Noradrenergic Slow Vasomotion: The Hidden Fluid Pump Linking Sleep, Brain Clearance, and Dementia Pathogenesis
Abstract
1. Introduction—Noradrenergic Vasomotion and the Hidden Dynamics of Brain Clearance
- Section 2 describes the anatomical and neurovascular organization of the noradrenergic vasomotion axis.
- Section 3 documents experimental evidence demonstrating modulation of and causality in clearance of this axis.
- Section 4 describes dysfunction of this axis in aging and disease.
- Section 5 reviews therapeutic approaches to enhance or restore the vasomotor pump.
- Section 6 outlines future directions, technological developments, and implications for translational neurobiology.
2. Architecture of the Noradrenergic Vasomotion Axis
2.1. Neural Origins and Neuro-Modulatory Dynamics
2.2. Vascular Mechanics, Perivascular Hydrodynamics, and Glial Interfaces
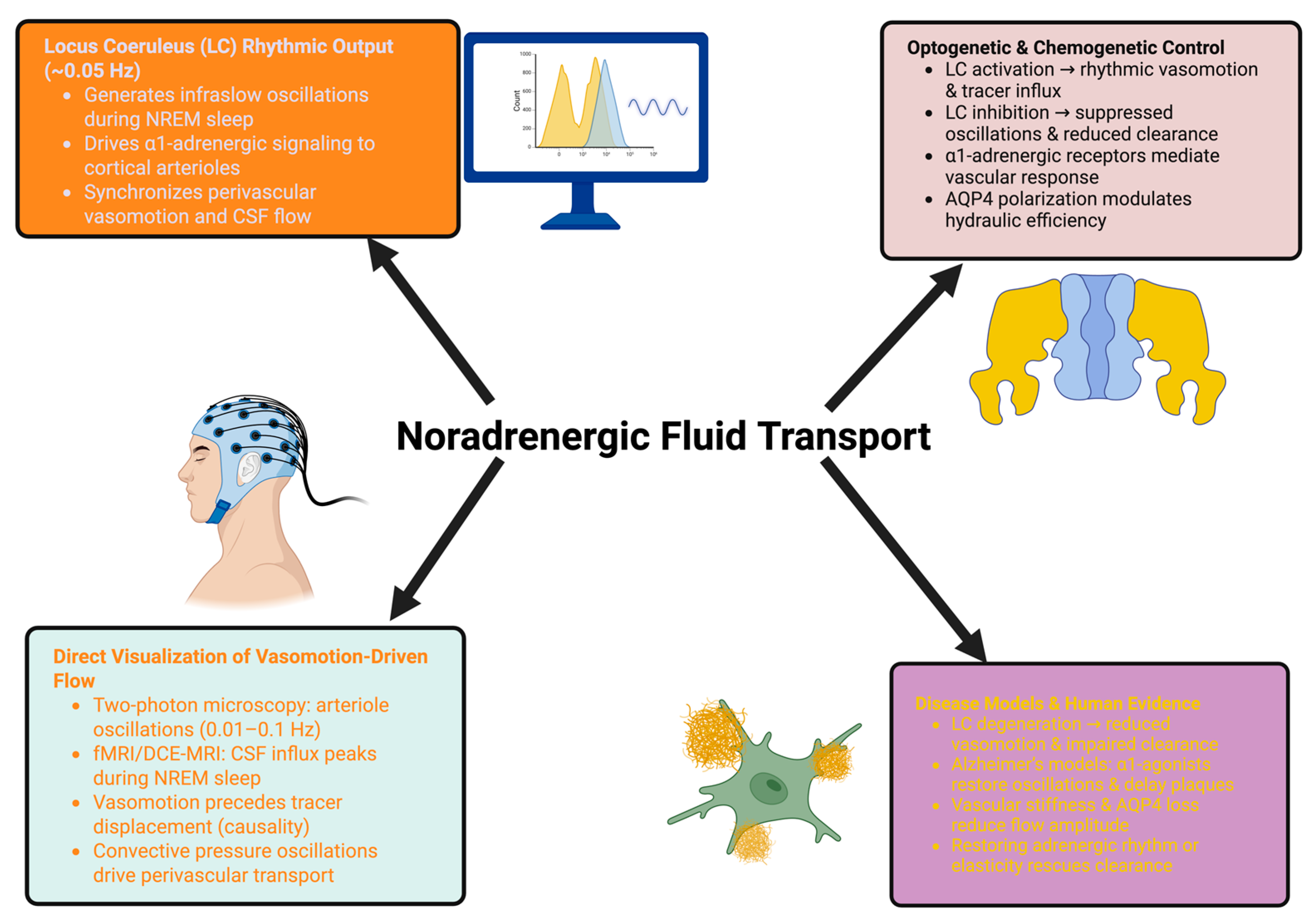
3. Experimental Evidence for a Tunable Noradrenergic Fluid Pump
3.1. Direct Manipulation of the Noradrenergic System Demonstrates Causality
3.2. Imaging, Biophysical, and Pharmacological Evidence of State-Dependent Fluid Transport
3.3. Pathological Disruption, Human Evidence, and Therapeutic Tunability
- Causal level: LC manipulation is sufficient to drive perivascular flow.
- Mechanistic level: Imaging and modeling show temporal precedence, frequency dependence, and biophysical feasibility.
4. Collapse of the Vasomotor Pump in Aging and Disease
4.1. Degeneration of Noradrenergic Rhythms: The Erosion of Systemic Drive
4.2. Vascular Stiffening and Network Remodeling: Mechanical Decoupling of the Pump
4.3. Astro-Glial Disruption and Perivascular Interface Breakdown: The Final Bottleneck
Conclusion of Section 4
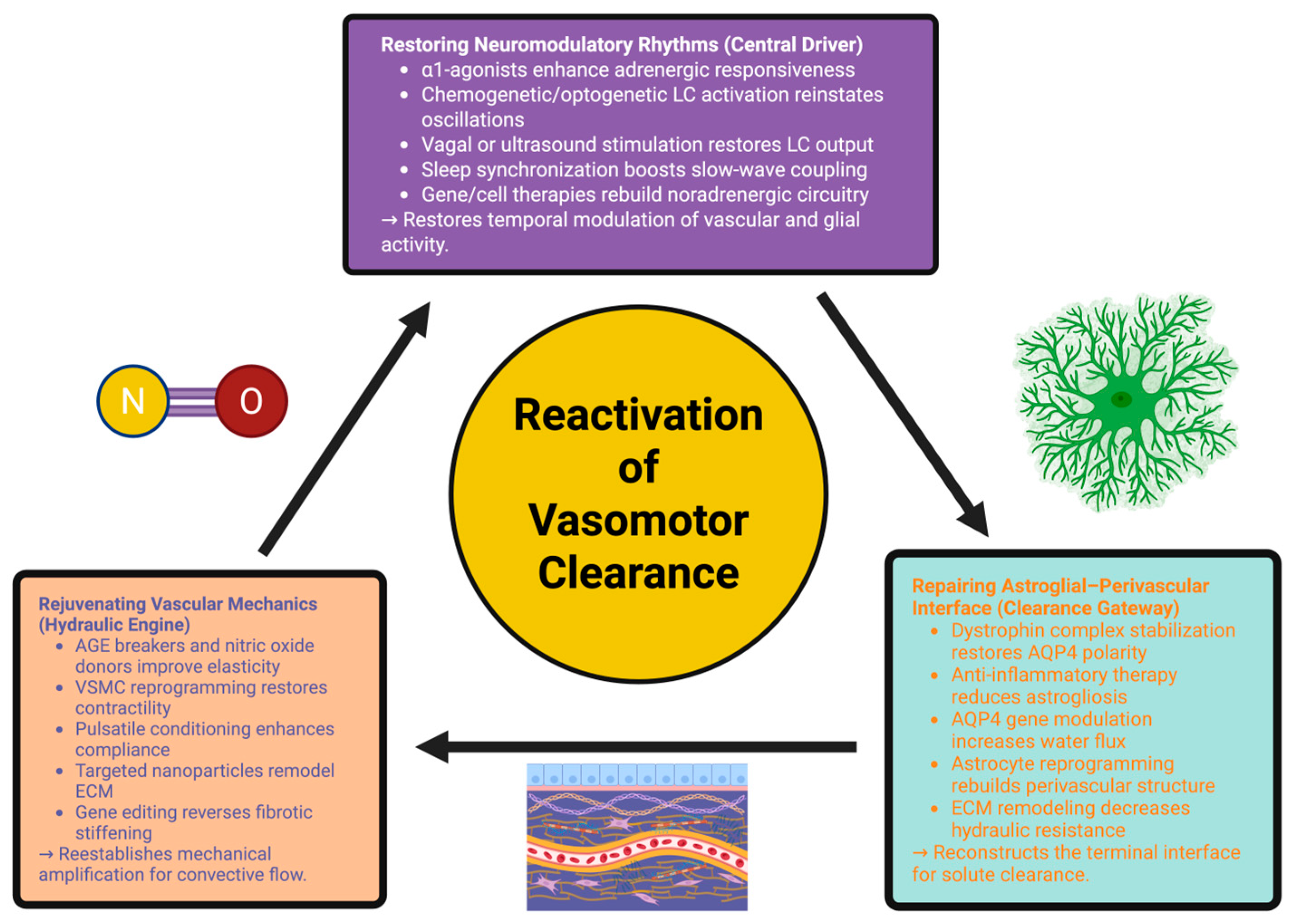
5. Therapeutic Reactivation of the Vasomotor Clearance System
5.1. Restoring Neuro-Modulatory Rhythms: Reviving the Central Driver
5.2. Rejuvenating Vascular Mechanics: Restoring the Hydraulic Engine
5.3. Repairing the Astro-Glial–Perivascular Interface: Rebuilding the Clearance Gateway
5.4. Clinical Feasibility, Risks, and Translational Steps
Conclusion of Section 5
6. Translational Horizons and Future Directions
6.1. Clearance as a Tunable Systems-Level Function: A Paradigm Shift
6.2. Tools to Measure and Modulate the Pump in Humans
6.3. Precision Neurotherapeutics and the Rebuilding of Brain Homeostasis
Conclusion of Section 6
7. Systems-Level Implications Beyond Disease
7.1. Cognitive Homeostasis and Network Stability: Clearance as a Prerequisite for Information Processing
7.2. Sleep Architecture, Memory Consolidation, and the Temporal Orchestration of Clearance
7.3. Neuroimmune Communication, Systemic Signaling, and the Expansion of the Clearance Paradigm
Conclusion of Section 7
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang-Xie, L.-F.; Drieu, A.; Bhasiin, K.; Quintero, D.; Smirnov, I.; Kipnis, J. Neuronal Dynamics Direct Cerebrospinal Fluid Perfusion and Brain Clearance. Nature 2024, 627, 157–164. [Google Scholar] [CrossRef]
- Ma, J.; Chen, M.; Liu, G.-H.; Gao, M.; Chen, N.-H.; Toh, C.H.; Hsu, J.-L.; Wu, K.-Y.; Huang, C.-M.; Lin, C.-M.; et al. Effects of Sleep on the Glymphatic Functioning and Multimodal Human Brain Network Affecting Memory in Older Adults. Mol. Psychiatry 2025, 30, 1717–1729. [Google Scholar] [CrossRef]
- Mestre, H.; Hablitz, L.M.; Xavier, A.L.; Feng, W.; Zou, W.; Pu, T.; Monai, H.; Murlidharan, G.; Castellanos Rivera, R.M.; Simon, M.J.; et al. Aquaporin-4-Dependent Glymphatic Solute Transport in the Rodent Brain. eLife 2018, 7, e40070. [Google Scholar] [CrossRef]
- Vasciaveo, V.; Iadarola, A.; Casile, A.; Dante, D.; Morello, G.; Minotta, L.; Tamagno, E.; Cicolin, A.; Guglielmotto, M. Sleep Fragmentation Affects Glymphatic System through the Different Expression of AQP4 in Wild Type and 5xFAD Mouse Models. Acta Neuropathol. Commun. 2023, 11, 16. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Alterations in Brain Fluid Physiology during the Early Stages of Development of Ischaemic Oedema. Fluids Barriers CNS 2024, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Forero, A.; Foustoukos, G.; Cardis, R.; Cherrad, N.; Devenoges, C.; Fernandez, L.M.J.; Lüthi, A. Infraslow Noradrenergic Locus Coeruleus Activity Fluctuations Are Gatekeepers of the NREM-REM Sleep Cycle. Nat. Neurosci. 2025, 28, 84–96. [Google Scholar] [CrossRef]
- Osorio-Forero, A.; Cherrad, N.; Banterle, L.; Fernandez, L.M.J.; Lüthi, A. When the Locus Coeruleus Speaks Up in Sleep: Recent Insights, Emerging Perspectives. Int. J. Mol. Sci. 2022, 23, 5028. [Google Scholar] [CrossRef] [PubMed]
- Mayrovitz, H.N. Consideration of the Role of Vasomotion-Induced Flowmotion on Microvascular Blood Flow. Cureus 2025, 17, e89919. [Google Scholar] [CrossRef]
- Kedarasetti, R.T.; Drew, P.J.; Costanzo, F. Arterial Pulsations Drive Oscillatory Flow of CSF but Not Directional Pumping. Sci. Rep. 2020, 10, 10102. [Google Scholar] [CrossRef]
- Kedarasetti, R.T.; Drew, P.J.; Costanzo, F. Arterial Vasodilation Drives Convective Fluid Flow in the Brain: A Poroelastic Model. Fluids Barriers CNS 2022, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Dabiri, S.; Ardekani, A.M. Perivascular Interactions and Tissue Properties Modulate Directional Glymphatic Transport in the Brain. Fluids Barriers CNS 2025, 22, 63. [Google Scholar] [CrossRef]
- Pikuleva, I.A.; Curcio, C.A. Cholesterol in the Retina: The Best Is yet to Come. Prog. Retin. Eye Res. 2014, 41, 64–89. [Google Scholar] [CrossRef]
- Tseng, C.-T.; Welch, H.F.; Gi, A.L.; Kang, E.M.; Mamidi, T.; Pydimarri, S.; Ramesh, K.; Sandoval, A.; Ploski, J.E.; Thorn, C.A. Frequency Specific Optogenetic Stimulation of the Locus Coeruleus Induces Task-Relevant Plasticity in the Motor Cortex. J. Neurosci. 2024, 44, e1528232023. [Google Scholar] [CrossRef]
- Masamoto, K.; Vazquez, A. Optical Imaging and Modulation of Neurovascular Responses. J. Cereb. Blood Flow Metab. 2018, 38, 2057–2072. [Google Scholar] [CrossRef] [PubMed]
- Broggini, T.; Duckworth, J.; Ji, X.; Liu, R.; Xia, X.; Mächler, P.; Shaked, I.; Munting, L.P.; Iyengar, S.; Kotlikoff, M.; et al. Long-Wavelength Traveling Waves of Vasomotion Modulate the Perfusion of Cortex. Neuron 2024, 112, 2349–2367.e8. [Google Scholar] [CrossRef] [PubMed]
- Rábago-Monzón, Á.R.; Osuna-Ramos, J.F.; Armienta-Rojas, D.A.; Camberos-Barraza, J.; Camacho-Zamora, A.; Magaña-Gómez, J.A.; De la Herrán-Arita, A.K. Stress-Induced Sleep Dysregulation: The Roles of Astrocytes and Microglia in Neurodegenerative and Psychiatric Disorders. Biomedicines 2025, 13, 1121. [Google Scholar] [CrossRef]
- Caestecker, S.; Lescrauwaet, E.; Boon, P.; Carrette, E.; Raedt, R.; Vonck, K. The Locus Coeruleus—Noradrenergic System in the Healthy and Diseased Brain: A Narrative Review. Eur. J. Neurol. 2025, 32, e70337. [Google Scholar] [CrossRef]
- MacVicar, B.A.; Newman, E.A. Astrocyte Regulation of Blood Flow in the Brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a020388. [Google Scholar] [CrossRef]
- Giri, P.M.; Banerjee, A.; Ghosal, A.; Layek, B. Neuroinflammation in Neurodegenerative Disorders: Current Knowledge and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 3995. [Google Scholar] [CrossRef] [PubMed]
- Eagleman, S.L.; Drover, C.M.; Drover, D.R.; Ouellette, N.T.; MacIver, M.B. Remifentanil and Nitrous Oxide Anesthesia Produces a Unique Pattern of EEG Activity During Loss and Recovery of Response. Front. Hum. Neurosci. 2018, 12, 173. [Google Scholar] [CrossRef]
- Mohs, R.C.; Greig, N.H. Drug Discovery and Development: Role of Basic Biological Research. Alzheimers Dement. Transl. Res. Clin. Interv. 2017, 3, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, B.; Pauza, D.H.; Limback, C.; Ottaviani, G.; Thiene, G. From Psychostasis to the Discovery of Cardiac Nerves: The Origins of the Modern Cardiac Neuromodulation Concept. Biology 2024, 13, 266. [Google Scholar] [CrossRef]
- Billwiller, F.; Castillo, L.; Elseedy, H.; Ivanov, A.I.; Scapula, J.; Ghestem, A.; Carponcy, J.; Libourel, P.A.; Bras, H.; Abdelmeguid, N.E.; et al. GABA–Glutamate Supramammillary Neurons Control Theta and Gamma Oscillations in the Dentate Gyrus during Paradoxical (REM) Sleep. Brain Struct. Funct. 2020, 225, 2643–2668. [Google Scholar] [CrossRef]
- Toyoda, H.; Won, J.; Kim, W.; Kim, H.; Davy, O.; Saito, M.; Kim, D.; Tanaka, T.; Kang, Y.; Oh, S.B. The Nature of Noradrenergic Volume Transmission From Locus Coeruleus to Brainstem Mesencephalic Trigeminal Sensory Neurons. Front. Cell. Neurosci. 2022, 16, 841239. [Google Scholar] [CrossRef]
- Osorio-Forero, A.; Cardis, R.; Vantomme, G.; Guillaume-Gentil, A.; Katsioudi, G.; Devenoges, C.; Fernandez, L.M.J.; Lüthi, A. Noradrenergic Circuit Control of Non-REM Sleep Substates. Curr. Biol. 2021, 31, 5009–5023.e7. [Google Scholar] [CrossRef]
- Parichatikanond, W.; Duangrat, R.; Kurose, H.; Mangmool, S. Regulation of β-Adrenergic Receptors in the Heart: A Review on Emerging Therapeutic Strategies for Heart Failure. Cells 2024, 13, 1674. [Google Scholar] [CrossRef] [PubMed]
- Watson, B.O. Cognitive and Physiologic Impacts of the Infraslow Oscillation. Front. Syst. Neurosci. 2018, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.W.; Hannigan, K.I.; Ghosh, D.; Xu, B.; Del Villar, S.G.; Xiang, Y.K.; Dickson, E.J.; Navedo, M.F.; Dixon, R.E. β-Adrenergic-Mediated Dynamic Augmentation of Sarcolemmal CaV 1.2 Clustering and Co-Operativity in Ventricular Myocytes. J. Physiol. 2019, 597, 2139–2162. [Google Scholar] [CrossRef]
- Speggiorin, M.; Chiavegato, A.; Zonta, M.; Gómez-Gonzalo, M. Characterization of the Astrocyte Calcium Response to Norepinephrine in the Ventral Tegmental Area. Cells 2025, 14, 24. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, W. Bidirectional Role of Pericytes in Ischemic Stroke. Brain Sci. 2025, 15, 605. [Google Scholar] [CrossRef]
- Tóth, R.; Farkas, A.E.; Krizbai, I.A.; Makra, P.; Bari, F.; Farkas, E.; Menyhárt, Á. Astrocyte Ca2+ Waves and Subsequent Non-Synchronized Ca2+ Oscillations Coincide with Arteriole Diameter Changes in Response to Spreading Depolarization. Int. J. Mol. Sci. 2021, 22, 3442. [Google Scholar] [CrossRef]
- Nuszkiewicz, J.; Rzepka, W.; Markiel, J.; Porzych, M.; Woźniak, A.; Szewczyk-Golec, K. Circadian Rhythm Disruptions and Cardiovascular Disease Risk: The Special Role of Melatonin. Curr. Issues Mol. Biol. 2025, 47, 664. [Google Scholar] [CrossRef]
- Sulaman, B.A.; Zhang, Y.; Matosevich, N.; Kjærby, C.; Foustoukos, G.; Andersen, M.; Eban-Rothschild, A. Emerging Functions of Neuromodulation during Sleep. J. Neurosci. 2024, 44, e1277242024. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Fan, X.; Xiang, C.; Lei, X. Altering Temporal Dynamics of Sleepiness and Mood During Sleep Deprivation: Evidence from Resting-State EEG Microstates. Brain Sci. 2025, 15, 423. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Sun, Z.; Hill, M.A.; Meininger, G.A. A Calcium Mediated Mechanism Coordinating Vascular Smooth Muscle Cell Adhesion During KCl Activation. Front. Physiol. 2018, 9, 1810. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Tataru, C.P.; Florian, I.-A.; Covache-Busuioc, R.-A.; Dumitrascu, D.-I.; Glavan, L.A.; Costin, H.P.; Bratu, B.-G.; Ciurea, A.V. From Homeostasis to Pathology: Decoding the Multifaceted Impact of Aquaporins in the Central Nervous System. Int. J. Mol. Sci. 2023, 24, 14340. [Google Scholar] [CrossRef]
- Gu, X.; Chen, W.; Volkow, N.D.; Koretsky, A.P.; Du, C.; Pan, Y. Synchronized Astrocytic Ca2+ Responses in Neurovascular Coupling during Somatosensory Stimulation and for the Resting State. Cell Rep. 2018, 23, 3878–3890. [Google Scholar] [CrossRef]
- Bojarskaite, L.; Bjørnstad, D.M.; Pettersen, K.H.; Cunen, C.; Hermansen, G.H.; Åbjørsbråten, K.S.; Chambers, A.R.; Sprengel, R.; Vervaeke, K.; Tang, W.; et al. Astrocytic Ca2+ Signaling Is Reduced during Sleep and Is Involved in the Regulation of Slow Wave Sleep. Nat. Commun. 2020, 11, 3240. [Google Scholar] [CrossRef]
- Aldea, R.; Weller, R.O.; Wilcock, D.M.; Carare, R.O.; Richardson, G. Cerebrovascular Smooth Muscle Cells as the Drivers of Intramural Periarterial Drainage of the Brain. Front. Aging Neurosci. 2019, 11, 1. [Google Scholar] [CrossRef]
- Yamamoto, E.A.; Bagley, J.H.; Geltzeiler, M.; Sanusi, O.R.; Dogan, A.; Liu, J.J.; Piantino, J. The Perivascular Space Is a Conduit for Cerebrospinal Fluid Flow in Humans: A Proof-of-Principle Report. Proc. Natl. Acad. Sci. USA 2024, 121, e2407246121. [Google Scholar] [CrossRef]
- Daversin-Catty, C.; Vinje, V.; Mardal, K.-A.; Rognes, M.E. The Mechanisms behind Perivascular Fluid Flow. PLoS ONE 2020, 15, e0244442. [Google Scholar] [CrossRef]
- Wichmann, T.O.; Damkier, H.H.; Pedersen, M. A Brief Overview of the Cerebrospinal Fluid System and Its Implications for Brain and Spinal Cord Diseases. Front. Hum. Neurosci. 2022, 15, 737217. [Google Scholar] [CrossRef] [PubMed]
- Claassen, J.A.H.R.; Thijssen, D.H.J.; Panerai, R.B.; Faraci, F.M. Regulation of Cerebral Blood Flow in Humans: Physiology and Clinical Implications of Autoregulation. Physiol. Rev. 2021, 101, 1487–1559. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Owashi, K.; Monnier, H.; Metanbou, S.; Capel, C.; Balédent, O. Cardiac and Respiratory Activities Induce Temporal Changes in Cerebral Blood Volume, Balanced by a Mirror CSF Volume Displacement in the Spinal Canal. NeuroImage 2025, 305, 120988. [Google Scholar] [CrossRef]
- Lehrer, P.M.; Vaschillo, E.G.; Vidali, V. Heart Rate and Breathing Are Not Always in Phase During Resonance Frequency Breathing. Appl. Psychophysiol. Biofeedback 2020, 45, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.M.; McFarland White, K.; Fass, S.B.; Chen, S.; Shi, Z.; Ge, X.; Engelbach, J.A.; Gaines, S.H.; Bice, A.R.; Vasek, M.J.; et al. Evaluation of Gliovascular Functions of AQP4 Readthrough Isoforms. Front. Cell. Neurosci. 2023, 17, 1272391. [Google Scholar] [CrossRef]
- Divecha, Y.A.; Rampes, S.; Tromp, S.; Boyanova, S.T.; Fleckney, A.; Fidanboylu, M.; Thomas, S.A. The Microcirculation, the Blood-Brain Barrier, and the Neurovascular Unit in Health and Alzheimer Disease: The Aberrant Pericyte Is a Central Player. Pharmacol. Rev. 2025, 77, 100052. [Google Scholar] [CrossRef]
- ElSankari, S.; Balédent, O.; van Pesch, V.; Sindic, C.; de Broqueville, Q.; Duprez, T. Concomitant Analysis of Arterial, Venous, and CSF Flows Using Phase-Contrast MRI: A Quantitative Comparison between MS Patients and Healthy Controls. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2013, 33, 1314–1321. [Google Scholar] [CrossRef]
- Li, Y.; Que, M.; Wang, X.; Zhan, G.; Zhou, Z.; Luo, X.; Li, S. Exploring Astrocyte-Mediated Mechanisms in Sleep Disorders and Comorbidity. Biomedicines 2023, 11, 2476. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, R.; Xie, M.; Ding, F.; Wang, M.; Wang, W. Updated Understanding of the Glial-Vascular Unit in Central Nervous System Disorders. Neurosci. Bull. 2022, 39, 503–518. [Google Scholar] [CrossRef]
- Xiang, L.; Harel, A.; Todorova, R.; Gao, H.; Sara, S.J.; Wiener, S.I. Locus Coeruleus Noradrenergic Neurons Phase-Lock to Prefrontal and Hippocampal Infra-Slow Rhythms That Synchronize to Behavioral Events. Front. Cell. Neurosci. 2023, 17, 1131151. [Google Scholar] [CrossRef]
- Wehbe, N.; Nasser, S.A.; Al-Dhaheri, Y.; Iratni, R.; Bitto, A.; El-Yazbi, A.F.; Badran, A.; Kobeissy, F.; Baydoun, E.; Eid, A.H. EPAC in Vascular Smooth Muscle Cells. Int. J. Mol. Sci. 2020, 21, 5160. [Google Scholar] [CrossRef]
- Cappelli, H.C.; Kanugula, A.K.; Adapala, R.K.; Amin, V.; Sharma, P.; Midha, P.; Paruchuri, S.; Thodeti, C.K. Mechanosensitive TRPV4 Channels Stabilize VE-Cadherin Junctions to Regulate Tumor Vascular Integrity and Metastasis. Cancer Lett. 2019, 442, 15–20. [Google Scholar] [CrossRef]
- Patabendige, A.; Chen, R. Astrocytic Aquaporin 4 Subcellular Translocation as a Therapeutic Target for Cytotoxic Edema in Ischemic Stroke. Neural Regen. Res. 2022, 17, 2666–2668. [Google Scholar] [CrossRef] [PubMed]
- Gouveia-Freitas, K.; Bastos-Leite, A.J. Perivascular Spaces and Brain Waste Clearance Systems: Relevance for Neurodegenerative and Cerebrovascular Pathology. Neuroradiology 2021, 63, 1581–1597. [Google Scholar] [CrossRef]
- das Neves, S.P.; Delivanoglou, N.; Da Mesquita, S. CNS-Draining Meningeal Lymphatic Vasculature: Roles, Conundrums and Future Challenges. Front. Pharmacol. 2021, 12, 655052. [Google Scholar] [CrossRef] [PubMed]
- Flores-Valle, A.; Vishniakou, I.; Seelig, J.D. Dynamics of Glia and Neurons Regulate Homeostatic Rest, Sleep and Feeding Behavior in Drosophila. Nat. Neurosci. 2025, 28. Correction in Drosophila. Nat. Neurosci. 2025, 28, 1361. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.S.; Sbaih, O.; Habermann, S.; Shahrour, N.; Weatherdon, E.; Tornatore, C. GlymphoVasomotor Field (GVF) Theory: A Non-Neuronal Scaffolding for Brain Rhythms and Consciousness. Med. Hypotheses 2025, 203, 111763. [Google Scholar] [CrossRef]
- Grimm, C.; Duss, S.N.; Privitera, M.; Munn, B.R.; Karalis, N.; Frässle, S.; Wilhelm, M.; Patriarchi, T.; Razansky, D.; Wenderoth, N.; et al. Tonic and Burst-like Locus Coeruleus Stimulation Distinctly Shift Network Activity across the Cortical Hierarchy. Nat. Neurosci. 2024, 27, 2167–2177. [Google Scholar] [CrossRef]
- Stevens, L.; Vonck, K.; Larsen, L.E.; Van Lysebettens, W.; Germonpré, C.; Baekelandt, V.; Van den Haute, C.; Carrette, E.; Wadman, W.J.; Boon, P.; et al. A Feasibility Study to Investigate Chemogenetic Modulation of the Locus Coeruleus by Means of Single Unit Activity. Front. Neurosci. 2020, 14, 162. [Google Scholar] [CrossRef]
- Torrillas-de la Cal, A.; Torres-Sanchez, S.; Bravo, L.; Llorca-Torralba, M.; Garcia-Partida, J.A.; Arroba, A.I.; Berrocoso, E. Chemogenetic Activation of Locus Coeruleus Neurons Ameliorates the Severity of Multiple Sclerosis. J. Neuroinflammation 2023, 20, 198. [Google Scholar] [CrossRef]
- Toader, C.; Brehar, F.-M.; Radoi, M.P.; Serban, M.; Covache-Busuioc, R.-A.; Glavan, L.-A.; Ciurea, A.V.; Dobrin, N. The Microsurgical Resection of an Arteriovenous Malformation in a Patient with Thrombophilia: A Case Report and Literature Review. Diagnostics 2024, 14, 2613. [Google Scholar] [CrossRef]
- Dyer-Reaves, K.; Goodman, A.M.; Nelson, A.R.; McMahon, L.L. Alpha1-Adrenergic Receptor Mediated Long-Term Depression at CA3-CA1 Synapses Can Be Induced via Accumulation of Endogenous Norepinephrine and Is Preserved Following Noradrenergic Denervation. Front. Synaptic Neurosci. 2019, 11, 27. [Google Scholar] [CrossRef]
- Perez, D.M. Current Developments on the Role of A1-Adrenergic Receptors in Cognition, Cardioprotection, and Metabolism. Front. Cell Dev. Biol. 2021, 9, 652152. [Google Scholar] [CrossRef]
- Gjedde, A.; Wong, D.F. Four Decades of Mapping and Quantifying Neuroreceptors at Work in Vivo by Positron Emission Tomography. Front. Neurosci. 2022, 16, 943512. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.W.; Lin, X.; Cao, C. Enhanced Brain Clearance of Tau and Amyloid-β in Alzheimer’s Disease Patients by Transcranial Radiofrequency Wave Treatment: A Central Role of Vascular Endothelial Growth Factor (VEGF). J. Alzheimers Dis. 2024, 100, S223–S241. [Google Scholar] [CrossRef] [PubMed]
- Helakari, H.; Järvelä, M.; Väyrynen, T.; Tuunanen, J.; Piispala, J.; Kallio, M.; Ebrahimi, S.M.; Poltojainen, V.; Kananen, J.; Elabasy, A.; et al. Effect of Sleep Deprivation and NREM Sleep Stage on Physiological Brain Pulsations. Front. Neurosci. 2023, 17, 1275184. [Google Scholar] [CrossRef] [PubMed]
- Fasaeiyan, N.; Soltani, M.; Moradi Kashkooli, F.; Taatizadeh, E.; Rahmim, A. Computational Modeling of PET Tracer Distribution in Solid Tumors Integrating Microvasculature. BMC Biotechnol. 2021, 21, 67. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Li, J.-Z.; Xie, H.-Q.; Jin, Y.-X.; Wang, W.-T.; Zhao, B.; Jia, J.-M. High-Resolution Vasomotion Analysis Reveals Novel Arteriole Physiological Features and Progressive Modulation of Cerebral Vascular Networks by Stroke. J. Cereb. Blood Flow Metab. 2024, 44, 1330–1348. [Google Scholar] [CrossRef] [PubMed]
- Voicu, V.; Toader, C.; Șerban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V. Systemic Neurodegeneration and Brain Aging: Multi-Omics Disintegration, Proteostatic Collapse, and Network Failure Across the CNS. Biomedicines 2025, 13, 2025. [Google Scholar] [CrossRef]
- Hauglund, N.L.; Andersen, M.; Tokarska, K.; Radovanovic, T.; Kjaerby, C.; Sørensen, F.L.; Bojarowska, Z.; Untiet, V.; Ballestero, S.B.; Kolmos, M.G.; et al. Norepinephrine-Mediated Slow Vasomotion Drives Glymphatic Clearance during Sleep. Cell 2025, 188, 606–622.e17. [Google Scholar] [CrossRef]
- Archer, M.; Dogra, N.; Dovey, Z.; Ganta, T.; Jang, H.-S.; Khusid, J.A.; Lantz, A.; Mihalopoulos, M.; Stockert, J.A.; Zahalka, A.; et al. Role of α- and β-Adrenergic Signaling in Phenotypic Targeting: Significance in Benign and Malignant Urologic Disease. Cell Commun. Signal. 2021, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Durante, A.; Mazzapicchi, A.; Baiardo Redaelli, M. Systemic and Cardiac Microvascular Dysfunction in Hypertension. Int. J. Mol. Sci. 2024, 25, 13294. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Wang, M.X.; Ismail, O.; Braun, M.; Schindler, A.G.; Reemmer, J.; Wang, Z.; Haveliwala, M.A.; O’Boyle, R.P.; Han, W.Y.; et al. Loss of Perivascular Aquaporin-4 Localization Impairs Glymphatic Exchange and Promotes Amyloid β Plaque Formation in Mice. Alzheimers Res. Ther. 2022, 14, 59. [Google Scholar] [CrossRef]
- Çolak, E.; Ekici, Ö.; Erdener, Ş.E. In Silico Investigation of the RBC Velocity Fluctuations in Ex Vivo Capillaries. Appl. Sci. 2025, 15, 7796. [Google Scholar] [CrossRef]
- Giannakidou, S.; Radoglou-Grammatikis, P.; Lagkas, T.; Argyriou, V.; Goudos, S.; Markakis, E.K.; Sarigiannidis, P. Leveraging the Power of Internet of Things and Artificial Intelligence in Forest Fire Prevention, Detection, and Restoration: A Comprehensive Survey. Internet Things 2024, 26, 101171. [Google Scholar] [CrossRef]
- Hirata, A.; Castro-Alamancos, M.A. Effects of Cortical Activation on Sensory Responses in Barrel Cortex. J. Neurophysiol. 2011, 105, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lv, Q.-K.; Liu, J.-Y.; Wang, F.; Liu, C.-F. New Perspectives on the Glymphatic System and the Relationship between Glymphatic System and Neurodegenerative Diseases. Neurobiol. Dis. 2025, 205, 106791. [Google Scholar] [CrossRef]
- Publik, M.A.; Filipoiu, F.M.; Dumitru, A.V.; Precup, A.; Petrescu, I.-A.; Slavu, I.; Tulin, R.F.; Tulin, A.; Baloiu, A.I.; Cirstoiu, M.M.; et al. An Extensive Study Regarding the Microscopic Anatomy of the Early Fetal Human Optic Nerve. Neurol. Int. 2024, 16, 470–482. [Google Scholar] [CrossRef]
- Labastida-Ramirez, A.; Codadu, N.K.; Agan, K.; Wykes, R.C. State-of-the-Art Preclinical Techniques to Study the Impact of Spreading Depolarizations in Awake Rodents. J. Headache Pain 2025, 26, 188. [Google Scholar] [CrossRef]
- Thakkar, R.N.; Kioutchoukova, I.P.; Griffin, I.; Foster, D.T.; Sharma, P.; Valero, E.M.; Lucke-Wold, B. Mapping the Glymphatic Pathway Using Imaging Advances. J 2023, 6, 477–491. [Google Scholar] [CrossRef]
- Vasan, S.; Lim, M.H.; Eikelis, N.; Lambert, E. Investigating the Relationship between Early Cardiovascular Disease Markers and Loneliness in Young Adults. Sci. Rep. 2024, 14, 14221. [Google Scholar] [CrossRef] [PubMed]
- Hatano, A.; Izu, L.T.; Chen-Izu, Y.; Sato, D. Modeling Autoregulation of Cardiac Excitation-Ca-Contraction and Arrhythmogenic Activities in Response to Mechanical Load Changes. iScience 2025, 28, 111788. [Google Scholar] [CrossRef] [PubMed]
- Fructuoso, M.; Vermeiren, Y.; Boluda, S.; Stimmer, L.; Crans, R.A.J.; Xicota, L.; Eisel, U.; Casan, N.O.; Bun, P.; Duyckaerts, C.; et al. Disease-Specific Neuropathological Alterations of the Locus Coeruleus in Alzheimer’s Disease, Down Syndrome, and Parkinson’s Disease. Alzheimers Dement. J. Alzheimers Assoc. 2025, 21, e70262. [Google Scholar] [CrossRef]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Serban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V.; Enyedi, M. Decoding Neurodegeneration: A Review of Molecular Mechanisms and Therapeutic Advances in Alzheimer’s, Parkinson’s, and ALS. Int. J. Mol. Sci. 2024, 25, 12613. [Google Scholar] [CrossRef]
- Song, Q.; Wang, M.; Zhou, T.; Sun, D.; Ma, H.; Li, X.; Heianza, Y.; Qi, L. The Lifestyle Related Cardiovascular Risk Is Modified by the Sleep Patterns. Mayo Clin. Proc. 2022, 97, 519–530. [Google Scholar] [CrossRef]
- Elorza Ridaura, I.; Sorrentino, S.; Moroni, L. Parallels between the Developing Vascular and Neural Systems: Signaling Pathways and Future Perspectives for Regenerative Medicine. Adv. Sci. 2021, 8, 2101837. [Google Scholar] [CrossRef] [PubMed]
- Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. Anatomy-Guided Microsurgical Resection of a Dominant Frontal Lobe Tumor Without Intraoperative Adjuncts: A Case Report from a Resource-Limited Context. Diagnostics 2025, 15, 2393. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. From Microbial Switches to Metabolic Sensors: Rewiring the Gut–Brain Kynurenine Circuit. Biomedicines 2025, 13, 2020. [Google Scholar] [CrossRef]
- Lambers, H.; Wachsmuth, L.; Lippe, C.; Faber, C. The Impact of Vasomotion on Analysis of Rodent fMRI Data. Front. Neurosci. 2023, 17, 1064000. [Google Scholar] [CrossRef]
- Huddleston, D.E.; Chen, X.; Hwang, K.; Langley, J.; Tripathi, R.; Tucker, K.; McKay, J.L.; Hu, X.; Factor, S.A. Neuromelanin-Sensitive MRI Correlates of Cognitive and Motor Function in Parkinson’s Disease with Freezing of Gait. Front. Dement. 2023, 2, 1215505. [Google Scholar] [CrossRef]
- Sprick, J.D.; Morison, D.L.; Stein, C.M.; Li, Y.; Paranjape, S.; Fonkoue, I.T.; DaCosta, D.R.; Park, J. Vascular A1-Adrenergic Sensitivity Is Enhanced in Chronic Kidney Disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R485–R490. [Google Scholar] [CrossRef]
- Wilckens, K.A.; Ferrarelli, F.; Walker, M.P.; Buysse, D.J. Slow-Wave Activity Enhancement to Improve Cognition. Trends Neurosci. 2018, 41, 470–482. [Google Scholar] [CrossRef]
- Dunaway, L.S.; Mills, W.A.; Eyo, U.B.; Isakson, B.E. The Cells of the Vasculature: Advances in the Regulation of Vascular Tone in the Brain and Periphery. Basic Clin. Pharmacol. Toxicol. 2025, 136, e70023. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Brehar, F.-M.; Radoi, M.P.; Serban, M.; Covache-Busuioc, R.-A.; Aljboor, G.S.; Gorgan, R.M. Stroke and Pulmonary Thromboembolism Complicating a Kissing Aneurysm in the M1 Segment of the Right MCA. J. Clin. Med. 2025, 14, 564. [Google Scholar] [CrossRef] [PubMed]
- Galgani, A.; Bartolini, E.; D’Amora, M.; Faraguna, U.; Giorgi, F.S. The Central Noradrenergic System in Neurodevelopmental Disorders: Merging Experimental and Clinical Evidence. Int. J. Mol. Sci. 2023, 24, 5805. [Google Scholar] [CrossRef]
- Zhang, S.-S.; Meng, F.-G.; Rong, Y.-Y.; Zhang, Y.-W.; Liu, H.-Q. Dexmedetomidine and the Glymphatic System: A New Perspective in Managing Postoperative Cognitive Dysfunction. Front. Pharmacol. 2025, 16, 1648308. [Google Scholar] [CrossRef]
- Coroleucă, R.; Filipoiu, F.M.; Cherecheanu, A.P.; Enyedi, M.; Bucșan, R.; Bostan, M.; Coroleucă, C.-A.; Ladea, L.; Vrînceanu, D.; Moraru, O.E.; et al. Laser Scanning Morphometric Measurements of the Main Orbital Communications in Dry Human Skulls. Diagnostics 2024, 14, 2168. [Google Scholar] [CrossRef]
- Reimann, H.M.; Niendorf, T. The (Un)Conscious Mouse as a Model for Human Brain Functions: Key Principles of Anesthesia and Their Impact on Translational Neuroimaging. Front. Syst. Neurosci. 2020, 14, 8. [Google Scholar] [CrossRef]
- Toader, C.; Serban, M.; Covache-Busuioc, R.-A.; Radoi, M.P.; Ciurea, A.V.; Dobrin, N. Comprehensive Management of a Giant Left Frontal AVM Coexisting with a Bilobed PComA Aneurysm: A Case Report Highlighting Multidisciplinary Strategies and Advanced Neurosurgical Techniques. J. Clin. Med. 2025, 14, 1232. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, P.; Aumann, M.A.; Claassen, D.O. Neuromelanin-Sensitive MRI as a Promising Biomarker of Catecholamine Function. Brain J. Neurol. 2024, 147, 337–351. [Google Scholar] [CrossRef]
- Kaplan, L.; Chow, B.W.; Gu, C. Neuronal Regulation of the Blood–Brain Barrier and Neurovascular Coupling. Nat. Rev. Neurosci. 2020, 21, 416–432. [Google Scholar] [CrossRef]
- Li, B.; Cao, Y.; Yuan, H.; Yu, Z.; Miao, S.; Yang, C.; Gong, Z.; Xie, W.; Li, C.; Bai, W.; et al. The Crucial Role of Locus Coeruleus Noradrenergic Neurons in the Interaction between Acute Sleep Disturbance and Headache. J. Headache Pain 2024, 25, 31. [Google Scholar] [CrossRef]
- Toader, C.; Brehar, F.M.; Radoi, M.P.; Covache-Busuioc, R.A.; Serban, M.; Ciurea, A.V.; Dobrin, N. Challenging Management of a Rare Complex Cerebral Arteriovenous Malformation in the Corpus Callosum and Post-Central Gyrus: A Case Study of a 41-Year-Old Female. J. Clin. Med. 2024, 13, 7494. [Google Scholar] [CrossRef]
- Djuric, M.; Nenadic, I.; Radisavljevic, N.; Todorovic, D.; Stojanovic, M.; Dimic, N.; Bobos, M.; Bojic, S.; Stevanovic, P.; Savic, P.; et al. The Influence of Anesthetics on the Functions of the Endothelium and Oxidative Stress: A Critical Review. Biomedicines 2025, 13, 2357. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.; Lancelot, S.; Brosse, S.; Mérida, I.; Redouté, J.; Greusard, E.; Lamberet, L.; Liotier, V.; Le Bars, D.; Costes, N.; et al. Noradrenergic Alterations in Parkinson’s Disease: A Combined 11C-Yohimbine PET/Neuromelanin MRI Study. Brain 2023, 147, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Mudge, J.D.; Kasole, M.; Chen, R.C.; Blanz, S.L.; Trevathan, J.K.; Lovett, E.G.; Williams, J.C.; Ludwig, K.A. Auricular Vagus Neuromodulation—A Systematic Review on Quality of Evidence and Clinical Effects. Front. Neurosci. 2021, 15, 664740. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Friedman, S.F.; Khurshid, S.; Ng, K.; Batra, P.; Lubitz, S.A.; Philippakis, A.A.; Uhler, C. Cross-Modal Autoencoder Framework Learns Holistic Representations of Cardiovascular State. Nat. Commun. 2023, 14, 2436. [Google Scholar] [CrossRef]
- Brynildsen, J.K.; Rajan, K.; Henderson, M.X.; Bassett, D.S. Network Models to Enhance the Translational Impact of Cross-Species Studies. Nat. Rev. Neurosci. 2023, 24, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Lyra e Silva, N.M.; Gonçalves, R.A.; Pascoal, T.A.; Lima-Filho, R.A.S.; Resende, E.d.P.F.; Vieira, E.L.M.; Teixeira, A.L.; de Souza, L.C.; Peny, J.A.; Fortuna, J.T.S.; et al. Pro-Inflammatory Interleukin-6 Signaling Links Cognitive Impairments and Peripheral Metabolic Alterations in Alzheimer’s Disease. Transl. Psychiatry 2021, 11, 251. [Google Scholar] [CrossRef]
- da Fonseca, A.C.C.; Matias, D.; Garcia, C.; Amaral, R.; Geraldo, L.H.; Freitas, C.; Lima, F.R.S. The Impact of Microglial Activation on Blood-Brain Barrier in Brain Diseases. Front. Cell. Neurosci. 2014, 8, 362. [Google Scholar] [CrossRef]
- Mishra, P.; Narayanan, R. The Enigmatic HCN Channels: A Cellular Neurophysiology Perspective. Proteins 2025, 93, 72–92. [Google Scholar] [CrossRef]
- Tiryaki, E.S.; Arslan, G.; Günaydın, C.; Ayyıldız, M.; Ağar, E. The Role of HCN Channels on the Effects of T-Type Calcium Channels and GABAA Receptors in the Absence Epilepsy Model of WAG/Rij Rats. Pflugers Arch. 2024, 476, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Covelo, J.; Cortada, M.; Vinci, G.V.; Mattia, M.; Sanchez-Vives, M.V. Network Desynchronization with Sine Waves: From Synchrony to Asynchrony by Periodic Stimulation. Adv. Sci. 2025, 12, e14602. [Google Scholar] [CrossRef]
- Korte, N.; James, G.; You, H.; Hirunpattarasilp, C.; Christie, I.; Sethi, H.; Attwell, D. Noradrenaline Released from Locus Coeruleus Axons Contracts Cerebral Capillary Pericytes via A2 Adrenergic Receptors. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2023, 43, 1142–1152. [Google Scholar] [CrossRef]
- Sharon, O.; Zhelezniakov, V.; Gat, Y.; Falach, R.; Narbayev, D.; Shiner, T.; Walker, M.P.; Tauman, R.; Bregman, N.; Nir, Y. Slow Wave Synchrony during NREM Sleep Tracks Cognitive Impairment in Prodromal Alzheimer’s Disease. Alzheimers Dement. 2025, 21, e70247. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Serban, M.; Eva, L.; Costea, D.; Covache-Busuioc, R.-A.; Radoi, M.P.; Ciurea, A.V.; Dumitru, A.V. Large Pontine Cavernoma with Hemorrhage: Case Report on Surgical Approach and Recovery. J. Clin. Med. 2025, 14, 2358. [Google Scholar] [CrossRef] [PubMed]
- Benkirane, O.; Delwiche, B.; Mairesse, O.; Peigneux, P. Impact of Sleep Fragmentation on Cognition and Fatigue. Int. J. Environ. Res. Public. Health 2022, 19, 15485. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Zhou, Q.A. Aging Hallmarks and Progression and Age-Related Diseases: A Landscape View of Research Advancement. ACS Chem. Neurosci. 2024, 15, 1–30. [Google Scholar] [CrossRef]
- Suphamungmee, W.; Lehman, W.; Morgan, K.G. Functional Remodeling of the Contractile Smooth Muscle Cell Cortex, a Provocative Concept, Supported by Direct Visualization of Cortical Remodeling. Biology 2022, 11, 662. [Google Scholar] [CrossRef]
- Faghih, M.M.; Keith Sharp, M. Mechanisms of Tracer Transport in Cerebral Perivascular Spaces. J. Biomech. 2021, 118, 110278. [Google Scholar] [CrossRef]
- Pinkiewicz, M.; Zaczyński, A.; Walecki, J.; Zawadzki, M. Beyond the Walls of Troy: A Scoping Review on Pharmacological Strategies to Enhance Drug Delivery Across the Blood–Brain Barrier and Blood–Tumor Barrier. Int. J. Mol. Sci. 2025, 26, 7050. [Google Scholar] [CrossRef]
- Voorter, P.H.; van Dinther, M.; Drenthen, G.S.; Elschot, E.P.; Staals, J.; van Oostenbrugge, R.J.; Jansen, J.F.; Backes, W.H.; CRUCIAL consortium. Vessel Architecture Imaging Reveals Microvascular Rarefaction and Capillary-to-Arteriole Shift in Cerebral Small Vessel Disease. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2025, 45, 2092–2103. [Google Scholar] [CrossRef]
- Toader, C.; Serban, M.; Covache-Busuioc, R.-A.; Radoi, M.P.; Aljboor, G.S.R.; Glavan, L.-A.; Corlatescu, A.D.; Ilie, M.-M.; Gorgan, R.M. Navigating the Rare and Dangerous: Successful Clipping of a Superior Cerebellar Artery Aneurysm Against the Odds of Uncontrolled Hypertension. J. Clin. Med. 2024, 13, 7430. [Google Scholar] [CrossRef]
- Chiu, J.-J.; Chien, S. Effects of Disturbed Flow on Vascular Endothelium: Pathophysiological Basis and Clinical Perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R. Peripheral Artery Disease: Atherosclerosis, Decreased Nitric Oxide, and Vascular Arterial Stiffening. J. Vasc. Dis. 2025, 4, 21. [Google Scholar] [CrossRef]
- Che, J.; Sun, Y.; Deng, Y.; Zhang, J. Blood-Brain Barrier Disruption: A Culprit of Cognitive Decline? Fluids Barriers CNS 2024, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.N.; Nienborg, H. Monoaminergic Neuromodulation of Sensory Processing. Front. Neural Circuits 2018, 12, 51. [Google Scholar] [CrossRef]
- Booth, L.C.; May, C.N.; Yao, S.T. The Role of the Renal Afferent and Efferent Nerve Fibers in Heart Failure. Front. Physiol. 2015, 6, 270. [Google Scholar] [CrossRef]
- Cohen-Salmon, M.; Guille, N.; Boulay, A.-C. Development of Perivascular Astrocyte Processes. Front. Neurosci. 2025, 19, 1585340. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, G.P.; Zhuo, F.; Yu, W.H.; Sun, S.Q.; Li, F.H.; Yang, M. Lost Polarization of Aquaporin4 and Dystroglycan in the Core Lesion after Traumatic Brain Injury Suggests Functional Divergence in Evolution. BioMed Res. Int. 2015, 2015, 471631. [Google Scholar] [CrossRef] [PubMed]
- Baloiu, A.I.; Filipoiu, F.; Toader, C.; Covache-Busuioc, R.-A.; Munteanu, O.; Serban, M. Sphenoid Sinus Hyperpneumatization: Anatomical Variants, Molecular Blueprints, and AI-Augmented Roadmaps for Skull Base Surgery. Front. Endocrinol. 2025, 16, 1634206. [Google Scholar] [CrossRef]
- Hermanova, Z.; Valihrach, L.; Kriska, J.; Maheta, M.; Tureckova, J.; Kubista, M.; Anderova, M. The Deletion of AQP4 and TRPV4 Affects Astrocyte Swelling/Volume Recovery in Response to Ischemia-Mimicking Pathologies. Front. Cell. Neurosci. 2024, 18, 1393751. [Google Scholar] [CrossRef]
- Li, H.; Xie, Y.; Zhang, N.; Yu, Y.; Zhang, Q.; Ding, S. Disruption of IP3R2-Mediated Ca2+ Signaling Pathway in Astrocytes Ameliorates Neuronal Death and Brain Damage While Reducing Behavioral Deficits after Focal Ischemic Stroke. Cell Calcium 2015, 58, 565–576. [Google Scholar] [CrossRef]
- Vellucci, L.; Mazza, B.; Barone, A.; Nasti, A.; De Simone, G.; Iasevoli, F.; de Bartolomeis, A. The Role of Astrocytes in the Molecular Pathophysiology of Schizophrenia: Between Neurodevelopment and Neurodegeneration. Biomolecules 2025, 15, 615. [Google Scholar] [CrossRef]
- Ineichen, B.V.; Okar, S.V.; Proulx, S.T.; Engelhardt, B.; Lassmann, H.; Reich, D.S. Perivascular Spaces and Their Role in Neuroinflammation. Neuron 2022, 110, 3566–3581. [Google Scholar] [CrossRef]
- Serbanoiu, A.; Ion, R.T.; Filipoiu, F.; Tulin, A.; Enyedi, M.; Serbanoiu, A.; Ion, R.T.; Sr, F.F.; Tulin, A.; Enyedi, M. Dissection of the Male Urethra Demonstrating Its Topographical Specificity. Cureus 2024, 16, e65946. [Google Scholar] [CrossRef] [PubMed]
- Okar, S.V.; Hu, F.; Shinohara, R.T.; Beck, E.S.; Reich, D.S.; Ineichen, B.V. The Etiology and Evolution of Magnetic Resonance Imaging-Visible Perivascular Spaces: Systematic Review and Meta-Analysis. Front. Neurosci. 2023, 17, 1038011. [Google Scholar] [CrossRef] [PubMed]
- Fornari Laurindo, L.; Aparecido Dias, J.; Cressoni Araújo, A.; Torres Pomini, K.; Machado Galhardi, C.; Rucco Penteado Detregiachi, C.; Santos de Argollo Haber, L.; Donizeti Roque, D.; Dib Bechara, M.; Vialogo Marques de Castro, M.; et al. Immunological Dimensions of Neuroinflammation and Microglial Activation: Exploring Innovative Immunomodulatory Approaches to Mitigate Neuroinflammatory Progression. Front. Immunol. 2024, 14, 1305933. [Google Scholar] [CrossRef]
- Tang, C.; Yang, J.; Lei, X.; Zhang, M.; Chen, Y.; Peng, X.; He, D. Unraveling the Complexity of Neurodegeneration: Heterogeneous Damage Patterns of Locus Coeruleus and Substantia Nigra in Alzheimer’s Disease. Alzheimers Dement. 2025, 21, e70605. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Yano, S.; Tabassum, S.; Nagai, A. The Role of the Vascular System in Degenerative Diseases: Mechanisms and Implications. Int. J. Mol. Sci. 2024, 25, 2169. [Google Scholar] [CrossRef]
- Morgos, D.-T.; Eftimie, L.-G.; Nicolae, H.; Nica, R.I.; Stefani, C.; Miricescu, D.; Hristu, R.; Stanciu, G.A.; Tulin, A.; Filipoiu, F. The Micro-Structure of the Celiac Ganglia—A Two-Photon Microscopy Study on Parkinson’s Disease. Diagnostics 2025, 15, 659. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Serban, M.; Covache-Busuioc, R.-A.; Radoi, M.P.; Aljboor, G.S.R.; Costin, H.P.; Corlatescu, A.D.; Glavan, L.-A.; Gorgan, R.M. Cerebellar Cavernoma Resection: Case Report with Long-Term Follow-Up. J. Clin. Med. 2024, 13, 7525. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-X.; Seo, B.A.; Kim, D.; Xiong, Y.; Kwon, S.-H.; Brahmachari, S.; Kim, S.; Kam, T.-I.; Nirujogi, R.S.; Kwon, S.H.; et al. Complement and Coagulation Cascades Are Potentially Involved in Dopaminergic Neurodegeneration in α-Synuclein-Based Mouse Models of Parkinson’s Disease. J. Proteome Res. 2021, 20, 3428–3443. [Google Scholar] [CrossRef]
- Senador, D.; Kaur, J.; Alvarez, A.; Hanna, H.W.; Krishnan, A.C.; Altamimi, Y.H.; O’Leary, D.S. Role of Endothelial Nitric Oxide in Control of Peripheral Vascular Conductance during Muscle Metaboreflex Activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R29–R34. [Google Scholar] [CrossRef]
- Jing, L.; Hou, L.; Zhang, D.; Li, S.; Ruan, Z.; Zhang, X.; Hong, J.-S.; Wang, Q. Microglial Activation Mediates Noradrenergic Locus Coeruleus Neurodegeneration via Complement Receptor 3 in a Rotenone-Induced Parkinson’s Disease Mouse Model. J. Inflamm. Res. 2021, 14, 1341–1356. [Google Scholar] [CrossRef]
- Torrente, D.; Su, E.J.; Schielke, G.P.; Warnock, M.; Mann, K.; Lawrence, D.A. Opposing Effects of β-2 and β-1 Adrenergic Receptor Signaling on Neuroinflammation and Dopaminergic Neuron Survival in α-Synuclein-Mediated Neurotoxicity. J. Neuroinflammation 2023, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, K. A Loss of Nuclear—Cytoskeletal Interactions in Vascular Smooth Muscle Cell Differentiation Induced by a Micro-Grooved Collagen Substrate Enabling the Modeling of an In Vivo Cell Arrangement. Bioengineering 2021, 8, 124. [Google Scholar] [CrossRef]
- Dixon, A.J.; Osei-Owusu, P. Elastin Haploinsufficiency Accelerates Age-Related Structural and Functional Changes in the Renal Microvasculature and Impairment of Renal Hemodynamics in Female Mice. Front. Physiol. 2023, 14, 1141094. [Google Scholar] [CrossRef]
- Simmonds, S.J.; Grootaert, M.O.J.; Cuijpers, I.; Carai, P.; Geuens, N.; Herwig, M.; Baatsen, P.; Hamdani, N.; Luttun, A.; Heymans, S.; et al. Pericyte Loss Initiates Microvascular Dysfunction in the Development of Diastolic Dysfunction. Eur. Heart J. Open 2024, 4, oead129. [Google Scholar] [CrossRef]
- Eide, P.K.; Hansson, H.-A. Astrogliosis and Impaired Aquaporin-4 and Dystrophin Systems in Idiopathic Normal Pressure Hydrocephalus. Neuropathol. Appl. Neurobiol. 2018, 44, 474–490. [Google Scholar] [CrossRef] [PubMed]
- Menze, I.; Bernal, J.; Kaya, P.; Aki, Ç.; Pfister, M.; Geisendörfer, J.; Yakupov, R.; Coello, R.D.; Valdés-Hernández, M.d.C.; Heneka, M.T.; et al. Perivascular Space Enlargement Accelerates in Ageing and Alzheimer’s Disease Pathology: Evidence from a Three-Year Longitudinal Multicentre Study. Alzheimers Res. Ther. 2024, 16, 242. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, G.M.; Conte, C. Alpha Synuclein Toxicity and Non-Motor Parkinson’s. Cells 2024, 13, 1265. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, H.; Bayraktutan, U. Cerebral Small Vessel Disease: Therapeutic Approaches Targeting Neuroinflammation, Oxidative Stress, and Endothelial Dysfunction. Curr. Issues Mol. Biol. 2025, 47, 232. [Google Scholar] [CrossRef]
- Toader, C.; Brehar, F.M.; Radoi, M.P.; Serban, M.; Covache-Busuioc, R.-A.; Aljboor, G.S.; Gorgan, R.M. The Management of a Giant Convexity En Plaque Anaplastic Meningioma with Gerstmann Syndrome: A Case Report of Surgical Outcomes in a 76-Year-Old Male. Diagnostics 2024, 14, 2566. [Google Scholar] [CrossRef]
- Ouellette, J.; Lacoste, B. From Neurodevelopmental to Neurodegenerative Disorders: The Vascular Continuum. Front. Aging Neurosci. 2021, 13, 749026. [Google Scholar] [CrossRef]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Covache-Busuioc, R.-A.; Serban, M.; Ciurea, A.V.; Enyedi, M. Revolutionizing Neuroimmunology: Unraveling Immune Dynamics and Therapeutic Innovations in CNS Disorders. Int. J. Mol. Sci. 2024, 25, 13614. [Google Scholar] [CrossRef]
- Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. The Collapse of Brain Clearance: Glymphatic-Venous Failure, Aquaporin-4 Breakdown, and AI-Empowered Precision Neurotherapeutics in Intracranial Hypertension. Int. J. Mol. Sci. 2025, 26, 7223. [Google Scholar] [CrossRef]
- Zaccone, V.; Falsetti, L.; Contegiacomo, S.; Cataldi, S.; Benfaremo, D.; Moroncini, G. Systemic Sclerosis: A Key Model of Endothelial Dysfunction. Biomedicines 2025, 13, 1771. [Google Scholar] [CrossRef]
- Zhou, D.; Lin, Y.; Han, Z.; Zhang, Z.; Lin, L.; Lin, S.; Yang, Q. The Impact of Aging on Neurological Diseases in the Elderly: Molecular Mechanisms and Therapeutic Perspectives. Aging Dis. 2024, 16, 2953–2978. [Google Scholar] [CrossRef]
- Costea, D.; Dobrin, N.; Tataru, C.-I.; Toader, C.; Șerban, M.; Covache-Busuioc, R.-A.; Munteanu, O.; Diaconescu, I.B. The Glymphatic–Venous Axis in Brain Clearance Failure: Aquaporin-4 Dysfunction, Biomarker Imaging, and Precision Therapeutic Frontiers. Int. J. Mol. Sci. 2025, 26, 10546. [Google Scholar] [CrossRef]
- Lecordier, S.; Manrique-Castano, D.; El Moghrabi, Y.; ElAli, A. Neurovascular Alterations in Vascular Dementia: Emphasis on Risk Factors. Front. Aging Neurosci. 2021, 13, 727590. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.Y.; Lew, W.-C.L.; Ang, K.K. Review of EEG Affective Recognition with a Neuroscience Perspective. Brain Sci. 2024, 14, 364. [Google Scholar] [CrossRef] [PubMed]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.-J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar] [CrossRef]
- Yang, Y.; Tao, Y. Regenerating Locus Coeruleus-Norepinephrine (LC-NE) Function: A Novel Approach for Neurodegenerative Diseases. Cell Prolif. 2025, 58, e13807. [Google Scholar] [CrossRef]
- Fermoyle, C.C.; La Salle, D.T.; Alpenglow, J.K.; Craig, J.C.; Jarrett, C.L.; Broxterman, R.M.; McKenzie, A.I.; Morgan, D.E.; Birgenheier, N.M.; Wray, D.W.; et al. Pharmacological Modulation of Adrenergic Tone Alters the Vasodilatory Response to Passive Leg Movement in Young but Not in Old Adults. J. Appl. Physiol. 2023, 134, 1124–1134. [Google Scholar] [CrossRef]
- Toader, C.; Dumitru, A.V.; Eva, L.; Serban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V. Nanoparticle Strategies for Treating CNS Disorders: A Comprehensive Review of Drug Delivery and Theranostic Applications. Int. J. Mol. Sci. 2024, 25, 13302. [Google Scholar] [CrossRef]
- Davidson, B.; Bhattacharya, A.; Sarica, C.; Darmani, G.; Raies, N.; Chen, R.; Lozano, A.M. Neuromodulation Techniques—From Non-Invasive Brain Stimulation to Deep Brain Stimulation. Neurotherapeutics 2024, 21, e00330. [Google Scholar] [CrossRef]
- Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. The Redox Revolution in Brain Medicine: Targeting Oxidative Stress with AI, Multi-Omics and Mitochondrial Therapies for the Precision Eradication of Neurodegeneration. Int. J. Mol. Sci. 2025, 26, 7498. [Google Scholar] [CrossRef]
- Covache-Busuioc, R.-A.; Toader, C.; Rădoi, M.P.; Șerban, M. Precision Recovery After Spinal Cord Injury: Integrating CRISPR Technologies, AI-Driven Therapeutics, Single-Cell Omics, and System Neuroregeneration. Int. J. Mol. Sci. 2025, 26, 6966. [Google Scholar] [CrossRef]
- Navarrete, M.; Schneider, J.; Ngo, H.-V.V.; Valderrama, M.; Casson, A.J.; Lewis, P.A. Examining the Optimal Timing for Closed-Loop Auditory Stimulation of Slow-Wave Sleep in Young and Older Adults. Sleep 2019, 43, zsz315. [Google Scholar] [CrossRef]
- Laurent, M.; Geoffroy, M.; Pavani, G.; Guiraud, S. CRISPR-Based Gene Therapies: From Preclinical to Clinical Treatments. Cells 2024, 13, 800. [Google Scholar] [CrossRef]
- Slavu, I.M.; Munteanu, O.; Filipoiu, F.; Tulin, R.; Oprescu, A.M.M.; Dima, I.; Dogaru, I.A.; Tulin, A.; Slavu, I.M.; Munteanu, O.; et al. A Review of Neoadjuvant Therapy and the Watch-and-Wait Protocol in Rectal Cancer: Current Evidence and Future Directions. Cureus 2024, 16, e68461. [Google Scholar] [CrossRef] [PubMed]
- Jover, E.; Silvente, A.; Marín, F.; Martínez-González, J.; Orriols, M.; Martinez, C.M.; Puche, C.M.; Valdés, M.; Rodriguez, C.; Hernández-Romero, D. Inhibition of Enzymes Involved in Collagen Cross-Linking Reduces Vascular Smooth Muscle Cell Calcification. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 4459–4469. [Google Scholar] [CrossRef]
- Herzog, M.J.; Müller, P.; Lechner, K.; Stiebler, M.; Arndt, P.; Kunz, M.; Ahrens, D.; Schmeißer, A.; Schreiber, S.; Braun-Dullaeus, R.C. Arterial Stiffness and Vascular Aging: Mechanisms, Prevention, and Therapy. Signal Transduct. Target. Ther. 2025, 10, 282. [Google Scholar] [CrossRef]
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How Vascular Smooth Muscle Cell Phenotype Switching Contributes to Vascular Disease. Cell Commun. Signal. CCS 2022, 20, 180. [Google Scholar] [CrossRef]
- Braga, G.C.D.; Ribeiro-Silva, J.C.; Boaro, A.; Martins, F.L.; Mauad, T.; Tavares, C.A.M.; Teixeira, L.R.; Caramelli, B.; Girardi, A.C.C. Restoring Lung Renin-Angiotensin System Balance through Blood Pressure Control. Clin. Sci. 2025, 139, 215–227. [Google Scholar] [CrossRef]
- Horta, M.; Soares, P.; Leite Pereira, C.; Lima, R.T. Emerging Approaches in Glioblastoma Treatment: Modulating the Extracellular Matrix Through Nanotechnology. Pharmaceutics 2025, 17, 142. [Google Scholar] [CrossRef]
- Miranda Hurtado, M.; Steinback, C.D.; Davenport, M.H.; Rodriguez-Fernandez, M. Increased Respiratory Modulation of Cardiovascular Control Reflects Improved Blood Pressure Regulation in Pregnancy. Front. Physiol. 2023, 14, 1070368. [Google Scholar] [CrossRef]
- Behringer, E.J. Impact of Aging on Vascular Ion Channels: Perspectives and Knowledge Gaps across Major Organ Systems. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H1012–H1038. [Google Scholar] [CrossRef]
- Palazzo, C.; Buccoliero, C.; Mola, M.G.; Abbrescia, P.; Nicchia, G.P.; Trojano, M.; Frigeri, A. AQP4ex Is Crucial for the Anchoring of AQP4 at the Astrocyte End-Feet and for Neuromyelitis Optica Antibody Binding. Acta Neuropathol. Commun. 2019, 7, 51. [Google Scholar] [CrossRef]
- Markou, A.; Kitchen, P.; Aldabbagh, A.; Repici, M.; Salman, M.M.; Bill, R.M.; Balklava, Z. Mechanisms of Aquaporin-4 Vesicular Trafficking in Mammalian Cells. J. Neurochem. 2024, 168, 100–114. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, H.; Dai, Z.; He, C.; Qin, S.; Su, Z. From Physiology to Pathology of Astrocytes: Highlighting Their Potential as Therapeutic Targets for CNS Injury. Neurosci. Bull. 2024, 41, 131–154. [Google Scholar] [CrossRef]
- Fernández de la Puebla, M.; Zhang, X.; Nagelhus, E.A.; Bjørås, M.; Tang, W. IP3R2-Mediated Astrocytic Ca2+ Transients Are Critical to Sustain Modulatory Effects of Locomotion on Neurons in Mouse Somatosensory Cortex. Cells 2025, 14, 1103. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; D’Ascola, A.; Scuruchi, M.; Prestipino, V.; Nastasi, G.; Calatroni, A.; Campo, S. The Inhibition of Hyaluronan Degradation Reduced Pro-Inflammatory Cytokines in Mouse Synovial Fibroblasts Subjected to Collagen-Induced Arthritis. J. Cell. Biochem. 2012, 113, 1852–1867. [Google Scholar] [CrossRef]
- Xin, W.; Pan, Y.; Wei, W.; Tatenhorst, L.; Graf, I.; Popa-Wagner, A.; Gerner, S.T.; Huber, S.; Kilic, E.; Hermann, D.M.; et al. Preconditioned Extracellular Vesicles from Hypoxic Microglia Reduce Poststroke AQP4 Depolarization, Disturbed Cerebrospinal Fluid Flow, Astrogliosis, and Neuroinflammation. Theranostics 2023, 13, 4197–4216. [Google Scholar] [CrossRef]
- Sahin, M.Z.; Yildirim, A. Gonadal Hormones and Aquaporin-4: Preclinical Insights into Glymphatic Regulation and Amyloid Clearance. J. Alzheimer’s Dis. 2025, 108, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Lu, K. Bidirectional Regulation of Nitric Oxide and Endothelin-1 in Cerebral Vasospasm: Mechanisms and Therapeutic Perspectives. Future Pharmacol. 2025, 5, 59. [Google Scholar] [CrossRef]
- Cobos-Puc, L.E.; Rodríguez-Salazar, M.d.C.; Silva-Belmares, S.Y.; Aguayo-Morales, H. Nanoparticle-Based Strategies to Enhance Catecholaminergic Drug Delivery for Neuropsychiatric Disorders: Advances, Challenges, and Therapeutic Opportunities. Future Pharmacol. 2025, 5, 51. [Google Scholar] [CrossRef]
- Li, K.-P.; Wu, J.-J.; Zhou, Z.-L.; Xu, D.-S.; Zheng, M.-X.; Hua, X.-Y.; Xu, J.-G. Noninvasive Brain Stimulation for Neurorehabilitation in Post-Stroke Patients. Brain Sci. 2023, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.-R.; Kim, D.H.; Abdelhamid, M.A.A.; Pack, S.P. Cancer and Aging Biomarkers: Classification, Early Detection Technologies and Emerging Research Trends. Biosensors 2025, 15, 737. [Google Scholar] [CrossRef]
- Terol-Úbeda, A.C.; Fernández-González, J.F.; Morán, A.; García-Domingo, M.; García-Pedraza, J.Á. Angiotensin II and EDH Pathways Underlie the Vascular Sympatho-Modulation by 5-HT in Female Rats. Int. J. Mol. Sci. 2025, 26, 9614. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Shin, S.-R.; Bañobre-López, M. Brain-on-a-Chip: An Emerging Platform for Studying the Nanotechnology-Biology Interface for Neurodegenerative Disorders. J. Nanobiotechnology 2024, 22, 573. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Li, J.-Z.; Wang, W.-T.; Xie, H.-Q.; Ruan, J.-Y.; Jia, J.-M. Vasomotion Delineates Cerebral Vascular Dynamic Features and Participates in the Homeostatic Cerebral Blood Flow Regulation. Sci. Rep. 2025, 15, 36210. [Google Scholar] [CrossRef]
- Lee, J.; Mun, J.; Choo, M.; Park, S.-M. Predictive Modeling of Hemodynamics during Viscerosensory Neurostimulation via Neural Computation Mechanism in the Brainstem. Npj Digit. Med. 2025, 8, 220. [Google Scholar] [CrossRef]
- Marsh, B.; Chauvette, S.; Huang, M.; Timofeev, I.; Bazhenov, M. Network Effects of Traumatic Brain Injury: From Infra Slow to High Frequency Oscillations and Seizures. J. Comput. Neurosci. 2025, 53, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Eliezeck, M.; Guedes Jesus, I.C.; Scalzo, S.A.; Sanches, B.d.L.; Silva, K.S.C.; Costa, M.; Mesquita, T.; Rocha-Resende, C.; Szawka, R.E.; Guatimosim, S. β-Adrenergic Signaling Drives Structural and Functional Maturation of Mouse Cardiomyocytes. Am. J. Physiol. Cell Physiol. 2024, 326, C1334–C1344. [Google Scholar] [CrossRef]
- Schirrmacher, V. Brain and Immune System: Intercellular Communication During Homeostasis and Neuroimmunomodulation upon Dysfunction. Int. J. Mol. Sci. 2025, 26, 6552. [Google Scholar] [CrossRef]
- Su, M.; Nizamutdinov, D.; Liu, H.; Huang, J.H. Recent Mechanisms of Neurodegeneration and Photobiomodulation in the Context of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 9272. [Google Scholar] [CrossRef]
- Sheng, L.; Zheng, X.; Ding, Z.; Liu, J.; Song, W. Neurovascular Coupling Dysfunction in Encephalopathy: Pathophysiological Advances and Clinical Implications. Front. Neurol. 2025, 16, 1522485. [Google Scholar] [CrossRef]
- Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. Ruptured Posterior Inferior Cerebellar Artery Aneurysms: Integrating Microsurgical Expertise, Endovascular Challenges, and AI-Driven Risk Assessment. J. Clin. Med. 2025, 14, 5374. [Google Scholar] [CrossRef]
- Gold, K.; Gaharwar, A.K.; Jain, A. Emerging Trends in Multiscale Modeling of Vascular Pathophysiology: Organon-a-Chip and 3D Printing. Biomaterials 2019, 196, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Song, Y.; Zhu, Z.; Huang, X.; Fan, J.; Qiao, J.; Mao, F. Cell–Cell Communication: New Insights and Clinical Implications. Signal Transduct. Target. Ther. 2024, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Simonetta, I.; Riolo, R.; Todaro, F.; Tuttolomondo, A. New Insights on Metabolic and Genetic Basis of Migraine: Novel Impact on Management and Therapeutical Approach. Int. J. Mol. Sci. 2022, 23, 3018. [Google Scholar] [CrossRef]
- Toader, C.; Serban, M.; Dobrin, N.; Covache-Busuioc, R.-A.; Radoi, M.P.; Ciurea, A.V.; Munteanu, O. Complex Anatomy, Advanced Techniques: Microsurgical Clipping of a Ruptured Hypophyseal Artery Aneurysm. J. Clin. Med. 2025, 14, 2361. [Google Scholar] [CrossRef]
- Drew, P.J.; Mateo, C.; Turner, K.L.; Yu, X.; Kleinfeld, D. Ultra-Slow Oscillations in fMRI and Resting-State Connectivity: Neuronal and Vascular Contributions and Technical Confounds. Neuron 2020, 107, 782–804. [Google Scholar] [CrossRef]
- Schrank, F.; Warmuth, C.; Tzschätzsch, H.; Kreft, B.; Hirsch, S.; Braun, J.; Elgeti, T.; Sack, I. Cardiac-Gated Steady-State Multifrequency Magnetic Resonance Elastography of the Brain: Effect of Cerebral Arterial Pulsation on Brain Viscoelasticity. J. Cereb. Blood Flow Metab. 2020, 40, 991–1001. [Google Scholar] [CrossRef]
- Noh, M.-Y.; Kwon, H.S.; Kwon, M.-S.; Nahm, M.; Jin, H.K.; Bae, J.; Kim, S.H. Biomarkers and Therapeutic Strategies Targeting Microglia in Neurodegenerative Diseases: Current Status and Future Directions. Mol. Neurodegener. 2025, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- Jigo, M.; Carmel, J.B.; Wang, Q.; Rodenkirch, C. Transcutaneous Cervical Vagus Nerve Stimulation Improves Sensory Performance in Humans: A Randomized Controlled Crossover Pilot Study. Sci. Rep. 2024, 14, 3975. [Google Scholar] [CrossRef]
- Van den Bulcke, L.; Peeters, A.-M.; Heremans, E.; Davidoff, H.; Borzée, P.; De Vos, M.; Emsell, L.; Van den Stock, J.; De Roo, M.; Tournoy, J.; et al. Acoustic Stimulation as a Promising Technique to Enhance Slow-Wave Sleep in Alzheimer’s Disease: Results of a Pilot Study. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2023, 19, 2107–2112. [Google Scholar] [CrossRef]
- Mota, K.O.; de Vasconcelos, C.M.L.; Kirshenbaum, L.A.; Dhalla, N.S. The Role of Advanced Glycation End-Products in the Pathophysiology and Pharmacotherapy of Cardiovascular Disease. Int. J. Mol. Sci. 2025, 26, 7311. [Google Scholar] [CrossRef]
- O’Connor, S.M.; Wang, R.; Sharp, P.S.; Shabir, O.; Shaw, K.; Okun, M.; Howarth, C.; Martin, C.; Berwick, J. Hemodynamic and Neuronal Contributions to Low-Frequency Vascular Oscillations in a Preclinical Model of Alzheimer’s Disease. Neurophotonics 2025, 12, S14615. [Google Scholar] [CrossRef]
- Ait Benichou, S.; Jauvin, D.; De Serres-Bérard, T.; Pierre, M.; Ling, K.K.; Bennett, C.F.; Rigo, F.; Gourdon, G.; Chahine, M.; Puymirat, J. Antisense Oligonucleotides as a Potential Treatment for Brain Deficits Observed in Myotonic Dystrophy Type 1. Gene Ther. 2022, 29, 698–709. [Google Scholar] [CrossRef]
- Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. Precision Neuro-Oncology in Glioblastoma: AI-Guided CRISPR Editing and Real-Time Multi-Omics for Genomic Brain Surgery. Int. J. Mol. Sci. 2025, 26, 7364. [Google Scholar] [CrossRef]
- Bader, K.B.; Padilla, F.; Haworth, K.J.; Ellens, N.; Dalecki, D.; Miller, D.L.; Wear, K.A. Overview of Therapeutic Ultrasound Applications and Safety Considerations: 2024 Update. J. Ultrasound Med. 2025, 44, 381–433. [Google Scholar] [CrossRef]
- Rafii, M.S.; Aisen, P.S. Detection and Treatment of Alzheimer’s Disease in Its Preclinical Stage. Nat. Aging 2023, 3, 520–531. [Google Scholar] [CrossRef]
- D’Amore, F.M.; Moscatelli, M.; Malvaso, A.; D’Antonio, F.; Rodini, M.; Panigutti, M.; Mirino, P.; Carlesimo, G.A.; Guariglia, C.; Caligiore, D. Explainable Machine Learning on Clinical Features to Predict and Differentiate Alzheimer’s Progression by Sex: Toward a Clinician-Tailored Web Interface. J. Neurol. Sci. 2025, 468, 123361. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Serban, M.; Munteanu, O.; Covache-Busuioc, R.-A.; Enyedi, M.; Ciurea, A.V.; Tataru, C.P. From Synaptic Plasticity to Neurodegeneration: BDNF as a Transformative Target in Medicine. Int. J. Mol. Sci. 2025, 26, 4271. [Google Scholar] [CrossRef]
- Malvaso, A.; Cerne, D.; Bernini, S.; Bottiroli, S.; Marchioni, E.; Businaro, P.; Masciocchi, S.; Morandi, C.; Scaranzin, S.; Mobilia, E.M.; et al. Retrograde Amnesia in LGI1 and CASPR2 Limbic Encephalitis: Two Case Reports and a Systematic Literature Review. Eur. J. Neurol. 2025, 32, e70113. [Google Scholar] [CrossRef]
- Wrzus, C.; Luong, G.; Wagner, G.G.; Riediger, M. Longitudinal Coupling of Momentary Stress Reactivity and Trait Neuroticism: Specificity of States, Traits, and Age Period. J. Pers. Soc. Psychol. 2021, 121, 691–706. [Google Scholar] [CrossRef]
- Wang, S.; Qin, L. Homeostatic Medicine: A Strategy for Exploring Health and Disease. Curr. Med. 2022, 1, 16. [Google Scholar] [CrossRef]
- Miliotou, A.N.; Kotsoni, A.; Zacharia, L.C. Deciphering the Role of Adrenergic Receptors in Alzheimer’s Disease: Paving the Way for Innovative Therapies. Biomolecules 2025, 15, 128. [Google Scholar] [CrossRef]
- Carè, M.; Chiappalone, M.; Cota, V.R. Personalized Strategies of Neurostimulation: From Static Biomarkers to Dynamic Closed-Loop Assessment of Neural Function. Front. Neurosci. 2024, 18, 1363128. [Google Scholar] [CrossRef]
- Ng, K.W.; Chaturvedi, N.; Coté, G.L.; Fisher, S.A.; Mabbott, S. Biomarkers and Point of Care Screening Approaches for the Management of Preeclampsia. Commun. Med. 2024, 4, 208. [Google Scholar] [CrossRef]
- Badea, T.G.; Dogaru, I.A.; Filipoiu, Z.F.; Mutu, D.E.G.; Filipoiu, F.; Badea, T.G.; Dogaru, I.A.; Filipoiu, Z.F.; Mutu, D.E.G.; Sr, F.F. Dissection of the Sympathetic Nerves Around the Mesorectum at the Abdominopelvic Border. Cureus 2024, 16, e69091. [Google Scholar] [CrossRef]
- Kovacs, G.G. Molecular Pathological Classification of Neurodegenerative Diseases: Turning towards Precision Medicine. Int. J. Mol. Sci. 2016, 17, 189. [Google Scholar] [CrossRef] [PubMed]
- Csala, D.; Ádám, Z.; Wilhelm, M. The Role of miRNAs and Extracellular Vesicles in Adaptation After Resistance Exercise: A Review. Curr. Issues Mol. Biol. 2025, 47, 583. [Google Scholar] [CrossRef] [PubMed]
- van Veluw, S.J.; Hou, S.S.; Calvo-Rodriguez, M.; Arbel-Ornath, M.; Snyder, A.C.; Frosch, M.P.; Greenberg, S.M.; Bacskai, B.J. Vasomotion as a Driving Force for Paravascular Clearance in the Awake Mouse Brain. Neuron 2020, 105, 549–561.e5. [Google Scholar] [CrossRef]
- Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. CRISPR and Artificial Intelligence in Neuroregeneration: Closed-Loop Strategies for Precision Medicine, Spinal Cord Repair, and Adaptive Neuro-Oncology. Int. J. Mol. Sci. 2025, 26, 9409. [Google Scholar] [CrossRef]
- Joseph, U.G.; Oyovwi, M.O.; Jeroh, E.; Esuku, D.T.; Ben-Azu, B. Dysfunctional Astrocyte Metabolism: A Driver of Imbalanced Excitatory/Inhibitory Tone and Support for Therapeutic Intervention Targets. J. Mol. Pathol. 2025, 6, 12. [Google Scholar] [CrossRef]
- Delbono, O.; Wang, Z.-M.; Messi, M.L. Brainstem Noradrenergic Neurons: Identifying a Hub at the Intersection of Cognition, Motility, and Skeletal Muscle Regulation. Acta Physiol. Oxf. Engl. 2022, 236, e13887. [Google Scholar] [CrossRef]
- Nasb, M.; Tao, W.; Chen, N. Alzheimer’s Disease Puzzle: Delving into Pathogenesis Hypotheses. Aging Dis. 2024, 15, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. Blueprint of Collapse: Precision Biomarkers, Molecular Cascades, and the Engineered Decline of Fast-Progressing ALS. Int. J. Mol. Sci. 2025, 26, 8072. [Google Scholar] [CrossRef]
- Zhong, J.; Li, G.; Lv, Z.; Chen, J.; Wang, C.; Shao, A.; Gong, Z.; Wang, J.; Liu, S.; Luo, J.; et al. Neuromodulation of Cerebral Blood Flow: A Physiological Mechanism and Methodological Review of Neurovascular Coupling. Bioengineering 2025, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Luo, L.-D.; Feng, I.; Ma, S. Molecular Mechanisms of Synaptogenesis. Front. Synaptic Neurosci. 2022, 14, 939793. [Google Scholar] [CrossRef]
- Bączyńska, E.; Pels, K.K.; Basu, S.; Włodarczyk, J.; Ruszczycki, B. Quantification of Dendritic Spines Remodeling under Physiological Stimuli and in Pathological Conditions. Int. J. Mol. Sci. 2021, 22, 4053. [Google Scholar] [CrossRef]
- Joshi, R.B.; Duckrow, R.B.; Goncharova, I.I.; Hirsch, L.J.; Spencer, D.D.; Godwin, D.W.; Zaveri, H.P. Stability of Infraslow Correlation Structure in Time-Shifted Intracranial EEG Signals. Front. Netw. Physiol. 2024, 4, 1441294. [Google Scholar] [CrossRef]
- Bellot-Saez, A.; Stevenson, R.; Kékesi, O.; Samokhina, E.; Ben-Abu, Y.; Morley, J.W.; Buskila, Y. Neuromodulation of Astrocytic K+ Clearance. Int. J. Mol. Sci. 2021, 22, 2520. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, G.; Ahmadian, Y.; Rubin, D.B.; Lengyel, M.; Miller, K.D. The Dynamical Regime of Sensory Cortex: Stable Dynamics around a Single Stimulus-Tuned Attractor Account for Patterns of Noise Variability. Neuron 2018, 98, 846–860.e5. [Google Scholar] [CrossRef]
- Conte, F.; Malloggi, S.; De Rosa, O.; Ficca, G.; Righi, S.; Viggiano, M.P.; Giganti, F. Sleep Benefits Prose Memory Consolidation in University Students. Brain Sci. 2025, 15, 265. [Google Scholar] [CrossRef]
- Dickey, C.W.; Verzhbinsky, I.A.; Jiang, X.; Rosen, B.Q.; Kajfez, S.; Eskandar, E.N.; Gonzalez-Martinez, J.; Cash, S.S.; Halgren, E. Cortical Ripples during NREM Sleep and Waking in Humans. J. Neurosci. 2022, 42, 7931–7946. [Google Scholar] [CrossRef]
- Mateo, C.; Knutsen, P.M.; Tsai, P.S.; Shih, A.Y.; Kleinfeld, D. Entrainment of Arteriole Vasomotor Fluctuations by Neural Activity Is a Basis of Blood-Oxygenation-Level-Dependent “Resting-State” Connectivity. Neuron 2017, 96, 936–948.e3. [Google Scholar] [CrossRef]
- Hermann, D.M.; Peruzzotti-Jametti, L.; Giebel, B.; Pluchino, S. Extracellular Vesicles Set the Stage for Brain Plasticity and Recovery by Multimodal Signalling. Brain 2023, 147, 372–389. [Google Scholar] [CrossRef]
- Reyes-Resina, I.; Samer, S.; Kreutz, M.R.; Oelschlegel, A.M. Molecular Mechanisms of Memory Consolidation That Operate During Sleep. Front. Mol. Neurosci. 2021, 14, 767384. [Google Scholar] [CrossRef]
- Runge, N.; Ahmed, I.; Saueressig, T.; Perea, J.; Labie, C.; Mairesse, O.; Nijs, J.; Malfliet, A.; Verschueren, S.; Van Assche, D.; et al. The Bidirectional Relationship between Sleep Problems and Chronic Musculoskeletal Pain: A Systematic Review with Meta-Analysis. Pain 2024, 165, 2455–2467. [Google Scholar] [CrossRef]
- Zou, J.; Li, J.; Wang, X.; Tang, D.; Chen, R. Neuroimmune Modulation in Liver Pathophysiology. J. Neuroinflammation 2024, 21, 188. [Google Scholar] [CrossRef]
- Kim, M.W.; Kipnis, J. Glymphatics and Meningeal Lymphatics Unlock the Brain-Immune Code. Immunity 2025, 58, 1040–1051. [Google Scholar] [CrossRef]
- Pisoni, L.; Donini, L.; Gagni, P.; Pennuto, M.; Ratti, A.; Verde, F.; Ticozzi, N.; Mandrioli, J.; Calvo, A.; Basso, M. Barriers in the Nervous System: Challenges and Opportunities for Novel Biomarkers in Amyotrophic Lateral Sclerosis. Cells 2025, 14, 848. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Z.; Chai, Z.; Ma, S.; Li, A.; Li, Y. Central Nervous System-Derived Extracellular Vesicles as Biomarkers in Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 8272. [Google Scholar] [CrossRef] [PubMed]
- Golub, V.M.; Reddy, D.S. Post-Traumatic Epilepsy and Comorbidities: Advanced Models, Molecular Mechanisms, Biomarkers, and Novel Therapeutic Interventions. Pharmacol. Rev. 2022, 74, 387–438. [Google Scholar] [CrossRef]
- Chai, A. Pleiotropic Neurotransmitters: Neurotransmitter-Receptor Crosstalk Regulates Excitation-Inhibition Balance in Social Brain Functions and Pathologies. Front. Neurosci. 2025, 19, 1552145. [Google Scholar] [CrossRef] [PubMed]
- van Veluw, S.J.; Benveniste, H.; Bakker, E.N.T.P.; Carare, R.O.; Greenberg, S.M.; Iliff, J.J.; Lorthois, S.; Van Nostrand, W.E.; Petzold, G.C.; Shih, A.Y.; et al. Is CAA a Perivascular Brain Clearance Disease? A Discussion of the Evidence to Date and Outlook for Future Studies. Cell. Mol. Life Sci. 2024, 81, 239. [Google Scholar] [CrossRef] [PubMed]
- Murase, S.; Sakitani, N.; Maekawa, T.; Yoshino, D.; Takano, K.; Konno, A.; Hirai, H.; Saito, T.; Tanaka, S.; Shinohara, K.; et al. Interstitial-Fluid Shear Stresses Induced by Vertically Oscillating Head Motion Lower Blood Pressure in Hypertensive Rats and Humans. Nat. Biomed. Eng. 2023, 7, 1350–1373, Correction in Nat. Biomed. Eng. 2023, 7, 1530. [Google Scholar] [CrossRef] [PubMed]
| Level/Component | Structural/Molecular Basis | Primary Clearance Function | Dominant Frequency | Dynamic Mechanism | Physiological Output | Pathological Consequence | References |
|---|---|---|---|---|---|---|---|
| Locus Coeruleus (LC) | Noradrenergic neurons with tonic + infralow firing (α1, β-AR projections) | Central oscillator coordinating vascular + glial rhythms | 0.01–0.1 Hz | NE volume transmission entrains vascular and astrocytic activity | Synchronized vascular tone and perivascular conductance | LC degeneration, sleep loss, inflammation → loss of vasomotor drive | [51] |
| Vascular Smooth Muscle Cells (VSMCs) | α1-AR → Gq/PLC/IP3/Ca2+; β2-AR–cAMP modulation | Generates vasomotion (contraction–relaxation cycles) | 0.01–0.1 Hz | Periodic Ca2+ cycling drives actomyosin contraction | Diameter oscillations generate convective pressure gradients | Stiffening → damped vasomotion, reduced hydraulic force | [52] |
| Pericytes | α1-AR, TRPV4; actin–myosin contractility | Fine-tuning of capillary resistance | 0.01–0.05 Hz | Local contractility propagates arteriolar waves | Phase-coherent microvascular perfusion | Pericyte loss → heterogeneous capillary constriction, impaired clearance | [53] |
| Astrocytic End-feet | AQP4, α1/β-AR, Ca2+-dependent cytoskeletal remodeling | Controls perivascular permeability + geometry | 0.03–0.1 Hz | NE-driven Ca2+ waves modulate AQP4 and end-foot volume | Adjusts perivascular resistance; CSF–ISF coupling | AQP4 depolarization → increased resistance, glymphatic failure | [54] |
| Perivascular Spaces (PVS) | Basement-membrane channels (10–30 μm) | Conduit for CSF influx and ISF mixing | Composite: cardiac (5–10 Hz), respiratory (0.2–0.3 Hz), vasomotor (0.01–0.1 Hz) | Integrated multi-frequency pressure waves sustain flow | Efficient solute transport periarterial → perivenous | Loss of compliance → low mixing, metabolite retention | [55] |
| Venular and Lymphatic Outflow | Perivenous pathways + meningeal lymphatics | Mediates ISF efflux and immune drainage | 0.01–0.2 Hz | Passive venous oscillations + lymphatic pumping | Metabolite and antigen clearance | Venous rigidity/lymphatic obstruction → neuroinflammation | [56] |
| Network Integration | Coupled LC–vascular–glial feedback loops | Aligns neuro-modulatory, mechanical, hydrodynamic rhythms | Hierarchical (5–0.01 Hz) | Phase coupling across brain states | Sleep-dependent clearance optimization | Sleep fragmentation/aging → loss of coherence, clearance collapse | [57] |
| Approach | Key Findings | Main Limitations |
|---|---|---|
| Optogenetic LC stimulation | Infra-slow (~0.05 Hz) activation drives synchronized arteriole dilation and tracer influx, establishing a causal link between LC rhythm and perivascular flow. | Invasive cranial windows; rodent-only; anesthesia and stimulation may not fully reflect physiological LC activity. |
| Chemo-genetic (DREADD) manipulation | Excitatory DREADDs enhance vasomotion and solute movement; inhibitory DREADDs suppress both, confirming adrenergic control of clearance. | Alters LC excitability with prolonged use; slower temporal precision; possible off-target effects. |
| α1-adrenergic pharmacology | α1-agonists restore vasomotion after LC suppression; α1-blockers abolish oscillations despite intact LC firing, identifying α1 receptors as essential effectors. | Limited receptor selectivity; systemic cardiovascular effects; pharmacologic tone may differ from physiological conditions. |
| Two-photon microscopy | Directly visualizes diameter oscillations and tracer motion at 0.01–0.1 Hz, demonstrating spatiotemporal coupling of mechanics and solute transport. | Highly invasive; restricted field of view; anesthesia alters noradrenergic tone and vessel compliance. |
| Dynamic contrast/phase-contrast MRI | Reveals whole-brain slow CSF–ISF oscillations peaking during NREM sleep, supporting large-scale vasomotor synchronization. | Flow measurements are indirect; limited temporal resolution; cardiac and respiratory confounds. |
| Biophysical and mechanical modeling | Accurate tracer kinetics reproduced only when slow vasomotion is included, supporting mechanical sufficiency of LC-driven pressure waves. | Simplified assumptions; lacks biological feedback; species-specific parameter variability. |
| Transgenic disease models | LC loss or reduced vasomotion precedes impaired clearance; restoring LC rhythmicity delays pathology and improves recovery. | Species differences; anesthesia and genetic background affect progression; short lifespan restricts long-term study. |
| Human neuroimaging | Detects infra-slow vasomotion linked to sleep; LC signal declines with aging and Alzheimer’s disease, paralleling reduced clearance. | Correlational; insufficient resolution to define cellular mechanisms or establish causality. |
| Domain/Component | Primary Pathological Process | Molecular and Cellular Mechanisms | Functional Consequence | Quantitative/Experimental Evidence | Potential Reversibility/Therapeutic Target | References |
|---|---|---|---|---|---|---|
| Locus Coeruleus (LC) | Degeneration of noradrenergic neurons and rhythm desynchronization | Mitochondrial dysfunction, oxidative stress, impaired mitophagy, microglial activation, reduced HCN/T-type Ca2+ currents | Loss of infra-slow oscillations (0.01–0.1 Hz); weakened NE drive | LC neuron loss: >40% by 7th decade; reduced NE tone precedes AD pathology by 10–20 years | Chemo-genetic activation or NE reuptake inhibitors restore vasomotor coupling | [51,146] |
| Adrenergic Signaling Cascade | Receptor desensitization and impaired α1/β2 balance | Downregulation of α1-AR on VSMCs and β2-AR on astrocytes; G-protein uncoupling | Reduced vasomotor amplitude and phase coherence | In aged mice, vasomotor amplitude ↓ ~70%; α1-AR mRNA ↓ ~60% | α1 agonists, β2 modulators, LC–astrocyte co-stimulation | [147] |
| Vascular Smooth Muscle Cells (VSMCs) | Phenotypic shift and cytoskeletal degeneration | Contractile-to-synthetic transition; actin loss; Ca2+ handling defects; ROS accumulation | Damped vasomotion; decreased compliance; transmission failure | Penetrating arteriole oscillations ↓ to <30% of young controls | NO donors, ROCK inhibitors, TGF-β blockade | [148] |
| Vascular Matrix and Elasticity | Elastin fragmentation, collagen crosslinking, calcification | AGE accumulation, MMP activation, angiotensin II signaling | Elevated stiffness, loss of low-frequency resonance | Arterial compliance ↓ 40–60% with age; loss of 0.01–0.1 Hz response | Crosslink breakers (ALT-711), sirtuin activators | [149] |
| Pericytes/Capillary Network | Loss of contractility and dropout | TRPV4 desensitization, oxidative DNA damage, apoptosis | Impaired microvascular resistance tuning; flow heterogeneity | Pericyte density ↓ ~25–40% in aging cortex | PDGF-BB therapy, pericyte reprogramming | [150] |
| Astrocytic End-feet and AQP4 Polarity | Depolarization and cytoskeletal rigidity | Disruption of dystrophin–dystroglycan complex; IL-1β/TNF-α–driven astrogliosis | Increased hydraulic resistance, reduced ISF–CSF exchange | AQP4 mislocalization ↑ 200–300% in AD cortex; tracer clearance ↓ > 50% | Dystrophin restoration, AQP4 modulators, astrocyte reprogramming | [131,151] |
| Perivascular Space Geometry | Fibrosis and basement membrane thickening | Collagen IV accumulation, perivascular inflammation, MMP imbalance | Narrowed lumen, elevated impedance, stagnation zones | PVS cross-section ↓ ~35% in aged rodents; diffusion times ↑ 2–3× | Anti-fibrotic, anti-MMP, or lymphatic drainage enhancers | [152] |
| Network-Level Integration | Phase decoupling and loss of multiscale synchrony | Desynchronization between LC rhythms, vascular oscillations, astrocytic Ca2+ waves | Transition from convective to diffusion-limited clearance | Net CSF flow velocity ↓ > 60% in aged animals; clearance half-time ↑ 3× | Combined neuro-modulatory–vascular–astro-glial restoration | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabija, M.G.; Tataru, C.-I.; Dumitru, A.V.; Munteanu, O.; Radoi, M.P.; Ciurea, A.V.; Petrescu, I.-A. Noradrenergic Slow Vasomotion: The Hidden Fluid Pump Linking Sleep, Brain Clearance, and Dementia Pathogenesis. Int. J. Mol. Sci. 2025, 26, 11444. https://doi.org/10.3390/ijms262311444
Dabija MG, Tataru C-I, Dumitru AV, Munteanu O, Radoi MP, Ciurea AV, Petrescu I-A. Noradrenergic Slow Vasomotion: The Hidden Fluid Pump Linking Sleep, Brain Clearance, and Dementia Pathogenesis. International Journal of Molecular Sciences. 2025; 26(23):11444. https://doi.org/10.3390/ijms262311444
Chicago/Turabian StyleDabija, Marius Gabriel, Catalina-Ioana Tataru, Adrian Vasile Dumitru, Octavian Munteanu, Mugurel Petrinel Radoi, Alexandru Vlad Ciurea, and Ioan-Andrei Petrescu. 2025. "Noradrenergic Slow Vasomotion: The Hidden Fluid Pump Linking Sleep, Brain Clearance, and Dementia Pathogenesis" International Journal of Molecular Sciences 26, no. 23: 11444. https://doi.org/10.3390/ijms262311444
APA StyleDabija, M. G., Tataru, C.-I., Dumitru, A. V., Munteanu, O., Radoi, M. P., Ciurea, A. V., & Petrescu, I.-A. (2025). Noradrenergic Slow Vasomotion: The Hidden Fluid Pump Linking Sleep, Brain Clearance, and Dementia Pathogenesis. International Journal of Molecular Sciences, 26(23), 11444. https://doi.org/10.3390/ijms262311444






