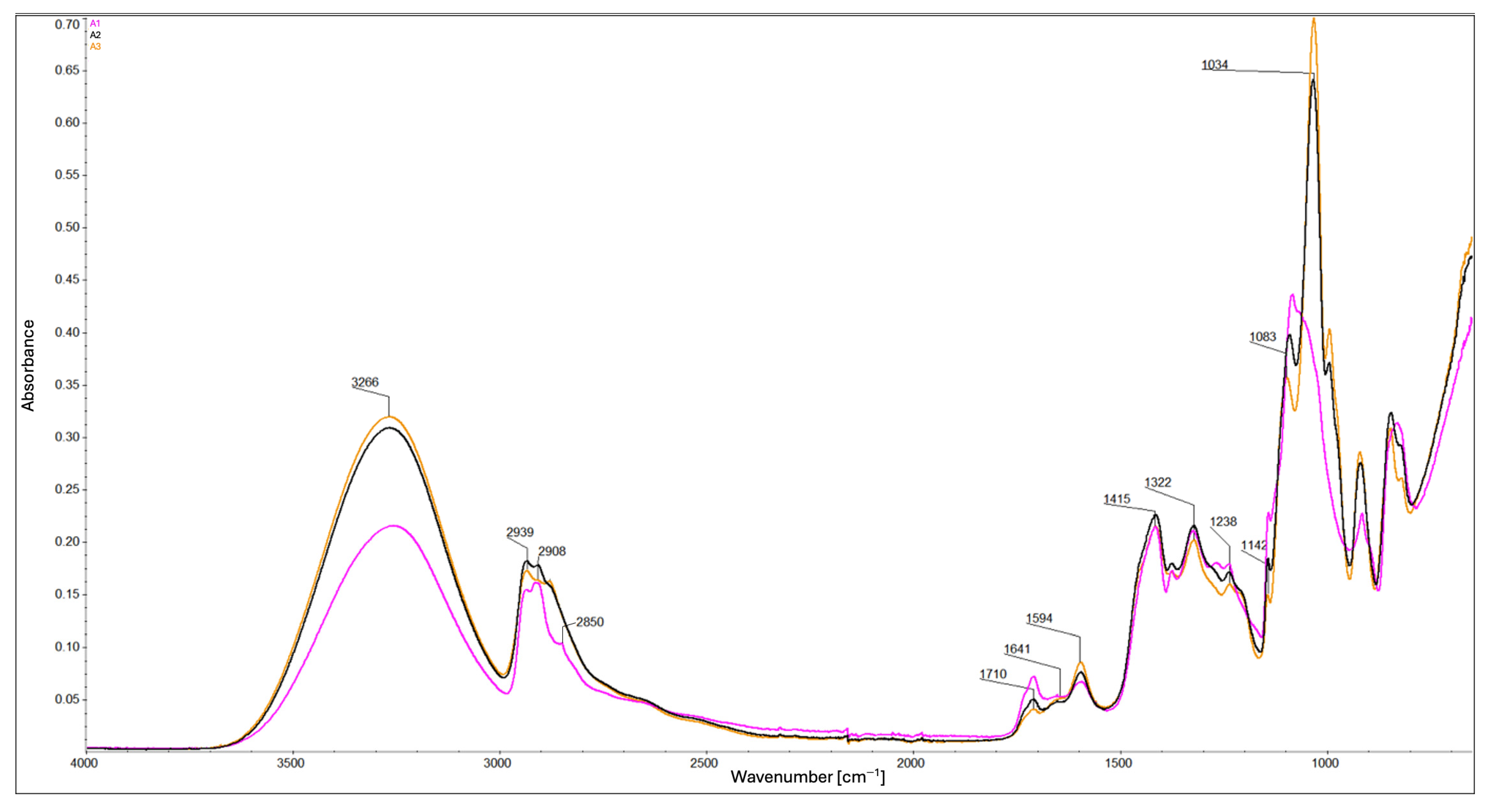
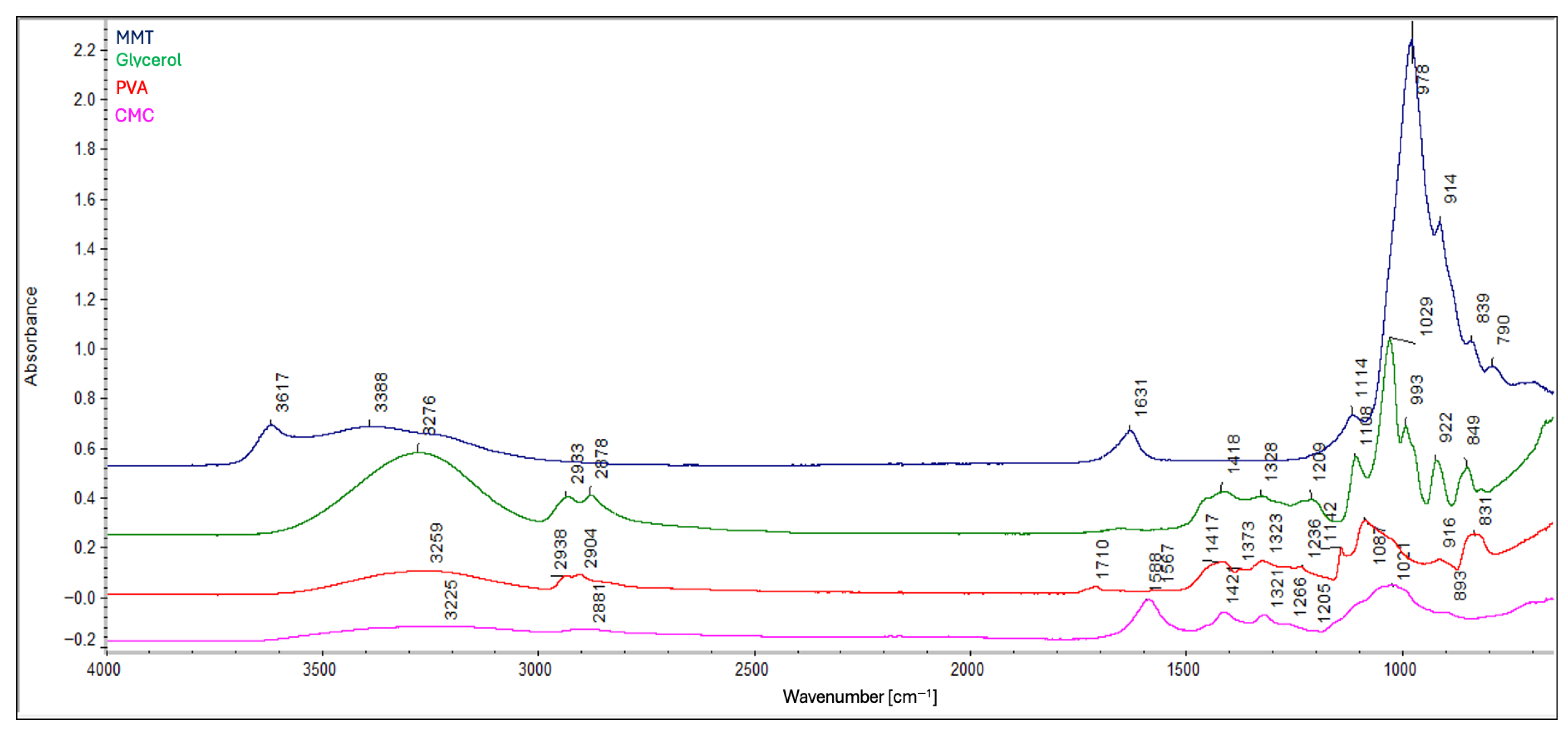
2.2. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
- Glycerol reduces the degree of cross-linking by competing for reactive hydroxyl and carboxyl groups through hydrogen bonding.
- Glycerol promotes polymer chain cleavage, leading to shorter chains and distinct structural organization compared with glycerol-free composites.
- The protonation state of carboxyl groups depends on composition: in glycerol-containing composites, esterification-driven consumption of –OH groups promotes deprotonation (–COO−), whereas in A1 (no MMT) and B7 (1% m/v MMT concentration) the protonated form (–COOH) is predominant. This reflects the dual role of MMT as a site for ion exchange and a potential “proton trap”.
- MMT content enhances molecular interactions and increases structural density by providing exchangeable surfaces and promotes hydrogen bonding through accessible –OH groups; however, this effect is partly counteracted by glycerol.
- MMT stiffens the polymer matrix and restricts chain mobility, while glycerol interacts with MMT layers through hydrogen bonding, reducing this stiffening effect (softens the structure) and stabilizing the spatial arrangement.
- Both glycerol and MMT simultaneously reduce crystallinity and amorphous content, disrupting conventional structural organization.
- The preceding point suggests the formation of intermediate, interfacial structures that do not correspond to purely crystalline or amorphous phases.
- Additional stiffening by MMT results in smaller or less ordered crystals.
- Consequently, glycerol and MMT partially neutralize each other’s adverse effects, leading to composites with balanced structural properties.
- Spectral similarities between composites C5 and C7 indicate that glycerol contributes to a more coherent chemical structure, in contrast to the greater variation observed in glycerol-free composites.
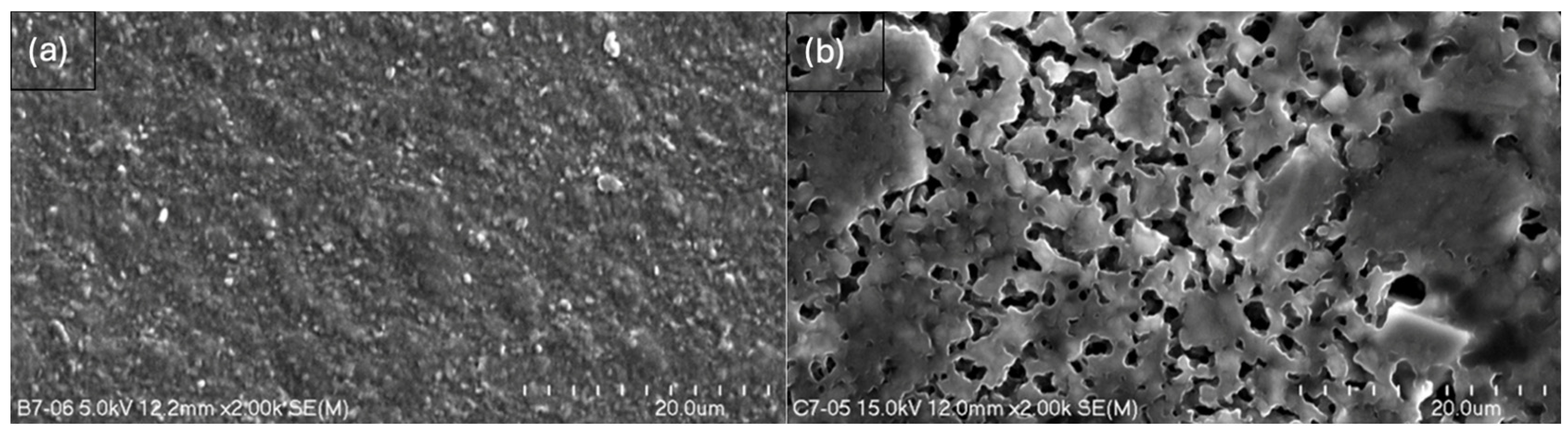
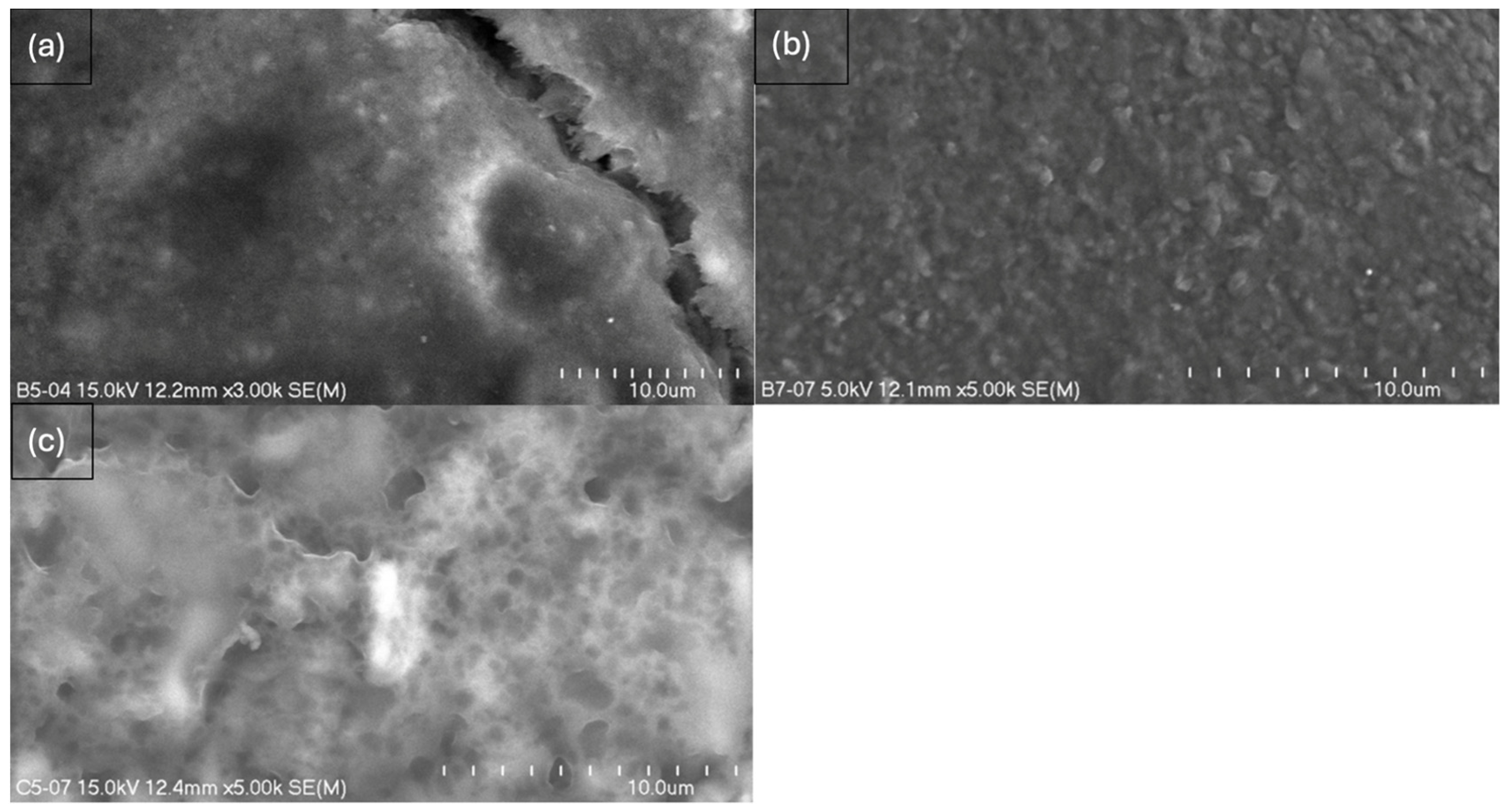
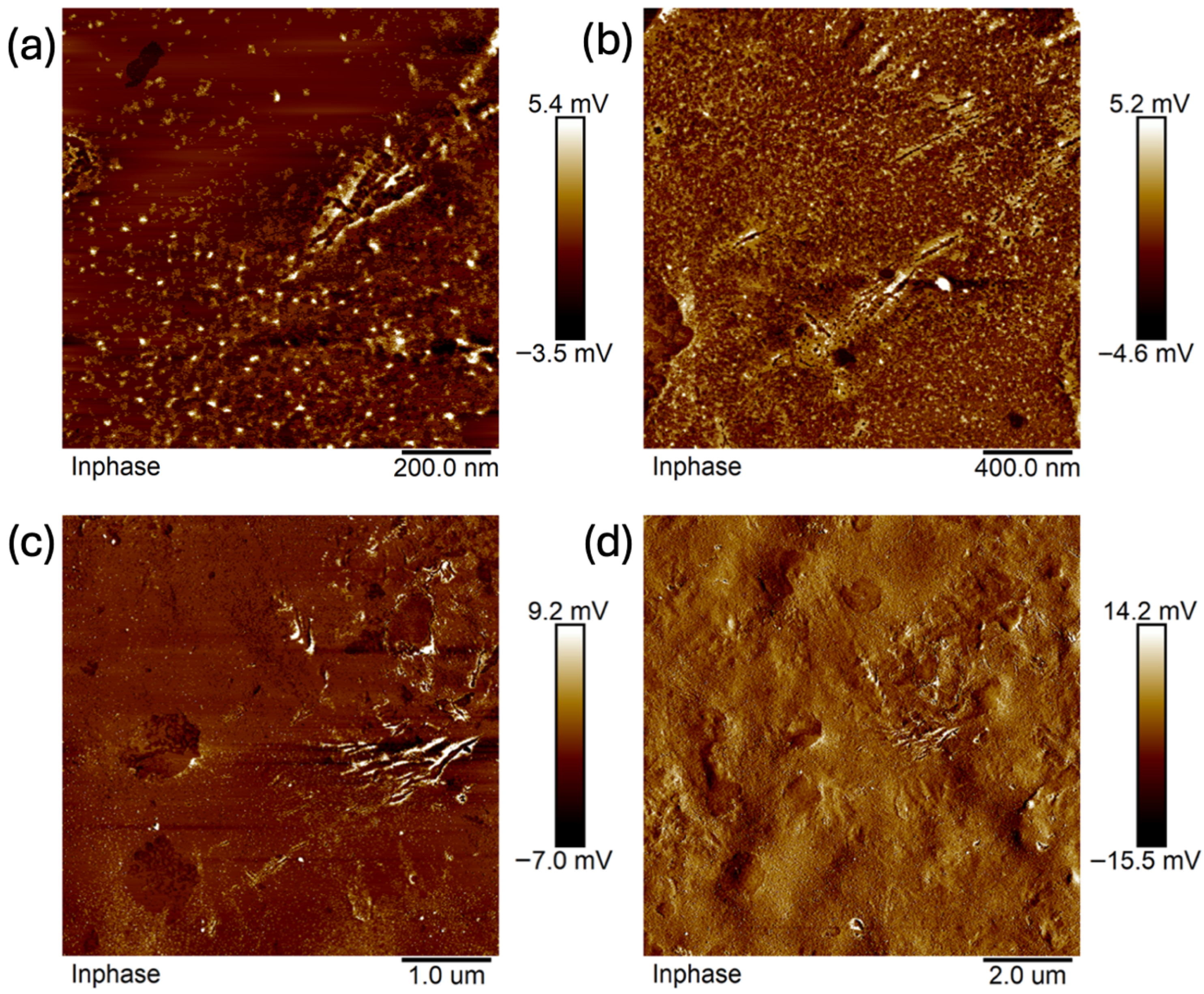
2.3. Scanning Electron Microscopy (SEM)
- Composites without glycerol (B5, B7) exhibited greater homogeneity and an absence of cavities, pores, or larger spaces, while the addition of glycerol altered the spatial configuration of the composite, leading to the formation of pores or even larger spaces, as observed in C7.
- The results confirm the synergistic effect of glycerol and MMT; nevertheless, the dominant role of glycerol in promoting porosity formation is evident.
- The more homogeneous surface of composite B7 creates the possibility of the adhesion of active molecules on the surface—higher MMT content enhances the potential for hydrogen bond formation compared to B5, which is consistent with FTIR evidence of greater molecular interactions.
- The structure of C7 suggests the possibility of stronger particle binding and potential molecule entrapment within the matrix, which is especially relevant in the context of modified drug release.
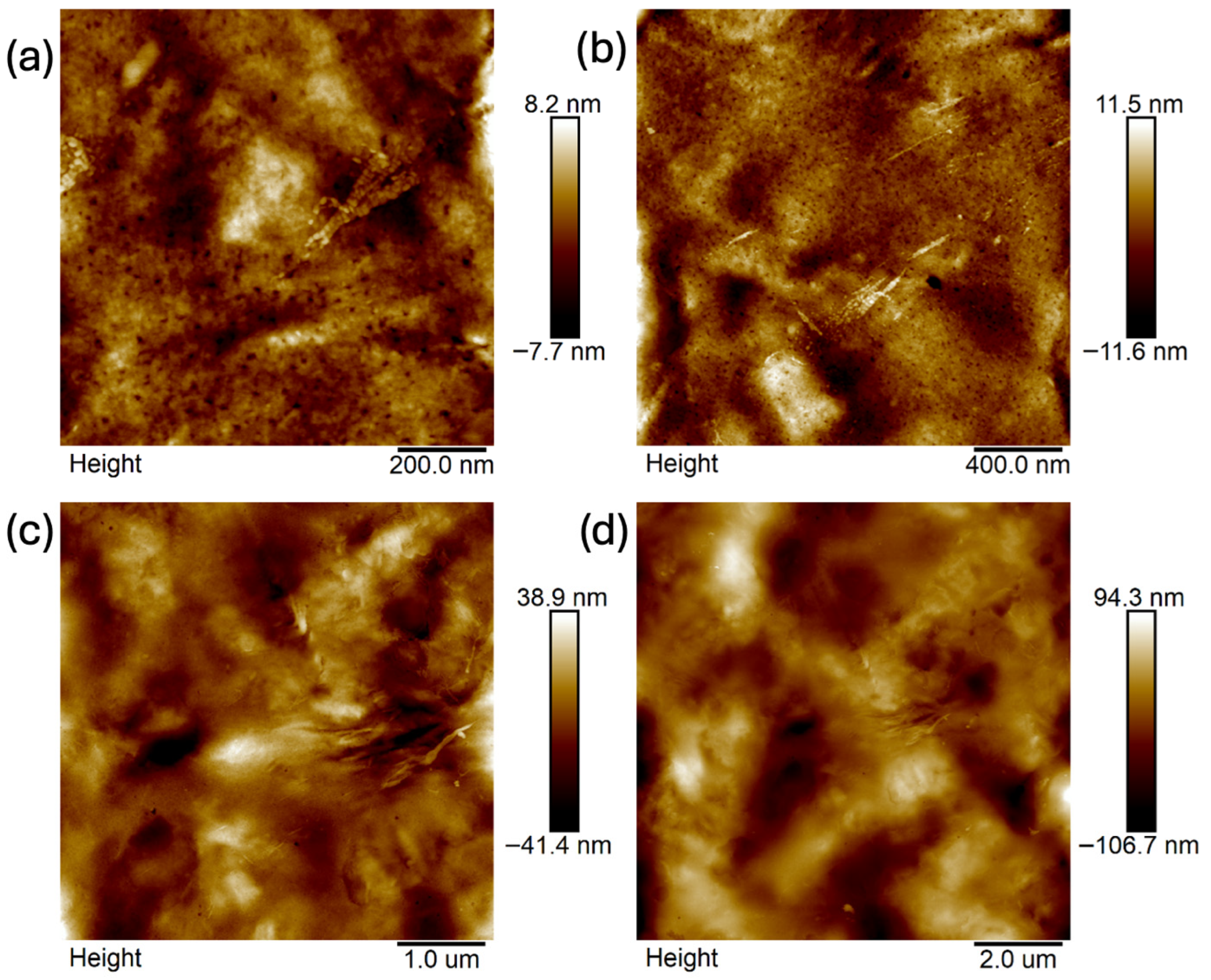
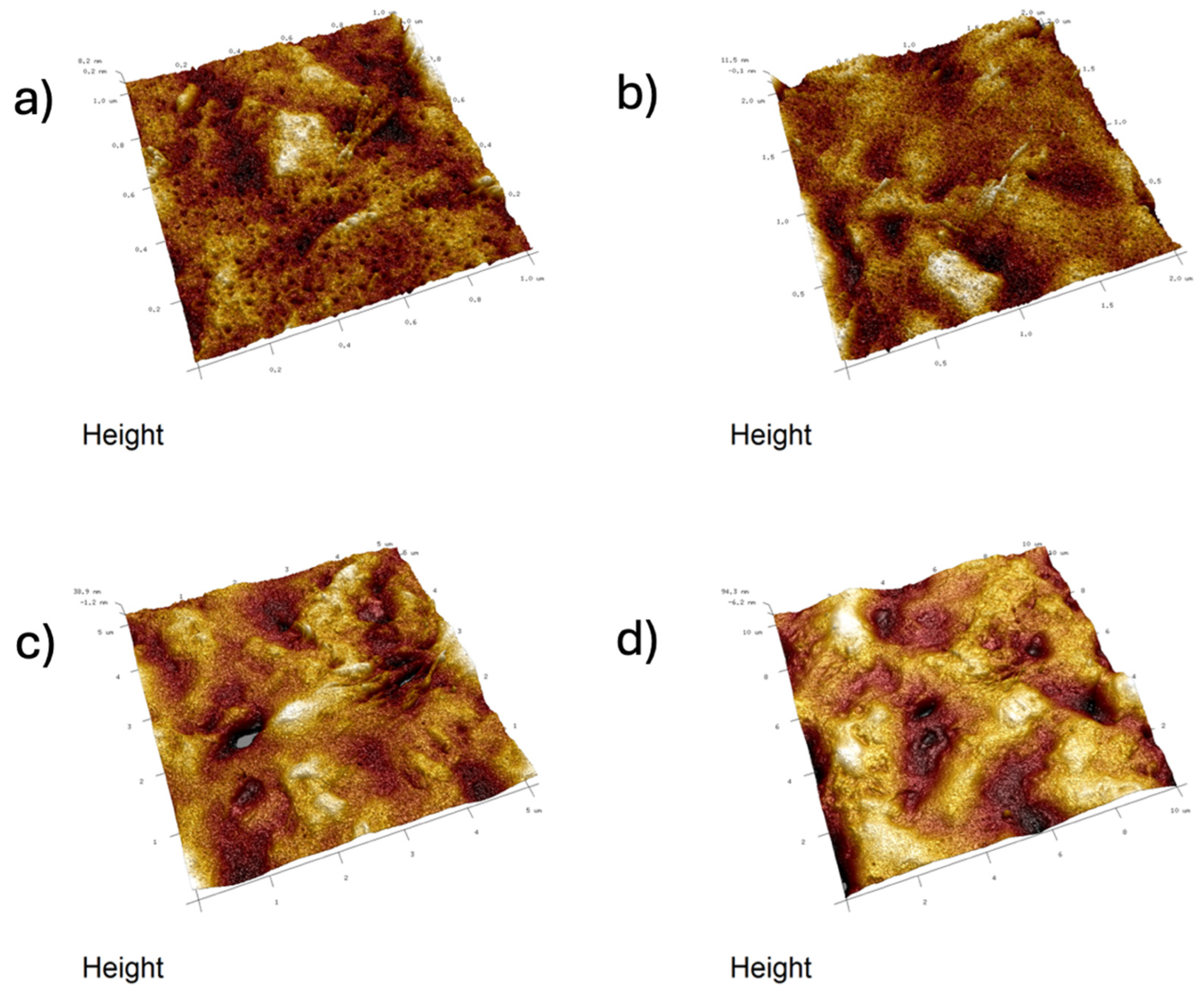
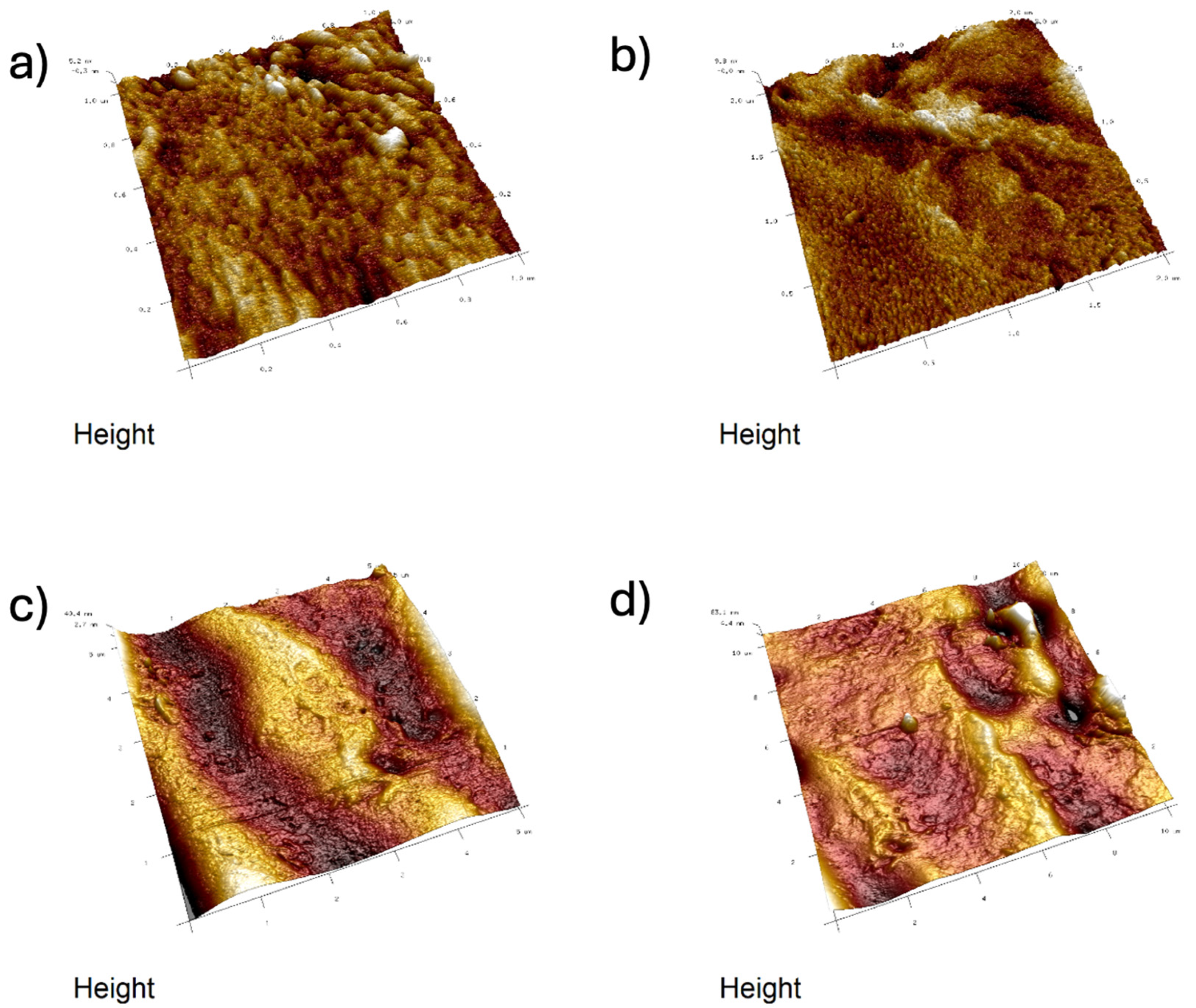
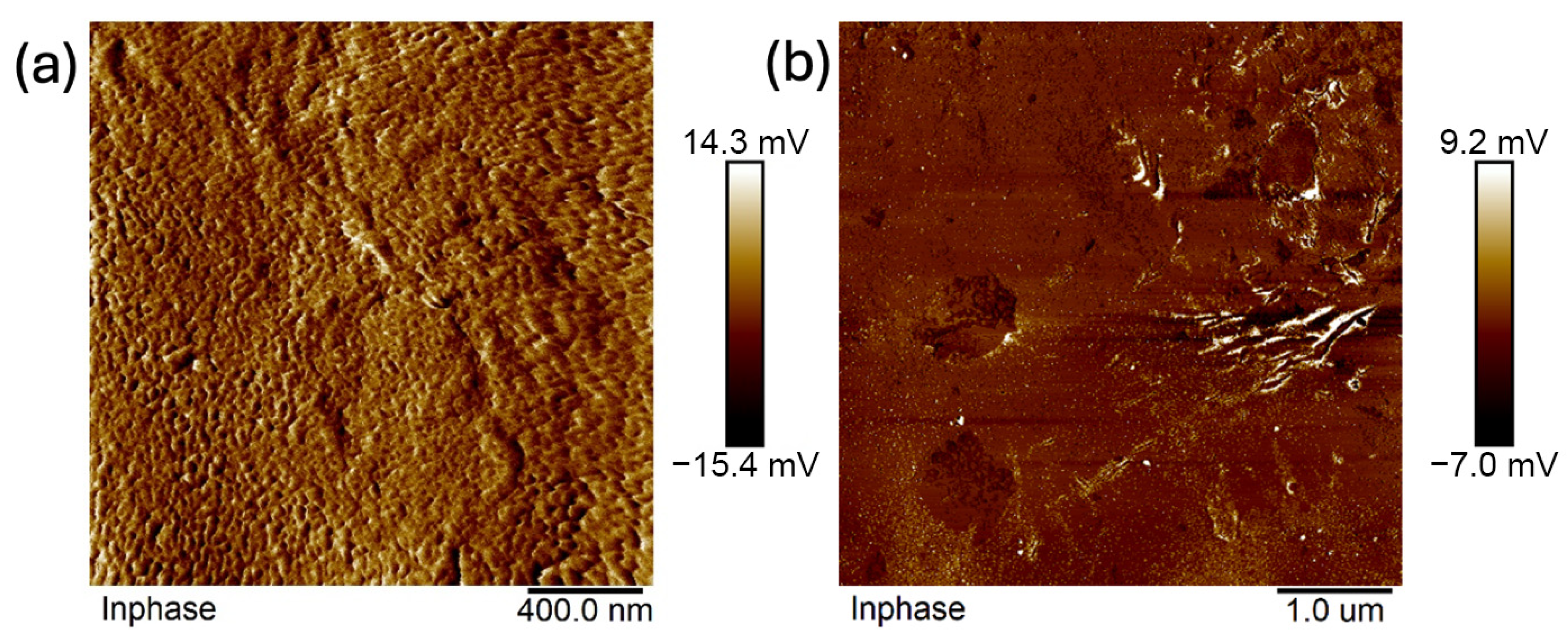
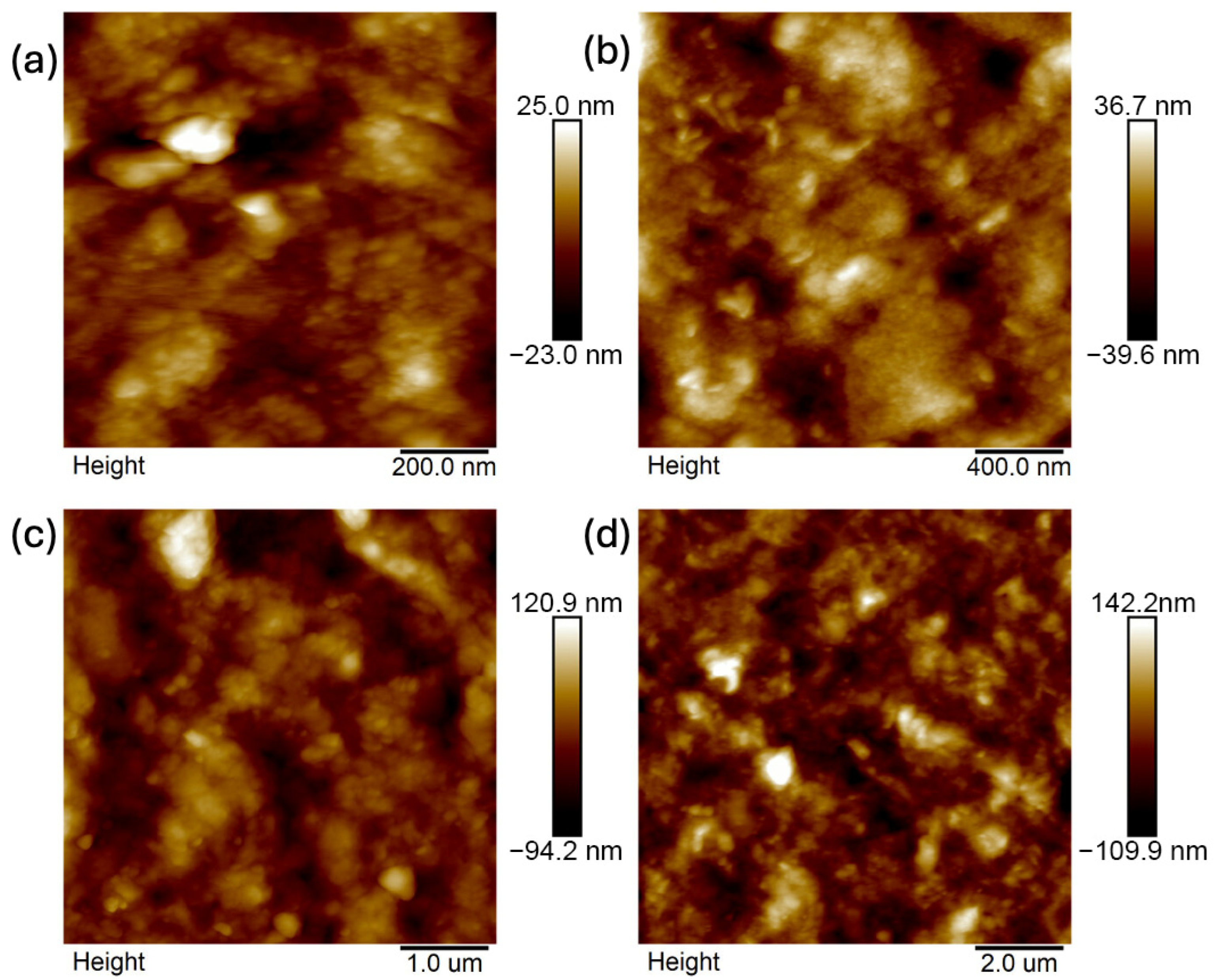
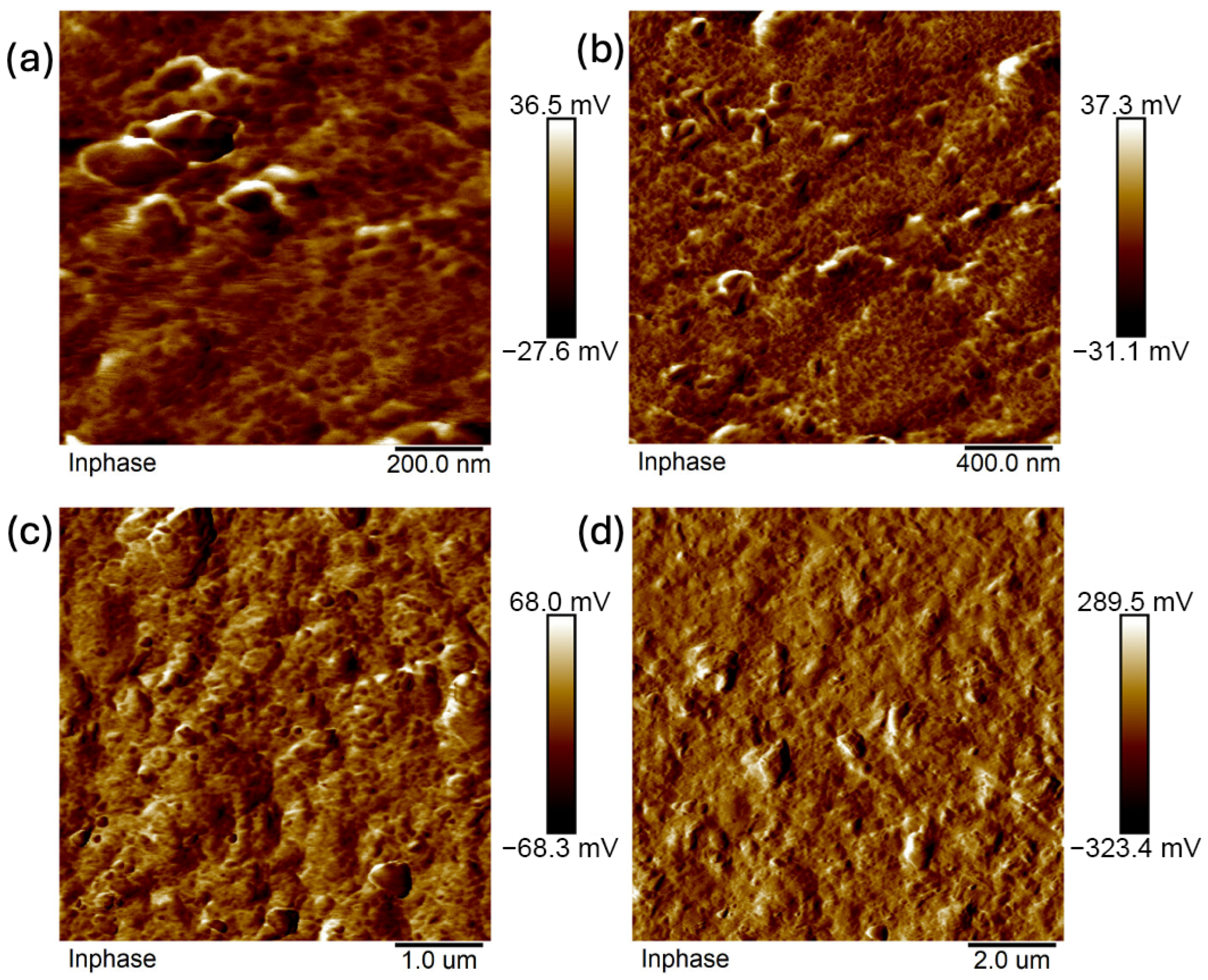
2.4. Atomic Force Microscopy (AFM)
- Analysis confirmed an increase in surface roughness, as well as an appearance of larger grains, pores, and spaces in the material after the addition of glycerol. These are positive phenomena both in terms of adhesion of the dressing to the wound and enabling drug incorporation.
- The occurrence of MMT aggregates in the C7 matrix (with higher MMT content and glycerol) was also indicated. While MMT aggregation is generally considered a disadvantage, in this case, it may represent an opportunity for the targeted incorporation or immobilization of active molecules within the matrix.
3. Materials and Methods
3.1. Materials
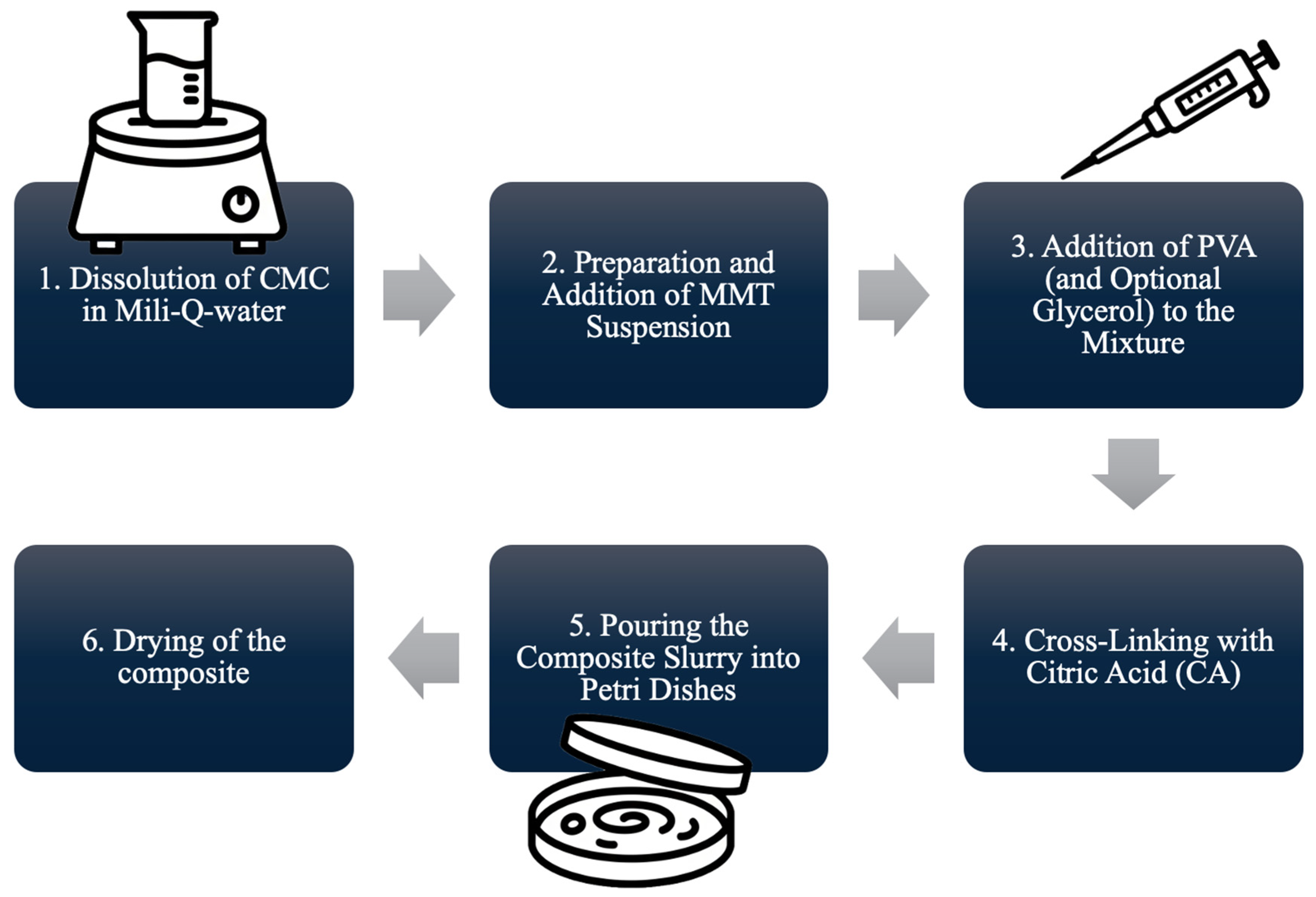
3.2. Preparation Method
3.3. Instrumentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niedźwiecka, A.; Talik, P. Przegląd I Ocena Perspektywicznych Modyfikacji Kompozytów Hydrożelowych Karboksymetylocelulozy Stosowanych W Materiałach Opatrunkowych: Review And Evaluation Of Prospective Modifications Of Carboxymethyl Cellulose Hydrogel Composites Used In Dressing Materials. Wiadomości Chem. 2024, 78, 609–623. [Google Scholar]
- Cullen, B.; Gefen, A. The Biological and Physiological Impact of the Performance of Wound Dressings. Int. Wound J. 2023, 20, 1292–1303. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Ngoc Le, T.T.; Nguyen, A.T.; Thien Le, H.N.; Pham, T.T. Biomedical Materials for Wound Dressing: Recent Advances and Applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Yudaev, P.; Butorova, I.; Chuev, V.; Posokhova, V.; Klyukin, B.; Chistyakov, E. Wound Gel with Antimicrobial Effects Based on Polyvinyl Alcohol and Functional Aryloxycyclotriphosphazene. Polymers 2023, 15, 2831. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Song, W.; Wang, Y.; Zhou, J.; Tan, Z. Silanized Cellulose Nanocrystals-Based Dual-Network Ionically Crosslinked Hydrogels with Enhanced Mechanical Strength, Antibacterial Activity, and Biocompatibility. Colloids Surf. A Physicochem. Eng. Asp. 2025, 727, 138236. [Google Scholar] [CrossRef]
- Qi, X.; Li, Y.; Xiang, Y.; Chen, Y.; Shi, Y.; Ge, X.; Zeng, B.; Shen, J. Hyperthermia-Enhanced Immunoregulation Hydrogel for Oxygenation and ROS Neutralization in Diabetic Foot Ulcers. Cell Biomater. 2025, 1, 100020. [Google Scholar] [CrossRef]
- Qi, X.; Lan, Y.; Chen, J.; Xiang, Y.; Wang, Y.; Jiang, L.; Dong, Y.; Li, J.; Liao, Z.; Li, Z.; et al. An Endogenous Adenosine Triphosphate-Activated Hydrogel Prodrug System for Healing Multidrug-Resistant Bacteria Infected Diabetic Foot Ulcers. Adv. Healthc. Mater. 2025, 14, 2500688. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl Cellulose-Based Materials for Infection Control and Wound Healing: A Review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef]
- Mansur, A.a.P.; Rodrigues, M.A.; Capanema, N.S.V.; Carvalho, S.M.; Gomes, D.A.; Mansur, H.S. Functionalized Bioadhesion-Enhanced Carboxymethyl Cellulose/Polyvinyl Alcohol Hybrid Hydrogels for Chronic Wound Dressing Applications. RSC Adv. 2023, 13, 13156–13168. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, I.C.; Carvalho, S.M.; Mansur, H.S. Bioengineered Water-Responsive Carboxymethyl Cellulose/Poly(Vinyl Alcohol) Hydrogel Hybrids for Wound Dressing and Skin Tissue Engineering Applications. Gels 2023, 9, 166. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.; Ge, G.; Dai, N.; Rao, J.; Ran, H.; Zhang, Y. Montmorillonite-Based Two-Dimensional Nanocomposites: Preparation and Applications. Molecules 2021, 26, 2521. [Google Scholar] [CrossRef]
- Wang, W.; Wu, L.; Chang, L.; Yang, W.; Si, L.; Nan, H.; Peng, W.; Cao, Y. Functionality Developments in Montmorillonite Nanosheet: Properties, Preparation, and Applications. Chem. Eng. J. 2024, 499, 156186. [Google Scholar] [CrossRef]
- Pagacz, J.; Pielichowski, K. Modyfication Of Layeres Silicates For Applications In Nanotechnology. Tech. Transactions. Chem. 2007, 104, 133–147. [Google Scholar]
- Grabowska, B.; Kurleto-Kozioł, Ż. Spectral study (FTIR, UV-Vis) of montmorillonite modified by ultrasound and potassium cations. Trans. Foundry Res. Inst. 2017, 57, 125–136. [Google Scholar]
- Karwal, A.; Kumar, A.; Yadav, A.K. Montmorillonite Revolution: A Unified Exploration of Exfoliation Techniques and Its Emerging Role as an Advanced Drug Delivery Carrier. Appl. Clay Sci. 2025, 273, 107845. [Google Scholar] [CrossRef]
- Yousefi, H.; Fasihi, M.; Rasouli, S. Tailoring Carboxymethyl Cellulose-Based Food Packaging Films Blended with Polyvinyl Alcohol and Nano-MMT for Enhanced Performance and Shelf Life. Cellulose 2025, 32, 999–1015. [Google Scholar] [CrossRef]
- Dudeja, I.; Mankoo, R.K.; Singh, A.; Kaur, J. Citric Acid: An Ecofriendly Cross-Linker for the Production of Functional Biopolymeric Materials. Sustain. Chem. Pharm. 2023, 36, 101307. [Google Scholar] [CrossRef]
- Uyanga, K.A.; Okpozo, O.P.; Onyekwere, O.S.; Daoud, W.A. Citric Acid Crosslinked Natural Bi-Polymer-Based Composite Hydrogels: Effect of Polymer Ratio and Beta-Cyclodextrin on Hydrogel Microstructure. React. Funct. Polym. 2020, 154, 104682. [Google Scholar] [CrossRef]
- Pratinthong, K.; Punyodom, W.; Jantrawut, P.; Jantanasakulwong, K.; Tongdeesoontorn, W.; Sriyai, M.; Panyathip, R.; Thanakkasaranee, S.; Worajittiphon, P.; Tanadchangsaeng, N.; et al. Modification of a Carboxymethyl Cellulose/Poly(Vinyl Alcohol) Hydrogel Film with Citric Acid and Glutaraldehyde Crosslink Agents to Enhance the Anti-Inflammatory Effectiveness of Triamcinolone Acetonide in Wound Healing. Polymers 2024, 16, 1798. [Google Scholar] [CrossRef]
- Thakare, N.R.; Gogoi, P.; Bharali, P.; Hazarika, S. Influence of Copper Ion Cross-Linked CMC-PVA Film on Cell Viability and Cell Proliferation Study. Int. J. Biol. Macromol. 2024, 282 Pt 2, 136645. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, S.M.; Martins, T.; Gonçalves, M.S.; Andrade, R.S.; Dorneles, E.M.S.; Lima, L.C.D.; de Alvarenga, É.L.F.C.; da Fonseca, E.V.B.; et al. Nanosilver-Functionalized Hybrid Hydrogels of Carboxymethyl Cellulose/Poly(Vinyl Alcohol) with Antibacterial Activity for Prevention and Therapy of Infections of Diabetic Chronic Wounds. Polymers 2023, 15, 4542. [Google Scholar] [CrossRef]
- Mapossa, A.B.; da Silva Júnior, A.H.; de Oliveira, C.R.S.; Mhike, W. Thermal, Morphological and Mechanical Properties of Multifunctional Composites Based on Biodegradable Polymers/Bentonite Clay: A Review. Polymers 2023, 15, 3443. [Google Scholar] [CrossRef]
- Weerawan, N.; Chalitangkoon, J.; Monvisade, P. Self-Healing Hydrogels Based on Sodium Carboxymethyl Cellulose/Poly(Vinyl Alcohol) Reinforced with Montmorillonite. Biointerface Res. Appl. Chem. 2022, 12, 4770–4779. [Google Scholar]
- Wrzecionek, M.; Matyszczak, G.; Bandzerewicz, A.; Ruśkowski, P.; Gadomska-Gajadhur, A. Kinetics of Polycondensation of Citric Acid with Glycerol Based on a Genetic Algorithm. Org. Process Res. Dev. 2021, 25, 271–281. [Google Scholar] [CrossRef]
- Joshi, J.; Homburg, S.V.; Ehrmann, A. Atomic Force Microscopy (AFM) on Biopolymers and Hydrogels for Biotechnological Applications—Possibilities and Limits. Polymers 2022, 14, 1267. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, G.; Casas, D.; Cuberes, T. Influence of Glycerol on the Surface Morphology and Crystallinity of Polyvinyl Alcohol Films. Polymers 2024, 16, 2421. [Google Scholar] [CrossRef]
- Kelar, K.; Jurkowski, B.; Mencel, K. Montmorillonite Separated from Bentonite—Its Modification and Potential Use in Anionic Polymerisation of ∊-Caprolactam for Preparation of Nanocomposites. Polimery 2005, 50, 449–454. [Google Scholar] [CrossRef]







| Wavenumber [cm−1] | Characteristic Bond and Movement | Ref. |
|---|---|---|
| 3266 | O–H stretching vibrations | [21,22] |
| 2939, 2908 | stretching vibrations of the –CH2 | [11,18,21,22,24] |
| 2850 | stretching vibrations of the –CH3 | [11] |
| 1710 | stretching vibrations of the C=O bond in ester groups that were formed by CA | [21,22,23] |
| 1641, 1594 | asymmetric vibrations of the COO− group | [11,23] |
| 1415, 1322 | symmetric vibrations of the COO− group | [11,22,23] |
| 1238 | stretching vibrations related to ester bonds | [23] |
| 1142, 1083, 1034 | stretching vibrations of the C–O bond | [11,18,23,24] |
| Composite | 1 × 1 μm | 2 × 2 μm | 5 × 5 μm | 10 × 10 μm | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ra [nm] | Rq [nm] | Rmax [nm] | Ra [nm] | Rq [nm] | Rmax [nm] | Ra [nm] | Rq [nm] | Rmax [nm] | Ra [nm] | Rq [nm] | Rmax [nm] | |
| B5 | 1.798 (0.196) | 2.366 (0.278) | 21.68 (2.167) | 2.47 (0.089) | 3.247 (0.127) | 40.97 (2.702) | 8.18 (0.078) | 10.67 (0.058) | 92.87 (2.04) | 22.43 (0.058) | 28.2 (0.01) | 228 (0.0) |
| B7 | 0.979 (0.091) | 1.297 (0.121) | 15.6 (2.629) | 3.417 (1.606) | 4.27 (1.806) | 33.15 (8.458) | 14.636 (5.670) | 17.775 (6.458) | 118.35 (14.911) | 16.95 (3.748) | 23.45 (4.738) | 263.5 (47.376) |
| C7 | 5.3 (1.788) | 6.797 (2.231) | 51.6 (8.404) | 9.077 (0.985) | 11.567 (1.172) | 88.8 (14.031) | 25.4 (3.559) | 32.7 (4.423) | 235 (36.116) | 27.05 (1.345) | 36.0 (2.121) | 389 (16.971) |
| Composite | CMC [g] | PVA [g] | 0.3% CA [mL] | Glycerin [g] | MMT Concentration [m/v %] |
|---|---|---|---|---|---|
| A1 | 0.25 | 1.00 | 10.00 | 0.00 | 0.00 |
| A2 | 0.25 | 1.00 | 10.00 | 1.00 | 0.00 |
| A3 | 0.25 | 1.00 | 10.00 | 2.00 | 0.00 |
| B5 | 0.25 | 1.00 | 10.00 | 0.00 | 0.25 |
| B7 | 0.25 | 1.00 | 10.00 | 0.00 | 1.00 |
| C5 | 0.25 | 1.00 | 10.00 | 1.00 | 0.25 |
| C7 | 0.25 | 1.00 | 10.00 | 1.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedźwiecka, A.; Brunarska, I.; Adamczyk, A.; Talik, P. Physicochemical Characterization and Structural Tuning of PVA/CMC Composites Modified with Montmorillonite and Glycerol: Toward Sustainable Wound Dressings. Int. J. Mol. Sci. 2025, 26, 11445. https://doi.org/10.3390/ijms262311445
Niedźwiecka A, Brunarska I, Adamczyk A, Talik P. Physicochemical Characterization and Structural Tuning of PVA/CMC Composites Modified with Montmorillonite and Glycerol: Toward Sustainable Wound Dressings. International Journal of Molecular Sciences. 2025; 26(23):11445. https://doi.org/10.3390/ijms262311445
Chicago/Turabian StyleNiedźwiecka, Aleksandra, Irena Brunarska, Anna Adamczyk, and Przemysław Talik. 2025. "Physicochemical Characterization and Structural Tuning of PVA/CMC Composites Modified with Montmorillonite and Glycerol: Toward Sustainable Wound Dressings" International Journal of Molecular Sciences 26, no. 23: 11445. https://doi.org/10.3390/ijms262311445
APA StyleNiedźwiecka, A., Brunarska, I., Adamczyk, A., & Talik, P. (2025). Physicochemical Characterization and Structural Tuning of PVA/CMC Composites Modified with Montmorillonite and Glycerol: Toward Sustainable Wound Dressings. International Journal of Molecular Sciences, 26(23), 11445. https://doi.org/10.3390/ijms262311445





