A Multidimensional Definition of Pre-Osteoarthritis: Toward 21st-Century Subclinical Detection and Targeted Intervention
Abstract
1. Background, Rationale and Objectives
2. Pre-OA as a Distinct Pre-Disease State: Insights from Pre-Diabetes and Pre-Hypertension
3. Structural and Functional Complexity of Articular Cartilage: Implications for Tissue Regeneration and OA Management
4. Challenging Misconceptions and Enhancing Awareness for Early OA Prevention
5. Pre-OA: Defining the Window Before Structural Damage
6. Matrix Degradation-Associated Secretory Endotype: Unveiling Its Latent Effects on Normal Gait
7. Load-Induced Release of ECM Fragments: Mechanisms, Signaling, and Biomarker Potential
8. Breaking the Balance: Ultra-Early Cartilage Dysregulation in Pre-OA
9. Mapping Temporospatial Patterns of Pre-OA Onset and Its Transition to Early OA
9.1. Circadian Oscillations of Chondrocyte Clock Genes and Reciprocal Regulation of Matrix Turnover
9.2. Metabolic Heterogeneity in Cartilage: Implications of the Thick Phenotype for Early Catabolic Onset
9.3. Mechanoinflammatory Cascades and Circadian Dysregulation in Cartilage Catabolic Progression
10. A Tetrahedral Framework for Pre-OA: Integrating Mechanical, Structural, Behavioral, and Circadian Drivers of Early Joint Degeneration
11. Targeting Pre-OA: Multimodal Strategies for Chondro-, Mechano-, and Osmoprotection
11.1. The Cornerstones of Pre-OA Intervention: Modulating Cartilage Metabolic Dysregulation
11.2. Integrating the CADENCE Chrono-Framework into OA Prevention: Rationale and Translational Roadmap
11.3. Should Prophylactic Pharmacotherapy Be Considered in the Pre-OA Window? Opportunities, Challenges, and Translational Potential
11.4. Additional Ethical, Economic, and Societal Considerations in Early OA Prevention Strategies
12. Limitations and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAOS | American Academy of Orthopaedic Surgeons |
| ACAN | Aggrecan |
| ADAMTS | A disintegrin and metalloproteinase with thrombospondin motifs |
| AMPK | AMP-activated protein kinase |
| ARGS | Aggrecanase-generated N-terminal neoepitope |
| ATP | Adenosine triphosphate |
| BHLHE40 | Basic helix–loop–helix family member e40 |
| BMAL1 (ARNTL) | Brain and muscle ARNT-like 1 |
| BML | Bone marrow lesion |
| CADENCE | Chondroprotection Advanced through Deliberate Exercise and Networked Circadian Engagement |
| CD44 | Cluster of differentiation 44 |
| cKO | Conditional knockout (tissue- or time-specific gene deletion) |
| CLOCK | Circadian locomotor output cycles kaput |
| COL2A1 | Type II collagen alpha-1 chain |
| COMP | Cartilage oligomeric matrix protein |
| CRY | Cryptochrome proteins |
| CS | Chondroitin sulfate |
| CTX-II | Cross-linked C-telopeptide fragments of type II collagen |
| DAMPs | Damage-associated molecular patterns |
| DMOADs | Disease-modifying osteoarthritis drugs |
| DNA | Deoxyribonucleic acid |
| DNBs | Dynamic Network Biomarkers |
| ECM | Extracellular matrix |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EMG | Electromyography |
| ERK | Extracellular signal-regulated kinase |
| FN | Fibronectin |
| Fn-fs | Fibronectin fragments |
| Glc | Glucose |
| GS | Glucosamine sulfate |
| HbA1c | Glycated haemoglobin |
| HA | Hyaluronic acid |
| HAPLN1 | Hyaluronan and proteoglycan link protein 1 |
| HELIX-II | Type-II collagen helical peptide |
| HK | Hexokinase |
| HSPA9 | Heat shock protein family A member 9 (mortalin) |
| HSP90 (HSP90AA1/HSP90AB1) | Heat shock protein 90 family |
| HST | Heel-strike transient |
| IGF-1 | Insulin-like growth factor 1 |
| IL-1β | Interleukin-1 beta |
| IL-1RAcP | Interleukin-1 receptor accessory protein |
| iNOS | Inducible nitric oxide synthase |
| iPSC | Induced pluripotent stem cells |
| JAK/STAT3 | Janus kinase/signal transducer and activator of transcription 3 |
| JSN | Joint space narrowing |
| JSW | Joint space width |
| KS-5D4 | KS-5D4 (biomarker/assay designation) |
| LMW-HA | Low-molecular-weight hyaluronan |
| L/P ratio | Lactate/pyruvat ratio |
| MAPK | Mitogen-activated protein kinase |
| MATN1 | Matrilin-1 |
| MDPs | Matrix-derived degradation products |
| MMPs | Matrix metalloproteinases |
| MRI | Magnetic resonance imaging |
| MSM | Methylsulfonylmethane |
| mRNA | Messenger ribonucleic acid |
| MyD88 | Myeloid differentiation primary response 88 |
| NAC | N-acetylcysteine |
| NADH | Nicotinamide adenine dinucleotide |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NFATC2 | Nuclear factor of activated T-cells |
| NIR | Near-Infrared |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 inflammasome |
| NR1D1 (REV-ERBα) | Nuclear receptor subfamily 1 group D member 1 |
| OA | Osteoarthritis |
| OARSI | Osteoarthritis Research Society International |
| OXPHOS | Oxidative phosphorylation |
| PAPS | 3′-phosphoadenosine-5′-phosphosulfate |
| PER | Period circadian proteins |
| PER2::LUC | PER2 luciferase reporter |
| sPIIANP | Procollagen type IIA N-terminal propeptide (serum PIIANP) |
| PRG4 | Proteoglycan 4 |
| PKM | Pyruvate kinase M |
| PLOD | Procollagen-lysine, 2-oxoglutarate 5-dioxygenase |
| PRP | Platelet-rich plasma |
| PRR | Pattern recognition receptor |
| PSMB/PSMD | Proteasome subunits B and D family |
| PTOA | Post-traumatic osteoarthritis |
| pre-OA | pre-osteoarthritis |
| RA | Rheumatoid arthritis |
| RNA | Ribonucleic acid |
| RNS | Reactive nitrogen species |
| RPL | Ribosomal protein |
| ROI | Regions of interest |
| ROM | Range of motion |
| RORA (RORα) | Retinoic acid-related orphan receptor alpha |
| ROS | Reactive oxygen species |
| SASP | Senescence-associated secretory phenotype |
| SCN | Suprachiasmatic nucleus |
| sDNB | single-sample Dynamic Network Biomarker |
| SERPINE1 | Serpin family E member 1 (plasminogen activator inhibitor-1, PAI-1) |
| SCS | Stagnant cartilage syndrome |
| SLC2A1 (GLUT1) | Solute carrier family 2 member 1 (glucose transporter 1, GLUT1) |
| SOX9 | SRY-box transcription factor 9 |
| SWIR | Short-wave infrared |
| TCA cycle | Tricarboxylic acid (Krebs) cycle |
| TGF-β | Transforming growth factor-beta |
| TIMP | Tissue inhibitor of metalloproteinases |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-alpha |
| TNFR1/2 | Tumor necrosis factor receptors 1 and 2 |
| UTE | Ultrashort echo time |
| vGRF | Vertical ground reaction force |
References
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef] [PubMed]
- del Río, E. 2050: An arthroplasty odyssey. Healthcare 2025, 13, 2730. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, C.; Oo, W.M.; Fu, K.; Risberg, M.A.; Bierma-Zeinstra, S.M.; Neogi, T.; Atukorala, I.; Malfait, A.M.; Ding, C.; et al. Osteoarthritis. Nat. Rev. Dis. Primers 2025, 11, 10. [Google Scholar] [CrossRef]
- Coleman, L.J.; Byrne, J.L.; Edwards, S.; O’Hara, R. Advancing early detection of osteoarthritis through biomarker profiling and predictive modelling: A review. Biologics 2025, 5, 27. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Im, G.I. The concept of early osteoarthritis and its significance in regenerative medicine. Tissue Eng. Regen. Med. 2022, 19, 431–436. [Google Scholar] [CrossRef]
- Ratneswaran, A.; Rockel, J.S.; Kapoor, M. Understanding osteoarthritis pathogenesis: A multiomics system-based approach. Curr. Opin. Rheumatol. 2020, 32, 80–91. [Google Scholar] [CrossRef]
- O’Brien, K.; Tailor, P.; Leonard, C.; DiFrancesco, L.M.; Hart, D.A.; Matyas, J.R.; Frank, C.B.; Krawetz, R.J. Enumeration and localization of mesenchymal progenitor cells and macrophages in synovium from normal individuals and patients with pre-osteoarthritis or clinically diagnosed osteoarthritis. Int. J. Mol. Sci. 2017, 18, 774. [Google Scholar] [CrossRef]
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; Di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst century: Risk factors and behaviours that influence disease onset and progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H.A. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef]
- Mehana, E.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Sengprasert, P.; Waitayangkoon, P.; Kamenkit, O.; Sawatpanich, A.; Chaichana, T.; Wongphoom, J.; Ngarmukos, S.; Taweevisit, M.; Lotinun, S.; Tumwasorn, S.; et al. Catabolic mediators from TLR2-mediated proteoglycan aggrecan peptide-stimulated chondrocytes are reduced by Lactobacillus-conditioned media. Sci. Rep. 2024, 14, 18043. [Google Scholar] [CrossRef]
- Batarfi, W.A.; Yunus, M.H.M.; Hamid, A.A.; Maarof, M.; Abdul Rani, R. Breaking down osteoarthritis: Exploring inflammatory and mechanical signaling pathways. Life 2025, 15, 1238. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Karsdal, M.A. Clinical monitoring in osteoarthritis: Biomarkers. Osteoarthr. Cartil. 2022, 30, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Sun, S.; Reed, A.; Soderblom, E.J.; Moseley, M.A.; Zhou, K.; Jain, V.; Arden, N.; Li, Y.J. An osteoarthritis pathophysiological continuum revealed by molecular biomarkers. Sci. Adv. 2024, 10, eadj6814. [Google Scholar] [CrossRef]
- Cope, P.J.; Ourradi, K.; Li, Y.; Sharif, M. Models of osteoarthritis: The good, the bad and the promising. Osteoarthr. Cartil. 2019, 27, 230–239. [Google Scholar] [CrossRef]
- Mukherjee, S.; Nazemi, M.; Jonkers, I.; Geris, L. Use of computational modeling to study joint degeneration: A review. Front. Bioeng. Biotechnol. 2020, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Ryd, L.; Brittberg, M.; Eriksson, K.; Jurvelin, J.S.; Lindahl, A.; Marlovits, S.; Moller, P.; Richardson, J.B.; Steinwachs, M.; Zenobi-Wong, M. Pre-osteoarthritis: Definition and diagnosis of an elusive clinical entity. Cartilage 2015, 6, 156–165. [Google Scholar] [CrossRef]
- Allen, K.D.; Choong, P.F.; Davis, A.M.; Dowsey, M.M.; Dziedzic, K.S.; Emery, C.; Hunter, D.J.; Losina, E.; Page, A.E.; Roos, E.M.; et al. Osteoarthritis: Models for appropriate care across the disease continuum. Best Pract. Res. Clin. Rheumatol. 2016, 30, 503–535. [Google Scholar] [CrossRef]
- Lv, Z.; Yang, Y.X.; Li, J.; Fei, Y.; Guo, H.; Sun, Z.; Lu, J.; Xu, X.; Jiang, Q.; Ikegawa, S.; et al. Molecular classification of knee osteoarthritis. Front. Cell Dev. Biol. 2021, 9, 725568. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Runhaar, J.; Bierma-Zeinstra, S.; Roos, E.M. A lifespan approach to osteoarthritis prevention. Osteoarthr. Cartil. 2021, 29, 1638–1653. [Google Scholar] [CrossRef]
- Hatzikotoulas, K.; Southam, L.; Stefansdottir, L.; Boer, C.G.; McDonald, M.L.; Pett, J.P.; Park, Y.C.; Tuerlings, M.; Mulders, R.; Barysenka, A.; et al. Translational genomics of osteoarthritis in 1,962,069 individuals. Nature 2025, 641, 1217–1224. [Google Scholar] [CrossRef]
- Oshima, Y.; Haruki, T.; Koizumi, K.; Yonezawa, S.; Taketani, A.; Kadowaki, M.; Saito, S. Practices, potential, and perspectives for detecting predisease using Raman spectroscopy. Int. J. Mol. Sci. 2023, 24, 12170. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, B.; Marchi, G.; Alberton, P.; Farkas, Z.; Aszodi, A.; Roths, J.; Clausen-Schaumann, H. Early detection of cartilage degeneration: A comparison of histology, fiber bragg grating-based micro-indentation, and atomic force microscopy-based nano-indentation. Int. J. Mol. Sci. 2020, 21, 7384. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Zhu, W.; Terai, Y.; Marin, E.; Boschetto, F.; Kawamoto, K.; Itaka, K. Raman spectroscopic insight into osteoarthritic cartilage regeneration by mRNA therapeutics encoding cartilage-anabolic transcription factor Runx1. Mater. Today Bio 2022, 13, 100210. [Google Scholar] [CrossRef]
- Hunter, D.J.; Collins, J.E.; Deveza, L.; Hoffmann, S.C.; Kraus, V.B. Biomarkers in osteoarthritis: Current status and outlook—The FNIH Biomarkers Consortium PROGRESS OA study. Skelet. Radiol. 2023, 52, 2323–2339. [Google Scholar] [CrossRef] [PubMed]
- Bay-Jensen, A.C.; Mobasheri, A.; Thudium, C.S.; Kraus, V.B.; Karsdal, M.A. Blood and urine biomarkers in osteoarthritis—An update on cartilage associated type II collagen and aggrecan markers. Curr. Opin. Rheumatol. 2022, 34, 54–60. [Google Scholar] [CrossRef]
- Viera, A.J. Predisease: When does it make sense? Epidemiol. Rev. 2011, 33, 122–134. [Google Scholar] [CrossRef]
- Chen, L.; Liu, R.; Liu, Z.P.; Li, M.; Aihara, K. Detecting early-warning signals for sudden deterioration of complex diseases by dynamical network biomarkers. Sci. Rep. 2012, 2, 342. [Google Scholar] [CrossRef]
- Liu, R.; Li, M.; Liu, Z.P.; Wu, J.; Chen, L.; Aihara, K. Identifying critical transitions and their leading biomolecular networks in complex diseases. Sci. Rep. 2012, 2, 813. [Google Scholar] [CrossRef]
- Liu, X.; Chang, X.; Liu, R.; Yu, X.; Chen, L.; Aihara, K. Quantifying critical states of complex diseases using single-sample dynamic network biomarkers. PLoS Comput. Biol. 2017, 13, e1005633. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S27–S49. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; Zhao, X.M.; Chen, L. Detecting early-warning signals of type 1 diabetes and its leading biomolecular networks by dynamical network biomarkers. BMC Med. Genom. 2013, 6 (Suppl. 2), S8. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.M.; Stevens-Fabry, S. Prehypertension—Prevalence, health risks, and management strategies. Nat. Rev. Cardiol. 2015, 12, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Haruki, T.; Yonezawa, S.; Koizumi, K.; Yoshida, Y.; Watanabe, T.M.; Fujita, H.; Oshima, Y.; Oku, M.; Taketani, A.; Yamazaki, M.; et al. Application of the dynamical network biomarker theory to Raman spectra. Biomolecules 2022, 12, 1730. [Google Scholar] [CrossRef]
- Aihara, K.; Liu, R.; Koizumi, K.; Liu, X.; Chen, L. Dynamical network biomarkers: Theory and applications. Gene 2022, 808, 145997. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.R.; Beynnon, B.D.; Buckwalter, J.A.; Garrett, W.E., Jr.; Katz, J.N.; Rodeo, S.A.; Spindler, K.P.; Stanton, R.A. Closing the gap between bench and bedside research for early arthritis therapies (EARTH): Report from the AOSSM/NIH U-13 Post-Joint Injury Osteoarthritis Conference II. Am. J. Sports Med. 2011, 39, 1569–1578. [Google Scholar] [CrossRef]
- Chu, C.R.; Williams, A.A.; Coyle, C.H.; Bowers, M.E. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res. Ther. 2012, 14, 212. [Google Scholar] [CrossRef]
- Chu, C.R.; Millis, M.B.; Olson, S.A. Osteoarthritis: From palliation to prevention: AOA critical issues. J. Bone Jt. Surg. Am. 2014, 96, e130. [Google Scholar] [CrossRef]
- Chu, C.R.; Andriacchi, T.P. Dance between biology, mechanics, and structure: A systems-based approach to developing osteoarthritis prevention strategies. J. Orthop. Res. 2015, 33, 939–947. [Google Scholar] [CrossRef]
- Edd, S.N.; Favre, J.; Blazek, K.; Omoumi, P.; Asay, J.L.; Andriacchi, T.P. Altered gait mechanics and elevated serum pro-inflammatory cytokines in asymptomatic patients with MRI evidence of knee cartilage loss. Osteoarthr. Cartil. 2017, 25, 899–906. [Google Scholar] [CrossRef]
- Chu, C.R.; Williams, A.A.; Erhart-Hledik, J.C.; Titchenal, M.R.; Qian, Y.; Andriacchi, T.P. Visualizing pre-osteoarthritis: Integrating MRI UTE-T2* with mechanics and biology to combat osteoarthritis—The 2019 Elizabeth Winston Lanier Kappa Delta Award. J. Orthop. Res. 2021, 39, 1585–1595. [Google Scholar] [CrossRef]
- Williams, A.A.; Koltsov, J.C.B.; Brett, A.; He, J.; Chu, C.R. Using 3D MRI bone shape to predict pre-osteoarthritis of the knee 2 years after anterior cruciate ligament reconstruction. Am. J. Sports Med. 2023, 51, 3677–3686. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.R.; Hochberg, M.; White, D.; Rodeo, S.; Huard, J.; Shapiro, S.; Lattermann, C.; Guilak, F. Transformative approaches for effective clinical trials to reduce the disease burden of osteoarthritis. Semin. Arthritis Rheum. 2025, 71, 152652. [Google Scholar] [CrossRef]
- Hawker, G.A.; King, L.K.; Liew, J.W.; Wang, Q.; Mahmoudian, A.; Jansen, N.E.J.; Stanaitis, I.; Berenbaum, F.; Das, S.; Ding, C.; et al. OARSI initiative to develop classification criteria for early-stage symptomatic knee OA (EsSKOA): What conditions should be considered in the differential diagnosis of EsSKOA? Osteoarthr. Cartil. 2025, 33, 1141–1146. [Google Scholar] [CrossRef]
- Rocha, F.A.C.; Ali, S.A. Soluble biomarkers in osteoarthritis in 2022: Year in review. Osteoarthr. Cartil. 2023, 31, 167–176. [Google Scholar] [CrossRef]
- Kok, Y.E.; Crisford, A.; Parkes, A.; Venkateswaran, S.; Oreffo, R.; Mahajan, S.; Pound, M. Classification of osteoarthritic and healthy cartilage using deep learning with Raman spectra. Sci. Rep. 2024, 14, 15902. [Google Scholar] [CrossRef]
- Mow, V.C.; Ratcliffe, A.; Poole, A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials 1992, 13, 67–97. [Google Scholar] [CrossRef]
- Mow, V.C.; Guo, X.E. Mechano-electrochemical properties of articular cartilage: Their inhomogeneities and anisotropies. Annu. Rev. Biomed. Eng. 2002, 4, 175–209. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.P. Articular cartilage repair. Am. J. Sports Med. 1998, 26, 309–324. [Google Scholar] [CrossRef]
- Cederlund, A.A.; Aspden, R.M. Walking on water: Revisiting the role of water in articular cartilage biomechanics in relation to tissue engineering and regenerative medicine. J. R. Soc. Interface 2022, 19, 20220364. [Google Scholar] [CrossRef]
- Oegema, T.R.; Carpenter, R.J.; Hofmeister, F.; Thompson, R.C. The interaction of the zone of calcified cartilage and subchondral bone in osteoarthritis. Microsc. Res. Tech. 1997, 37, 324–332. [Google Scholar] [CrossRef]
- Goldring, M.B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 269–285. [Google Scholar] [CrossRef]
- Javaheri, B.; Caetano-Silva, S.P.; Kanakis, I.; Bou-Gharios, G.; Pitsillides, A.A. The chondro-osseous continuum: Is it possible to unlock the potential assigned within? Front. Bioeng. Biotechnol. 2018, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Nichols, K.; Srivastava, A.; Weiss, D.J.; Eldridge, P.; Cuende, N.; Deans, R.J.; Rasko, J.E.; Levine, A.D.; Turner, L.; et al. Positioning a scientific community on unproven cellular therapies: The 2015 International Society for Cellular Therapy perspective. Cytotherapy 2015, 17, 1663–1666. [Google Scholar] [CrossRef]
- Sipp, D.; Caulfield, T.; Kaye, J.; Barfoot, J.; Blackburn, C.; Chan, S.; De Luca, M.; Kent, A.; McCabe, C.; Munsie, M.; et al. Marketing of unproven stem cell-based interventions: A call to action. Sci. Transl. Med. 2017, 9, eaag0426. [Google Scholar] [CrossRef]
- Chu, C.R.; Rodeo, S.; Bhutani, N.; Goodrich, L.R.; Huard, J.; Irrgang, J.; LaPrade, R.F.; Lattermann, C.; Lu, Y.; Mandelbaum, B.; et al. Optimizing clinical use of biologics in orthopaedic surgery: Consensus recommendations from the 2018 AAOS/NIH U-13 conference. J. Am. Acad. Orthop. Surg. 2019, 27, e50–e63. [Google Scholar] [CrossRef]
- Lammi, M.J.; Piltti, J.; Prittinen, J.; Qu, C. Challenges in fabrication of tissue-engineered cartilage with correct cellular colonization and extracellular matrix assembly. Int. J. Mol. Sci. 2018, 19, 2700. [Google Scholar] [CrossRef]
- Nukavarapu, S.P.; Dorcemus, D.L. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol. Adv. 2013, 31, 706–721. [Google Scholar] [CrossRef]
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioact. Mater. 2021, 6, 4830–4855. [Google Scholar] [CrossRef]
- Morouço, P.; Fernandes, C.; Lattanzi, W. Challenges and innovations in osteochondral regeneration: Insights from biology and inputs from bioengineering toward the optimization of tissue engineering strategies. J. Funct. Biomater. 2021, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Mennan, C.; Hopkins, T.; Channon, A.; Elliott, M.; Johnstone, B.; Kadir, T.; Loughlin, J.; Peffers, M.; Pitsillides, A.; Sofat, N.; et al. The use of technology in the subcategorisation of osteoarthritis: A Delphi study approach. Osteoarthr. Cartil. Open 2020, 2, 100081. [Google Scholar] [CrossRef] [PubMed]
- Darlow, B.; Brown, M.; Thompson, B.; Hudson, B.; Grainger, R.; McKinlay, E.; Abbott, J.H. Living with osteoarthritis is a balancing act: An exploration of patients’ beliefs about knee pain. BMC Rheumatol. 2018, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.; Holm, P.M.; Bricca, A.; Dideriksen, M.; Tang, L.H.; Skou, S.T. Clinicians’ beliefs and attitudes to physical activity and exercise therapy as treatment for knee and/or hip osteoarthritis: A scoping review. Osteoarthr. Cartil. 2022, 30, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; McAlindon, T.E.; Anderson, J.J.; Naimark, A.; Weissman, B.W.; Aliabadi, P.; Evans, S.; Levy, D.; LaValley, M.P. Defining radiographic osteoarthritis for the whole knee. Osteoarthr. Cartil. 1997, 5, 241–250. [Google Scholar] [CrossRef]
- Herrero-Manley, L.; Alabajos-Cea, A.; Suso-Martí, L.; Viosca-Herrero, E.; Vazquez-Arce, I. Early knee osteoarthritis classification and clinical evolution: A longitudinal observational pilot study. Biomedicines 2023, 11, 1670. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Sharma, A.R.; Chakraborty, C.; Saibaba, B.; Ahn, M.E.; Lee, S.S. Review of prospects of biological fluid biomarkers in osteoarthritis. Int. J. Mol. Sci. 2017, 18, 601. [Google Scholar] [CrossRef]
- Mobasheri, A.; Bay-Jensen, A.C.; Gualillo, O.; Larkin, J.; Levesque, M.C.; Henrotin, Y. Soluble biochemical markers of osteoarthritis: Are we close to using them in clinical practice? Best Pract. Res. Clin. Rheumatol. 2017, 31, 705–720. [Google Scholar] [CrossRef]
- Buck, R.J.; Wyman, B.T.; Le Graverand, M.P.; Hudelmaier, M.; Wirth, W.; Eckstein, F. Osteoarthritis may not be a one-way-road of cartilage loss—Comparison of spatial patterns of cartilage change between osteoarthritic and healthy knees. Osteoarthr. Cartil. 2010, 18, 329–335. [Google Scholar] [CrossRef]
- Bay-Jensen, A.C.; Hoegh-Madsen, S.; Dam, E.; Henriksen, K.; Sondergaard, B.C.; Pastoureau, P.; Qvist, P.; Karsdal, M.A. Which elements are involved in reversible and irreversible cartilage degradation in osteoarthritis? Rheumatol. Int. 2010, 30, 435–442. [Google Scholar] [CrossRef]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef]
- Chu, C.R. Defining pre-osteoarthritis is key to prevention. Cartilage 2016, 7, 204. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Schjerling, P.; Heinemeier, J.; Møller, M.B.; Krogsgaard, M.R.; Grum-Schwensen, T.; Petersen, M.M.; Kjaer, M. Radiocarbon dating reveals minimal collagen turnover in both healthy and osteoarthritic human cartilage. Sci. Transl. Med. 2016, 8, 346ra90. [Google Scholar] [CrossRef]
- Joseph, G.B.; Nevitt, M.C.; McCulloch, C.E.; Neumann, J.; Lynch, J.A.; Heilmeier, U.; Lane, N.E.; Link, T.M. Associations between molecular biomarkers and MR-based cartilage composition and knee joint morphology: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2018, 26, 1070–1077. [Google Scholar] [CrossRef]
- Kalogera, S.; Jansen, M.P.; Bay-Jensen, A.C.; Frederiksen, P.; Karsdal, M.A.; Thudium, C.S.; Mastbergen, S.C. Relevance of biomarkers in serum vs. synovial fluid in patients with knee osteoarthritis. Int. J. Mol. Sci. 2023, 24, 9483. [Google Scholar] [CrossRef]
- Styrkarsdottir, U.; Lund, S.H.; Thorleifsson, G.; Saevarsdottir, S.; Gudbjartsson, D.F.; Thorsteinsdottir, U.; Stefansson, K. Cartilage acidic protein 1 in plasma associates with prevalent osteoarthritis and predicts future risk as well as progression to joint replacements: Results from the UK Biobank resource. Arthritis Rheumatol. 2023, 75, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Jindal, D.; Khanna, R. sCTX II is a better biomarker than sMMP-3 to identify early knee osteoarthritis. J. Orthop. Res. 2023, 41, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, W.; Liu, Y.; Lu, Y.; Liu, M.; Cao, X.; Guo, D. Exploring biomarkers associated with severity of knee osteoarthritis in Southern China using widely targeted metabolomics. BMC Musculoskelet. Disord. 2023, 24, 953. [Google Scholar] [CrossRef]
- Mobasheri, A.; Thudium, C.S.; Bay-Jensen, A.C.; Maleitzke, T.; Geissler, S.; Duda, G.N.; Winkler, T. Biomarkers for osteoarthritis: Current status and future prospects. Best Pract. Res. Clin. Rheumatol. 2023, 37, 101852. [Google Scholar] [CrossRef]
- Kraus, V.B.; Hsueh, M.F. Molecular biomarker approaches to prevention of post-traumatic osteoarthritis. Nat. Rev. Rheumatol. 2024, 20, 272–289. [Google Scholar] [CrossRef] [PubMed]
- Armitano-Lago, C.; Evans-Pickett, A.; Davis-Wilson, H.; Munsch, A.; Longobardi, L.; Willcockson, H.; Schwartz, T.A.; Franz, J.R.; Pietrosimone, B. Modifying loading during gait leads to biochemical changes in serum cartilage oligomeric matrix protein concentrations in a subgroup of individuals with anterior cruciate ligament reconstruction. Clin. Rheumatol. 2024, 43, 1363–1373. [Google Scholar] [CrossRef]
- Baran, K.; Czechowska, A.; Kopacz, K.; Padula, G.; Migdalska-Sęk, M.; Tomaszewski, W.; Nowak, K.; Domżalski, M.; Brzeziańska-Lasota, E. MMP13 mRNA expression level as a potential marker for knee OA progression—An observational study. J. Clin. Med. 2025, 14, 1263. [Google Scholar] [CrossRef]
- Dreiner, M.; Godonou, E.T.; Mündermann, A.; Tascilar, K.; Schett, G.; Zaucke, F.; Liphardt, A.M.; Niehoff, A. Immobilization by 21-days of bed rest causes changes in biomarkers of cartilage homeostasis in healthy individuals. Osteoarthr. Cartil. Open 2025, 7, 100597. [Google Scholar] [CrossRef]
- Zibetti, M.V.W.; Menon, R.G.; de Moura, H.L.; Zhang, X.; Kijowski, R.; Regatte, R.R. Updates on compositional MRI mapping of the cartilage: Emerging techniques and applications. J. Magn. Reson. Imaging 2023, 58, 44–60. [Google Scholar] [CrossRef]
- Kaur, B.; Rana, D.; Sharma, R.; Konar, M.; Dhillon, M.S.; Chouhan, D.K.; Saini, U.C.; Prakash, M.; Arora, A.; Verma, I.; et al. Proteomic insights into early detection and progression of knee osteoarthritis: Unveiling molecular signatures. Arch. Med. Res. 2025, 56, 103206. [Google Scholar] [CrossRef]
- Gardner, D.L. The nature and causes of osteoarthrosis. Br. Med. J. 1983, 286, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Radin, E.L.; Yang, K.H.; Riegger, C.; Kish, V.L.; O’Connor, J.J. Relationship between lower limb dynamics and knee joint pain. J. Orthop. Res. 1991, 9, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Houard, X.; Goldring, M.B.; Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 2013, 15, 375. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Molnar, V.; Rod, E.; Jelec, Z.; Cukelj, F.; Matisic, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Boric, I. Knee osteoarthritis: A review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef] [PubMed]
- Terkawi, M.A.; Ebata, T.; Yokota, S.; Takahashi, D.; Endo, T.; Matsumae, G.; Shimizu, T.; Kadoya, K.; Iwasaki, N. Low-grade inflammation in the pathogenesis of osteoarthritis: Cellular and molecular mechanisms and strategies for future therapeutic intervention. Biomedicines 2022, 10, 1109. [Google Scholar] [CrossRef]
- Man, G.S.; Mologhianu, G. Osteoarthritis pathogenesis—A complex process that involves the entire joint. J. Med. Life 2014, 7, 37–41. [Google Scholar]
- Akagi, K.; Koizumi, K.; Kadowaki, M.; Kitajima, I.; Saito, S. New possibilities for evaluating the development of age-related pathologies using the dynamical network biomarkers theory. Cells 2023, 12, 2297. [Google Scholar] [CrossRef]
- Thorstensson, C.A.; Gooberman-Hill, R.; Adamson, J.; Williams, S.; Dieppe, P. Help-seeking behaviour among people living with chronic hip or knee pain in the community. BMC Musculoskelet. Disord. 2009, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Coutinho de Almeida, R.; Mahfouz, A.; Mei, H.; Houtman, E.; den Hollander, W.; Soul, J.; Suchiman, E.; Lakenberg, N.; Meessen, J.; Huetink, K.; et al. Identification and characterization of two consistent osteoarthritis subtypes by transcriptome and clinical data integration. Rheumatology 2021, 60, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.A. Osteoarthritis as an umbrella term for different subsets of humans undergoing joint degeneration: The need to address the differences to develop effective conservative treatments and prevention strategies. Int. J. Mol. Sci. 2022, 23, 15365. [Google Scholar] [CrossRef]
- Zhang, M.; Theleman, J.L.; Lygrisse, K.A.; Wang, J. Epigenetic mechanisms underlying the aging of articular cartilage and osteoarthritis. Gerontology 2019, 65, 387–396. [Google Scholar] [CrossRef]
- Mobasheri, A.; van Spil, W.E.; Budd, E.; Uzieliene, I.; Bernotiene, E.; Bay-Jensen, A.C.; Larkin, J.; Levesque, M.C.; Gualillo, O.; Henrotin, Y. Molecular taxonomy of osteoarthritis for patient stratification, disease management and drug development: Biochemical markers associated with emerging clinical phenotypes and molecular endotypes. Curr. Opin. Rheumatol. 2019, 31, 80–89. [Google Scholar] [CrossRef]
- Deveza, L.A.; Nelson, A.E.; Loeser, R.F. Phenotypes of osteoarthritis: Current state and future implications. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 64–72. [Google Scholar]
- Eisenberg, L. Disease and illness. Distinctions between professional and popular ideas of sickness. Cult. Med. Psychiatry 1977, 1, 9–23. [Google Scholar] [CrossRef]
- Caplan, A.L.; McCartney, J.J.; Sisti, D.A. Health, Disease, and Illness: Concepts in Medicine; Georgetown University Press: Washington, DC, USA, 2004. [Google Scholar]
- Deshpande, B.R.; Katz, J.N.; Solomon, D.H.; Yelin, E.H.; Hunter, D.J.; Messier, S.P.; Suter, L.G.; Losina, E. Number of persons with symptomatic knee osteoarthritis in the US: Impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res. 2016, 68, 1743–1750. [Google Scholar] [CrossRef]
- Brown, T.D.; Johnston, R.C.; Saltzman, C.L.; Marsh, J.L.; Buckwalter, J.A. Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma 2006, 20, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Mankin, H.J.; Lippiello, L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J. Bone Jt. Surg. Am. 1970, 52, 424–434. [Google Scholar] [CrossRef]
- Mankin, H.J.; Dorfman, H.; Lippiello, L.; Zarins, A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Jt. Surg. Am. 1971, 53, 523–537. [Google Scholar] [CrossRef]
- Khella, C.M.; Horvath, J.M.; Asgarian, R.; Rolauffs, B.; Hart, M.L. Anti-inflammatory therapeutic approaches to prevent or delay post-traumatic osteoarthritis (PTOA) of the knee joint with a focus on sustained delivery approaches. Int. J. Mol. Sci. 2021, 22, 8005. [Google Scholar] [CrossRef]
- Chow, Y.Y.; Chin, K.Y. The role of inflammation in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef]
- Maldonado, M.; Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. BioMed Res. Int. 2013, 2013, 284873. [Google Scholar] [CrossRef]
- Hosseininia, S.; Lindberg, L.R.; Dahlberg, L.E. Cartilage collagen damage in hip osteoarthritis similar to that seen in knee osteoarthritis; a case-control study of relationship between collagen, glycosaminoglycan and cartilage swelling. BMC Musculoskelet. Disord. 2013, 14, 18. [Google Scholar] [CrossRef]
- Gottardi, R.; Hansen, U.; Raiteri, R.; Loparic, M.; Duggelin, M.; Mathys, D.; Friederich, N.F.; Bruckner, P.; Stolz, M. Supramolecular organization of collagen fibrils in healthy and osteoarthritic human knee and hip joint cartilage. PLoS ONE 2016, 11, e0163552. [Google Scholar] [CrossRef]
- Sharma, A.; Jagga, S.; Lee, S.-S.; Nam, J.-S. Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int. J. Mol. Sci. 2013, 14, 19805–19830. [Google Scholar] [CrossRef]
- Kim, J.R.; Yoo, J.J.; Kim, H.A. Therapeutics in osteoarthritis based on an understanding of its molecular pathogenesis. Int. J. Mol. Sci. 2018, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Goldring, M.B. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin. Orthop. Relat. Res. 2004, 427, S27–S36. [Google Scholar] [CrossRef]
- Troeberg, L.; Nagase, H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim. Biophys. Acta 2011, 1824, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, T.; Yuh, C.; Wimmer, M.A.; Schmid, T.M.; Espinosa-Marzal, R.M. Nanoscale insight into the degradation mechanisms of the cartilage articulating surface preceding OA. Biomater. Sci. 2020, 8, 3944–3955. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.T.; Neu, C.P.; Drissi, H.; Emery, N.C.; Pierce, D.M. Cyclic loading of human articular cartilage: The transition from compaction to fatigue. J. Mech. Behav. Biomed. Mater. 2017, 65, 734–742. [Google Scholar] [CrossRef]
- Moo, E.K.; Tanska, P.; Federico, S.; Al-Saffar, Y.; Herzog, W.; Korhonen, R.K. Collagen fibres determine the crack morphology in articular cartilage. Acta Biomater. 2021, 126, 301–314. [Google Scholar] [CrossRef]
- Tschaikowsky, M.; Brander, S.; Barth, V.; Thomann, R.; Rolauffs, B.; Balzer, B.N.; Hugel, T. The articular cartilage surface is impaired by a loss of thick collagen fibers and formation of type I collagen in early osteoarthritis. Acta Biomater. 2022, 146, 274–283. [Google Scholar] [CrossRef]
- Verzijl, N.; DeGroot, J.; Thorpe, S.R.; Bank, R.A.; Shaw, J.N.; Lyons, T.J.; Bijlsma, J.W.J.; Lafeber, F.P.J.G.; Baynes, J.W.; TeKoppele, J.M. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 2000, 275, 39027–39031. [Google Scholar] [CrossRef]
- Felson, D.T.; Hodgson, R. Identifying and treating pre-clinical and early osteoarthritis. Rheum. Dis. Clin. N. Am. 2014, 40, 699–710. [Google Scholar] [CrossRef]
- Madry, H.; Kon, E.; Condello, V.; Peretti, G.M.; Steinwachs, M.; Seil, R.; Berruto, M.; Engebretsen, L.; Filardo, G.; Angele, P. Early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1753–1762. [Google Scholar] [CrossRef]
- Lohmander, L.S.; Atley, L.M.; Pietka, T.A.; Eyre, D.R. The release of crosslinked peptides from type II collagen into human synovial fluid is increased soon after joint injury and in osteoarthritis. Arthritis Rheum. 2003, 48, 3130–3139. [Google Scholar] [CrossRef]
- Quintana, D.J.; Garnero, P.; Huebner, J.L.; Charni-Ben Tabassi, N.; Kraus, V.B. PIIANP and HELIXII diurnal variation. Osteoarthr. Cartil. 2008, 16, 1192–1195. [Google Scholar] [CrossRef]
- Guilak, F. Biomechanical factors in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 815–823. [Google Scholar] [CrossRef]
- Williams, P.T. Effects of running and walking on osteoarthritis and hip replacement risk. Med. Sci. Sports Exerc. 2013, 45, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Henao-Murillo, L.; Pastrama, M.I.; Ito, K.; van Donkelaar, C.C. The relationship between proteoglycan loss, overloading-induced collagen damage, and cyclic loading in articular cartilage. Cartilage 2021, 13, 1501S–1512S. [Google Scholar] [CrossRef]
- Simon, S.R.; Paul, I.L.; Mansour, J.; Munro, M.; Abernethy, P.J.; Radin, E.L. Peak dynamic force in human gait. J. Biomech. 1981, 14, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Berger, W.; Dietz, V.; Quintern, J. Corrective reactions to stumbling in man: Neuronal co-ordination of bilateral leg muscle activity during gait. J. Physiol. 1984, 357, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.J.; Whittle, M.W. Impulsive forces during walking and their clinical implications. Clin. Biomech. 1989, 4, 179–187. [Google Scholar] [CrossRef]
- Whittle, M.W. Generation and attenuation of transient impulsive forces beneath the foot: A review. Gait Posture 1999, 10, 264–275. [Google Scholar] [CrossRef]
- Verdini, F.; Marcucci, M.; Benedetti, M.G.; Leo, T. Identification and characterisation of heel strike transient. Gait Posture 2006, 24, 77–84. [Google Scholar] [CrossRef]
- Liikavainio, T.; Isolehto, J.; Helminen, H.J.; Perttunen, J.; Lepola, V.; Kiviranta, I.; Arokoski, J.P.A.; Komi, P.V. Loading and gait symmetry during level and stair walking in asymptomatic subjects with knee osteoarthritis: Importance of quadriceps femoris in reducing impact force during heel strike? Knee 2007, 14, 231–238. [Google Scholar] [CrossRef]
- Zelik, K.E.; Kuo, A.D. Human walking isn’t all hard work: Evidence of soft tissue contributions to energy dissipation and return. J. Exp. Biol. 2010, 213, 4257–4264. [Google Scholar] [CrossRef]
- Collins, A.; Blackburn, J.T.; Olcott, C.; Yu, B.; Weinhold, P. The impact of stochastic resonance electrical stimulation and knee sleeve on impulsive loading and muscle co-contraction during gait in knee osteoarthritis. Clin. Biomech. 2011, 26, 853–858. [Google Scholar] [CrossRef]
- Nilsson, J.; Thorstensson, A. Ground reaction forces at different speeds of human walking and running. Acta Physiol. Scand. 1989, 136, 217–227. [Google Scholar] [CrossRef]
- Jefferson, R.J.; Collins, J.J.; Whittle, M.W.; Radin, E.L.; O’Connor, J.J. The role of the quadriceps in controlling impulsive forces around heel strike. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1990, 204, 21–28. [Google Scholar] [CrossRef]
- Andriacchi, T.P. Dynamics of knee malalignment. Orthop. Clin. N. Am. 1994, 25, 395–403. [Google Scholar] [CrossRef]
- Maly, M.R. Abnormal and cumulative loading in knee osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 547–552. [Google Scholar] [CrossRef]
- Lieberman, D.E.; Venkadesan, M.; Werbel, W.A.; Daoud, A.I.; D’Andrea, S.; Davis, I.S.; Mang’Eni, R.O.; Pitsiladis, Y. Foot strike patterns and collision forces in habitually barefoot versus shod runners. Nature 2010, 463, 531–535. [Google Scholar] [CrossRef]
- Fu, W.; Fang, Y.; Gu, Y.; Huang, L.; Li, L.; Liu, Y. Shoe cushioning reduces impact and muscle activation during landings from unexpected, but not self-initiated, drops. J. Sci. Med. Sport 2017, 20, 915–920. [Google Scholar] [CrossRef]
- Wang, X.; Deng, L.; Lam, W.K.; Yang, Y.; Zhang, X.; Fu, W. Wearing cushioning shoes reduce load rates more effectively in post-fatigue than in pre-fatigue during landings. Biology 2021, 10, 962. [Google Scholar] [CrossRef]
- Palazzo, F.; Lamouchideli, N.; Caronti, A.; Tufi, F.; Padua, E.; Annino, G. Neuromuscular response to the stimulation of plantar cutaneous during walking at different speeds. Gait Posture 2022, 95, 84–92. [Google Scholar] [CrossRef]
- Tarkka, I.M. Short and long latency reflexes in human muscles following electrical and mechanical stimulation. Acta Physiol. Scand. Suppl. 1986, 557, 1–32. [Google Scholar]
- Gielen, C.C.; Ramaekers, L.; van Zuylen, E.J. Long-latency stretch reflexes as co-ordinated functional responses in man. J. Physiol. 1988, 407, 275–292. [Google Scholar] [CrossRef]
- Michel, B.A.; Hauselmann, H.J.; Neidhart, M.; Paulsson, M.; Hauser, N.; Di Cesare, P.E. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br. J. Rheumatol. 1997, 36, 1151–1160. [Google Scholar] [CrossRef]
- Nardone, A.; Schieppati, M. Medium-latency response to muscle stretch in human lower limb: Estimation of conduction velocity of group II fibres and central delay. Neurosci. Lett. 1998, 249, 29–32. [Google Scholar] [CrossRef]
- Jung, Y.K.; Park, H.R.; Cho, H.J.; Jang, J.A.; Lee, E.J.; Han, M.S.; Kim, G.W.; Han, S. Degrading products of chondroitin sulfate can induce hypertrophy-like changes and MMP-13/ADAMTS5 production in chondrocytes. Sci. Rep. 2019, 9, 15846. [Google Scholar] [CrossRef]
- Lambert, C.; Zappia, J.; Sanchez, C.; Florin, A.; Dubuc, J.E.; Henrotin, Y. The damage-associated molecular atterns (DAMPs) as potential targets to treat osteoarthritis: Perspectives from a review of the literature. Front. Med. 2021, 7, 607186. [Google Scholar] [CrossRef]
- Smeathers, J.E. Transient vibrations caused by heel strike. Proc. Inst. Mech. Eng. H 1989, 203, 181–186. [Google Scholar] [CrossRef]
- Barr, D.A.; Kernohan, W.G.; Mollan, R.A. Transient vibrations caused by heel strike. Proc. Inst. Mech. Eng. H 1990, 204, 135–137. [Google Scholar]
- Zelik, K.E.; Takahashi, K.Z.; Sawicki, G.S. Six degree-of-freedom analysis of hip, knee, ankle and foot provides updated understanding of biomechanical work during human walking. J. Exp. Biol. 2015, 218, 876–886. [Google Scholar] [CrossRef]
- Smeathers, J.E.; Wright, V. Response of the human body to impact dynamics and vibration. Proc. Inst. Mech. Eng. H 1989, 203, 179–180. [Google Scholar] [CrossRef]
- Wakeling, J.M.; Liphardt, A.M.; Nigg, B.M. Muscle activity reduces soft-tissue resonance at heel-strike during walking. J. Biomech. 2003, 36, 1761–1769. [Google Scholar] [CrossRef]
- DeVita, P.; Helseth, J.; Hortobagyi, T. Muscles do more positive than negative work in human locomotion. J. Exp. Biol. 2007, 210, 3361–3373. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Zwarts, M. Measurement of muscle fiber conduction velocity in humans: Techniques and applications. J. Clin. Neurophysiol. 1989, 6, 173–190. [Google Scholar] [CrossRef]
- Hildebrand, C.; Oqvist, G.; Brax, L.; Tuisku, F. Anatomy of the rat knee joint and fibre composition of a major articular nerve. Anat. Rec. 1991, 229, 545–555. [Google Scholar] [CrossRef]
- Bloem, B.R.; Allum, J.H.; Carpenter, M.G.; Honegger, F. Is lower leg proprioception essential for triggering human automatic postural responses? Exp. Brain Res. 2000, 130, 375–391. [Google Scholar] [CrossRef]
- Grassel, S.G. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res. Ther. 2014, 16, 485. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular cartilage and osteoarthritis. Instr. Course Lect. 2005, 54, 465–480. [Google Scholar]
- Pearle, A.D.; Warren, R.F.; Rodeo, S.A. Basic science of articular cartilage and osteoarthritis. Clin. Sports Med. 2005, 24, 1–12. [Google Scholar] [CrossRef]
- Sofat, N. Analysing the role of endogenous matrix molecules in the development of osteoarthritis. Int. J. Exp. Pathol. 2009, 90, 463–479. [Google Scholar] [CrossRef]
- Stanton, H.; Melrose, J.; Little, C.B.; Fosang, A.J. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim. Biophys. Acta 2011, 1812, 1616–1629. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Li, T.; Peng, J.; Li, Q.; Shu, Y.; Zhu, P.; Hao, L. The mechanism and role of ADAMTS protein family in osteoarthritis. Biomolecules 2022, 12, 959. [Google Scholar] [CrossRef]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar] [CrossRef]
- Kim, B.; Bonassar, L.J. Understanding the influence of local physical stimuli on chondrocyte behavior. Adv. Exp. Med. Biol. 2023, 1402, 31–44. [Google Scholar]
- Schaefer, L. Complexity of danger: The diverse nature of damage-associated molecular patterns. J. Biol. Chem. 2014, 289, 35237–35245. [Google Scholar] [CrossRef]
- Neill, T.; Schaefer, L.; Iozzo, R.V. Decoding the matrix: Instructive roles of proteoglycan receptors. Biochemistry 2015, 54, 4583–4598. [Google Scholar] [CrossRef]
- Homandberg, G.A.; Hui, F.; Wen, C.; Purple, C.; Bewsey, K.; Koepp, H.; Huch, K.; Harris, A. Fibronectin-fragment-induced cartilage chondrolysis is associated with release of catabolic cytokines. Biochem. J. 1997, 321 Pt 3, 751–757. [Google Scholar] [CrossRef]
- Homandberg, G.A. Potential regulation of cartilage metabolism in osteoarthritis by fibronectin fragments. Front. Biosci. 1999, 4, D713–D730. [Google Scholar] [CrossRef]
- Di Cesare, P.E.; Chen, F.S.; Moergelin, M.; Carlson, C.S.; Leslie, M.P.; Perris, R.; Fang, C. Matrix-matrix interaction of cartilage oligomeric matrix protein and fibronectin. Matrix Biol. 2002, 21, 461–470. [Google Scholar] [CrossRef]
- Stanton, H.; Ung, L.; Fosang, A.J. The 45 kDa collagen-binding fragment of fibronectin induces matrix metalloproteinase-13 synthesis by chondrocytes and aggrecan degradation by aggrecanases. Biochem. J. 2002, 364, 181–190. [Google Scholar] [CrossRef]
- Mundermann, A.; Dyrby, C.O.; Andriacchi, T.P.; King, K.B. Serum concentration of cartilage oligomeric matrix protein (COMP) is sensitive to physiological cyclic loading in healthy adults. Osteoarthr. Cartil. 2005, 13, 34–38. [Google Scholar] [CrossRef]
- Yasuda, T. Cartilage destruction by matrix degradation products. Mod. Rheumatol. 2006, 16, 197–205. [Google Scholar] [CrossRef]
- Niehoff, A.; Kersting, U.G.; Helling, S.; Dargel, J.; Maurer, J.; Thevis, M.; Bruggemann, G.P. Different mechanical loading protocols influence serum cartilage oligomeric matrix protein levels in young healthy humans. Eur. J. Appl. Physiol. 2010, 110, 651–657. [Google Scholar] [CrossRef]
- Celik, O.; Salci, Y.; Ak, E.; Kalaci, A.; Korkusuz, F. Serum cartilage oligomeric matrix protein accumulation decreases significantly after 12 weeks of running but not swimming and cycling training—A randomised controlled trial. Knee 2013, 20, 19–25. [Google Scholar] [CrossRef]
- Hwang, H.S.; Park, S.J.; Cheon, E.J.; Lee, M.H.; Kim, H.A. Fibronectin fragment-induced expression of matrix metalloproteinases is mediated by MyD88-dependent TLR-2 signaling pathway in human chondrocytes. Arthritis Res. Ther. 2015, 17, 320. [Google Scholar] [CrossRef]
- Roberts, H.M.; Law, R.J.; Thom, J.M. The time course and mechanisms of change in biomarkers of joint metabolism in response to acute exercise and chronic training in physiologic and pathological conditions. Eur. J. Appl. Physiol. 2019, 119, 2401–2420. [Google Scholar] [CrossRef]
- Pérez-García, S.; Carrión, M.; Gutiérrez-Cañas, I.; Villanueva-Romero, R.; Castro, D.; Martínez, C.; González-Álvaro, I.; Blanco, F.J.; Juarranz, Y.; Gomariz, R.P. Profile of matrix-remodeling proteinases in osteoarthritis: Impact of fibronectin. Cells 2019, 9, 40. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, J. Cartilage oligomeric matrix protein, diseases, and therapeutic opportunities. Int. J. Mol. Sci. 2022, 23, 9253. [Google Scholar] [CrossRef]
- Dreiner, M.; Munk, T.; Zaucke, F.; Liphardt, A.M.; Niehoff, A. Relationship between different serum cartilage biomarkers in the acute response to running and jumping in healthy male individuals. Sci. Rep. 2022, 12, 6434. [Google Scholar]
- Fukai, F.; Ohtaki, M.; Fujii, N.; Yajima, H.; Ishii, T.; Nishizawa, Y.; Miyazaki, K.; Katayama, T. Release of biological activities from quiescent fibronectin by a conformational change and limited proteolysis by matrix metalloproteinases. Biochemistry 1995, 34, 11453–11459. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Roth, J. Mechanisms of disease: A ‘DAMP’ view of inflammatory arthritis. Nat. Clin. Pract. Rheumatol. 2007, 3, 382–390. [Google Scholar] [CrossRef]
- Rosenberg, J.H.; Rai, V.; Dilisio, M.F.; Sekundiak, T.D.; Agrawal, D.K. Increased expression of damage-associated molecular patterns (DAMPs) in osteoarthritis of human knee joint compared to hip joint. Mol. Cell Biochem. 2017, 436, 59–69. [Google Scholar] [CrossRef]
- Rosenberg, J.H.; Rai, V.; Dilisio, M.F.; Agrawal, D.K. Damage-associated molecular patterns in the pathogenesis of osteoarthritis: Potentially novel therapeutic targets. Mol. Cell Biochem. 2017, 434, 171–179. [Google Scholar] [CrossRef]
- Andersson, M.L.E.; Thorstensson, C.A.; Roos, E.M.; Petersson, I.F.; Heinegard, D.; Saxne, T. Serum levels of cartilage oligomeric matrix protein (COMP) increase temporarily after physical exercise in patients with knee osteoarthritis. BMC Musculoskelet. Disord. 2006, 7, 98. [Google Scholar] [CrossRef]
- Mundermann, A.; King, K.B.; Smith, R.L.; Andriacchi, T.P. Change in serum COMP concentration due to ambulatory load is not related to knee OA status. J. Orthop. Res. 2009, 27, 1408–1413. [Google Scholar] [CrossRef]
- Andriacchi, T.P.; Favre, J.; Erhart-Hledik, J.C.; Chu, C.R. A systems view of risk factors for knee osteoarthritis reveals insights into the pathogenesis of the disease. Ann. Biomed. Eng. 2015, 43, 376–387. [Google Scholar] [CrossRef]
- Dreiner, M.; Willwacher, S.; Kramer, A.; Kummel, J.; Frett, T.; Zaucke, F.; Liphardt, A.M.; Gruber, M.; Niehoff, A. Short-term response of serum cartilage oligomeric matrix protein to different types of impact loading under normal and artificial gravity. Front. Physiol. 2020, 11, 1032. [Google Scholar] [CrossRef]
- Scanzello, C.R.; Plaas, A.; Crow, M.K. Innate immune system activation in osteoarthritis: Is osteoarthritis a chronic wound? Curr. Opin. Rheumatol. 2008, 20, 565–572. [Google Scholar] [CrossRef]
- Belcher, C.; Yaqub, R.; Fawthrop, F.; Bayliss, M.; Doherty, M. Synovial fluid chondroitin and keratan sulphate epitopes, glycosaminoglycans, and hyaluronan in arthritic and normal knees. Ann. Rheum. Dis. 1997, 56, 299–307. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Madsen, S.H.; Christiansen, C.; Henriksen, K.; Fosang, A.J.; Sondergaard, B.C. Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis Res. Ther. 2008, 10, R63. [Google Scholar] [CrossRef]
- Merrild, N.G.; Holzmann, V.; Ariosa-Morejon, Y.; Faull, P.A.; Coleman, J.; Barrell, W.B.; Young, G.; Fischer, R.; Kelly, D.J.; Addison, O.; et al. Local depletion of proteoglycans mediates cartilage tissue repair in an ex vivo integration model. Acta Biomater. 2022, 149, 179–188. [Google Scholar] [CrossRef]
- del Río, E. Thick or thin? Implications of cartilage architecture for osteoarthritis risk in sedentary lifestyles. Biomedicines 2025, 13, 1650. [Google Scholar] [CrossRef]
- del Río, E. A novel etiological approach for the development of knee osteoarthritis in sedentary adults. Med. Hypotheses 2024, 185, 111291. [Google Scholar] [CrossRef]
- Craddock, R.J.; Hodson, N.W.; Ozols, M.; Shearer, T.; Hoyland, J.A.; Sherratt, M.J. Extracellular matrix fragmentation in young, healthy cartilaginous tissues. Eur. Cells Mater. 2018, 35, 34–53. [Google Scholar] [CrossRef]
- Tiku, M.L.; Gupta, S.; Deshmukh, D.R. Aggrecan degradation in chondrocytes is mediated by reactive oxygen species and protected by antioxidants. Free Radic. Res. 1999, 30, 395–405. [Google Scholar] [CrossRef]
- Henrotin, Y.E.; Bruckner, P.; Pujol, J.P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 2003, 11, 747–755. [Google Scholar] [CrossRef]
- Henrotin, Y.; Kurz, B.; Aigner, T. Oxygen and reactive oxygen species in cartilage degradation: Friends or foes? Osteoarthr. Cartil. 2005, 13, 643–654. [Google Scholar] [CrossRef]
- Yudoh, K.; van Trieu, N.; Nakamura, H.; Hongo-Masuko, K.; Kato, T.; Nishioka, K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: Oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res. Ther. 2005, 7, R380–R391. [Google Scholar] [CrossRef]
- Gibson, J.S.; Milner, P.I.; White, R.; Fairfax, T.P.; Wilkins, R.J. Oxygen and reactive oxygen species in articular cartilage: Modulators of ionic homeostasis. Pflug. Arch. 2008, 455, 563–573. [Google Scholar] [CrossRef]
- Blanco, F.J.; Rego, I.; Ruiz-Romero, C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 161–169. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Liu, L.; Luo, P.; Yang, M.; Wang, J.; Hou, W.; Xu, P. The role of oxidative stress in the development of knee osteoarthritis: A comprehensive research review. Front. Mol. Biosci. 2022, 9, 1001212. [Google Scholar] [CrossRef]
- Danalache, M.; Umrath, F.; Riester, R.; Schwitalle, M.; Guilak, F.; Hofmann, U.K. Proteolysis of the pericellular matrix: Pinpointing the role and involvement of matrix metalloproteinases in early osteoarthritic remodeling. Acta Biomater. 2024, 181, 297–307. [Google Scholar] [CrossRef]
- Ji, Q.; Zheng, Y.; Zhang, G.; Hu, Y.; Fan, X.; Hou, Y.; Wen, L.; Li, L.; Xu, Y.; Wang, Y.; et al. Single-cell RNA-seq analysis reveals the progression of human osteoarthritis. Ann. Rheum. Dis. 2019, 78, 100–110. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Gossan, N.; Zeef, L.; Hensman, J.; Hughes, A.; Bateman, J.F.; Rowley, L.; Little, C.B.; Piggins, H.D.; Rattray, M.; Boot-Handford, R.P.; et al. The circadian clock in murine chondrocytes regulates genes controlling key aspects of cartilage homeostasis. Arthritis Rheum. 2013, 65, 2334–2345. [Google Scholar] [CrossRef]
- Dudek, M.; Meng, Q.-J. Running on time: The role of circadian clocks in the musculoskeletal system. Biochem. J. 2014, 463, 1–8. [Google Scholar] [CrossRef]
- Snelling, S.J.; Forster, A.; Mukherjee, S.; Price, A.J.; Poulsen, R.C. The chondrocyte-intrinsic circadian clock is disrupted in human osteoarthritis. Chronobiol. Int. 2016, 33, 574–579. [Google Scholar] [CrossRef]
- Yang, G.; Chen, L.; Grant, G.R.; Paschos, G.; Song, W.L.; Musiek, E.S.; Lee, V.; McLoughlin, S.C.; Grosser, T.; Cotsarelis, G.; et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci. Transl. Med. 2016, 8, 324ra16. [Google Scholar] [CrossRef]
- Dudek, M.; Gossan, N.; Yang, N.; Im, H.-J.; Ruckshanthi, J.P.D.; Yoshitane, H.; Li, X.; Jin, D.; Wang, P.; Boudiffa, M.; et al. The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity. J. Clin. Investig. 2016, 126, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Akagi, R.; Akatsu, Y.; Fisch, K.M.; Alvarez-Garcia, O.; Teramura, T.; Muramatsu, Y.; Saito, M.; Sasho, T.; Su, A.I.; Lotz, M.K. Dysregulated circadian rhythm pathway in human osteoarthritis: NR1D1 and BMAL1 suppression alters TGF-β signaling in chondrocytes. Osteoarthr. Cartil. 2017, 25, 943–951. [Google Scholar] [CrossRef]
- Bekki, H.; Duffy, T.; Okubo, N.; Olmer, M.; Alvarez-Garcia, O.; Lamia, K.; Kay, S.; Lotz, M. Suppression of circadian clock protein cryptochrome 2 promotes osteoarthritis. Osteoarthr. Cartil. 2020, 28, 966–976. [Google Scholar] [CrossRef]
- Song, X.; Hu, H.; Zhao, M.; Ma, T.; Gao, L. Prospects of circadian clock in joint cartilage development. FASEB J. 2020, 34, 14120–14135. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Angelucci, C.; Pathiranage, D.; Wang, P.; Mallikarjun, V.; Lawless, C.; Swift, J.; Kadler, K.E.; Boot-Handford, R.P.; Hoyland, J.A.; et al. Circadian time series proteomics reveals daily dynamics in cartilage physiology. Osteoarthr. Cartil. 2021, 29, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, R.C.; Hearn, J.I.; Dalbeth, N. The circadian clock: A central mediator of cartilage maintenance and osteoarthritis development? Rheumatology 2021, 60, 3048–3057. [Google Scholar] [CrossRef]
- Du, Z.; You, X.; Wu, D.; Huang, S.; Zhou, Z. Rhythm disturbance in osteoarthritis. Cell Commun. Signal. 2022, 20, 70. [Google Scholar] [CrossRef]
- Qian, Z.; Liu, Z.; Feng, Z.; Cai, Z.; Qiu, Y.; Zhu, Z. Blocking circadian clock factor Rev-erbα inhibits growth plate chondrogenesis via up-regulating MAPK-ERK1/2 pathway. Cell Cycle 2022, 22, 73–84. [Google Scholar] [CrossRef]
- Hosokawa, H.; Akagi, R.; Watanabe, S.; Horii, M.; Shinohara, M.; Mikami, Y.; Toguchi, K.; Kimura, S.; Yamaguchi, S.; Ohtori, S.; et al. Nuclear receptor subfamily 1 group D member 1 in the pathology of obesity-induced osteoarthritis progression. J. Orthop. Res. 2023, 41, 930–941. [Google Scholar] [CrossRef]
- Shen, J.; Abu-Amer, Y.; O’Keefe, R.J.; McAlinden, A. Inflammation and epigenetic regulation in osteoarthritis. Connect. Tissue Res. 2017, 58, 49–63. [Google Scholar] [CrossRef]
- Guo, B.; Yang, N.; Borysiewicz, E.; Dudek, M.; Williams, J.L.; Li, J.; Maywood, E.S.; Adamson, A.; Hastings, M.H.; Bateman, J.F.; et al. Catabolic cytokines disrupt the circadian clock and the expression of clock-controlled genes in cartilage via an NFкB-dependent pathway. Osteoarthr. Cartil. 2015, 23, 1981–1988. [Google Scholar] [CrossRef]
- Bunger, M.K.; Walisser, J.A.; Sullivan, R.; Manley, P.A.; Moran, S.M.; Kalscheur, V.L.; Colman, R.J.; Bradfield, C.A. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis 2005, 41, 122–132. [Google Scholar] [CrossRef]
- Yang, W.; Kang, X.; Liu, J.; Li, H.; Ma, Z.; Jin, X.; Qian, Z.; Xie, T.; Qin, N.; Feng, D.; et al. Clock gene Bmal1 modulates human cartilage gene expression by crosstalk with Sirt1. Endocrinology 2016, 157, 3096–3107. [Google Scholar] [CrossRef]
- Dudek, M.; Pathiranage, D.R.J.; Bano-Otalora, B.; Paszek, A.; Rogers, N.; Goncalves, C.F.; Lawless, C.; Wang, D.; Luo, Z.; Yang, L.; et al. Mechanical loading and hyperosmolarity as a daily resetting cue for skeletal circadian clocks. Nat. Commun. 2023, 14, 7237. [Google Scholar] [CrossRef]
- Yang, N.; Williams, J.; Pekovic-Vaughan, V.; Wang, P.; Olabi, S.; McConnell, J.; Gossan, N.; Hughes, A.; Cheung, J.; Streuli, C.H.; et al. Cellular mechano-environment regulates the mammary circadian clock. Nat. Commun. 2017, 8, 14287. [Google Scholar] [CrossRef]
- Streuli, C.H.; Meng, Q.J. Influence of the extracellular matrix on cell-intrinsic circadian clocks. J. Cell Sci. 2019, 132, jcs207498. [Google Scholar] [CrossRef]
- Vágó, J.; Katona, É.; Takács, R.; Dócs, K.; Hajdú, T.; Kovács, P.; Zákány, R.; van der Veen, D.R.; Matta, C. Cyclic uniaxial mechanical load enhances chondrogenesis through entraining the molecular circadian clock. J. Pineal Res. 2022, 73, e12827. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Jeon, S.; Park, J.; Ryu, J.H.; Mobasheri, A.; Matta, C.; Jin, E.J. Progressing future osteoarthritis treatment toward precision medicine: Integrating regenerative medicine, gene therapy and circadian biology. Exp. Mol. Med. 2025, 57, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Maroudas, A.; Bullough, P.; Swanson, S.A.V.; Freeman, M.A.R. The permeability of articular cartilage. J. Bone Jt. Surg. 1968, 50, 166–177. [Google Scholar] [CrossRef]
- Stockwell, R.A. Biology of cartilage cells; Cambridge University Press: Cambridge, UK; London, UK; New York, NY, USA; Melbourne, Australia, 1979. [Google Scholar]
- Otte, P. Basic cell metabolism of articular cartilage. Manometric studies. Z. Rheumatol. 1991, 50, 304–312. [Google Scholar]
- Mobasheri, A. Glucose: An energy currency and structural precursor in articular cartilage and bone with emerging roles as an extracellular signaling molecule and metabolic regulator. Front. Endocrinol. 2012, 3, 153. [Google Scholar] [CrossRef]
- Mech, D.J.; Rizvi, M.S. Modeling the role of ATP metabolism in articular cartilage and osteoarthritis. Biosystems 2025, 257, 105597. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.; Hall, A.C.; Gehl, K.A. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J. Cell. Physiol. 1993, 154, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Cui, Z.; Urban, J.P.G. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: A modeling study. Arthritis Rheum. 2004, 50, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Jung, A.; Murphy, A.; Andreyev, A.; Dykens, J.; Terkeltaub, R. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheum. 2000, 43, 1560–1570. [Google Scholar] [CrossRef]
- Johnson, Z.I.; Shapiro, I.M.; Risbud, M.V. Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: Evolving role of TonEBP. Matrix Biol. 2014, 40, 10–16. [Google Scholar] [CrossRef]
- Lai, W.M.; Hou, J.S.; Mow, V.C. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J. Biomech. Eng. 1991, 113, 245–258. [Google Scholar] [CrossRef]
- Mow, V.C.; Wang, C.C.; Hung, C.T. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthr. Cartil. 1999, 7, 41–58. [Google Scholar] [CrossRef]
- Chen, S.S.; Falcovitz, Y.H.; Schneiderman, R.; Maroudas, A.; Sah, R.L. Depth-dependent compressive properties of normal aged human femoral head articular cartilage: Relationship to fixed charge density. Osteoarthr. Cartil. 2001, 9, 561–569. [Google Scholar] [CrossRef]
- Govindaraj, K.; Meteling, M.; van Rooij, J.; Becker, M.; van Wijnen, A.J.; van den Beucken, J.; Ramos, Y.F.M.; van Meurs, J.; Post, J.N.; Leijten, J. Osmolarity-induced altered intracellular molecular crowding drives osteoarthritis pathology. Adv. Sci. 2024, 11, e2306722. [Google Scholar] [CrossRef] [PubMed]
- Beg, Q.K.; Vazquez, A.; Ernst, J.; de Menezes, M.A.; Bar-Joseph, Z.; Barabási, A.L.; Oltvai, Z.N. Intracellular crowding defines the mode and sequence of substrate uptake by Escherichia coli and constrains its metabolic activity. Proc. Natl. Acad. Sci. USA 2007, 104, 12663–12668. [Google Scholar] [CrossRef]
- Zhou, H.X.; Rivas, G.; Minton, A.P. Macromolecular crowding and confinement: Biochemical, biophysical, and potential physiological consequences. Annu. Rev. Biophys. 2008, 37, 375–397. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Ding, X.; Liu, S.; Sun, T. Osmolarity influences chondrocyte repair after injury in human articular cartilage. J. Orthop. Surg. Res. 2015, 10, 19. [Google Scholar] [CrossRef]
- Nettesheim, G.; Nabti, I.; Murade, C.U.; Jaffe, G.R.; King, S.J.; Shubeita, G.T. Macromolecular crowding acts as a physical regulator of intracellular transport. Nat. Phys. 2020, 16, 1144–1151. [Google Scholar] [CrossRef]
- Buenaventura, A.; Saito, T.; Kanao, T.; Matsunaga, D.; Matsui, T.S.; Deguchi, S. Intracellular macromolecular crowding within individual stress fibers analyzed by fluorescence correlation spectroscopy. Cell. Mol. Bioeng. 2024, 17, 165–176. [Google Scholar] [CrossRef]
- O’Hara, B.P.; Urban, J.P.; Maroudas, A. Influence of cyclic loading on the nutrition of articular cartilage. Ann. Rheum. Dis. 1990, 49, 536–539. [Google Scholar] [CrossRef]
- Bonassar, L.J.; Grodzinsky, A.J.; Frank, E.H.; Davila, S.G.; Bhaktav, N.R.; Trippel, S.B. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J. Orthop. Res. 2001, 19, 11–17. [Google Scholar] [CrossRef]
- Paschos, G.K.; Ibrahim, S.; Song, W.L.; Kunieda, T.; Grant, G.; Reyes, T.M.; Bradfield, C.A.; Vaughan, C.H.; Eiden, M.; Masoodi, M.; et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 2012, 18, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Chadha, R. Revealed aspect of metabolic osteoarthritis. J. Orthop. 2016, 13, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Dickson, B.M.; Roelofs, A.J.; Rochford, J.J.; Wilson, H.M.; De Bari, C. The burden of metabolic syndrome on osteoarthritic joints. Arthritis Res. Ther. 2019, 21, 289. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Lu, K.; Umar, M.; Zhu, Z.; Lu, W.W.; Speakman, J.R.; Chen, Y.; Tong, L.; Chen, D. Risk of metabolic abnormalities in osteoarthritis: A new perspective to understand its pathological mechanisms. Bone Res. 2023, 11, 63. [Google Scholar] [CrossRef]
- Adam, M.S.; Zhuang, H.; Ren, X.; Zhang, Y.; Zhou, P. The metabolic characteristics and changes of chondrocytes in vivo and in vitro in osteoarthritis. Front. Endocrinol. 2024, 15, 1393550. [Google Scholar] [CrossRef]
- Guo, P.; Alhaskawi, A.; Adel Abdo Moqbel, S.; Pan, Z. Recent development of mitochondrial metabolism and dysfunction in osteoarthritis. Front. Pharmacol. 2025, 16, 1538662. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Di, J.; Guo, Z.; Chen, S.; Xiang, C. Association of organs-crosstalk with the pathogenesis of osteoarthritis: Cartilage as a key player. Front. Endocrinol. 2025, 16, 1593658. [Google Scholar] [CrossRef]
- Shepherd, D.; Seedhom, B. Thickness of human articular cartilage in joints of the lower limb. Ann. Rheum. Dis. 1999, 58, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Oei, E.H.G.; Runhaar, J. Imaging of early-stage osteoarthritis: The needs and challenges for diagnosis and classification. Skeletal Radiol. 2023, 52, 2031–2036. [Google Scholar] [CrossRef]
- Xu, X.; Wang, D.; Ni, B.; Xu, H.; Wu, Z.; He, T.; Zhang, Y.; Hao, X.; Ding, G.; Zhang, X.; et al. Inhibiting the REV-ERBα expression protects against mechanical overloading-induced cartilage clock disruption and osteoarthritis progression. J. Orthop. Transl. 2025, 53, 112–125. [Google Scholar] [CrossRef]
- Ze, Y.; Wu, Y.; Tan, Z.; Li, R.; Li, R.; Gao, W.; Zhao, Q. Signaling pathway mechanisms of circadian clock gene Bmal1 regulating bone and cartilage metabolism: A review. Bone Res. 2025, 13, 19. [Google Scholar] [CrossRef]
- Mortimer, T. Getting the clock back on its feet: Targeting the circadian clock to treat osteoarthritis. FEBS J. 2022, 289, 6640–6642. [Google Scholar] [CrossRef]
- He, T.; Pang, S.; Wang, H.; Yun, H.; Hao, X.; Jia, L.; Liu, H.; Wang, D.; Wang, D.; Xu, H.; et al. Drugging the circadian clock feedback cycle to ameliorate cartilage degeneration. FEBS J. 2022, 289, 6643–6658. [Google Scholar] [CrossRef]
- Roemer, F.W.; Jansen, M.; Marijnissen, A.C.A.; Guermazi, A.; Heiss, R.; Maschek, S.; Lalande, A.; Blanco, F.J.; Berenbaum, F.; van de Stadt, L.A.; et al. Structural tissue damage and 24-month progression of semi-quantitative MRI biomarkers of knee osteoarthritis in the IMI-APPROACH cohort. BMC Musculoskelet. Disord. 2022, 23, 988. [Google Scholar] [CrossRef]
- del Río, E. Rethinking osteoarthritis management: Synergistic effects of chronoexercise, circadian rhythm, and chondroprotective agents. Biomedicines 2025, 13, 598. [Google Scholar] [CrossRef]
- del Río, E. Pharmacist-driven chondroprotection in osteoarthritis: A multifaceted approach using patient education, information visualization, and lifestyle integration. Pharmacy 2025, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- del Río, E.; Vergés, J. Exploring the influence of physical activity on the efficacy of chondroprotective agents for osteoarthritis: The role of diffusion conditions. Med. Hypotheses 2024, 182, 111244. [Google Scholar] [CrossRef]
- Leong, D.J.; Choudhury, M.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; Sun, H.B. Nutraceuticals: Potential for chondroprotection and molecular targeting of osteoarthritis. Int. J. Mol. Sci. 2013, 14, 23063–23085. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical supplements in the management and prevention of osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Saravana Bhavan, P.; Sheu, J.-R. Molecular targets of natural products for chondroprotection in destructive joint diseases. Int. J. Mol. Sci. 2020, 21, 4931. [Google Scholar] [CrossRef] [PubMed]
- Sukhikh, S.; Noskova, S.; Ivanova, S.; Ulrikh, E.; Izgaryshev, A.; Babich, O. Chondroprotection and molecular mechanism of action of phytonutraceuticals on osteoarthritis. Molecules 2021, 26, 2391. [Google Scholar] [CrossRef]
- Colletti, A.; Cicero, A.F.G. Nutraceutical approach to chronic osteoarthritis: From molecular research to clinical evidence. Int. J. Mol. Sci. 2021, 22, 12920. [Google Scholar] [CrossRef]
- Fernández-Martín, S.; González-Cantalapiedra, A.; Muñoz, F.; García-González, M.; Permuy, M.; López-Peña, M. Glucosamine and chondroitin sulfate: Is there any scientific evidence for their effectiveness as disease-modifying drugs in knee osteoarthritis preclinical studies? A systematic review from 2000 to 2021. Animals 2021, 11, 1608. [Google Scholar] [CrossRef] [PubMed]
- Tenório, M.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.; Goulart, M.O.F. N-acetylcysteine (NAC): Impacts on human health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Kawashima, H.; Tanaka, T.; Hirose, M.; Toyama-Sorimachi, N.; Matsuzawa, Y.; Miyasaka, M. CD44 binds a chondroitin sulfate proteoglycan, aggrecan. Int. Immunol. 2001, 13, 359–366. [Google Scholar] [CrossRef]
- Embry, J.J.; Knudson, W. G1 domain of aggrecan cointernalizes with hyaluronan via a CD44-mediated mechanism in bovine articular chondrocytes. Arthritis Rheum. 2003, 48, 3431–3441. [Google Scholar] [CrossRef]
- Danielson, B.T.; Knudson, C.B.; Knudson, W. Extracellular processing of the cartilage proteoglycan aggregate and its effect on CD44-mediated internalization of hyaluronan. J. Biol. Chem. 2015, 290, 9555–9570. [Google Scholar] [CrossRef]
- Loeser, R.F. Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol. 2014, 39, 11–16. [Google Scholar] [CrossRef]
- Jin, H.; Jiang, S.; Wang, R.; Zhang, Y.; Dong, J.; Li, Y. Mechanistic insight into the roles of integrins in osteoarthritis. Front. Cell Dev. Biol. 2021, 9, 693484. [Google Scholar] [CrossRef]
- Derfoul, A.; Miyoshi, A.D.; Freeman, D.E.; Tuan, R.S. Glucosamine promotes chondrogenic phenotype in both chondrocytes and mesenchymal stem cells and inhibits MMP-13 expression and matrix degradation. Osteoarthr. Cartil. 2007, 15, 646–655. [Google Scholar] [CrossRef]
- Nagaoka, I.; Igarashi, M.; Sakamoto, K. Biological activities of glucosamine and its related substances. Adv. Food Nutr. Res. 2012, 65, 337–352. [Google Scholar] [PubMed]
- Gallagher, B.; Tjoumakaris, F.P.; Harwood, M.I.; Good, R.P.; Ciccotti, M.G.; Freedman, K.B. Chondroprotection and the prevention of osteoarthritis progression of the knee: A systematic review of treatment agents. Am. J. Sports Med. 2015, 43, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzo, E.; Faggian, A.; Venerando, R.; Benetti, A.; Belluzzi, E.; Abatangelo, G.; Ruggieri, P.; Brun, P. In vitro effects of low doses of β-caryophyllene, ascorbic acid and d-glucosamine on human chondrocyte viability and inflammation. Pharmaceuticals 2021, 14, 286. [Google Scholar] [CrossRef]
- Valsamidou, E.; Gioxari, A.; Amerikanou, C.; Zoumpoulakis, P.; Skarpas, G.; Kaliora, A.C. Dietary interventions with polyphenols in osteoarthritis: A systematic review directed from the preclinical data to randomized clinical studies. Nutrients 2021, 13, 1420. [Google Scholar] [CrossRef]
- Cholet, J.; Decombat, C.; Delort, L.; Gainche, M.; Berry, A.; Ogeron, C.; Ripoche, I.; Vareille-Delarbre, M.; Vermerie, M.; Fraisse, D.; et al. Potential anti-inflammatory and chondroprotective effect of Luzula sylvatica. Int. J. Mol. Sci. 2022, 24, 127. [Google Scholar] [CrossRef] [PubMed]
- Hridayanka, K.S.N.; Duttaroy, A.K.; Basak, S. Bioactive compounds and their chondroprotective effects for osteoarthritis amelioration: A focus on nanotherapeutic strategies, epigenetic modifications, and gut microbiota. Nutrients 2024, 16, 3587. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sang, L.; Wu, D.; Rong, J.; Jiang, L. Effectiveness and safety of glucosamine and chondroitin for the treatment of osteoarthritis: A meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2018, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Ashruf, O.S.; Ansari, M.Y. Natural compounds: Potential therapeutics for the inhibition of cartilage matrix degradation in osteoarthritis. Life 2023, 13, 102. [Google Scholar] [CrossRef]
- Brandt, M.D.; Malone, J.B.; Kean, T.J. Advances and challenges in the pursuit of disease-modifying osteoarthritis drugs: A review of 2010–2024 clinical trials. Biomedicines 2025, 13, 355. [Google Scholar] [CrossRef]
- van der Graaff, S.J.A.; Oei, E.H.G.; Reijman, M.; Steenbekkers, L.; van Middelkoop, M.; van der Heijden, R.A.; Meuffels, D.E. Post-traumatic and OA-related lesions in the knee at baseline and 2 years after traumatic meniscal injury: Secondary analysis of a randomized controlled trial. Osteoarthr. Cartil. 2025, 33, 647–655. [Google Scholar] [CrossRef]
- Moynihan, R.; Heath, I.; Henry, D. Selling sickness: The pharmaceutical industry and disease mongering. BMJ 2002, 324, 886–891. [Google Scholar] [CrossRef]
- Reeve, E.; Shakib, S.; Hendrix, I.; Roberts, M.S.; Wiese, M.D. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br. J. Clin. Pharmacol. 2014, 78, 738–747. [Google Scholar] [CrossRef]
- Scott, I.A.; Hilmer, S.N.; Reeve, E.; Potter, K.; Le Couteur, D.; Rigby, D.; Gnjidic, D.; Del Mar, C.B.; Roughead, E.E.; Page, A.; et al. Reducing inappropriate polypharmacy: The process of deprescribing. JAMA Intern. Med. 2015, 175, 827–834. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Zhou, T.; Sun, D.; Liang, Z.; Li, Y.; Heianza, Y.; Qi, L. Glucosamine use, inflammation, and genetic susceptibility, and incidence of type 2 diabetes: A prospective study in UK Biobank. Diabetes Care 2020, 43, 719–725. [Google Scholar] [CrossRef]
- Esfandiari, H.; Pakravan, M.; Zakeri, Z.; Ziaie, S.; Pakravan, P.; Ownagh, V. Effect of glucosamine on intraocular pressure: A randomized clinical trial. Eye 2017, 31, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Losina, E.; Burbine, S.A.; Suter, L.G.; Hunter, D.J.; Solomon, D.H.; Daigle, M.E.; Dervan, E.E.; Jordan, J.M.; Katz, J.N. Pharmacologic regimens for knee osteoarthritis prevention: Can they be cost-effective? Osteoarthr. Cartil. 2014, 22, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Levaggi, L.; Levaggi, R. Timely, cheap, or risk-free? The effect of regulation on the price and availability of new drugs. Pharmacy 2024, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, E.D.; Flahault, A. A scoping review on the economic impacts of healthy ageing promotion and disease prevention in OECD member countries. Int. J. Environ. Res. Public Health 2025, 22, 1161. [Google Scholar] [CrossRef]
- Eichler, H.G.; Baird, L.G.; Barker, R.; Bloechl-Daum, B.; Børlum-Kristensen, F.; Brown, J.; Chua, R.; Del Signore, S.; Dugan, U.; Ferguson, J.; et al. From adaptive licensing to adaptive pathways: Delivering a flexible life-span approach to bring new drugs to patients. Clin. Pharmacol. Ther. 2015, 97, 234–246. [Google Scholar] [CrossRef]
- Charles, C.; Gafni, A.; Whelan, T. Shared decision-making in the medical encounter: What does it mean? (or it takes at least two to tango). Soc. Sci. Med. 1997, 44, 681–692. [Google Scholar] [CrossRef]
- Emanuel, E.J.; Wendler, D.; Grady, C. What makes clinical research ethical? JAMA 2000, 283, 2701–2711. [Google Scholar] [CrossRef]
- Gonçalves, J.R.; Magalhães, N.; Machado, S.; Ramalhinho, I.; Cavaco, A.M. Pharmacist-mediated deprescribing in long-term care facilities: A systematic review. Pharmacy 2025, 13, 3. [Google Scholar] [CrossRef]
- Gu, Y.; Hu, Y.; Zhang, H.; Wang, S.; Xu, K.; Su, J. Single-cell RNA sequencing in osteoarthritis. Cell Prolif. 2023, 56, e13517. [Google Scholar] [CrossRef] [PubMed]
- Boffa, A.; Merli, G.; Andriolo, L.; Lattermann, C.; Salzmann, G.M.; Filardo, G. Synovial fluid biomarkers in knee osteoarthritis: A systematic review and quantitative evaluation using BIPEDs criteria. Cartilage 2021, 13, 82S–103S. [Google Scholar] [CrossRef]
- Lynskey, S.J.; Macaluso, M.J.; Gill, S.D.; McGee, S.L.; Page, R.S. Biomarkers of osteoarthritis-A narrative review on causal links with metabolic syndrome. Life 2023, 13, 730. [Google Scholar] [CrossRef]
- Oliinyk, M.; Mastbergen, S.C.; Marijnissen, A.; Felson, D.T.; Hunter, D.J.; Nevitt, M.C.; Weinans, H.; Jansen, M.P. Comparing five major knee osteoarthritis cohort studies: Similarities, differences, and unique aspects of CHECK, OAI, FNIH, IMI-APPROACH, and MOST. Cartilage 2025, 19, 19476035251326276. [Google Scholar] [CrossRef]
- Xue, M.; Huang, N.; Luo, Y.; Yang, X.; Wang, Y.; Fang, M. Combined transcriptomics and metabolomics identify regulatory mechanisms of porcine vertebral chondrocyte development in vitro. Int. J. Mol. Sci. 2024, 25, 1189. [Google Scholar] [CrossRef]
- Guo, W.; Jin, P.; Li, R.; Huang, L.; Liu, Z.; Li, H.; Zhou, T.; Fang, B.; Xia, L. Dynamic network biomarker identifies cdkn1a-mediated bone mineralization in the triggering phase of osteoporosis. Exp. Mol. Med. 2023, 55, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Roman-Blas, J.A.; Castañeda, S.; Largo, R.; Herrero-Beaumont, G. Osteoarthritis associated with estrogen deficiency. Arthritis Res. Ther. 2009, 11, 241. [Google Scholar] [CrossRef]
- Peshkova, M.; Lychagin, A.; Lipina, M.; Di Matteo, B.; Anzillotti, G.; Ronzoni, F.; Kosheleva, N.; Shpichka, A.; Royuk, V.; Fomin, V.; et al. Gender-related aspects in osteoarthritis development and progression: A review. Int. J. Mol. Sci. 2022, 23, 2767. [Google Scholar] [CrossRef]
- Franke, M.; Mancino, C.; Taraballi, F. Reasons for the sex bias in osteoarthritis research: A review of preclinical studies. Int. J. Mol. Sci. 2023, 24, 10386. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, K.; Li, Y.; Li, Y.; Li, X.; Lin, Y.; Huang, L.; Tian, G. Global, regional, and national burden of osteoarthritis from 1990 to 2021 and projections to 2035: A cross-sectional study for the Global Burden of Disease Study 2021. PLoS ONE 2025, 20, e0324296. [Google Scholar] [CrossRef] [PubMed]
- Felson David, T.; Kim, Y.-J. The futility of current approaches to chondroprotection. Arthritis Rheum. 2007, 56, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
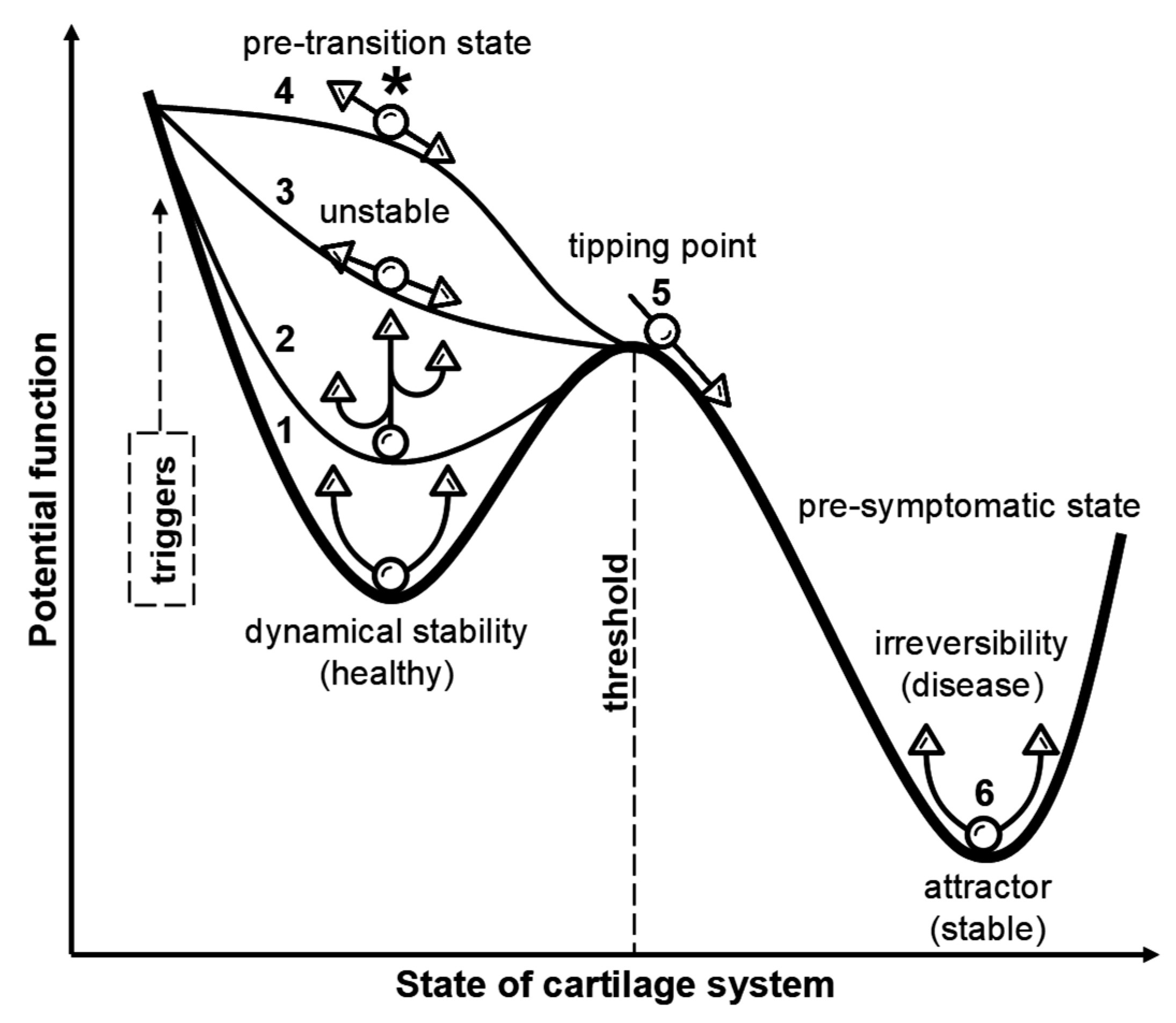
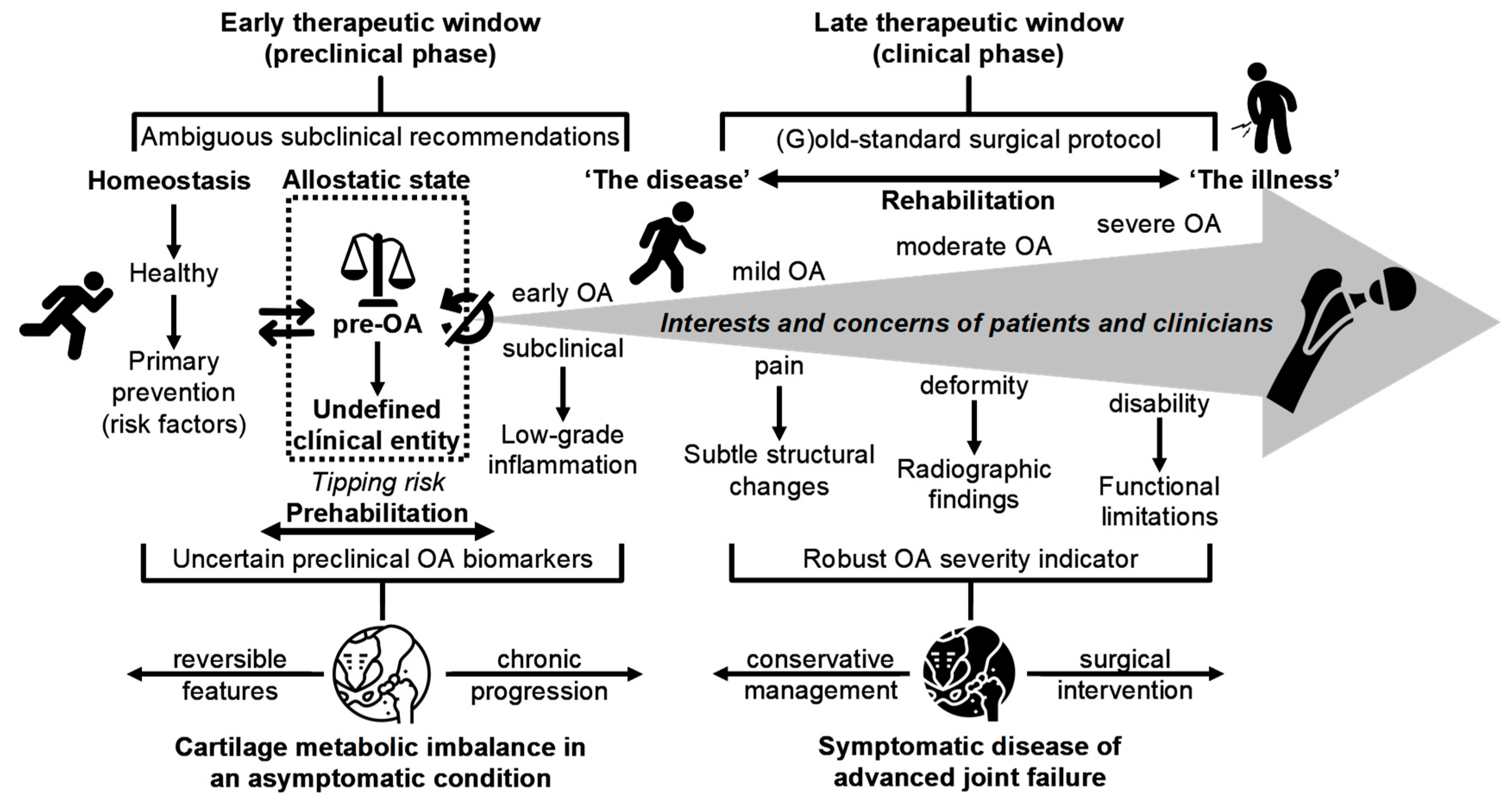
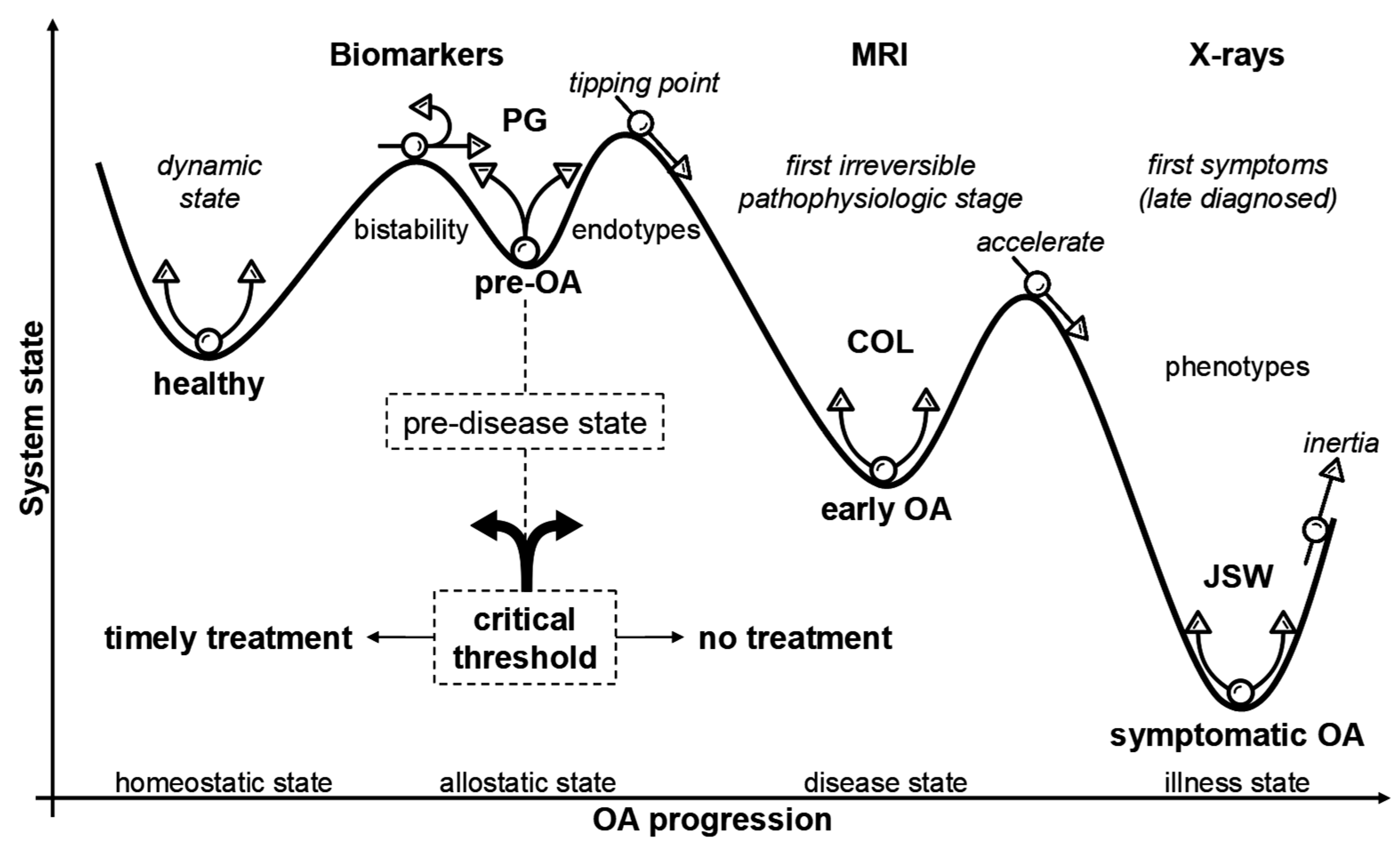
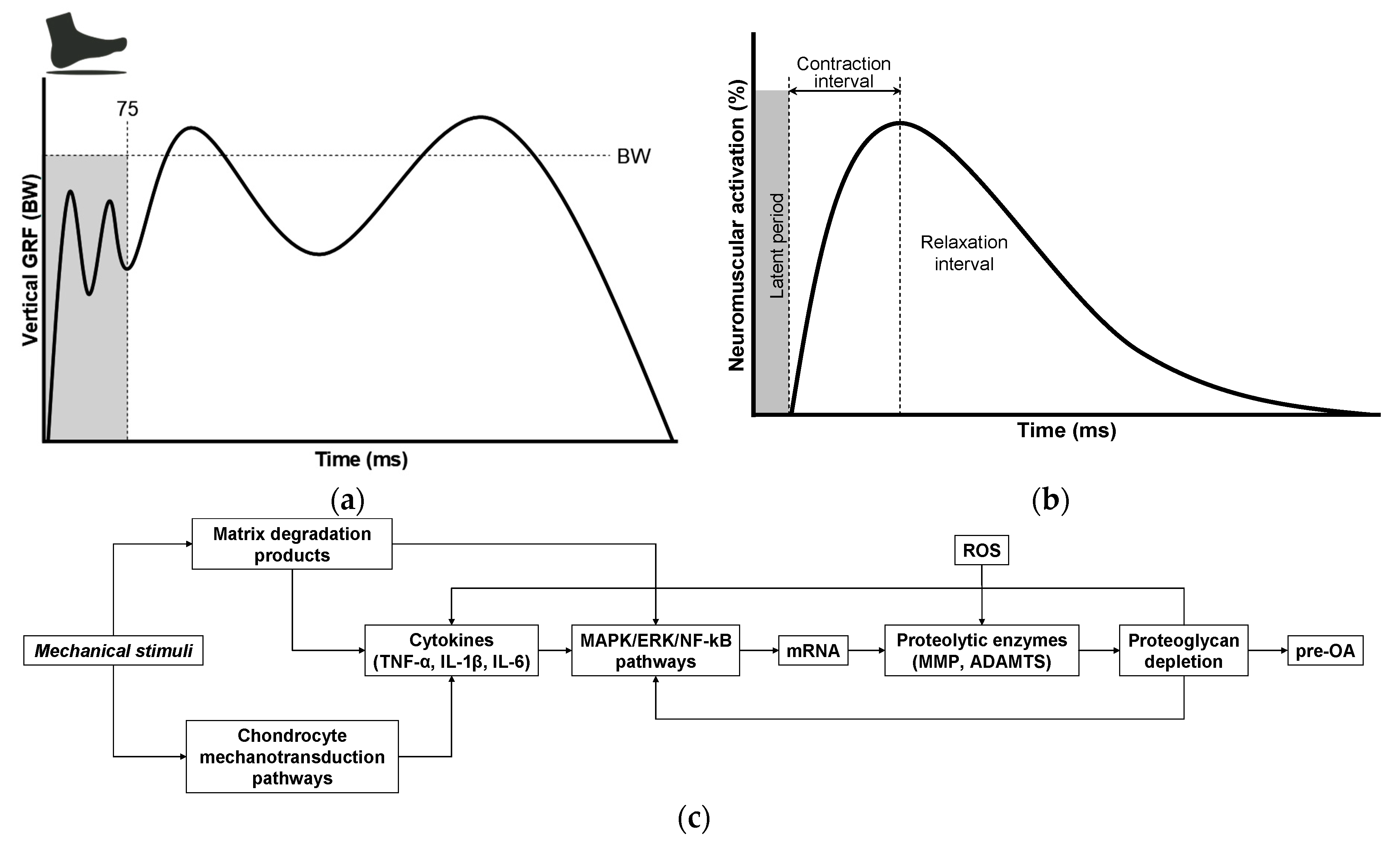
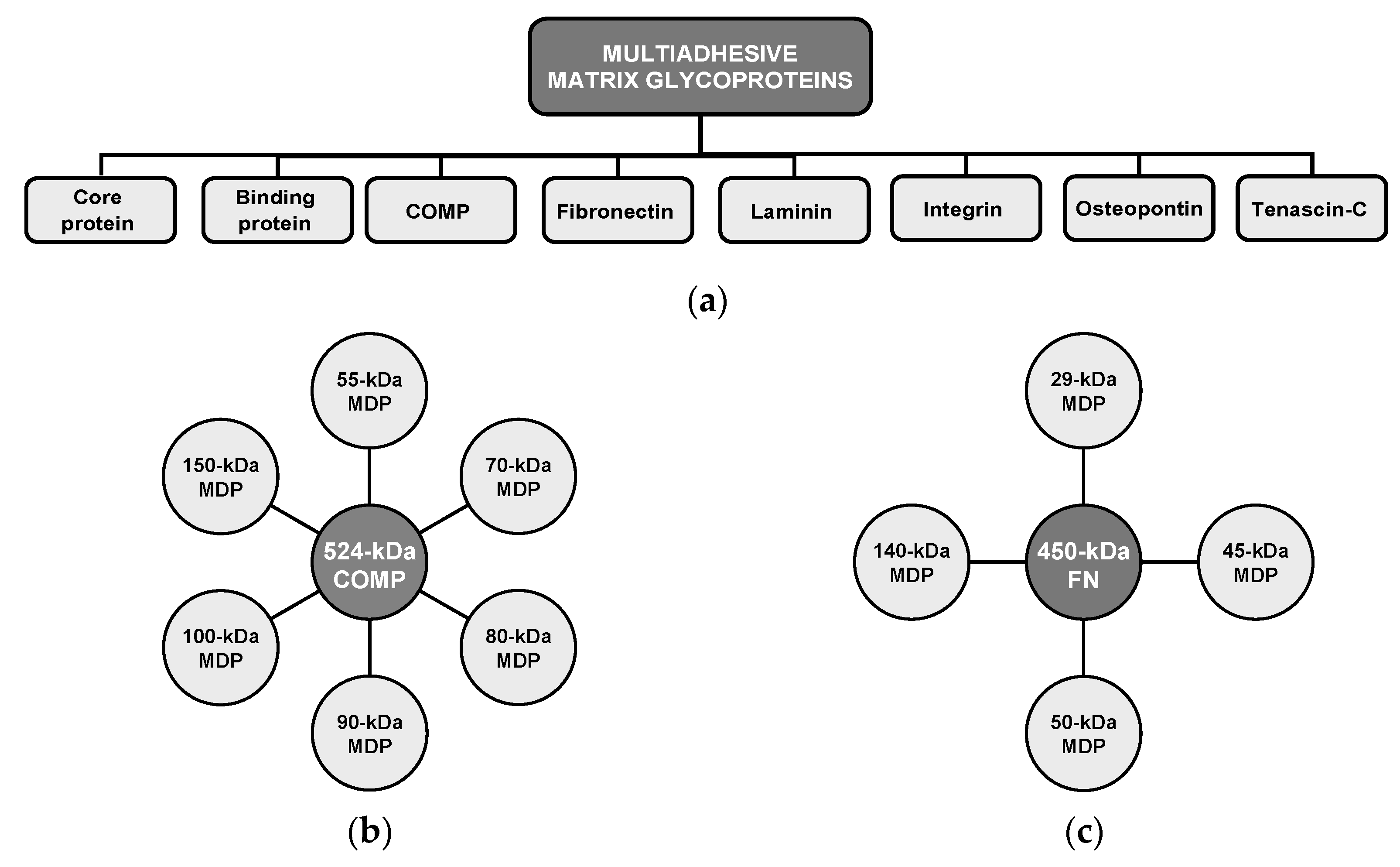
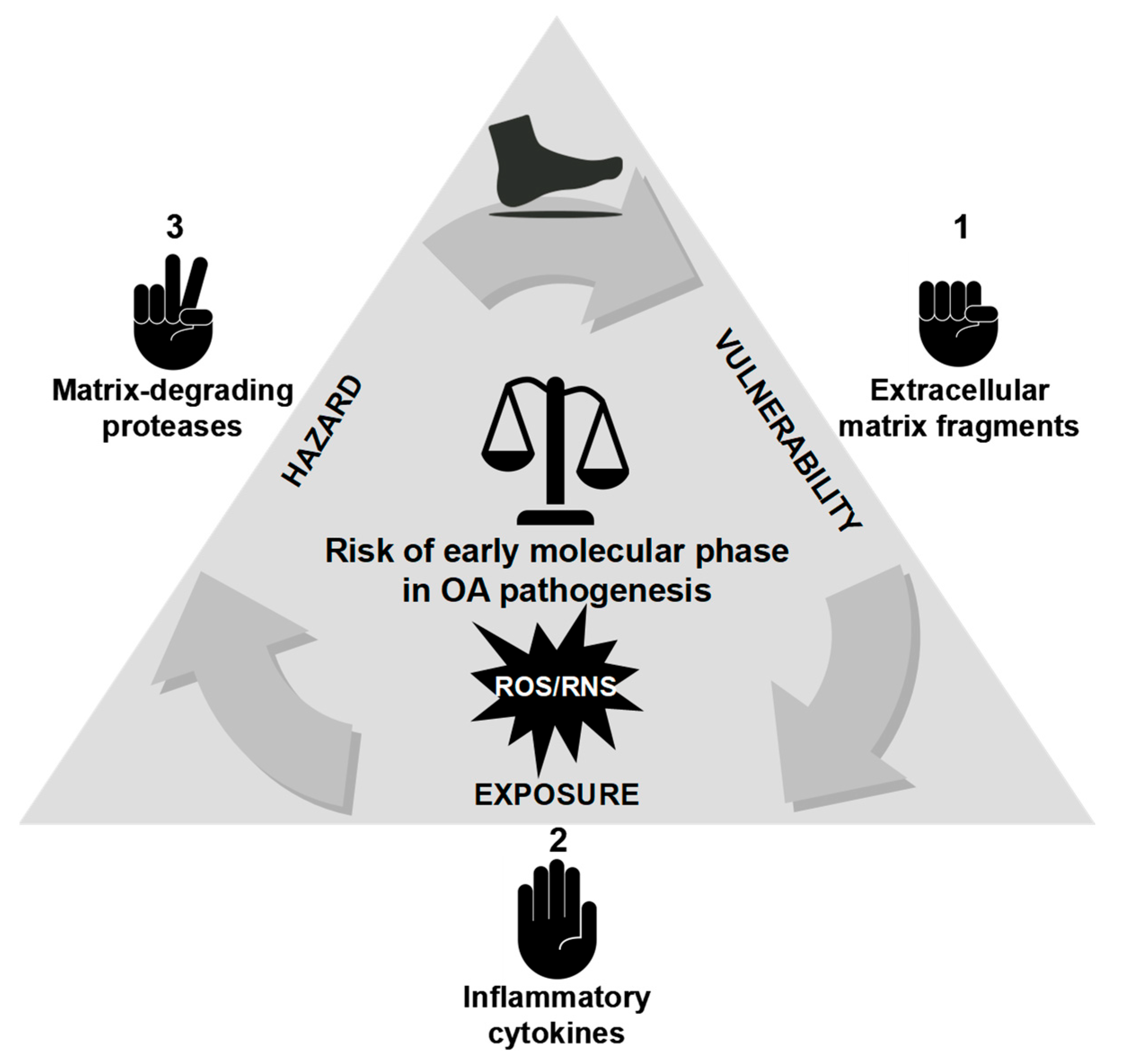
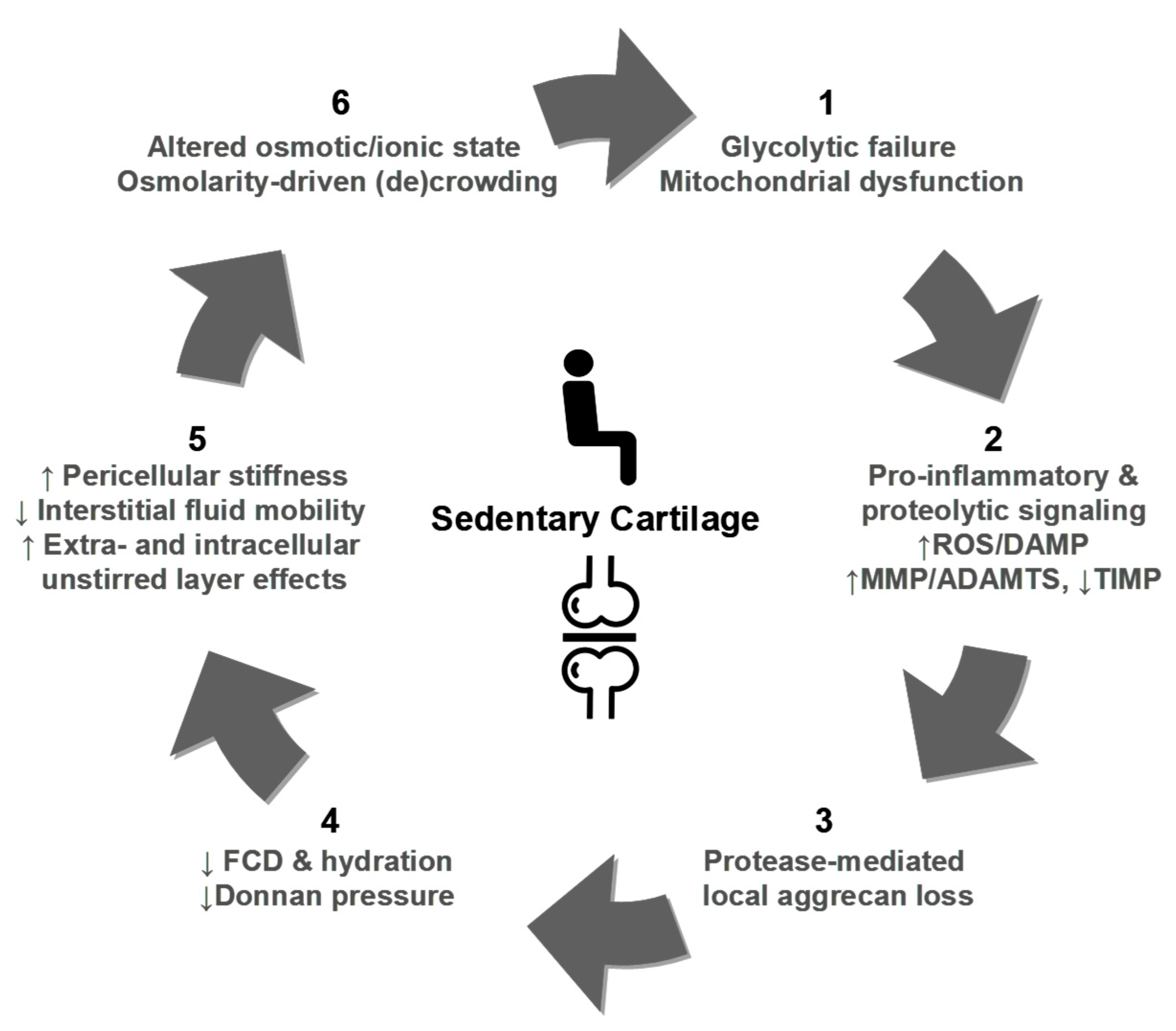
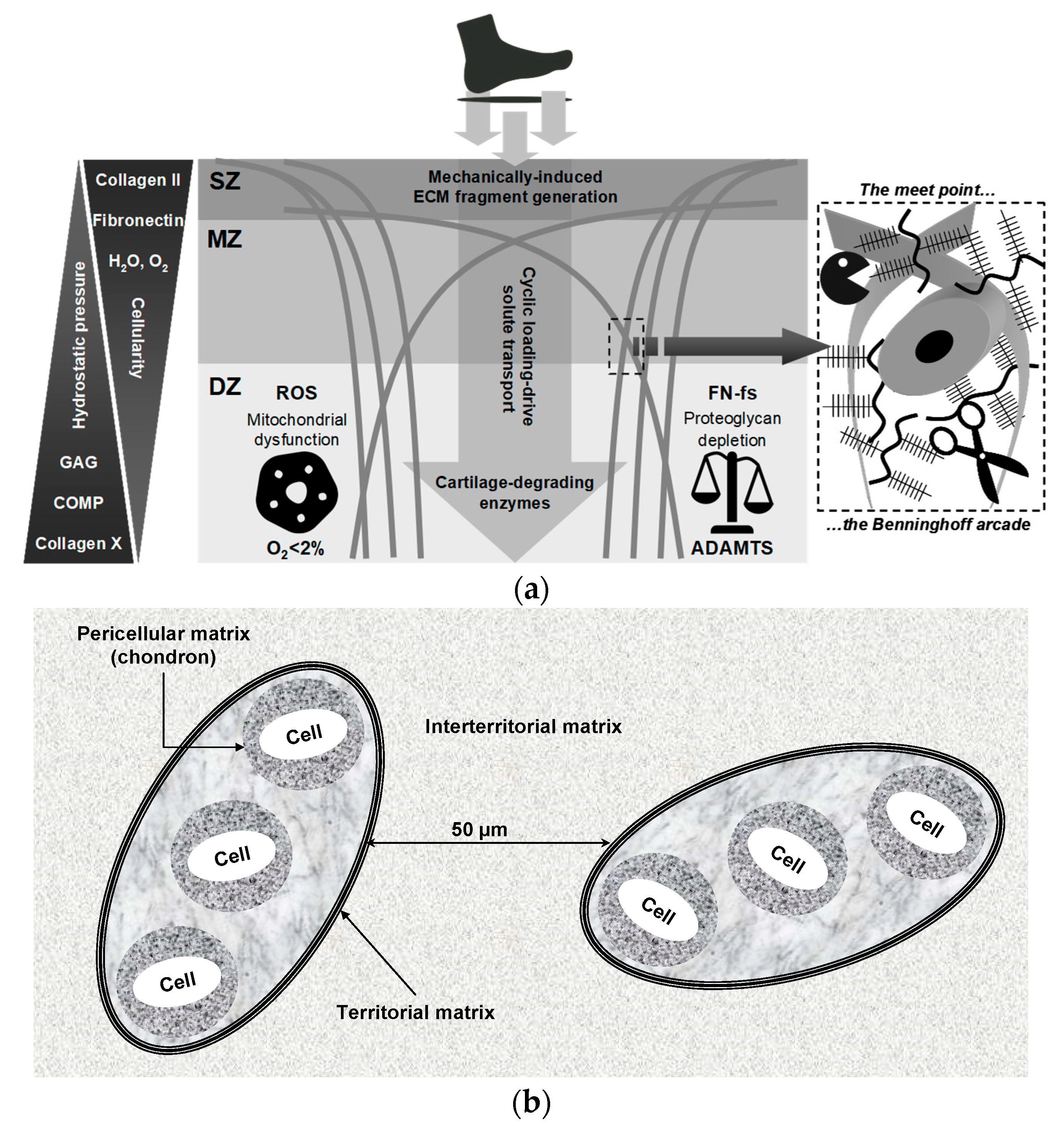
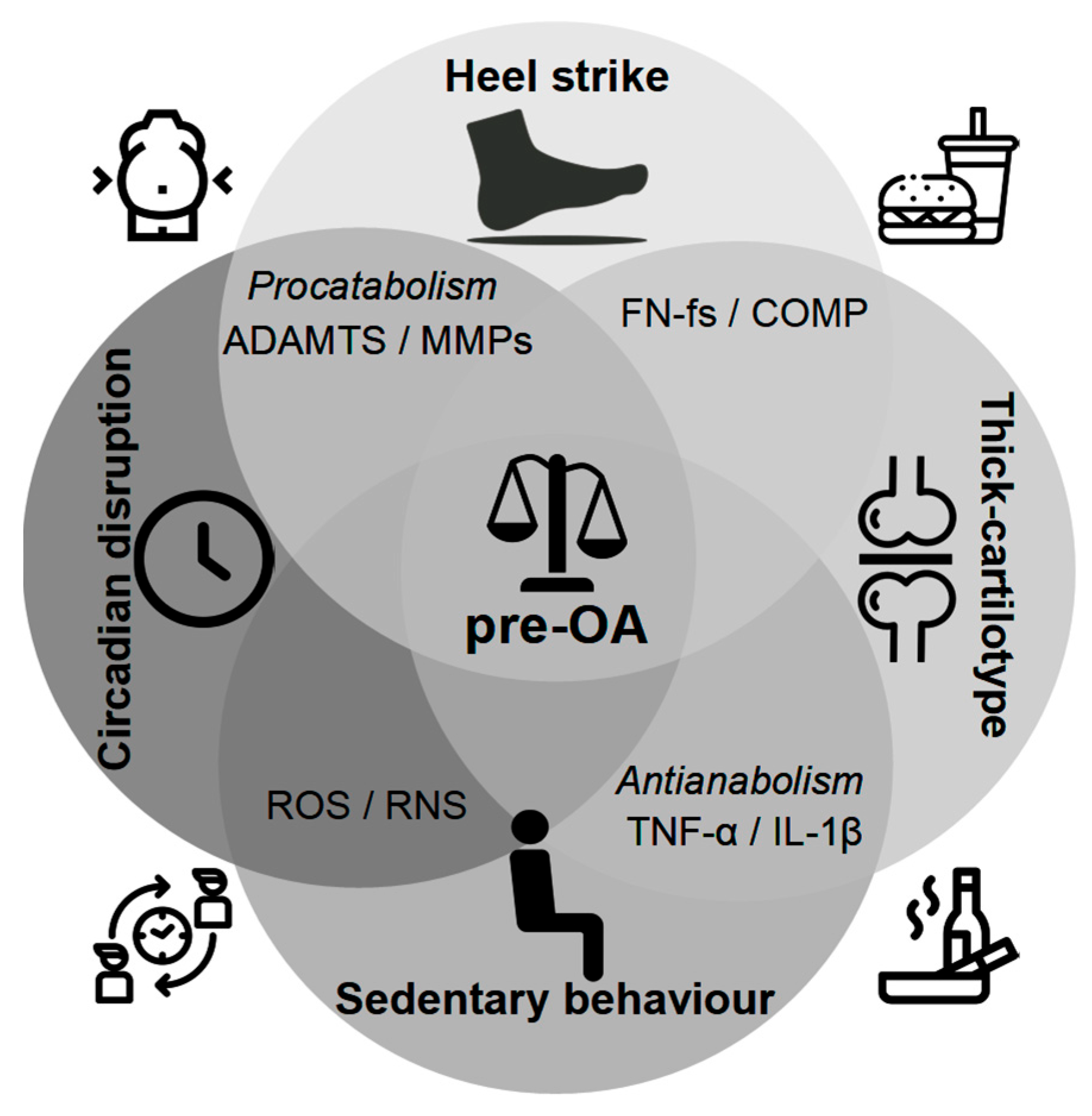
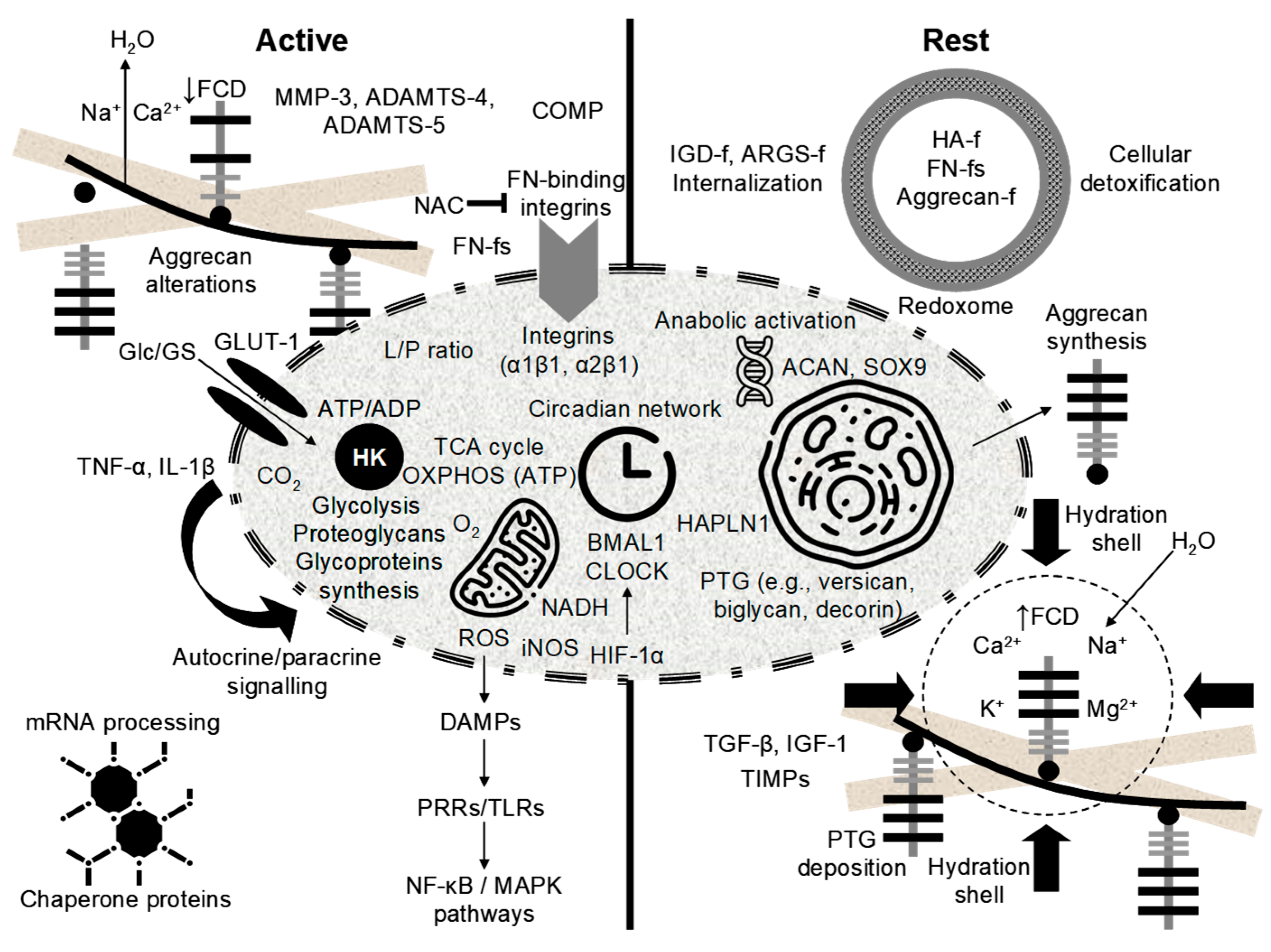
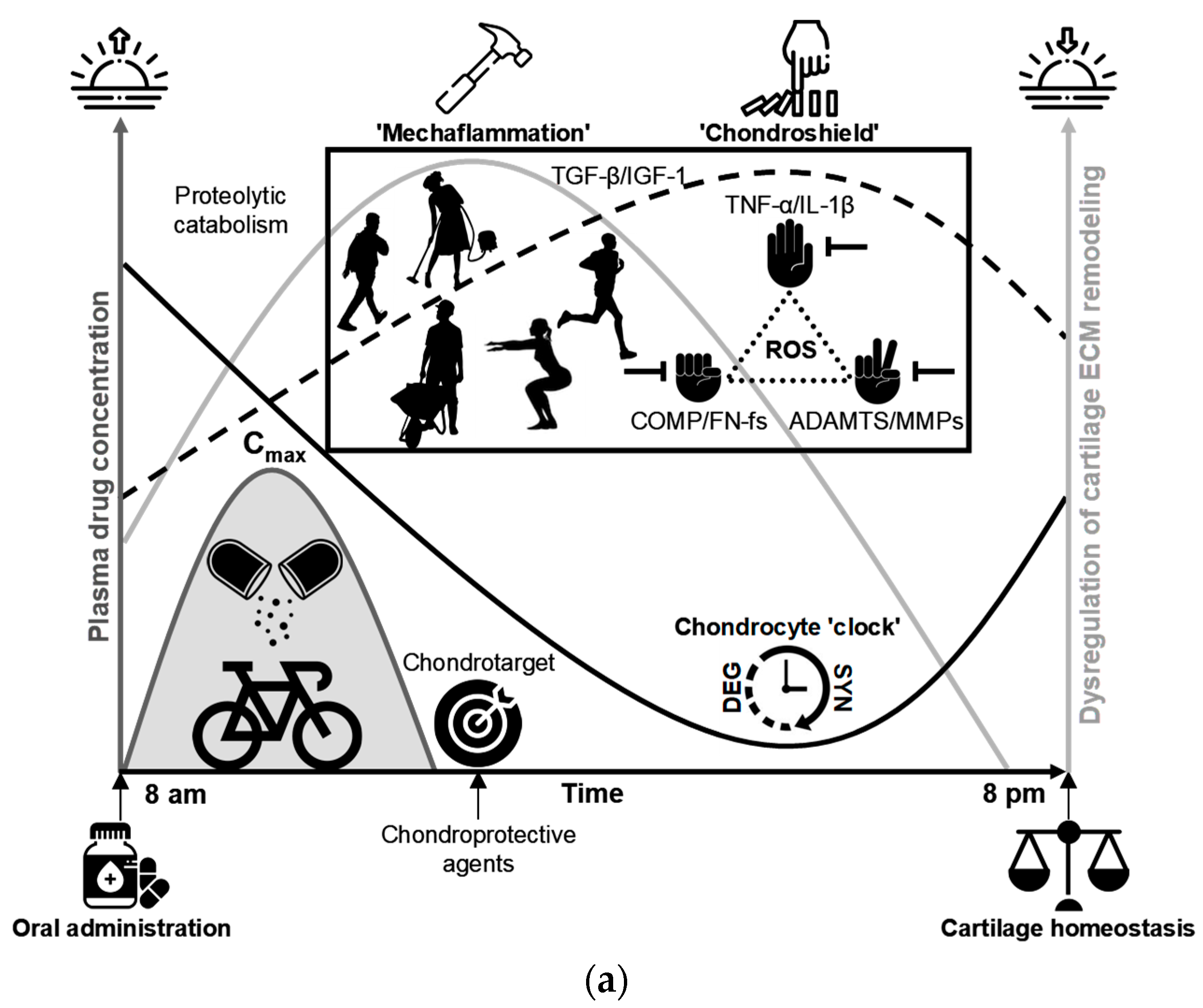
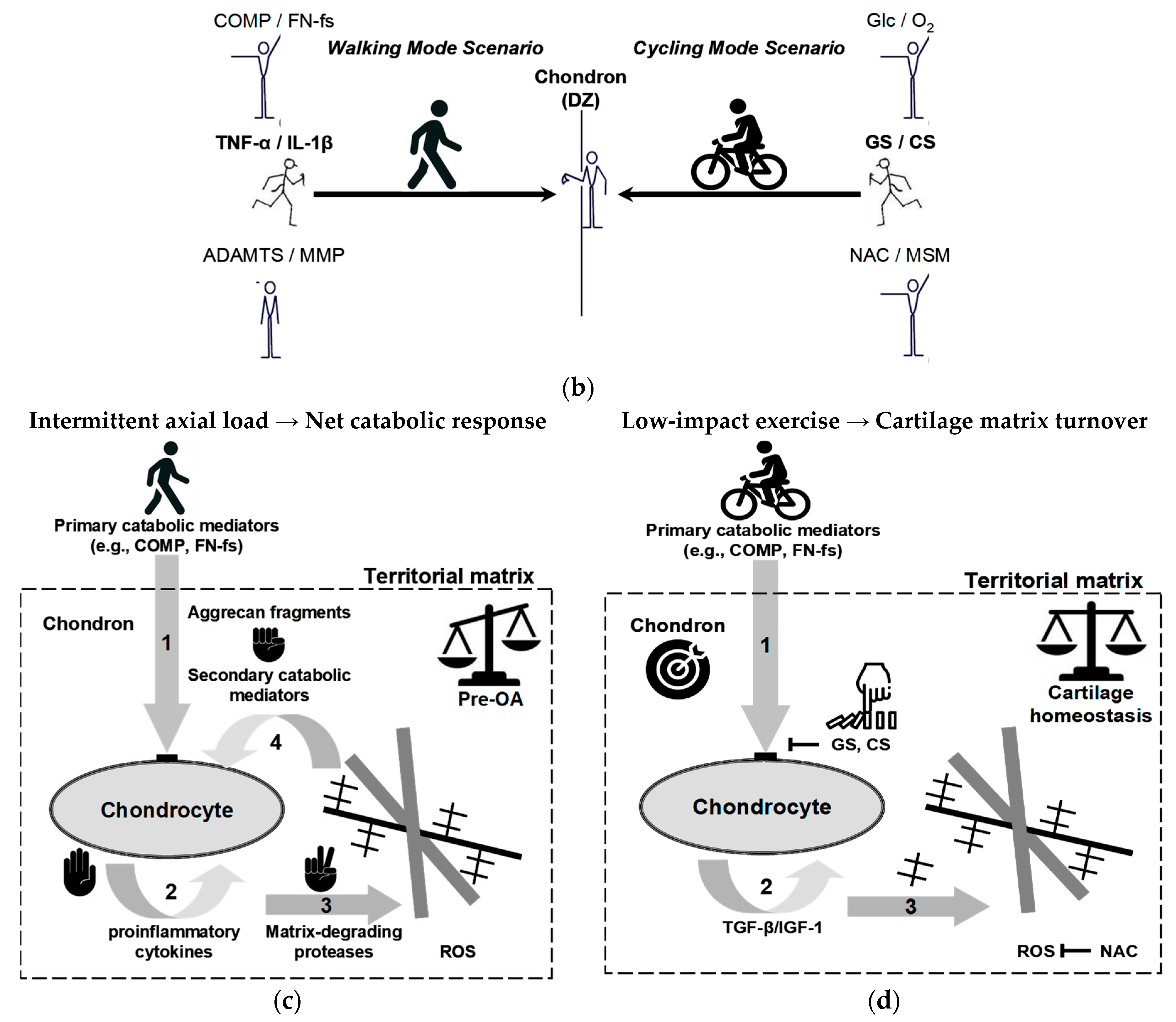
| Stage | Characteristics | Clinical Manifestations | Diagnostic Criteria |
|---|---|---|---|
| Pre-OA | No structural damage; at-risk joint with early molecular or biomechanical perturbations; imbalance in anabolic/catabolic signaling; metabolic dysregulation in predisposed individuals (injury, obesity, genetic or epigenetic factors) | No clinical signs; occasional joint sensitivity; risk stratification based on molecular biomarkers and imaging | Relative elevations in early turnover serum or synovial biomarkers (e.g., COMP, ARGS-aggrecan and hyaluronic acid fragments, CRTAC1 1) or low-grade inflammatory signals compared with controls (increased activity/expression of selected ILs and MMPs), indicating net catabolic balance; elevated leptin and adiponectin in obese individuals. Normal radiographs (KL 0); MRI T2 mapping showing altered cartilage hydration; dGEMRIC signal alteration without focal cartilage defect. Subtle gait changes or altered joint loading in at-risk groups (e.g., post-ACL injury, obesity-related overload). |
| Early OA | Initial cartilage breakdown; collagen disorganization and softening; emergence of SASP, chondrocyte hypertrophy, and apoptosis; low-grade inflammation; minor or often undetectable changes on imaging; minimal morphologic change | Asymptomatic or intermittent, activity-related pain or stiffness; no objective joint swelling or crepitus; no or minimal functional impairment; no effusion | Slightly elevated catabolic markers (sHA, sCOMP, and sMMP-3, Coll2-, uCTX-II, CPII, C2C, C1,2C); early inflammatory cytokines (TNF-α, IL-1β, IL-6) in synovial fluid/serum; altered cartilage methylation profiles. Normal radiographs (no JSN or osteophytes) or equivocal (KL 0–1); dGEMRIC/T1ρ signal change; MRI showing superficial fibrillation, focal cartilage softening. Abnormal gait kinetics; reduced shock absorption; asymmetrical loading pattern; subtle decline in muscle strength or proprioception. |
| Mild OA | Focal cartilage thinning; JSN visible on radiographs; early subchondral bone remodeling; small osteophytes | Activity-related joint discomfort; transient morning stiffness (<30 min); slight functional limitations | Radiographic KL 1–2 with focal JSN/osteophytes; MRI showing focal cartilage defect, small-to-moderate BMLs; ultrasound showing synovial thickening absent or mild. Elevated cartilage degradation markers (COMP, CTX-II), altered CPII/C2C ratios indicating increased catabolism; synovial cytokine profile variable. Minor decrease in walking speed or joint stability; quadriceps weakness, altered gait patterns. |
| Moderate OA | Marked cartilage loss; osteophyte enlargement; subchondral sclerosis; cartilage fissuring or partial thickness loss; synovial hypertrophy; synovitis | Persistent pain; crepitus; intermittent effusion; functional decline; reduced ROM | KL grade 2–3 with definite JSN, evident osteophytes, sclerosis; MRI showing full-thickness focal defects, larger BMLs, synovitis on contrast-enhanced sequences. High levels of matrix degradation markers (MMP-13, CTX-II, AGEs), increased inflammatory mediators (IL-6); synovial fluid shows inflammatory cell increases. Reduced loading tolerance; gait asymmetry; quadriceps weakness; reduced 6-min walk distance; decline in timed-up-and-go; WOMAC function score ≥40. |
| Severe OA | Near-complete cartilage loss or complete erosion; bone-on-bone contact; large osteophytes; joint deformity and fibrosis; synovial thickening and persistent inflammation | Constant pain (including at rest/night); marked stiffness; swelling; instability/deformity; severe functional limitation; disability | KL grade 3–4; marked JSN; large osteophytes; subchondral cysts; MRI showing diffuse cartilage loss and large BMLs; osteophytes; joint deformity; synovial hypertrophy/fibrosis. Plateau or decline in cartilage turnover markers with persistent systemic inflammation (hsCRP, IL-6); elevated N-α-acetyl-L-asparagine 2 as a metabolic marker of advanced OA; high viscosity changes, abundant inflammatory cells, and increased catabolic enzyme activity. Severe mobility limitation, need for assistive devices, or surgical indication (joint replacement candidate). |
| Key Activators | Primary Receptor | Major Signalling Pathways | Principal Downstream Effects |
|---|---|---|---|
| ECM fragments (MDPs)—e.g., FN-fs, COMP, aggrecan ARGS peptides, LMW-HA, tenascin-C | Integrins (α5β1, etc.), CD44, TLR2/TLR4 (and other innate sensors) | NF-κB; MAPKs (p38, ERK, JNK); can promote NLRP3 inflammasome | Induction of pro-inflammatory cytokines and chemokines; upregulation of MMPs/ADAMTS; synovial inflammation, chondrocyte catabolic shift, feed-forward ECM degradation and pain sensitization |
| Pro-inflammatory cytokines—e.g., IL-1β, TNF-α, IL-6 (classical & trans-signalling) | IL-1R1 (+IL-1RAcP); TNFR1/2; IL-6R/gp130 (±sIL-6R) | MyD88 → NF-κB; MAPK; JAK/STAT3 (IL-6); AP-1 | Strong induction of proteases (MMPs, ADAMTS), suppression of ECM biosynthesis (aggrecan, collagen II), chondrocyte hypertrophy/apoptosis, synovitis and bone remodeling |
| Matrix-degrading proteases—e.g., ADAMTS-5, MMP-13, cathepsin K | Secreted enzymes (their cleavage products engage CD44, TLRs, integrins on neighboring cells) | Proteolytic generation of DAMPs → secondary activation of NF-κB/MAPK and downstream transcriptional programs | Proteolysis of aggrecan and collagen → loss of cartilage biomechanical integrity, irreversible collagen network breakdown, progression of structural OA and amplification of inflammatory/proteolytic loops |
| Gene | Role (Anabolic/Catabolic/Clock/Signalling) | Effect of Clock Disruption or Manipulation | Model Sample/Reference |
|---|---|---|---|
| BMAL1 (ARNTL) | Clock/pro-homeostasis | Chondrocyte-specific Bmal1 ablation abolishes local cartilage circadian rhythms and reduces anabolic markers (SOX9, ACAN, COL2A1) while impairing TGF-β signaling (reduced p-SMAD2/3) and upregulates catabolic proteases (MMP1, MMP3, MMP13, MMP14, ADAMTS5, ADAMTS5), shifting chondrocytes into a catabolic, repair-deficient state that leads to progressive cartilage degeneration | Chondrocyte-specific Bmal1 KO (Col2a1-Cre; Bmal1), PER2::LUC cartilage; mouse and human cartilage [211,212,213,214,217] |
| CLOCK | Clock (activator with BMAL1) | Perturbation of the CLOCK:BMAL1 transcriptional axis dampens rhythmic expression of cartilage matrix and metabolic genes and shifts transcriptional programmes toward catabolic profiles | Murine cartilage time-series and cultured chondrocytes; circadian transcriptomics [209] |
| PER1 | Clock (negative limb/signalling) | Rhythm disruption or altered PER1 expression associates with increased inflammatory sensitivity and higher expression of matrix-degrading enzymes in cartilage/rhythm-disruption models | Primary chondrocytes, cartilage explants and in vivo rhythm-disruption (jet-lag/inflammatory stimuli) models [209,223] |
| PER2 | Clock (negative limb/reporter) | PER2::Luc reports robust cartilage rhythms that are dampened with ageing/OA; PER2 loss/dampening reduces protective rhythmic responses and modifies inflammatory/matrix outcomes | PER2::LUC reporter cartilage, primary chondrocytes (mouse/human); time-series bioluminescence and gene expression [209] |
| CRY1 | Clock (negative limb) | Reduced CRY1 amplitude reported in OA/damaged cartilage; decreased CRY1 correlates with heightened catabolic/inflammatory gene induction | Human OA chondrocytes, mouse cartilage (expression profiling/KO analyses) [215] |
| CRY2 | Clock (negative limb) | CRY2 expression/amplitude is reduced in OA; Cry2 deficiency increases OA severity in experimental models and is associated with upregulated MMP/ADAMTS catabolic programmes | Human OA samples and Cry2 KO mouse OA models; RNA-seq and histopathology [215] |
| NR1D1 (REV-ERBα) | Clock/signalling (repressor) | NR1D1/REV-ERBα downregulation in aging/OA cartilage; genetic or pharmacologic perturbation modifies TGF-β signalling and matrix gene expression and alters mechanotransduction responses | Human chondrocyte cultures, cartilage explants and OA models; knockdown/agonist studies [220,221] |
| RORα (RORA) | Clock/transcriptional regulator | RORα contributes to transcriptional control of inflammatory and metabolic targets in chondrocytes; modulation of RORα changes cytokine responsiveness and downstream catabolic signalling | In vitro human chondrocytes and experimental cartilage injury models; targeted perturbation studies [216] |
| BHLHE40 (DEC1)/BHLHE41 (DEC2) | Clock/signalling (repressors & stress-responsive) | Stress/inflammatory upregulation of DEC1/DEC2 perturbs clock outputs in chondrocytes and promotes pro-catabolic signalling; manipulation alters rhythmic matrix/inflammatory gene expression | Primary chondrocytes under inflammatory or mechanical stress; cartilage injury models and circadian studies [216,219] |
| ECM Cartilage | Location/Appearance | Major Components (Representative) | Primary Function/Mechanical Role |
|---|---|---|---|
| Pericellular | Immediately surrounds individual chondrocytes (chondron); thin capsule often highlighted by type VI collagen immunostain | Type VI collagen; perlecan (heparan sulfate proteoglycan); small leucine-rich proteoglycans (decorin, biglycan); hyaluronan; multi-adhesive link proteins or hyaluronan- and proteoglycan-binding proteins (e.g., HAPLN1) | Specialized pericellular microenvironment for mechanotransduction and cell–matrix signaling; attenuates cell-level strains and mediates biochemical exchange |
| Territorial | Encircles the pericellular matrix as an intensely basophilic “rim” (basket-like) around single cells or cell clusters | High local aggrecan concentration (proteoglycan-rich); type II collagen present in dense/disorganized bundles; hyaluronan; link proteins | Local compressive buffering and high fixed-charge density near cells; mechanical shielding of chondrons and modulation of ion/water movement |
| Interterritorial | Bulk ECM between chondrons; less basophilic than the territorial rim; shows organized collagen fibrils under polarized light | Type II collagen fibrillar network (major bulk collagen); aggrecan aggregates bound to hyaluronan; high tissue hydration | Primary tissue-level load bearing—tensile strength from collagen network and compressive resistance/resilience from proteoglycans and water |
| Phase | Peak Pathway (s) | Representative Molecules | Cartilage Function |
|---|---|---|---|
| Morning (early rest) | Cytoskeletal remodelling; proteasomal degradation | PSMB7, PSMD2, PSMD5; PLOD1/2 (collagen cross-linking enzymes); SERPINE1 (protease inhibitor) | Supports structural rearrangements; proteasomal turnover removes damaged proteins, while PLOD-mediated cross-linking and SERPINE1 activity stabilize collagens and prevent premature degradation, enhancing tensile strength |
| Afternoon (late rest) | Protein synthesis (e.g., ribosomal function) | RPL5, RPL23a, RPS3a1 | Upregulated translation machinery supports de novo synthesis of matrix components (e.g., glycoproteins) |
| Evening (early active) | ATP synthesis (energy metabolism) | HSPA9, HSP90AA1, HSP90AB1; MATN1; SERPINE1; PLOD2 | Chondrocytes increase glycolysis and oxidative phosphorylation to meet energy demands |
| Night (late active) | Glucose metabolism; proteostasis | SLC2A1 (GLUT1); PKM (pyruvate kinase); protein synthesis (e.g., molecular chaperones) | Increased glucose uptake, while chaperone expression support mechanical resilience |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Río, E. A Multidimensional Definition of Pre-Osteoarthritis: Toward 21st-Century Subclinical Detection and Targeted Intervention. Int. J. Mol. Sci. 2025, 26, 11447. https://doi.org/10.3390/ijms262311447
del Río E. A Multidimensional Definition of Pre-Osteoarthritis: Toward 21st-Century Subclinical Detection and Targeted Intervention. International Journal of Molecular Sciences. 2025; 26(23):11447. https://doi.org/10.3390/ijms262311447
Chicago/Turabian Styledel Río, Eloy. 2025. "A Multidimensional Definition of Pre-Osteoarthritis: Toward 21st-Century Subclinical Detection and Targeted Intervention" International Journal of Molecular Sciences 26, no. 23: 11447. https://doi.org/10.3390/ijms262311447
APA Styledel Río, E. (2025). A Multidimensional Definition of Pre-Osteoarthritis: Toward 21st-Century Subclinical Detection and Targeted Intervention. International Journal of Molecular Sciences, 26(23), 11447. https://doi.org/10.3390/ijms262311447






