Thyroid Hormone T4 Alleviates Traumatic Brain Injury by Enhancing Blood–Brain Barrier Integrity
Abstract
1. Introduction
2. Results
2.1. Effect of T4 on Vascular Leakage in Frontal Cortex and Hippocampus 7 Days Post-TBI
2.2. Effect of T4 on Thyroid Hormone Receptor and Transporters
2.3. Effect of T4 on Hyperpermeability and Angiogenesis Post-TBI
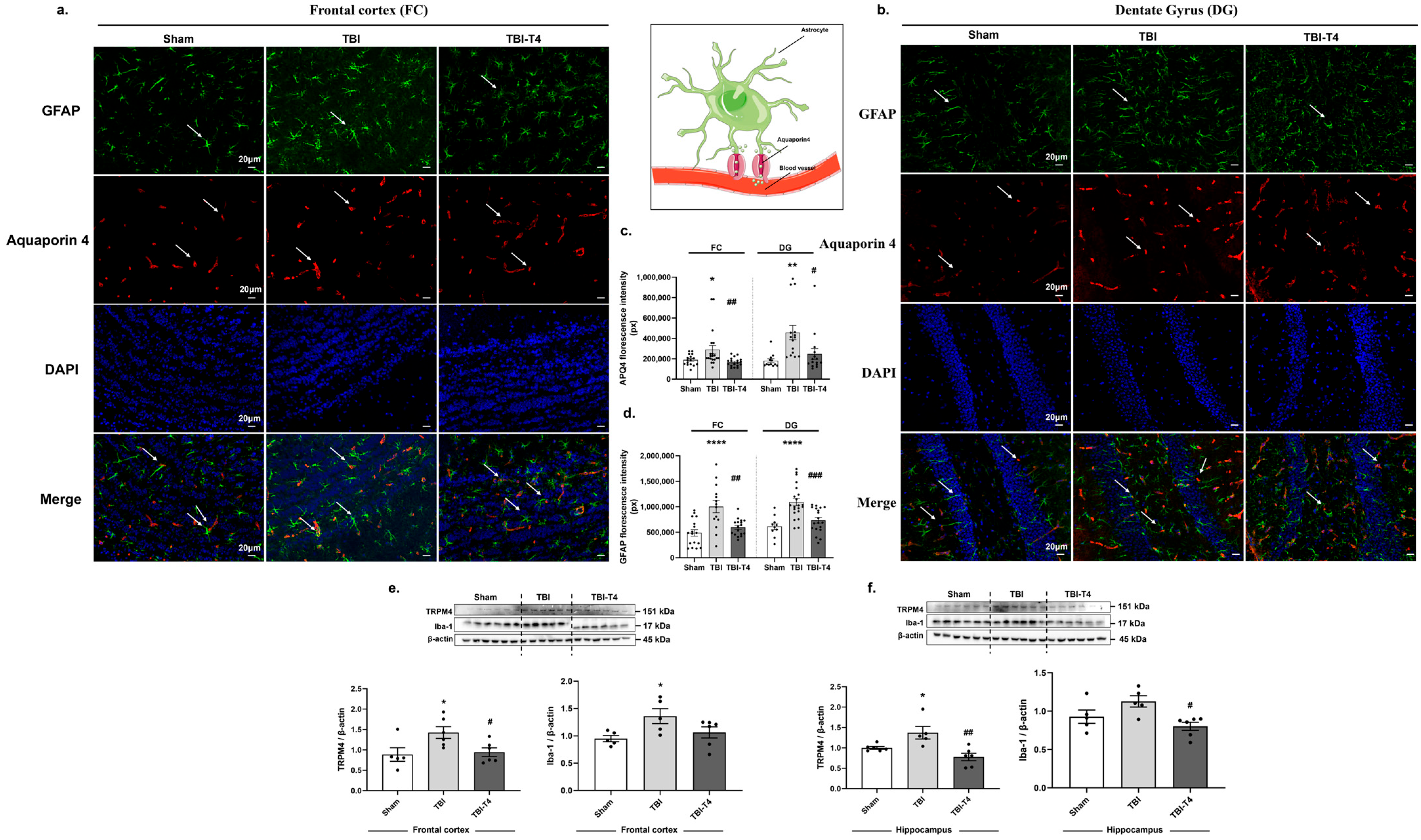
2.4. Post-TBI Effects of T4 on Aquaporin 4, Astrocyte Marker GFAP and Microglial Marker Iba-1 in Frontal Cortex and Dentate Gyrus (DG) Region of Hippocampus
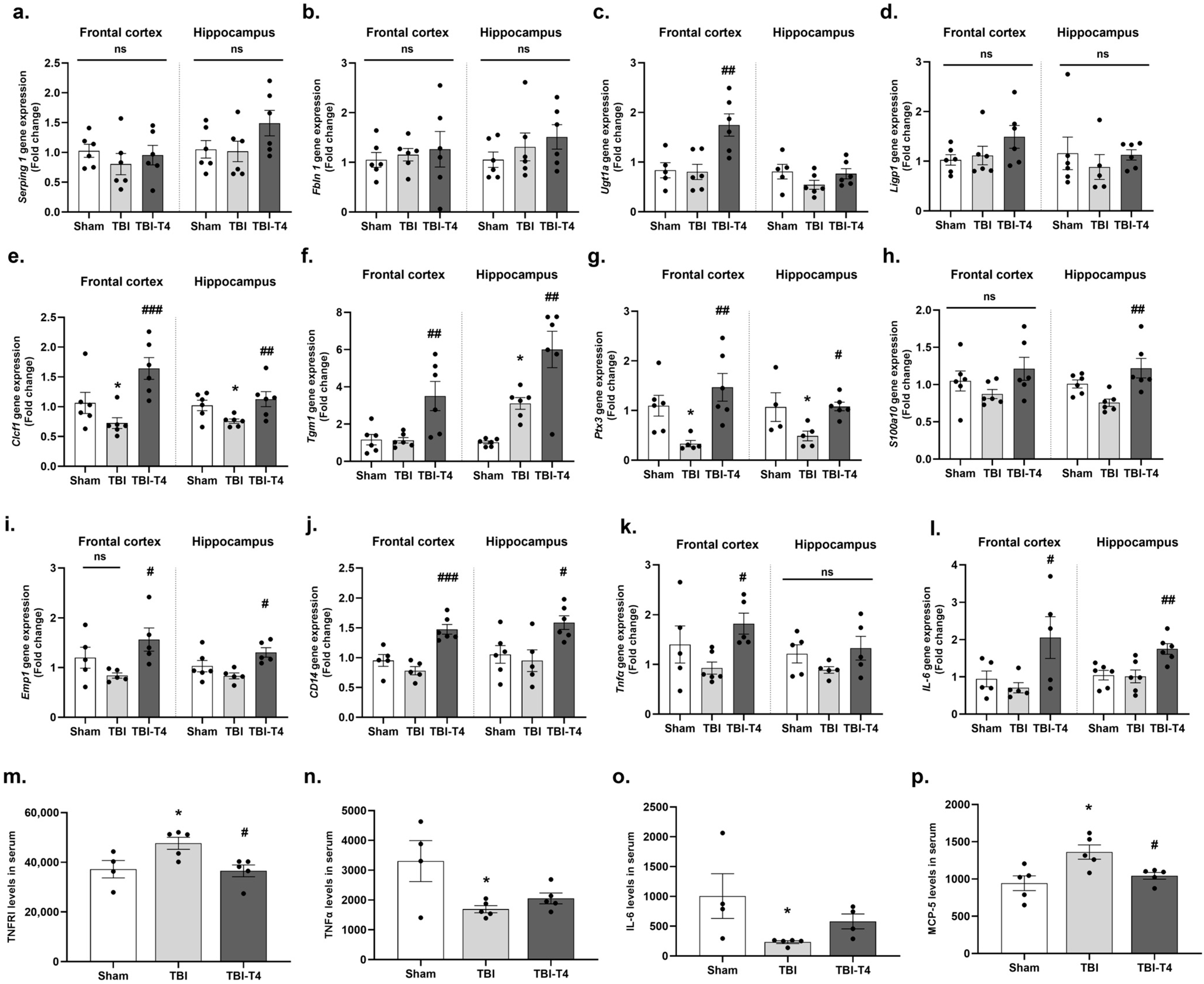
2.5. Post-TBI Effect of T4 on Phenotype-Specific Astrocytes and Inflammation in Frontal Cortex and Hippocampus
2.6. Effect of T4 on DHA and Brain Fatty Acid Binding Protein (FABP-B) After TBI
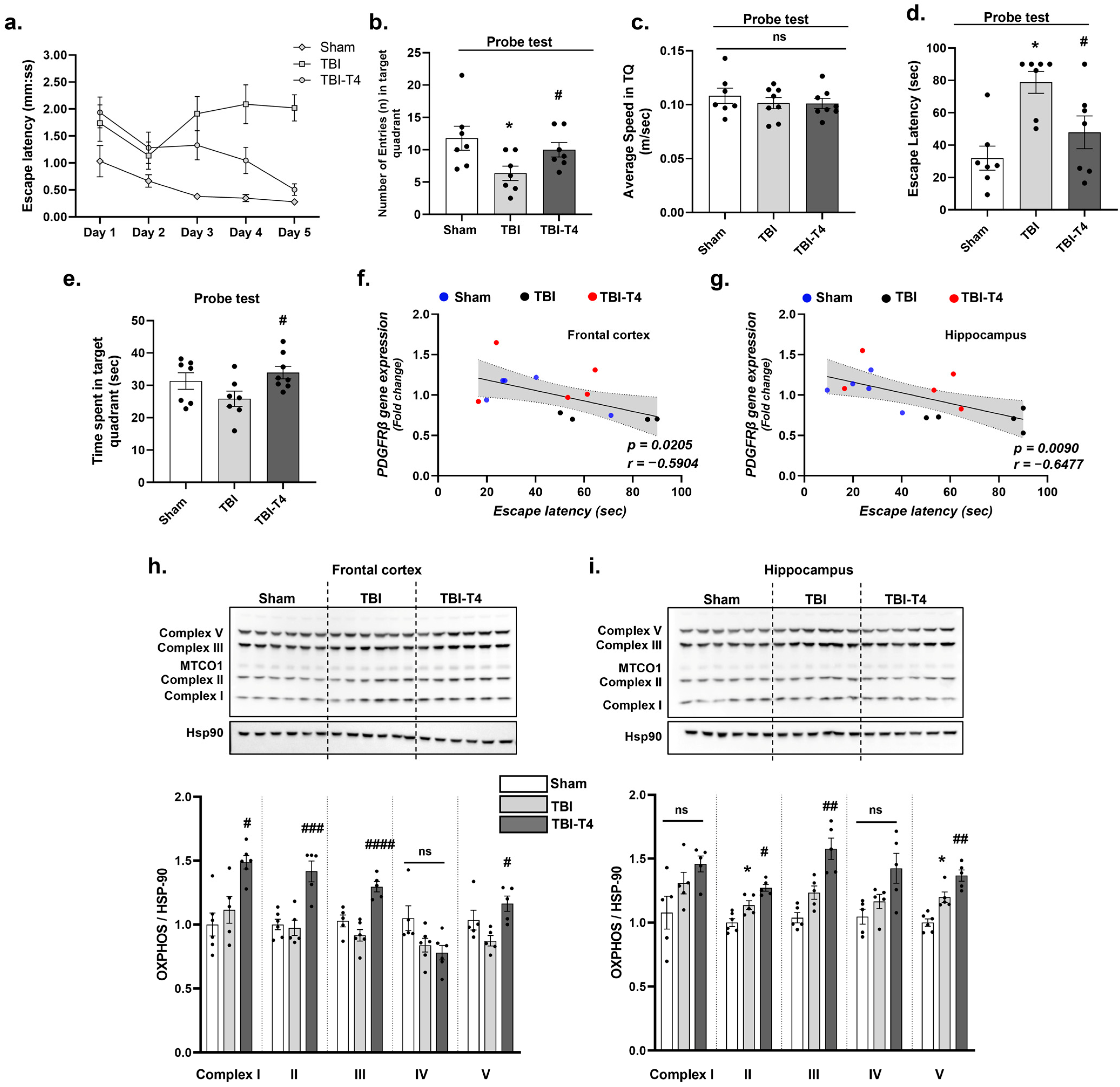
2.7. Effect of T4 on Mitochondrial Complex Post-TBI
2.8. Effect of T4 on Behavioral Parameters and Cognition Post-TBI
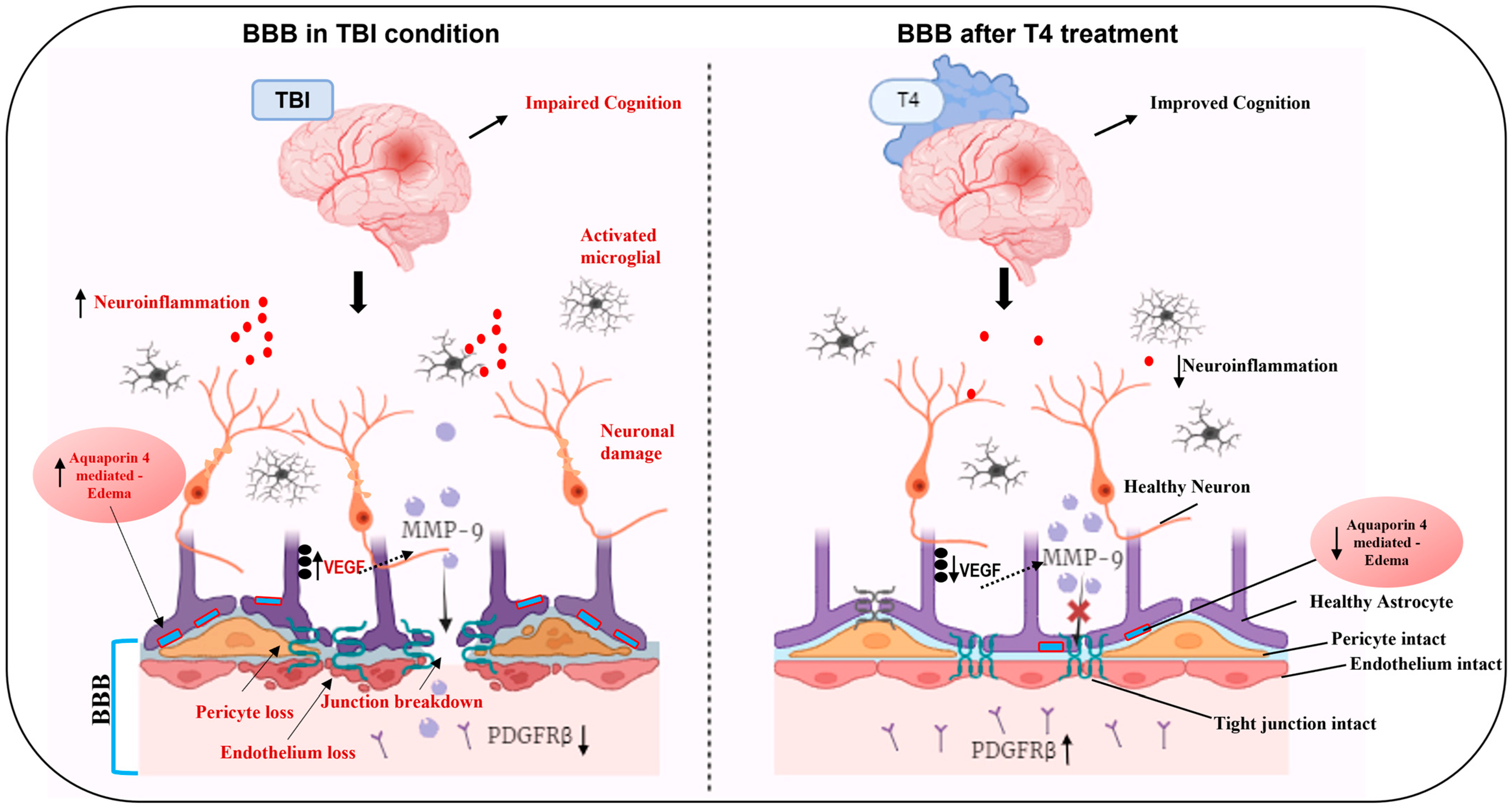
3. Discussion
3.1. Counteractive Action of T4 Treatment on BBB Integrity After TBI
3.2. Neuroprotective Role of T4 on Astrocytosis and Cerebral Edema
3.3. Impact of TBI and T4 Treatment on Vascular Rigidity
3.4. Impact of TBI and T4 Treatment on BBB-Related Angiogenesis
3.5. Influence of TBI on Thyroid Hormone Regulation
3.6. Impact of TBI and T4 Treatment on Mitochondrial Biogenesis After BBB Disruption
3.7. Cognitive Implications of TBI-Induced BBB Breakdown and the Therapeutic Potential of T4
3.8. Action of TBI and T4 Treatment on DHA
3.9. Translational Relevance and Clinical Implications of T4 Therapy in TBI
3.10. Limitations of Using T4
4. Material and Methods
4.1. Animal Studies
4.2. Fluid Percussion Injury
4.3. Barnes Maze
4.4. Evan’s Blue
4.5. Immunoblotting
4.6. Real-Time PCR
4.7. Gas Chromatography: Fatty Acid Analysis
4.8. Immunofluorescence for AQP4 and GFAP
4.9. Mouse Cytokine Array
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shackleton, B.; Crawford, F.; Bachmeier, C. Inhibition of ADAM10 Promotes the Clearance of Aβ across the BBB by Reducing LRP1 Ectodomain Shedding. Fluids Barriers CNS 2016, 13, 14. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood-Brain Barrier: Structure, Regulation, and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-Brain Barrier Breakdown in Alzheimer Disease and Other Neurodegenerative Disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular Pathways to Neurodegeneration in Alzheimer’s Disease and Other Disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, M.; Daneman, R. Formation and Maintenance of the BBB. Mech. Dev. 2015, 138 Pt 1, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Wang, L.; Zhang, L.; Qin, C.; Song, Y.; Ma, Y.; Chen, Y.; Chen, S.; Wang, Y.; Zhang, Z.; et al. Blood-Brain Barrier Disruption Induced Cognitive Impairment Is Associated With Increase of Inflammatory Cytokine. Front. Aging Neurosci. 2018, 10, 129. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-Endothelial Interactions at the Blood-Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Wolburg, H.; Noell, S.; Mack, A.; Wolburg-Buchholz, K.; Fallier-Becker, P. Brain Endothelial Cells and the Glio-Vascular Complex. Cell Tissue Res. 2009, 335, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Chodobski, A.; Zink, B.J.; Szmydynger-Chodobska, J. Blood-Brain Barrier Pathophysiology in Traumatic Brain Injury. Transl. Stroke Res. 2011, 2, 492–516. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, D.; Faustino, J.; Daneman, R.; Zhou, L.; Lee, S.Y.; Derugin, N.; Wendland, M.F.; Vexler, Z.S. Blood-Brain Barrier Permeability Is Increased after Acute Adult Stroke but Not Neonatal Stroke in the Rat. J. Neurosci. 2012, 32, 9588–9600. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes Regulate the Blood-Brain Barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.; Yeap, B.B. Thyroid Hormone: Influences on Mood and Cognition in Adults. Maturitas 2015, 81, 266–275. [Google Scholar] [CrossRef]
- Bernal, J.; Morte, B.; Diez, D. Thyroid Hormone Regulators in Human Cerebral Cortex Development. J. Endocrinol. 2022, 255, R27–R36. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xia, S.; Ge, T.; Lin, Y.; Hu, S.; Wu, H.; Xie, X.; Zhang, B.; Zhang, S.; Zeng, J.; et al. Atp13a5 Marker Reveals Pericyte Specification in the Mouse Central Nervous System. J. Neurosci. 2024, 44, e0727242024. [Google Scholar] [CrossRef]
- Ceballos, A.; Belinchon, M.M.; Sanchez-Mendoza, E.; Grijota-Martinez, C.; Dumitrescu, A.M.; Refetoff, S.; Morte, B.; Bernal, J. Importance of Monocarboxylate Transporter 8 for the Blood-Brain Barrier-Dependent Availability of 3,5,3’-Triiodo-L-Thyronine. Endocrinology 2009, 150, 2491–2496. [Google Scholar] [CrossRef]
- Ferrara, A.M.; Liao, X.-H.; Gil-Ibáñez, P.; Marcinkowski, T.; Bernal, J.; Weiss, R.E.; Dumitrescu, A.M.; Refetoff, S. Changes in Thyroid Status during Perinatal Development of MCT8-Deficient Male Mice. Endocrinology 2013, 154, 2533–2541. [Google Scholar] [CrossRef]
- Egashira, Y.; Zhao, H.; Hua, Y.; Keep, R.F.; Xi, G. White Matter Injury After Subarachnoid Hemorrhage: Role of Blood-Brain Barrier Disruption and Matrix Metalloproteinase-9. Stroke 2015, 46, 2909–2915. [Google Scholar] [CrossRef]
- Wu, M.; Gong, Y.; Jiang, L.; Zhang, M.; Gu, H.; Shen, H.; Dang, B. VEGF Regulates the Blood-brain Barrier through MMP-9 in a Rat Model of Traumatic Brain Injury. Exp. Ther. Med. 2022, 24, 728. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zheng, Y.; Wang, T.; Jiao, L.; Luo, Y. VEGF, a Key Factor for Blood Brain Barrier Injury After Cerebral Ischemic Stroke. Aging Dis. 2022, 13, 647–654. [Google Scholar] [CrossRef]
- Stokum, J.A.; Shim, B.; Negoita, S.; Tsymbalyuk, N.; Tsymbalyuk, O.; Ivanova, S.; Keledjian, K.; Bryan, J.; Blaustein, M.P.; Jha, R.M.; et al. Cation Flux through SUR1-TRPM4 and NCX1 in Astrocyte Endfeet Induces Water Influx through AQP4 and Brain Swelling after Ischemic Stroke. Sci. Signal. 2023, 16, eadd6364. [Google Scholar] [CrossRef]
- Laurent, C.; Dorothée, G.; Hunot, S.; Martin, E.; Monnet, Y.; Duchamp, M.; Dong, Y.; Légeron, F.-P.; Leboucher, A.; Burnouf, S.; et al. Hippocampal T Cell Infiltration Promotes Neuroinflammation and Cognitive Decline in a Mouse Model of Tauopathy. Brain 2017, 140, 184–200. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, H.; Zeng, J.; Pluimer, B.; Dong, S.; Xie, X.; Guo, X.; Ge, T.; Liang, X.; Feng, S.; et al. Mild Traumatic Brain Injury Induces Microvascular Injury and Accelerates Alzheimer-like Pathogenesis in Mice. Acta Neuropathol. Commun. 2021, 9, 74. [Google Scholar] [CrossRef]
- Baratz, R.; Tweedie, D.; Wang, J.-Y.; Rubovitch, V.; Luo, W.; Hoffer, B.J.; Greig, N.H.; Pick, C.G. Transiently Lowering Tumor Necrosis Factor-α Synthesis Ameliorates Neuronal Cell Loss and Cognitive Impairments Induced by Minimal Traumatic Brain Injury in Mice. J. Neuroinflammation 2015, 12, 45. [Google Scholar] [CrossRef]
- Corrigan, F.; Mander, K.A.; Leonard, A.V.; Vink, R. Neurogenic Inflammation after Traumatic Brain Injury and Its Potentiation of Classical Inflammation. J. Neuroinflammation 2016, 13, 264. [Google Scholar] [CrossRef]
- Lier, J.; Ondruschka, B.; Bechmann, I.; Dreßler, J. Fast Microglial Activation after Severe Traumatic Brain Injuries. Int. J. Legal Med. 2020, 134, 2187–2193. [Google Scholar] [CrossRef]
- Ralay Ranaivo, H.; Hodge, J.N.; Choi, N.; Wainwright, M.S. Albumin Induces Upregulation of Matrix Metalloproteinase-9 in Astrocytes via MAPK and Reactive Oxygen Species-Dependent Pathways. J. Neuroinflammation 2012, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, C.C.; Pennypacker, K.R. Neuroinflammation and MMPs: Potential Therapeutic Targets in Neonatal Hypoxic-Ischemic Injury. J. Neuroinflammation 2009, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Potential Utility of Aquaporin Modulators for Therapy of Brain Disorders. Prog. Brain Res. 2008, 170, 589–601. [Google Scholar] [CrossRef]
- Sugasini, D.; Yalagala, P.C.R.; Goggin, A.; Tai, L.M.; Subbaiah, P.V. Enrichment of Brain Docosahexaenoic Acid (DHA) Is Highly Dependent upon the Molecular Carrier of Dietary DHA: Lysophosphatidylcholine Is More Efficient than Either Phosphatidylcholine or Triacylglycerol. J. Nutr. Biochem. 2019, 74, 108231. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Suzuki, S.; Tagami, M. Docosahexaenoic Acid Prevented Tumor Necrosis Factor Alpha-Induced Endothelial Dysfunction and Senescence. Prostaglandins. Leukot. Essent. Fatty Acids 2016, 104, 11–18. [Google Scholar] [CrossRef]
- Chmurzyńska, A. The Multigene Family of Fatty Acid-Binding Proteins (FABPs): Function, Structure and Polymorphism. J. Appl. Genet. 2006, 47, 39–48. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood-Brain Barrier Breakdown Is an Early Biomarker of Human Cognitive Dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, Y.; Xu, S.; Wei, L.; Zhang, Y.; Chen, W.; Liu, M.; Zhong, C. HDAC Inhibitor Attenuates Rat Traumatic Brain Injury Induced Neurological Impairments. Heliyon 2023, 9, e18485. [Google Scholar] [CrossRef]
- Tang, J.; Kang, Y.; Zhou, Y.; Shang, N.; Li, X.; Wang, H.; Lan, J.; Wang, S.; Wu, L.; Peng, Y. TIMP2 Ameliorates Blood-Brain Barrier Disruption in Traumatic Brain Injury by Inhibiting Src-Dependent VE-Cadherin Internalization. J. Clin. Invest. 2023, 134, e164199. [Google Scholar] [CrossRef]
- Patabendige, A.; Janigro, D. The Role of the Blood-Brain Barrier during Neurological Disease and Infection. Biochem. Soc. Trans. 2023, 51, 613–626. [Google Scholar] [CrossRef]
- van Splunder, H.; Villacampa, P.; Martínez-Romero, A.; Graupera, M. Pericytes in the Disease Spotlight. Trends Cell Biol. 2024, 34, 58–71. [Google Scholar] [CrossRef]
- Liu, Q.; Radwanski, R.; Babadjouni, R.; Patel, A.; Hodis, D.M.; Baumbacher, P.; Zhao, Z.; Zlokovic, B.; Mack, W.J. Experimental Chronic Cerebral Hypoperfusion Results in Decreased Pericyte Coverage and Increased Blood-Brain Barrier Permeability in the Corpus Callosum. J. Cereb. Blood Flow Metab. 2019, 39, 240–250. [Google Scholar] [CrossRef]
- Sakata, M.; Yanamoto, H.; Hashimoto, N.; Iihara, K.; Tsukahara, T.; Taniguchi, T.; Kikuchi, H. Induction of Infarct Tolerance by Platelet-Derived Growth Factor against Reversible Focal Ischemia. Brain Res. 1998, 784, 250–255. [Google Scholar] [CrossRef]
- Tseng, H.C.; Dichter, M.A. Platelet-Derived Growth Factor-BB Pretreatment Attenuates Excitotoxic Death in Cultured Hippocampal Neurons. Neurobiol. Dis. 2005, 19, 77–83. [Google Scholar] [CrossRef]
- Kyyriäinen, J.; Ekolle Ndode-Ekane, X.; Pitkänen, A. Dynamics of PDGFRβ Expression in Different Cell Types after Brain Injury. Glia 2017, 65, 322–341. [Google Scholar] [CrossRef]
- Huang, M.; Long, A.; Hao, L.; Shi, Z.; Zhang, M. Astrocyte in Neurological Disease: Pathogenesis and Therapy. MedComm 2025, 6, e70299. [Google Scholar] [CrossRef]
- Dai, Y.; Dong, J.; Wu, Y.; Zhu, M.; Xiong, W.; Li, H.; Zhao, Y.; Hammock, B.D.; Zhu, X. Enhancement of the Liver’s Neuroprotective Role Ameliorates Traumatic Brain Injury Pathology. Proc. Natl. Acad. Sci. USA 2023, 120, e2301360120. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Qian, Z.; Wang, B.; Cao, J.; Zhang, S.; Jiang, F.; Kong, R.; Yu, X.; Cao, X.; Yang, L.; et al. Transplantation of A2 Type Astrocytes Promotes Neural Repair and Remyelination after Spinal Cord Injury. Cell Commun. Signal. 2023, 21, 37. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Huo, J. A1/A2 Astrocytes in Central Nervous System Injuries and Diseases: Angels or Devils? Neurochem. Int. 2021, 148, 105080. [Google Scholar] [CrossRef]
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.P.Q.; Perreau, V.M.; Shultz, S.R.; Brady, R.D.; Lei, E.; Dixit, S.; Taylor, J.M.; Beart, P.M.; Boon, W.C. Inflammation in Traumatic Brain Injury: Roles for Toxic A1 Astrocytes and Microglial–Astrocytic Crosstalk. Neurochem. Res. 2019, 44, 1410–1424. [Google Scholar] [CrossRef]
- Wang, J.; Hou, Y.; Zhang, L.; Liu, M.; Zhao, J.; Zhang, Z.; Ma, Y.; Hou, W. Estrogen Attenuates Traumatic Brain Injury by Inhibiting the Activation of Microglia and Astrocyte-Mediated Neuroinflammatory Responses. Mol. Neurobiol. 2021, 58, 1052–1061. [Google Scholar] [CrossRef]
- Wu, J.; Ren, R.; Chen, T.; Su, L.D.; Tang, T. Neuroimmune and Neuroinflammation Response for Traumatic Brain Injury. Brain Res. Bull. 2024, 217, 111066. [Google Scholar] [CrossRef] [PubMed]
- Kämper, A.; Rodemann, H.P. Alterations of Protein Degradation and 2-D Protein Pattern in Muscle Cells of MDX and DMD Origin. Biochem. Biophys. Res. Commun. 1992, 189, 1484–1490. [Google Scholar] [CrossRef]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; MacDonald, J.A.; Ishida, H.; Vogel, H.J.; Almutiri, S.; Logan, A.; Kreida, S.; et al. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell 2020, 181, 784–799. [Google Scholar] [CrossRef]
- Zhao, F.; Deng, J.; Xu, X.; Cao, F.; Lu, K.; Li, D.; Cheng, X.; Wang, X.; Zhao, Y. Aquaporin-4 Deletion Ameliorates Hypoglycemia-Induced BBB Permeability by Inhibiting Inflammatory Responses. J. Neuroinflammation 2018, 15, 157. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoo, B.H.; Won, S.J.; Choi, B.Y.; Lee, B.E.; Kim, I.Y.; Kho, A.; Lee, S.H.; Sohn, M.; Suh, S.W. Melatonin Reduces Hypoglycemia-Induced Neuronal Death in Rats. Neuroendocrinology 2015, 102, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Gianello, P.; Squifflet, J.P.; Carlier, M.; Jamart, J.; Pirson, Y.; Mahy, B.; Berbinschi, A.; Donckier, J.; Ketelslegers, J.M.; Lambotte, L. Evidence That Atrial Natriuretic Factor Is the Humoral Factor by Which Volume Loading or Mannitol Infusion Produces an Improved Renal Function after Acute Ischemia. An Experimental Study in Dogs. Transplantation 1989, 48, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ratter, J.M.; Rooijackers, H.M.M.; Tack, C.J.; Hijmans, A.G.M.; Netea, M.G.; de Galan, B.E.; Stienstra, R. Proinflammatory Effects of Hypoglycemia in Humans With or Without Diabetes. Diabetes 2017, 66, 1052–1061. [Google Scholar] [CrossRef]
- Pisani, F.; Simone, L.; Mola, M.G.; De Bellis, M.; Frigeri, A.; Nicchia, G.P.; Svelto, M. Regulation of Aquaporin-4 Expression in the Central Nervous System Investigated Using M23-AQP4 Null Mouse. Glia 2021, 69, 2235–2251. [Google Scholar] [CrossRef]
- Sapkota, D.; Florian, C.; Doherty, B.M.; White, K.M.; Reardon, K.M.; Ge, X.; Garbow, J.R.; Yuede, C.M.; Cirrito, J.R.; Dougherty, J.D. Aqp4 Stop Codon Readthrough Facilitates Amyloid-β Clearance from the Brain. Brain 2022, 145, 2982–2990. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.M.; McFarland White, K.; Fass, S.B.; Chen, S.; Shi, Z.; Ge, X.; Engelbach, J.A.; Gaines, S.H.; Bice, A.R.; Vasek, M.J.; et al. Evaluation of Gliovascular Functions of AQP4 Readthrough Isoforms. Front. Cell. Neurosci. 2023, 17, 1272391. [Google Scholar] [CrossRef]
- Palazzo, C.; Buccoliero, C.; Mola, M.G.; Abbrescia, P.; Nicchia, G.P.; Trojano, M.; Frigeri, A. AQP4ex Is Crucial for the Anchoring of AQP4 at the Astrocyte End-Feet and for Neuromyelitis Optica Antibody Binding. Acta Neuropathol. Commun. 2019, 7, 51. [Google Scholar] [CrossRef]
- Iliff, J.J.; Chen, M.J.; Plog, B.A.; Zeppenfeld, D.M.; Soltero, M.; Yang, L.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of Glymphatic Pathway Function Promotes Tau Pathology after Traumatic Brain Injury. J. Neurosci. 2014, 34, 16180–16193. [Google Scholar] [CrossRef]
- Seo, J.H.; Miyamoto, N.; Hayakawa, K.; Pham, L.-D.D.; Maki, T.; Ayata, C.; Kim, K.-W.; Lo, E.H.; Arai, K. Oligodendrocyte Precursors Induce Early Blood-Brain Barrier Opening after White Matter Injury. J. Clin. Investig. 2013, 123, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tsirka, S.E. Neuroprotection by Inhibition of Matrix Metalloproteinases in a Mouse Model of Intracerebral Haemorrhage. Brain 2005, 128, 1622–1633. [Google Scholar] [CrossRef]
- Zhao, B.-Q.; Wang, S.; Kim, H.-Y.; Storrie, H.; Rosen, B.R.; Mooney, D.J.; Wang, X.; Lo, E.H. Role of Matrix Metalloproteinases in Delayed Cortical Responses after Stroke. Nat. Med. 2006, 12, 441–445. [Google Scholar] [CrossRef]
- Hollidge, B.S.; Cohen, C.A.; Akuoku Frimpong, J.; Badger, C.V.; Dye, J.M.; Schmaljohn, C.S. Toll-like Receptor 4 Mediates Blood-Brain Barrier Permeability and Disease in C3H Mice during Venezuelan Equine Encephalitis Virus Infection. Virulence 2021, 12, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Zhang, L.; Jiang, Q.; Zhang, R.; Davies, K.; Powers, C.; Bruggen, N.V.; Chopp, M. VEGF Enhances Angiogenesis and Promotes Blood-Brain Barrier Leakage in the Ischemic Brain. J. Clin. Investig. 2000, 106, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.I.; Shields, D.J.; Barillas, S.G.; Acevedo, L.M.; Murphy, E.; Huang, J.; Scheppke, L.; Stockmann, C.; Johnson, R.S.; Angle, N.; et al. A Role for VEGF as a Negative Regulator of Pericyte Function and Vessel Maturation. Nature 2008, 456, 809–813. [Google Scholar] [CrossRef]
- Melgar, M.A.; Rafols, J.; Gloss, D.; Diaz, F.G. Postischemic Reperfusion: Ultrastructural Blood-Brain Barrier and Hemodynamic Correlative Changes in an Awake Model of Transient Forebrain Ischemia. Neurosurgery 2005, 56, 571–581. [Google Scholar] [CrossRef]
- Schroeder, A.C.; Privalsky, M.L. Thyroid Hormones, T3 and T4, in the Brain. Front. Endocrinol. (Lausanne) 2014, 5, 40. [Google Scholar] [CrossRef]
- Bernal, J. The Significance of Thyroid Hormone Transporters in the Brain. Endocrinology 2005, 146, 1698–1700. [Google Scholar] [CrossRef] [PubMed]
- Mele, C.; Pagano, L.; Franciotta, D.; Caputo, M.; Nardone, A.; Aimaretti, G.; Marzullo, P.; Pingue, V. Thyroid function in the subacute phase of traumatic brain injury: A potential predictor of post-traumatic neurological and functional outcomes. J. Endocrinol. Investig. 2022, 45, 379–389. [Google Scholar] [CrossRef]
- Feldt-Rasmussen, U.; Klose, M.C. Pathophysiology and diagnosis of neuroendocrine abnormalities in patients with traumatic brain injury. Best Pract. Res. Clin. Endocrinol. Metab. 2025, 39, 102020. [Google Scholar] [CrossRef]
- Lin, C.; Li, N.; Chang, H.; Shen, Y.; Li, Z.; Wei, W.; Chen, H.; Lu, H.; Ji, J.; Liu, N. Dual Effects of Thyroid Hormone on Neurons and Neurogenesis in Traumatic Brain Injury. Cell Death Dis. 2020, 11, 671. [Google Scholar] [CrossRef]
- Davis, G.M.; Juere, E.; Hayes, J.M.; Davey, G.P. Mitochondrial Complex I Controls Blood Brain Barrier Permeability. bioRxiv 2022. [Google Scholar] [CrossRef]
- Bénard, G.; Massa, F.; Puente, N.; Lourenço, J.; Bellocchio, L.; Soria-Gómez, E.; Matias, I.; Delamarre, A.; Metna-Laurent, M.; Cannich, A.; et al. Mitochondrial CB1 Receptors Regulate Neuronal Energy Metabolism. Nat. Neurosci. 2012, 15, 558–564. [Google Scholar] [CrossRef]
- Barisano, G.; Montagne, A.; Kisler, K.; Schneider, J.A.; Wardlaw, J.M.; Zlokovic, B.V. Blood-Brain Barrier Link to Human Cognitive Impairment and Alzheimer’s Disease. Nat. Cardiovasc. Res. 2022, 1, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.L.; Dayon, L.; Kirkland, R.; Wojcik, J.; Peyratout, G.; Severin, I.C.; Henry, H.; Oikonomidi, A.; Migliavacca, E.; Bacher, M.; et al. Blood-Brain Barrier Breakdown, Neuroinflammation, and Cognitive Decline in Older Adults. Alzheimers. Dement. 2018, 14, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Golob, E.J.; Su, M.-Y. Vascular Volume and Blood-Brain Barrier Permeability Measured by Dynamic Contrast Enhanced MRI in Hippocampus and Cerebellum of Patients with MCI and Normal Controls. J. Magn. Reson. Imaging 2006, 24, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Michael-Titus, A.T.; Priestley, J.V. Omega-3 Fatty Acids and Traumatic Neurological Injury: From Neuroprotection to Neuroplasticity? Trends Neurosci. 2014, 37, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pan, Z.; Fang, Z.; Lin, W.; Wu, S.; Yang, F.; Li, Y.; Fu, H.; Gao, H.; Li, S. Omega-3 Polyunsaturated Fatty Acid Attenuates Traumatic Brain Injury-Induced Neuronal Apoptosis by Inducing Autophagy through the Upregulation of SIRT1-Mediated Deacetylation of Beclin-1. J. Neuroinflammation 2018, 15, 310. [Google Scholar] [CrossRef]
- Hall, J.C.E.; Priestley, J.V.; Perry, V.H.; Michael-Titus, A.T. Docosahexaenoic Acid, but Not Eicosapentaenoic Acid, Reduces the Early Inflammatory Response Following Compression Spinal Cord Injury in the Rat. J. Neurochem. 2012, 121, 738–750. [Google Scholar] [CrossRef]
- Cao, D.; Li, M.; Xue, R.; Zheng, W.; Liu, Z.; Wang, X. Chronic Administration of Ethyl Docosahexaenoate Decreases Mortality and Cerebral Edema in Ischemic Gerbils. Life Sci. 2005, 78, 74–81. [Google Scholar] [CrossRef]
- Pan, H.-C.; Kao, T.-K.; Ou, Y.-C.; Yang, D.-Y.; Yen, Y.-J.; Wang, C.-C.; Chuang, Y.-H.; Liao, S.-L.; Raung, S.-L.; Wu, C.-W.; et al. Protective Effect of Docosahexaenoic Acid against Brain Injury in Ischemic Rats. J. Nutr. Biochem. 2009, 20, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.W.; On, N.H.; Del Bigio, M.R.; Miller, D.W.; Hatch, G.M. Fatty Acid Transport Protein Expression in Human Brain and Potential Role in Fatty Acid Transport across Human Brain Microvessel Endothelial Cells. J. Neurochem. 2011, 117, 735–746. [Google Scholar] [CrossRef]
- Balendiran, G.K.; Schnutgen, F.; Scapin, G.; Borchers, T.; Xhong, N.; Lim, K.; Godbout, R.; Spener, F.; Sacchettini, J.C. Crystal Structure and Thermodynamic Analysis of Human Brain Fatty Acid-Binding Protein. J. Biol. Chem. 2000, 275, 27045–27054. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Yip, P.K.; Adams, L.; Davies, M.; Lee, J.W.; Michael, G.J.; Priestley, J.V.; Michael-Titus, A.T. A Single Bolus of Docosahexaenoic Acid Promotes Neuroplastic Changes in the Innervation of Spinal Cord Interneurons and Motor Neurons and Improves Functional Recovery after Spinal Cord Injury. J. Neurosci. 2015, 35, 12733–12752. [Google Scholar] [CrossRef]
- Pan, Y.; Choy, K.H.C.; Marriott, P.J.; Chai, S.Y.; Scanlon, M.J.; Porter, C.J.H.; Short, J.L.; Nicolazzo, J.A. Reduced Blood-Brain Barrier Expression of Fatty Acid-Binding Protein 5 Is Associated with Increased Vulnerability of APP/PS1 Mice to Cognitive Deficits from Low Omega-3 Fatty Acid Diets. J. Neurochem. 2018, 144, 81–92. [Google Scholar] [CrossRef]
- Fullerton, J.L.; Hay, J.; Bryant-Craig, C.; Atkinson, J.; Smith, D.H.; Stewart, W. Pediatric Traumatic Brain Injury and Microvascular Blood-Brain Barrier Pathology. JAMA Netw. Open 2024, 7, e2446767. [Google Scholar] [CrossRef] [PubMed]
- McCrea, M.; Guskiewicz, K.; Doncevic, S.; Helmick, K.; Kennedy, J.; Boyd, C.; Asmussen, S.; Ahn, K.W.; Wang, Y.; Hoelzle, J.; et al. Day of Injury Cognitive Performance on the Military Acute Concussion Evaluation (MACE) by U.S. Military Service Members in OEF/OIF. Mil. Med. 2014, 179, 990–997. [Google Scholar] [CrossRef]
- Dixon, M.L.; Thiruchselvam, R.; Todd, R.; Christoff, K. Emotion and the Prefrontal Cortex: An Integrative Review. Psychol. Bull. 2017, 143, 1033–1081. [Google Scholar] [CrossRef] [PubMed]
- Opitz, B. Memory Function and the Hippocampus. Front. Neurol. Neurosci. 2014, 34, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kobeissy, F.H. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects, 1st ed.; CRC Press: London, UK, 2015. [Google Scholar]
- Arneson, D.; Zhang, G.; Ying, Z.; Zhuang, Y.; Byun, H.R.; Ahn, I.S.; Gomez-Pinilla, F.; Yang, X. Single Cell Molecular Alterations Reveal Target Cells and Pathways of Concussive Brain Injury. Nat. Commun. 2018, 9, 3894. [Google Scholar] [CrossRef]
- Zhang, G.; Diamante, G.; Ahn, I.S.; Palafox-Sanchez, V.; Cheng, J.; Cheng, M.; Ying, Z.; Wang, S.S.-M.; Abuhanna, K.D.; Phi, N.; et al. Thyroid Hormone T4 Mitigates Traumatic Brain Injury in Mice by Dynamically Remodeling Cell Type Specific Genes, Pathways, and Networks in Hippocampus and Frontal Cortex. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167344. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, M.; Krishna, G.; Ying, Z.; Gomez-Pinilla, F. Liver Acts as a Metabolic Gate for the Traumatic Brain Injury Pathology: Protective Action of Thyroid Hormone. Biochim. Biophys. Acta-Mol. Basis Dis. 2023, 1869, 166728. [Google Scholar] [CrossRef] [PubMed]
- Sackheim, A.M.; Stockwell, D.; Villalba, N.; Haines, L.; Scott, C.L.; Russell, S.; Hammack, S.E.; Freeman, K. Traumatic Brain Injury Impairs Sensorimotor Function in Mice. J. Surg. Res. 2017, 213, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Newell, E.A.; Todd, B.P.; Luo, Z.; Evans, L.P.; Ferguson, P.J.; Bassuk, A.G. A Mouse Model for Juvenile, Lateral Fluid Percussion Brain Injury Reveals Sex-Dependent Differences in Neuroinflammation and Functional Recovery. J. Neurotrauma 2020, 37, 635–646. [Google Scholar] [CrossRef]



| Antibody | Company | Catalog No. | Molecular Weight (kDa) |
|---|---|---|---|
| MMP-9 | Cell signaling (Danvers, MA, USA) | 13667 | 92 |
| TLR-4 | Santa Cruz (Dallas, TX, USA) | sc-293072 | 95 |
| pVEGFR2 | Cell signaling | 3770 | 230 |
| VEGFR2 | Cell signaling | 9698 | 210 and 230 |
| ZO-1 | Cell signaling | 8193 | 220 |
| OXPHOS | Invitrogen (Carlsbad, CA, USA) | 45-8099 | 55, 48, 40, 30, and 20 |
| Iba-1 | Fujifilm Wako (Minato, TYO, Japan) | 019-19741 | 17 |
| TRMP4 | Novus bio (Centennial, CO, USA) | NBP3-07990 | 151 |
| β-actin | Santa Cruz | sc-47778 | 45 |
| Gene | Accession Number | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| PDGF | NM_001411620.1 | AAGTGTGAGACAATAGTGACCCC | CATGGGTGTGCTTAAACTTTCG |
| PDGFRβ | NM_008809.2 | CAAGAAGCGGCCATGAATCAG | CGGCCCTAGTGAGTTGTTGT |
| PECAM | NM_001032378 | ACGCTGGTGCTCTATGCAAG | TCAGTTGCTGCCCATTCATCA |
| ATP13a5 | NM_012675.3 | GAGGTGTTTGGCTACCATACC | GGGATGCAACTGGTCCACA |
| GLUT 1 | NM_011400.3 | GCAGTTCGGCTATAACACTGG | GCGGTGGTTCCATGTTTGATTG |
| OATP1C1 | NM_021471 | GGGCCATCCTTTACAGTCGG | CCTTCTCTCTATCTGAGTCACGG |
| MCT8 | NM_009197 | CGGCTGGATAGTGGTGTTTG | TGGAGTAGAGGATACCAACAGAG |
| Dio2 | NM_010050 | CAGCTTCCTCCTAGATGCCTA | CTGATTCAGGATTGGAGACGTG |
| THα | NM_178060 | TTTCGCCGCACAATCCAGAA | GGTGATCTTGTCGATGACACAG |
| THβ | NM_001113417 | GGACAAGCACCCATCGTGAAT | CTCTGGTAATTGCTGGTGTGAT |
| FABP-B | NM_010634 | AAAGAGCTAGGAGTAGGACTGG | TGTTGCCATCACACGTAATGA |
| Serping1 | NM_009776.3 | ACAGCCCCCTCTGAATTCTT | GGATGCTCTCCAAGTTGCTC |
| Fbln5 | NM_001413785.1 | CTTCAGATGCAAGCAACAA | AGGCAGTGTCAGAGGCCTTA |
| Ugt1a | NM_201645.2 | CCTATGGGTCACTTGCCACT | AAAACCATGTTGGGCATGAT |
| Ligp1 | NM_021792.5 | GGGGCAATAGCTCATTGGTA | ACCTCGAAGACATCCCCTTT |
| Clcf1 | NM_019952.6 | CTTCAATCCTCCTCGACTGG | TACGTCGGAGTTCAGCTGTG |
| Tgm1 | NM_001161714.1 | CTGTTGGTCCCGTCCCAAA | GGACCTTCCATTGTGCCTGG |
| Ptx3 | NM_008987.3 | AACAAGCTCTGTTGCCCATT | TCCCAAATGGAACATTGGAT |
| S100a10 | NM_009112.2 | CCTCTGGCTGTGGACAAAAT | CTGCTCACAAGAAGCAGTGG |
| Emp1 | NM_001288628.1 | GAGACACTGGCCAGAAAAGC | TAAAAGGCAAGGGAATGCAC |
| CD14 | NM_009841.4 | GGACTGATCTCAGCCCTCTG | GCTTCAGCCCAGTGAAAGAC |
| Tnfα | NM 001278601.1 | CAGGCGGTGCCTATGTCTC | CGATCACCCCGAAGTTCAGTAG |
| IL-6 | NM_001314054.1 | TGAACAACGATGATGCACTTG | CTGAAGGACTCTGGCTTTGTC |
| GAPDH | NM_017008.4 | GGGCTCTCTGCTCCTCCCTGT | ACGGCCAAATCCGTTCACACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandelwal, M.; Ying, Z.; Gomez-Pinilla, F. Thyroid Hormone T4 Alleviates Traumatic Brain Injury by Enhancing Blood–Brain Barrier Integrity. Int. J. Mol. Sci. 2025, 26, 9632. https://doi.org/10.3390/ijms26199632
Khandelwal M, Ying Z, Gomez-Pinilla F. Thyroid Hormone T4 Alleviates Traumatic Brain Injury by Enhancing Blood–Brain Barrier Integrity. International Journal of Molecular Sciences. 2025; 26(19):9632. https://doi.org/10.3390/ijms26199632
Chicago/Turabian StyleKhandelwal, Mayuri, Zhe Ying, and Fernando Gomez-Pinilla. 2025. "Thyroid Hormone T4 Alleviates Traumatic Brain Injury by Enhancing Blood–Brain Barrier Integrity" International Journal of Molecular Sciences 26, no. 19: 9632. https://doi.org/10.3390/ijms26199632
APA StyleKhandelwal, M., Ying, Z., & Gomez-Pinilla, F. (2025). Thyroid Hormone T4 Alleviates Traumatic Brain Injury by Enhancing Blood–Brain Barrier Integrity. International Journal of Molecular Sciences, 26(19), 9632. https://doi.org/10.3390/ijms26199632





