Targeting Ferroptosis in Nasopharyngeal Carcinoma: Mechanisms, Resistance, and Precision Therapeutic Opportunities
Abstract
1. Introduction
2. Molecular Mechanisms Linking Ferroptosis to NPC
2.1. System xc−–GSH–GPX4 Axis
2.2. Iron Metabolism and Ferritinophagy
2.3. Lipid Metabolism and ACSL4
2.4. Epigenetic and Post-Transcriptional Regulation
2.5. Tumor Microenvironment
3. Ferroptosis in NPC Therapy Resistance
3.1. Ferroptosis and Radioresistance in NPC
3.2. Ferroptosis and Chemoresistance in NPC
4. Natural Compounds and Pharmacological Agents Inducing Ferroptosis in NPC
4.1. Canonical Ferroptosis Activators as Mechanistic Tools
4.2. Natural Products as Ferroptosis Inducers
4.3. Pharmacologic Modulation of Ferroptosis
4.4. Nanotechnology-Enabled Ferroptosis
4.5. Practical Considerations for Combination Therapy
5. Biomarkers and Patient Stratification for Ferroptosis-Targeted Therapy in NPC
5.1. Ferroptosis-Related Genes and Prognostic Signatures
5.2. Epigenetic and Post-Transcriptional Biomarkers
5.3. Tumor Microenvironment and Viral Factors
5.4. On-Treatment Biomarkers for Dynamic Stratification
5.5. Toward Precision Stratification
6. Therapeutic Implications
6.1. Ferroptosis-Based Radiosensitization
6.2. Ferroptosis in Overcoming Chemoresistance
6.3. Ferroptosis and Immunotherapy Synergy
6.4. Targeting Anti-Ferroptosis Pathways
6.5. Nanotechnology and Drug Delivery Systems
6.6. Clinical Translation and Challenges
6.7. Risk–Benefit Considerations and Translational Barriers
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chang, E.T.; Ye, W.; Zeng, Y.X.; Adami, H.O. The Evolving Epidemiology of Nasopharyngeal Carcinoma. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1035–1047. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, S.; Dai, M.; Wu, G.; Wang, F.; Zhang, K. Global burden of nasopharyngeal carcinoma attributable to alcohol use: A 1990-2021 analysis with projections to 2040. Front. Public Health 2025, 13, 1623089. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Shi, F.; Tang, M.; Li, Y.; Hu, J.; Zhao, L.; Zhao, L.; Yu, X.; Luo, X.; et al. Targeting the signaling in Epstein-Barr virus-associated diseases: Mechanism, regulation, and clinical study. Signal Transduct. Target. Ther. 2021, 6, 15. [Google Scholar] [CrossRef]
- Chua, M.L.K.; Zhang, X.; Wong, K.C.W.; Grégoire, M.; Spreafico, A.; Ma, B. Updates on Treatments and Management of Nasopharyngeal Carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2025, 45, e472460, Erratum in Am. Soc. Clin. Oncol. Educ. Book 2025, 45, e472460CX1. [Google Scholar] [CrossRef]
- Shah, A.B.; Nagalli, S. Nasopharyngeal Carcinoma. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554588/ (accessed on 30 October 2025).
- Yip, P.L.; You, R.; Chen, M.Y.; Chua, M.L.K. Embracing Personalized Strategies in Radiotherapy for Nasopharyngeal Carcinoma: Beyond the Conventional Bounds of Fields and Borders. Cancers 2024, 16, 383. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhang, L.L.; Kou, J.; Zhou, G.Q.; Wu, C.F.; Sun, Y.; Lin, L. Cisplatin-based concurrent chemoradiotherapy improved the survival of locoregionally advanced nasopharyngeal carcinoma after induction chemotherapy by reducing early treatment failure. BMC Cancer 2022, 22, 1230. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shen, H.; Zhang, L.; Huang, W.; Zhang, S.; Zhang, B. Immunotherapy for recurrent or metastatic nasopharyngeal carcinoma. NPJ Precis. Oncol. 2024, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Pandrangi, V.C.; Liao, J.J.; de Almeida, J.R.; El-Sayed, I.H.; Hanna, G.; Su, S.Y.; Tsang, R.; Won, T.B.; Witterick, I.; Choby, G.; et al. Current Trends in the Management of Recurrent Nasopharyngeal Carcinoma. Head Neck 2025, 47, 2611–2621. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Berndt, C.; Alborzinia, H.; Amen, V.S.; Ayton, S.; Barayeu, U.; Bartelt, A.; Bayir, H.; Bebber, C.M.; Birsoy, K.; Böttcher, J.P.; et al. Ferroptosis in health and disease. Redox Biol. 2024, 75, 103211. [Google Scholar] [CrossRef]
- Dai, E.; Chen, X.; Linkermann, A.; Jiang, X.; Kang, R.; Kagan, V.E.; Bayir, H.; Yang, W.S.; Garcia-Saez, A.J.; Ioannou, M.S.; et al. A guideline on the molecular ecosystem regulating ferroptosis. Nat. Cell Biol. 2024, 26, 1447–1457. [Google Scholar] [CrossRef]
- Alves, F.; Lane, D.; Nguyen, T.P.M.; Bush, A.I.; Ayton, S. In defence of ferroptosis. Signal Transduct. Target. Ther. 2025, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Roh, J.L. SLC7A11 as a Gateway of Metabolic Perturbation and Ferroptosis Vulnerability in Cancer. Antioxidants 2022, 11, 2444. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Roh, J.L. Targeting GPX4 in human cancer: Implications of ferroptosis induction for tackling cancer resilience. Cancer Lett. 2023, 559, 216119. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Wang, Z.; Jin, Y.; Gu, W. Ferroptosis as a new tool for tumor suppression through lipid peroxidation. Commun. Biol. 2024, 7, 1475. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Hu, R.; Li, T.; Shi, Z.; Feng, E.; Yang, X.; Yang, J.; Lin, F.; Ren, Y.; Li, X. Anti-ferroptosis: A Promising Therapeutic Approach in Nasopharyngeal Carcinoma. Front. Biosci. (Landmark Ed.) 2025, 30, 27115. [Google Scholar] [CrossRef]
- Li, H.L.; Deng, N.H.; Xiao, J.X.; He, X.S. Cross-link between ferroptosis and nasopharyngeal carcinoma: New approach to radiotherapy sensitization. Oncol. Lett. 2021, 22, 770. [Google Scholar] [CrossRef]
- Zhou, P.; Peng, X.; Zhang, K.; Cheng, J.; Tang, M.; Shen, L.; Zhou, Q.; Li, D.; Yang, L. HAT1/HDAC2 mediated ACSL4 acetylation confers radiosensitivity by inducing ferroptosis in nasopharyngeal carcinoma. Cell Death Dis. 2025, 16, 160. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Lan, Y.; Li, J.; Cui, Y.; Zhou, J.; Wen, H. The m6A modification reader protein IGF2BP2 regulates ferroptosis in nasopharyngeal carcinoma cells by stabilizing CP expression via an m6A-dependent mechanism. Biochem. Biophys. Res. Commun. 2025, 778, 152417. [Google Scholar] [CrossRef]
- Liu, H.; Fu, Y.; Tang, L.; Song, B.; Gu, W.; Yang, H.; Xiao, T.; Wang, H.; Chen, P. O-GlcNAc-modified HOXA9 suppresses ferroptosis via promoting UBR5-mediated SIRT6 degradation in nasopharyngeal carcinoma. Neoplasia 2025, 62, 101142. [Google Scholar] [CrossRef]
- Yuan, L.; Li, S.; Chen, Q.; Xia, T.; Luo, D.; Li, L.; Liu, S.; Guo, S.; Liu, L.; Du, C.; et al. EBV infection-induced GPX4 promotes chemoresistance and tumor progression in nasopharyngeal carcinoma. Cell Death Differ. 2022, 29, 1513–1527. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jia, Q.; Tang, Q.; Deng, H.; He, Y.; Tang, F. Berberine-mediated Ferroptosis through System Xc(-)/GSH/GPX4 Axis Inhibits Metastasis of Nasopharyngeal Carcinoma. J. Cancer 2024, 15, 685–698. [Google Scholar] [CrossRef]
- Huang, S.; Cao, B.; Zhang, J.; Feng, Y.; Wang, L.; Chen, X.; Su, H.; Liao, S.; Liu, J.; Yan, J.; et al. Induction of ferroptosis in human nasopharyngeal cancer cells by cucurbitacin B: Molecular mechanism and therapeutic potential. Cell Death Dis. 2021, 12, 237. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Ma, S.; He, B.; Lu, J.; Guo, Z. Solasodine suppresses nasopharyngeal carcinoma progression by inducing ferroptosis. Sci. Rep. 2025, 15, 17247. [Google Scholar] [CrossRef]
- Feng, T.; Hu, G.; Luo, Y.; Zhang, X.; Chen, H.; Liang, Z.; He, X.; Ma, S.; Wei, J.; Fang, X.; et al. The Combination of Celastrol and Curcumin Enhances the Antitumor Effect in Nasopharyngeal Carcinoma by Inducing Ferroptosis. Biol. Pharm. Bull. 2025, 48, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.; Lv, H.; Lin, L.; Zhang, Y.; Qiao, Y.; Zhang, X.; Li, Z.; Wang, X.; Dai, X.; Dong, J. Antitumor/anti-inflammatory effects/tissue healing as an all-in-one therapeutic strategy for nasopharyngeal carcinoma. J. Nanobiotechnol. 2025, 23, 431. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, Q.; Mo, Y.; Zhang, H.; Dang, R.; Lin, M.; Xiao, R.; Chen, Y.; Deng, X.; Wang, S.; et al. A Novel Peculiarity of TXNIP Reversing the Radioresistance of NPC and Inducing Ferroptosis by xCT-GSH-GPX4-ROS Axis. Head Neck, 2025; online ahead of print. [Google Scholar] [CrossRef]
- Li, W.; Liang, L.; Liu, S.; Zeng, F.; Cao, J.; Lei, Y.; Yuan, X.; He, Q.; Zhou, Y. CD38 inhibits ferroptosis to promote radiotherapy resistance in nasopharyngeal carcinoma by competitively binding to TRIM21 to stabilize SLC7A11 protein. Int. J. Biol. Macromol. 2025, 317, 144742. [Google Scholar] [CrossRef]
- Dai, Z.; Lin, B.; Qin, M.; Lin, Y.; Wang, L.; Liao, K.; Xie, G.; Wang, F.; Zhang, J. METTL3-mediated m6A modification of SLC7A11 enhances nasopharyngeal carcinoma radioresistance by inhibiting ferroptosis. Int. J. Biol. Sci. 2025, 21, 1837–1851. [Google Scholar] [CrossRef]
- He, Y.; Yan, L.; Zhang, R.; Yang, R.; Kong, Z.; Wang, X. Down-regulation of PCK2 enhanced the radioresistance phenotype of nasopharyngeal carcinoma. Int. J. Radiat. Biol. 2025, 101, 499–509. [Google Scholar] [CrossRef]
- Zhou, R.; Qiu, L.; Zhou, L.; Geng, R.; Yang, S.; Wu, J. P4HA1 activates HMGCS1 to promote nasopharyngeal carcinoma ferroptosis resistance and progression. Cell. Signal. 2023, 105, 110609. [Google Scholar] [CrossRef]
- Cheng, Z.; Huang, L.; Zhang, Y.; Yue, K.; Jia, S.; Fang, Z.; Lin, Z. Heme oxygenase-1 leads to cisplatin resistance in nasopharyngeal carcinoma by reducing oxidative stress and ferroptosis. Cancer Cell Int. 2025, 25, 302. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhou, Y.; Xie, C.; He, G.; Zhang, M. Identification of key ferroptosis-related genes and therapeutic target in nasopharyngeal carcinoma. Front. Genet. 2025, 16, 1595456. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wu, S.; Lin, Z.; Ming, X.; Yang, X.; Yang, M.; Chen, X. Hypoxia-induced BAP1 enhances erastin-induced ferroptosis in nasopharyngeal carcinoma by stabilizing H2A. Cancer Cell Int. 2024, 24, 307. [Google Scholar] [CrossRef]
- Pu, X.; Wu, H.; Liu, X.; Yang, F. PRMT4 Reduced Erastin-Induced Ferroptosis in Nasopharyngeal Carcinoma Cisplatin-Resistant Cells by Nrf2/GPX4 Pathway. J. Environ. Pathol. Toxicol. Oncol. 2025, 44, 57–71. [Google Scholar] [CrossRef]
- Xue, Z.; Xie, H.; Shan, Y.; Zhang, L.; Cheng, L.; Chen, W.; Zhu, R.; Zhang, K.; Ni, H.; Zhang, Z.; et al. NAT10 inhibition promotes ac4C-dependent ferroptosis to counteract sorafenib resistance in nasopharyngeal carcinoma. Cancer Sci. 2024, 115, 3256–3272. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tang, L.; Liu, H.; Chen, Y.; Xiao, T.; Gu, W.; Yang, H.; Wang, H.; Chen, P. Cancer-associated fibroblasts secrete FGF5 to inhibit ferroptosis to decrease cisplatin sensitivity in nasopharyngeal carcinoma through binding to FGFR2. Cell Death Dis. 2024, 15, 279. [Google Scholar] [CrossRef]
- Li, F.; Xu, T.; Chen, P.; Sun, R.; Li, C.; Zhao, X.; Ou, J.; Li, J.; Liu, T.; Zeng, M.; et al. Platelet-derived extracellular vesicles inhibit ferroptosis and promote distant metastasis of nasopharyngeal carcinoma by upregulating ITGB3. Int. J. Biol. Sci. 2022, 18, 5858–5872. [Google Scholar] [CrossRef]
- Lee, J.; Seo, Y.; Roh, J.L. Emerging Therapeutic Strategies Targeting GPX4-Mediated Ferroptosis in Head and Neck Cancer. Int. J. Mol. Sci. 2025, 26, 6452. [Google Scholar] [CrossRef]
- Yang, F.; Gong, H.; Chen, S.; Li, J.; Huang, N.; Wang, M. Depletion of SLC7A11 Sensitizes Nasopharyngeal Carcinoma Cells to Ionizing Radiation. Protein Pept. Lett. 2024, 31, 323–331. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.L.; Li, J.; Ye, Z.P.; Du, T.; Li, L.C.; Guo, Y.Q.; Yang, D.; Li, Z.L.; Cao, J.H.; et al. Tubastatin A potently inhibits GPX4 activity to potentiate cancer radiotherapy through boosting ferroptosis. Redox Biol. 2023, 62, 102677. [Google Scholar] [CrossRef]
- Wang, D.; Tang, L.; Chen, M.; Gong, Z.; Fan, C.; Qu, H.; Liu, Y.; Shi, L.; Mo, Y.; Wang, Y.; et al. Nanocarriers Targeting Circular RNA ADARB1 Boost Radiosensitivity of Nasopharyngeal Carcinoma through Synergically Promoting Ferroptosis. ACS Nano 2024, 18, 31055–31075. [Google Scholar] [CrossRef]
- Mi, J.; Wang, Y.; He, S.; Qin, X.; Li, Z.; Zhang, T.; Huang, W.; Wang, R. LncRNA HOTAIRM1 promotes radioresistance in nasopharyngeal carcinoma by modulating FTO acetylation-dependent alternative splicing of CD44. Neoplasia 2024, 56, 101034. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, F.; Chen, J.; Chan, S.; He, Y.; Liu, W.; Zhang, G. Disulfiram/Copper Induces Antitumor Activity against Both Nasopharyngeal Cancer Cells and Cancer-Associated Fibroblasts through ROS/MAPK and Ferroptosis Pathways. Cancers 2020, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in human diseases: Mechanisms and therapeutic prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Q.; Li, X.; Chen, Y.; Xu, G. Itraconazole attenuates the stemness of nasopharyngeal carcinoma cells via triggering ferroptosis. Environ. Toxicol. 2021, 36, 257–266. [Google Scholar] [CrossRef]
- He, X.; Yao, Q.; Fan, D.; Duan, L.; You, Y.; Liang, W.; Zhou, Z.; Teng, S.; Liang, Z.; Hall, D.D.; et al. Cephalosporin antibiotics specifically and selectively target nasopharyngeal carcinoma through HMOX1-induced ferroptosis. Life Sci. 2021, 277, 119457. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Zou, Y.; Wang, Y.; Duan, T.; Zhou, Z.; Huang, Y.; Ye, Q. EMC2 suppresses ferroptosis via regulating TFRC in nasopharyngeal carcinoma. Transl. Oncol. 2025, 52, 102251. [Google Scholar] [CrossRef]
- Magtanong, L.; Mueller, G.D.; Williams, K.J.; Billmann, M.; Chan, K.; Armenta, D.A.; Pope, L.E.; Moffat, J.; Boone, C.; Myers, C.L.; et al. Context-dependent regulation of ferroptosis sensitivity. Cell Chem. Biol. 2022, 29, 1409–1418.e1406. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Gan, B. Ferroptosis execution: Is it all about ACSL4? Cell Chem. Biol. 2022, 29, 1363–1365. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, S.; Wang, K.; Liu, Z.; Yu, T.; Zhang, Y. The COX-2 Inhibitor Celecoxib Sensitizes Nasopharyngeal Carcinoma Cells to Ferroptosis. Curr. Cancer Drug Targets, 2025; online ahead of print. [Google Scholar] [CrossRef]
- Huang, W.M.; Li, Z.X.; Wu, Y.H.; Shi, Z.L.; Mi, J.L.; Hu, K.; Wang, R.S. m6A demethylase FTO renders radioresistance of nasopharyngeal carcinoma via promoting OTUB1-mediated anti-ferroptosis. Transl. Oncol. 2023, 27, 101576. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wen, X.; Huang, C.; Lin, Z.; Xu, Z.; Sun, C.; Li, L.; Zhang, S.; Song, S.; Lou, J.; et al. RRFERV stabilizes TEAD1 expression to mediate nasopharyngeal cancer radiation resistance rendering tumor cells vulnerable to ferroptosis. Int. J. Surg. 2025, 111, 450–466. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Z.; Fu, Y.; Wu, S.; Zhu, Y.; Yuan, J.; Liu, Y. MiR-122-5p regulates erastin-induced ferroptosis via CS in nasopharyngeal carcinoma. Sci. Rep. 2024, 14, 10019. [Google Scholar] [CrossRef]
- Chen, P.; Wang, D.; Xiao, T.; Gu, W.; Yang, H.; Yang, M.; Wang, H. ACSL4 promotes ferroptosis and M1 macrophage polarization to regulate the tumorigenesis of nasopharyngeal carcinoma. Int. Immunopharmacol. 2023, 122, 110629. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Ding, B.; Li, Q.; Chen, X.; Liu, H.; Xu, M.; Lan, Y.; Li, Y. TNF-α inhibits Epstein Barr virus reactivation through the GPX4 mediated glutathione pathway. Sci. Rep. 2025, 15, 16448. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y.; Fan, R.; Gao, K.; Xie, S.; Wang, F.; Zhang, J.; Zhang, H.; He, Y.; Xie, Z.; et al. Treatment of Recurrent Nasopharyngeal Carcinoma: A Sequential Challenge. Cancers 2022, 14, 4111. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.M.; Chen, W.P.; Du, X.J.; Ou, C.X.; Luo, Z.K.; Wang, R.; Zhang, C.Q.; Ge, C.D.; Han, M.; et al. Tumor-repopulating cells evade ferroptosis via PCK2-dependent phospholipid remodeling. Nat. Chem. Biol. 2024, 20, 1341–1352. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Y.; Lin, Y.; Zhou, X.; Wang, L.; Zhou, Y.; Lin, K.; Cai, L. GSTM3 enhances radiosensitivity of nasopharyngeal carcinoma by promoting radiation-induced ferroptosis through USP14/FASN axis and GPX4. Br. J. Cancer 2024, 130, 755–768. [Google Scholar] [CrossRef]
- Huang, X.; Lin, K.; Chen, W.; Zhang, D.; Khan, M.; Ye, X.; Wang, B.; Chen, C.; Tian, Y.; Yuan, Y.; et al. Modulation of the local angiotensin II: Suppression of ferroptosis and radiosensitivity in nasopharyngeal carcinoma via the HIF-1α-HILPDA axis. Radiother. Oncol. 2025, 203, 110686. [Google Scholar] [CrossRef]
- Amos, A.; Jiang, N.; Zong, D.; Gu, J.; Zhou, J.; Yin, L.; He, X.; Xu, Y.; Wu, L. Depletion of SOD2 enhances nasopharyngeal carcinoma cell radiosensitivity via ferroptosis induction modulated by DHODH inhibition. BMC Cancer 2023, 23, 117, Erratum in BMC Cancer 2023, 23, 462. [Google Scholar] [CrossRef]
- Qiu, L.; Zhou, R.; Zhou, L.; Yang, S.; Wu, J. CAPRIN2 upregulation by LINC00941 promotes nasopharyngeal carcinoma ferroptosis resistance and metastatic colonization through HMGCR. Front. Oncol. 2022, 12, 931749. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, P.; Wu, D.; Tang, F.; Shen, N.; Hou, G. Deubiquitinating enzyme UCHL1 stabilizes CAV1 to inhibit ferroptosis and enhance docetaxel resistance in nasopharyngeal carcinoma. Anticancer Drugs 2025, 36, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Tan, G.; Lei, G.; Zhang, X.; Xie, Z. Programmed cell death in nasopharyngeal carcinoma: Mechanisms and therapeutic targets. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189265. [Google Scholar] [CrossRef]
- Liu, S.; Yan, S.; Zhu, J.; Lu, R.; Kang, C.; Tang, K.; Zeng, J.; Ding, M.; Guo, Z.; Lai, X.; et al. Combination RSL3 Treatment Sensitizes Ferroptosis- and EGFR-Inhibition-Resistant HNSCCs to Cetuximab. Int. J. Mol. Sci. 2022, 23, 9014. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, J.; Xiong, Y.; Su, K.; Liu, C.; Cheng, B.; Wu, T. Sulfasalazine combined with anti-IL-1β mAb induces ferroptosis and immune modulation in oral squamous cell carcinoma. Cell. Mol. Life Sci. 2025, 82, 216. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gu, Z. Ferroptosis in head and neck squamous cell carcinoma: From pathogenesis to treatment. Front. Pharmacol. 2024, 15, 1283465. [Google Scholar] [CrossRef]
- Wang, H.H.; Fan, S.Q.; Zhan, Y.T.; Peng, S.P.; Wang, W.Y. Suppression of the SLC7A11/glutathione axis causes ferroptosis and apoptosis and alters the mitogen-activated protein kinase pathway in nasopharyngeal carcinoma. Int. J. Biol. Macromol. 2024, 254, 127976. [Google Scholar] [CrossRef]
- Wu, Z.; Qu, Q. Mechanism of luteolin induces ferroptosis in nasopharyngeal carcinoma cells. J. Toxicol. Sci. 2024, 49, 399–408. [Google Scholar] [CrossRef]
- Luo, X.; Gong, Y.; Jiang, Q.; Wang, Q.; Li, S.; Liu, L. Isoquercitrin promotes ferroptosis and oxidative stress in nasopharyngeal carcinoma via the AMPK/NF-κB pathway. J. Biochem. Mol. Toxicol. 2024, 38, e23542. [Google Scholar] [CrossRef]
- Li, X.; Luo, J.Q.; Liao, X.Q.; Zhang, S.; Yang, L.F.; Wu, T.; Wang, L.; Xu, Q.; He, B.S.; Guo, Z. Allicin inhibits the growth of HONE-1 and HNE1 human nasopharyngeal carcinoma cells by inducing ferroptosis. Neoplasma 2024, 71, 243–254. [Google Scholar] [CrossRef]
- Zhou, J.C.; Wu, B.; Zhang, J.J.; Zhang, W. Lupeol triggers oxidative stress, ferroptosis, apoptosis and restrains inflammation in nasopharyngeal carcinoma via AMPK/NF-κB pathway. Immunopharmacol. Immunotoxicol. 2022, 44, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhou, X.; Liu, L.; Wang, Z.; Wang, C.; Luo, N.; Jin, G. Superparamagnetic Iron Oxide-Erastin-Polyethylene Glycol Nanotherapeutic Platform: A Ferroptosis-Based Approach for the Integrated Diagnosis and Treatment of Nasopharyngeal Cancer. Mol. Pharm. 2024, 21, 2767–2780. [Google Scholar] [CrossRef]
- Roh, J.L. Targeting ferroptosis suppressor protein 1 in cancer therapy: Implications and perspectives, with emphasis on head and neck cancer. Crit. Rev. Oncol. Hematol. 2024, 202, 104440. [Google Scholar] [CrossRef]
- Li, F.J.; Long, H.Z.; Zhou, Z.W.; Luo, H.Y.; Xu, S.G.; Gao, L.C. System X(c) (-)/GSH/GPX4 axis: An important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy. Front. Pharmacol. 2022, 13, 910292. [Google Scholar] [CrossRef]
- Yazar, M.; Sevindik, M.; Polat, A.; Koçer, O.; Karatepe, H.; Uysal, İ. General properties, biosynthesis, pharmacological properties, biological activities and daily uses of luteolin. Pros. Pharm. Sci. 2025, 22, 146–154. [Google Scholar] [CrossRef]
- Su, J.; Zhong, G.; Qin, W.; Zhou, L.; Ye, J.; Ye, Y.; Chen, C.; Liang, P.; Zhao, W.; Xiao, X.; et al. Integrating iron metabolism-related gene signature to evaluate prognosis and immune infiltration in nasopharyngeal carcinoma. Discov. Oncol. 2024, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Bao, L.; Ren, Q.; Zhang, Z.; Yi, L.; Lei, W.; Yang, Z.; Lu, Y.; You, B.; You, Y.; et al. SCARB1 in extracellular vesicles promotes NPC metastasis by co-regulating M1 and M2 macrophage function. Cell Death Discov. 2023, 9, 323. [Google Scholar] [CrossRef]
- Sun, S.; Shen, J.; Jiang, J.; Wang, F.; Min, J. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Signal Transduct. Target. Ther. 2023, 8, 372. [Google Scholar] [CrossRef]
- Wang, D.; Shen, Y.; Qian, H.; Jiang, J.; Xu, W. Emerging advanced approaches for liquid biopsy: In situ nucleic acid assays of extracellular vesicles. Theranostics 2024, 14, 7309–7332. [Google Scholar] [CrossRef]
- Shi, M.; Du, J.; Shi, J.; Huang, Y.; Zhao, Y.; Ma, L. Ferroptosis-related gene ATG5 is a novel prognostic biomarker in nasopharyngeal carcinoma and head and neck squamous cell carcinoma. Front. Bioeng. Biotechnol. 2022, 10, 1006535. [Google Scholar] [CrossRef]
- Chen, W.; Zuo, F.; Zhang, K.; Xia, T.; Lei, W.; Zhang, Z.; Bao, L.; You, Y. Exosomal MIF Derived From Nasopharyngeal Carcinoma Promotes Metastasis by Repressing Ferroptosis of Macrophages. Front. Cell Dev. Biol. 2021, 9, 791187. [Google Scholar] [CrossRef]
- Semeradtova, A.; Liegertova, M.; Herma, R.; Capkova, M.; Brignole, C.; Del Zotto, G. Extracellular vesicles in cancer´s communication: Messages we can read and how to answer. Mol. Cancer 2025, 24, 86. [Google Scholar] [CrossRef]
- Cai, H.; Ren, Y.; Chen, S.; Wang, Y.; Chu, L. Ferroptosis and tumor immunotherapy: A promising combination therapy for tumors. Front. Oncol. 2023, 13, 1119369. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Song, Y.; Wang, R.; Wang, T. Molecular mechanisms of tumor resistance to radiotherapy. Mol. Cancer 2023, 22, 96. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.F.; Chaudhary, K.R.; Zandkarimi, F.; Harken, A.D.; Kinslow, C.J.; Upadhyayula, P.S.; Dovas, A.; Higgins, D.M.; Tan, H.; Zhang, Y.; et al. Radiation-Induced Lipid Peroxidation Triggers Ferroptosis and Synergizes with Ferroptosis Inducers. ACS Chem. Biol. 2020, 15, 469–484. [Google Scholar] [CrossRef]
- Ma, C.; Hu, H.; Liu, H.; Zhong, C.; Wu, B.; Lv, C.; Tian, Y. Lipotoxicity, lipid peroxidation and ferroptosis: A dilemma in cancer therapy. Cell Biol. Toxicol. 2025, 41, 75. [Google Scholar] [CrossRef]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Sun, Z.; Wang, Y.; Wang, L.; Liu, Z.; Han, X. Ferroptosis: A double-edged sword mediating immune tolerance of cancer. Cell Death Dis. 2022, 13, 925. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Jin, D.Y.; Chen, X.; Liu, Y.; Williams, C.M.; Pedersen, L.C.; Stafford, D.W.; Tie, J.K. A genome-wide CRISPR-Cas9 knockout screen identifies FSP1 as the warfarin-resistant vitamin K reductase. Nat. Commun. 2023, 14, 828. [Google Scholar] [CrossRef]
- Sinha, B.K. Ferroptosis in Toxicology: Present and Future. Int. J. Mol. Sci. 2025, 26, 6658. [Google Scholar] [CrossRef]
- Du, X.; Dong, R.; Wu, Y.; Ni, B. Physiological Effects of Ferroptosis on Organ Fibrosis. Oxidative Med. Cell. Longev. 2022, 2022, 5295434. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhong, Y. Bioinformatics analysis based on ferroptosis-related lncRNAs: Construction of a clinical prognostic model for nasopharyngeal carcinoma and correlation analysis. Transl. Cancer Res. 2022, 11, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, J.; Hu, X. Ferroptosis regulators related scoring system by Gaussian finite mixture model to predict prognosis and immunotherapy efficacy in nasopharyngeal carcinoma. Front. Genet. 2022, 13, 975190. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, X.; Yang, B.; Li, M.; Du, Y.; Wang, J.; Liu, S.; Gong, L.; Li, L.; Gao, L. The role of ferroptosis in radiotherapy and combination therapy for head and neck squamous cell carcinoma (Review). Oncol. Rep. 2024, 51, 1–10. [Google Scholar] [CrossRef]
- Kerkhove, L.; Geirnaert, F.; Dufait, I.; De Ridder, M. Ferroptosis: Frenemy of Radiotherapy. Int. J. Mol. Sci. 2024, 25, 3641. [Google Scholar] [CrossRef]
- Ge, C.; Zhang, S.; Mu, H.; Zheng, S.; Tan, Z.; Huang, X.; Xu, C.; Zou, J.; Zhu, Y.; Feng, D.; et al. Emerging Mechanisms and Disease Implications of Ferroptosis: Potential Applications of Natural Products. Front. Cell Dev. Biol. 2021, 9, 774957. [Google Scholar] [CrossRef]

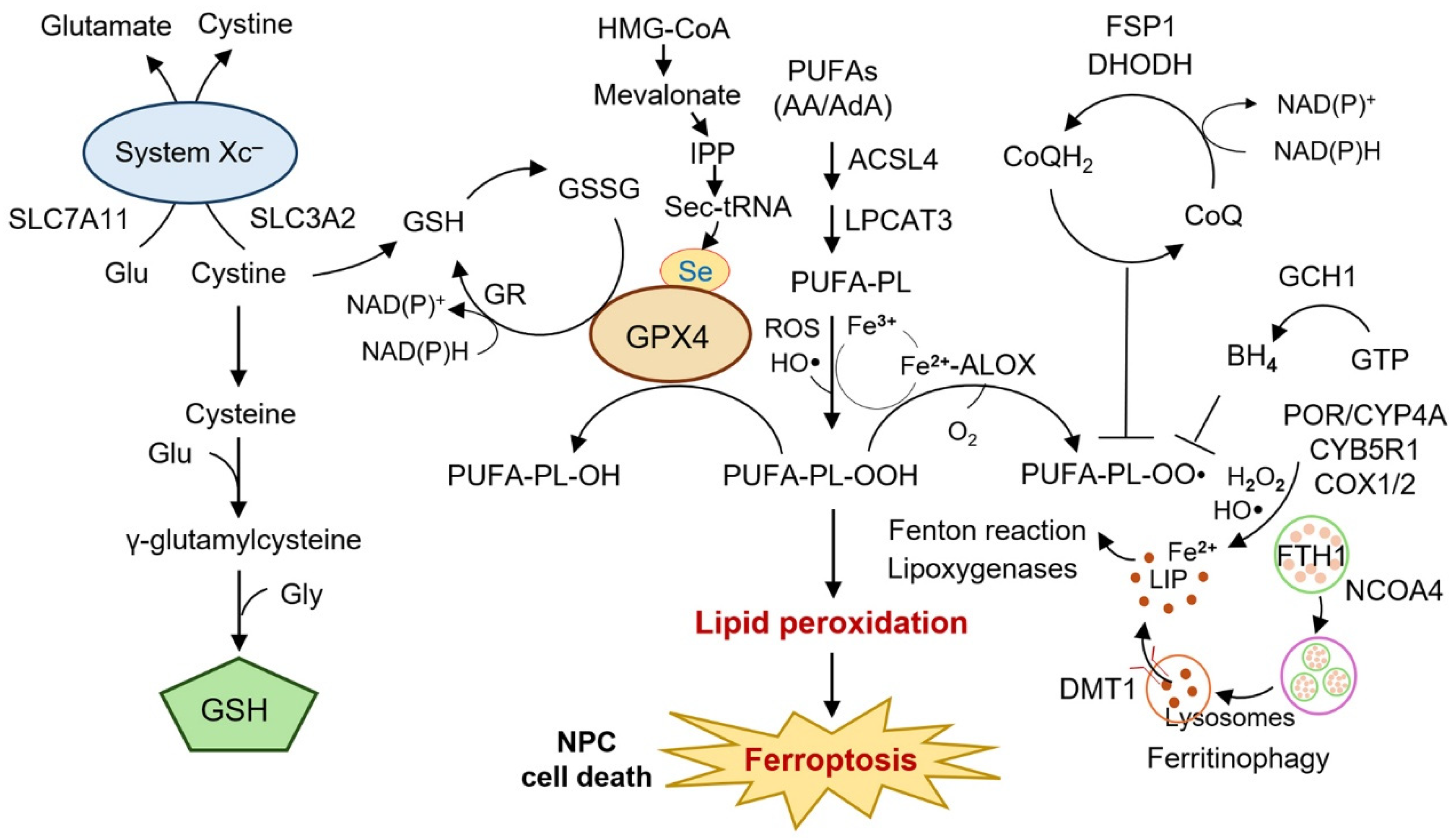
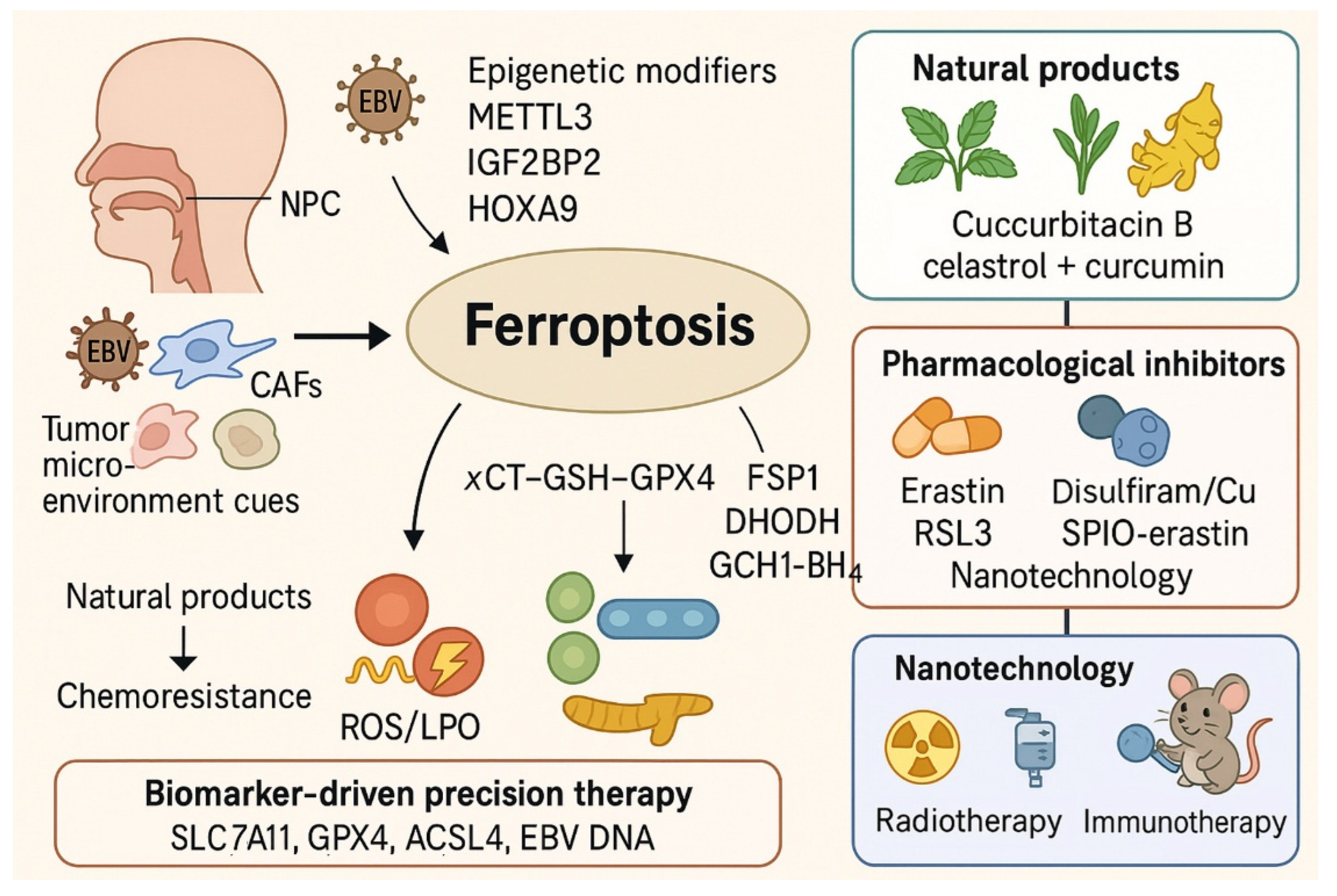
| Axis | Key Regulator(s) in NPC | Direction on Ferroptosis | Phenotypic Impact in NPC | Model(s) | Reference(s) |
|---|---|---|---|---|---|
| System xCT–GSH–GPX4 | SLC7A11, GPX4 | Anti-ferroptosis | Enhances radio/chemoresistance via ROS detoxification | Multiple NPC cell lines; patient samples | [20,21] |
| System xCT–ROS linkage | TXNIP ↓ in resistant NPC | Pro-ferroptosis | Overexpression restores radiosensitivity by suppressing xCT–GSH–GPX4 axis | Patient samples; xenografts | [31] |
| Ubiquitin control of xCT | CD38–TRIM21–SLC7A11 | Anti-ferroptosis | Stabilizes SLC7A11; confers radioresistance | In vitro NPC; co-immunoprecipitation validation | [32] |
| Epigenetic m6A regulation | METTL3–SLC7A11; IGF2BP2–CP | Anti-ferroptosis | m6A stabilization of SLC7A11 and CP suppresses ferroptosis, promotes radioresistance and progression | NPC lines; xenografts | [23,33] |
| Epigenetic post-translational control | HOXA9 (O-GlcNAcylated)–UBR5–SIRT6 | Anti-ferroptosis | SIRT6 degradation restrains ferroptosis; enhances RT resistance | NPC cells; xenografts | [24] |
| Lipid metabolism | ACSL4 (PUFA enrichment); ACSL4 acetylation; PCK2; P4HA1–HMGCS1 axis | ACSL4 acetylation: Pro-ferroptosis; PCK2 ↓ and P4HA1 ↑: Anti-ferroptosis | ACSL4 acetylation restores radiosensitivity; PCK2 downregulation suppresses ferroptosis; P4HA1 promotes proliferation | NPC lines; xenograft | [22,34,35] |
| Iron/heme stress | HO-1 (HMOX1); ferritinophagy (NCOA4); TFRC, FTH1, SLC40A1 | HO-1: Anti-ferroptosis; TFRC/NCOA4: Pro-ferroptosis | HO-1 upregulation confers cisplatin resistance; ZnPP inhibition restores ferroptosis | HK1, C666-1; xenografts | [36,37] |
| Chromatin/hypoxia link | BAP1–H2A–SLC7A11 | Pro-ferroptosis | BAP1 loss reduces erastin sensitivity under hypoxia; modifies RT response | NPC cell lines; xenografts | [38] |
| Protein methylation | PRMT4–Nrf2/GPX4 pathway | Anti-ferroptosis | Enhances cisplatin resistance; PRMT4 knockdown restores ferroptosis | NPC lines; xenografts | [39] |
| RNA acetylation | NAT10–SLC7A11 (ac4C modification) | Anti-ferroptosis | Stabilizes SLC7A11, confers resistance to sorafenib and platinum | In vitro NPC; xenograft | [40] |
| Viral oncogenesis | EBV-driven GPX4 upregulation; p62–Keap1–Nrf2 | Anti-ferroptosis | Enhances antioxidant defense; drives progression and chemoresistance | Clinical samples; xenografts | [25] |
| Microenvironmental signals | CAF–FGF5–FGFR2–Nrf2–HO-1; Platelet-EV ITGB3 | Anti-ferroptosis | Inhibits cisplatin-induced ferroptosis; promotes metastasis | NPC co-culture; in vivo | [41,42] |
| Agent/Class | Primary Target or Pathway | NPC Model/Setting | Key Outcome | Combination Read-Through | Reference(s) |
|---|---|---|---|---|---|
| Erastin | System xCT inhibitor → ↓ cystine/GSH | CDDP-resistant HK1, C666-1; xenografts | Restored cisplatin cytotoxicity; ↓ tumor growth | Pairs with cisplatin for re-sensitization | [36,48] |
| RSL3 | GPX4 inhibitor induced ↑ lipid peroxides | CNE2; H&N models | Inhibits survival; reverses resistance | Synergy with RT or COX-2/EGFR inhibitors | [55,69] |
| Sulfasalazine | xCT blockade (FDA-approved scaffold) | NPC cell lines | Depletes cystine/GSH; induces ferroptosis | Potential radiosensitizer; PD-1 synergy | [70,71] |
| Sorafenib | xCT/GSH depletion + kinase inhibition | NPC preclinical models | Sensitizes to RT and CDDP | Enhances RT/chemotherapy efficacy | [40,72] |
| Disulfiram/Cu | ROS/MAPK & ferroptosis via GSH depletion | NPC cell lines & CAFs | Antitumor activity against NPC cells + CAFs | Repurposed; combinable with CDDP | [48] |
| ZnPP (HO-1 inhibitor) | Blocks anti-ferroptotic HO-1 | Cisplatin-resistant NPC; xenografts | Restores cisplatin sensitivity; ↓ tumor burden | Combines effectively with cisplatin | [36] |
| Solasodine | ↑ Fe2+/ROS/MDA, ↓ GPX4/SLC40A1 | NPC cell lines | Induces ferroptosis, suppresses growth | Potential complement with RT/chemotherapy | [28] |
| Berberine | Inhibits xCT–GSH–GPX4 axis | NPC in vitro/in vivo | ↓ GPX4, ↓ SLC7A11; anti-metastatic | Radiosensitization reported | [26] |
| Cucurbitacin B | ↑ iron, ↓ GPX4/GSH | NPC xenografts | Induces ferroptosis; enhances cisplatin efficacy | Works synergistically with CDDP | [27] |
| Celastrol + Curcumin | ↑ ACSL4, ↓ GPX4/SLC7A11 | NPC xenografts | Synergistic tumor suppression; low toxicity | Potentiates chemotherapy efficacy | [29] |
| Luteolin | SOX4/GDF15 suppression induced ↓ GPX4 | NPC cell lines | Increases lipid ROS; enhances ferroptosis | Candidate radiosensitizer | [73] |
| Isoquercitrin | AMPK/NF-κB signaling | NPC cells; xenografts | Induces oxidative stress; tumor suppression | Dietary adjunct potential | [74] |
| Allicin | ↓ GPX4/GSH, ↑ lipid ROS | HONE-1, HNE1 cells | Suppresses proliferation via ferroptosis | Candidate dietary sensitizer | [75] |
| Lupeol | Inhibits GPX4; ↑ ROS | NPC cell lines | Induces ferroptosis + apoptosis | Promising natural sensitizer | [76] |
| Itraconazole | ↑ lysosomal iron; ferroptosis induction | NPC cells | Reduces stemness; ↑ lipid peroxidation | Maintenance therapy potential | [50] |
| Cephalosporins | HMOX1 activation → ferroptosis | NPC cells | Selective NPC killing | Drug repurposing candidate | [51] |
| PRMT4 inhibitors | Target PRMT4–Nrf2/GPX4 pathway | Cisplatin-resistant NPC | Restore ferroptosis; ↑ cisplatin efficacy | Synergy with CDDP | [39] |
| NAT10 inhibitors | Block ac4C–SLC7A11 stabilization | NPC models | Promote ferroptosis; overcome sorafenib resistance | Enhance platinum/sorafenib therapy | [40] |
| Nanoplatforms (Bi2Se3 hydrogels, SPIO-Erastin NPs) | ROS release, iron delivery, GPX4 suppression | NPC xenografts | Spatially controlled ferroptosis + apoptosis; tissue repair | Integrate with RT or PD-1 blockade | [30,77] |
| FSP1 inhibitors | Block ubiquinol regeneration | NPC models (preclinical) | Promote ferroptosis independent of GPX4 | Candidate for combination with RT/ICI | [20,78] |
| Biomarker | Assay/Sample Type | Directionality & Interpretation | Clinical Use Case | Reference(s) |
| SLC7A11 (xCT) | IHC/RNA-seq | High → anti-ferroptotic; resistance risk | Predict radio/chemoresistance; exclude monotherapy inducers | [32,33] |
| GPX4 | IHC/RNA-seq | High → resistance; Low → susceptibility | Select patients for GPX4-targeted strategies (e.g., RSL3) | [39,63] |
| ACSL4 | IHC/RNA-seq | High → pro-ferroptotic lipidome | Radiosensitization candidate; marker of ferroptotic vulnerability | [22] |
| TFRC, FTH1, SLC40A1 | RNA-seq/WGCNA | Iron metabolism-related FRGs; correlate with prognosis | Risk stratification based on iron metabolism | [37,81] |
| TXNIP | IHC in tumor biopsies | Low in resistant tumors; restoration ↑ ferroptosis | Predict radiosensitization potential | [31] |
| HO-1 (HMOX1) | IHC/qRT-PCR | High → cisplatin resistance via ferroptosis blockade | Add HO-1 inhibitor (ZnPP) with cisplatin | [36] |
| METTL3 (m6A writer) | RNA-seq/protein assay | Stabilizes SLC7A11 → suppresses ferroptosis | Predict RT resistance; candidate for epigenetic targeting | [33] |
| IGF2BP2 (m6A reader) | RNA-seq/IHC | Stabilizes CP mRNA; anti-ferroptotic | Prognostic biomarker; iron metabolism regulator | [23] |
| HOXA9–UBR5–SIRT6 axis | Protein assay/functional validation | Anti-ferroptotic; promotes progression | Therapy resistance marker; target for intervention | [24] |
| NAT10 (RNA acetyltransferase) | RNA/protein assays | Stabilizes SLC7A11 via ac4C → anti-ferroptotic | Marker for sorafenib/platinum resistance | [40] |
| EBV DNA (plasma) | qPCR | High baseline or slow decline → poor prognosis | Widely validated clinical biomarker; may reflect ferroptotic vulnerability | [4] |
| Immune infiltration signatures (M1 macrophages, neutrophils) | Bulk RNA-seq/IHC | High FRG activity linked to pro-inflammatory immune context | Predict response to ferroptosis-immunotherapy combinations | [37,59,82] |
| Dynamic PD markers (↓ GSH, ↑ MDA, ↑ Fe2+) | Tumor tissue assays/liquid biopsy | Real-time readout of ferroptosis induction | On-treatment monitoring and adaptive decision-making | [83,84] |
| FRG hub genes (TBK1, KIF20A, SLC16A1, QSOX1) | Multi-omics (WGCNA, RNA-seq) | Diagnostic/prognostic; link to immune infiltration | Baseline stratification for ferroptosis-targeted therapy | [37] |
| Setting | Concept Combo | Primary Endpoints | Stratifiers & On-Treatment Markers | Rationale |
|---|---|---|---|---|
| Locally advanced NPC (good induction responders) | CCRT ± low-dose erastin/sulfasalazine or HO-1 inhibitor during weeks 1–4 | 3-y DFS; ORN/late toxicity | Baseline: SLC7A11, GPX4; On-treatment: EBV DNA kinetics, GSH ↓/MDA ↑/Fe2+ ↑ | Radiosensitization by tipping redox balance toward ferroptosis while minimizing normal tissue toxicity [4,13] |
| Recurrent/metastatic NPC (first-line) | Platinum doublet + PD-1 ± ferroptosis enabler (e.g., ACSL4 upmodulator, FSP1 inhibitor) | PFS, ORR; immune-related AEs | PD-L1, EBV DNA load, ferroptosis-related gene (FRG) signature | PD-1 combinations are standardizing; ferroptosis enhances immunogenic cell death and T cell priming [20,88] |
| Cisplatin-resistant disease | Cisplatin ± ZnPP (HO-1 inhibitor) or NAT10/PRMT4 inhibitor | ORR, PFS; nephrotoxicity monitoring | HO-1 expression, NAT10/PRMT4 activity, EBV DNA kinetics | Restores ferroptosis by blocking anti-ferroptosis defenses; overcomes platinum resistance [36,39,40] |
| Post-operative/bed therapy or salvage RT site | NIR-triggered Bi2Se3 nanosheet–alginate hydrogel | Local control; wound complications | Local MDA/4-HNE staining; MRI radiomics | Spatially selective ferroptosis with concurrent anti-inflammatory/tissue healing benefits [30] |
| Maintenance/secondary prevention | Itraconazole or disulfiram/Cu with low-dose RT/chemo | Time to recurrence; QoL | Iron metabolism genes (TFRC, FTH1), GSH levels | Repurposed agents induce ferroptosis and reduce stemness, potentially delaying relapse [48,50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Roh, J.-L. Targeting Ferroptosis in Nasopharyngeal Carcinoma: Mechanisms, Resistance, and Precision Therapeutic Opportunities. Int. J. Mol. Sci. 2025, 26, 11439. https://doi.org/10.3390/ijms262311439
Lee J, Roh J-L. Targeting Ferroptosis in Nasopharyngeal Carcinoma: Mechanisms, Resistance, and Precision Therapeutic Opportunities. International Journal of Molecular Sciences. 2025; 26(23):11439. https://doi.org/10.3390/ijms262311439
Chicago/Turabian StyleLee, Jaewang, and Jong-Lyel Roh. 2025. "Targeting Ferroptosis in Nasopharyngeal Carcinoma: Mechanisms, Resistance, and Precision Therapeutic Opportunities" International Journal of Molecular Sciences 26, no. 23: 11439. https://doi.org/10.3390/ijms262311439
APA StyleLee, J., & Roh, J.-L. (2025). Targeting Ferroptosis in Nasopharyngeal Carcinoma: Mechanisms, Resistance, and Precision Therapeutic Opportunities. International Journal of Molecular Sciences, 26(23), 11439. https://doi.org/10.3390/ijms262311439







