Role of Explicit Hydration in Scavenging of CO3•− by Trolox: A DFT Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Conformational Analysis
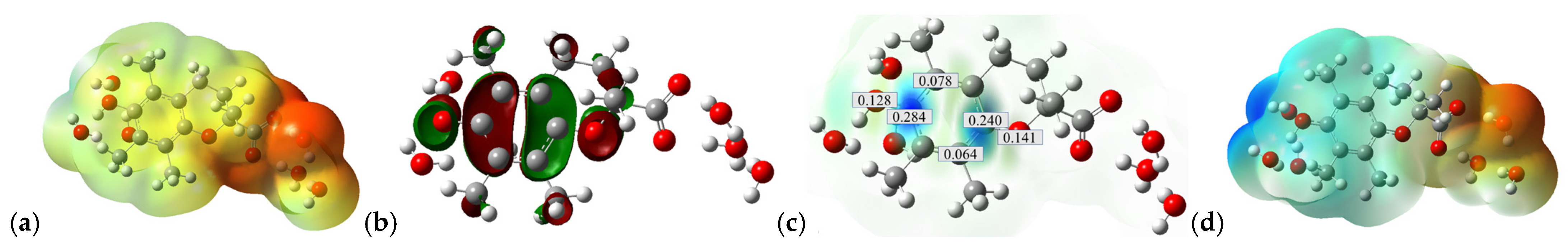
2.2. SET from Trolox Molecule (Trolox-COOH-OH) to CO3•− Species
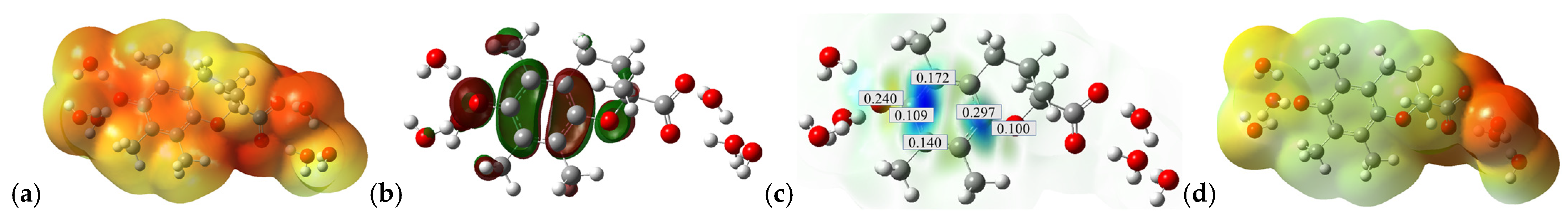
2.3. SET from Trolox Carboxylate Anion (Trolox-OH-COO−) to CO3•− Species
2.4. SET from Trolox Dianion (Trolox-COO−-O−) to Unhydrated and Hydrated CO3•− Species
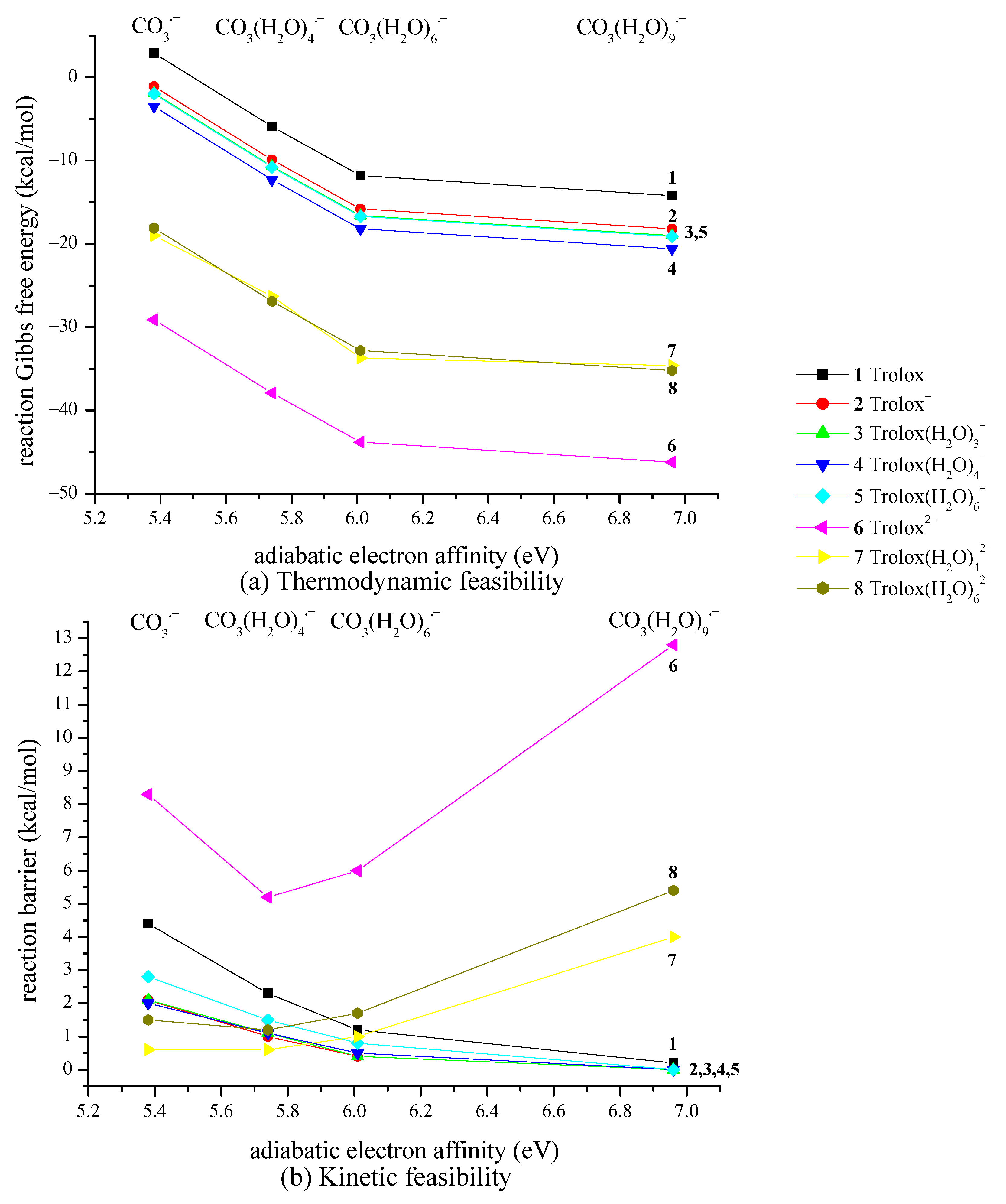
2.5. Overview of Explicit Hydration Impact on Thermodynamics and Kinetics of SET Reaction Between Carbonate Radical Anion and Trolox, and Estimation of Overall Rate Constant
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl radical is a significant player in oxidative DNA damage in vivo. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Repine, J.E.; Eaton, J.W.; Anders, M.W.; Hoidal, J.R.; Fox, R.B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J. Clin. Investig. 1979, 64, 1642–1651. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35, Erratum in Free Radic. Biol. Med. 2014, 74, 307. [Google Scholar] [CrossRef]
- Illés, E.; Mizrahi, A.; Marks, V.; Meyerstein, D. Carbonate-radical-anions, and not hydroxyl radicals, are the products of the Fenton reaction in neutral solutions containing bicarbonate. Free Radic. Biol. Med. 2019, 131, 1–6. [Google Scholar] [CrossRef]
- Fleming, A.M.; Burrows, C.J. On the irrelevancy of hydroxyl radical to DNA damage from oxidative stress and implications for epigenetics. Chem. Soc. Rev. 2020, 49, 6524–6528. [Google Scholar] [CrossRef]
- Meyerstein, D. Re-examining Fenton and Fenton-like reactions. Nat. Rev. Chem. 2021, 5, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Burrows, C.J. Why the ROS matters: One-electron oxidants focus DNA damage and repair on G-quadruplexes for gene regulation. DNA Repair 2025, 145, 103789. [Google Scholar] [CrossRef] [PubMed]
- Michelson, A.M.; Maral, J. Carbonate anions; effects on the oxidation of luminol, oxidative hemolysis, γ-irradiation and the reaction of activated oxygen species with enzymes containing various active centres. Biochimie 1983, 65, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Quinn, P.J. Vitamin E and its function in membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Azzi, A. Reflections on a century of vitamin E research: Looking at the past with an eye on the future. Free Radic. Biol. Med. 2021, 175, 155–160. [Google Scholar] [CrossRef]
- Albertini, R.; Abuja, P.M. Prooxidant and antioxidant properties of Trolox C, analogue of vitamin E, in oxidation of low-density lipoprotein. Free Radic. Res. 1999, 30, 181–188. [Google Scholar] [CrossRef]
- Alberto, M.E.; Russo, N.; Grand, A.; Galano, A. A physicochemical examination of the free radical scavenging activity of Trolox: Mechanism, kinetics and influence of the environment. Phys. Chem. Chem. Phys. 2013, 15, 4642–4650. [Google Scholar] [CrossRef]
- Saïd, A.E.-H.; Mekelleche, S.M. Antioxidant activity of Trolox derivatives toward methylperoxyl radicals: Thermodynamic and kinetic theoretical study. Theor. Chem. Acc. 2021, 140, 128. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Alvarez-Idaboy, J.R. A computational methodology for accurate predictions of rate constants in solution: Application to the assessment of primary antioxidant activity. J. Comput. Chem. 2013, 34, 2430–2445. [Google Scholar] [CrossRef]
- Wang, Y.; An, P.; Li, S.; Zhou, L. The oxidation mechanism and kinetics of 2′-deoxyguanosine by carbonate radical anion. Chem. Phys. Lett. 2020, 739, 136982. [Google Scholar] [CrossRef]
- McPherson, P.A.C. Evaluating theoretical rate constants for bimolecular antioxidant processes involving ionic species under physiological conditions. Comput. Theor. Chem. 2024, 1239, 114794. [Google Scholar] [CrossRef]
- Karmakar, S.; Datta, A. Understanding the reactivity of CO3•− and NO2• radicals toward S-containing and aromatic amino acids. J. Phys. Chem. B 2017, 121, 7621–7632. [Google Scholar] [CrossRef]
- Cao, H.; Xiong, S.-F.; Dong, L.-L.; Dai, Z.-T. Study on the mechanism of lipid peroxidation induced by carbonate radicals. Molecules 2024, 29, 1125. [Google Scholar] [CrossRef]
- Navarrete, M.; Rangel, C.; Espinosa-Garcia, J.; Corchado, J.C. Theoretical study of the antioxidant activity of vitamin E: Reactions of α-tocopherol with the hydroperoxy radical. J. Chem. Theory Comput. 2005, 1, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Crean, C.; Geacintov, N.E.; Shafirovich, V. Oxidation of guanine and 8-oxo-7,8-dihydroguanine by carbonate radical anions: Insight from oxygen-18 labeling experiments. Angew. Chem. Int. Ed. 2005, 44, 5057–5060. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Tóth, T.; Takács, E. Rate constants of carbonate radical anion reactions with molecules of environmental interest in aqueous solution: A review. Sci. Total Environ. 2020, 717, 137219. [Google Scholar] [CrossRef]
- Simic, M.G.; Hunter, E.P.L. The Reactivities of Organic Oxygen (oxy) Radicals. In Oxygen Radicals Chemistry and Biology; Bors, W., Saran, M., Tait, D., Eds.; de Gruyter: Berlin, Germany, 1984; pp. 109–121. [Google Scholar]
- Miao, J.-L.; Wang, W.-F.; Pan, J.-X.; Lu, C.-Y.; Li, R.-Q.; Yao, S.-D. The scavenging reactions of nitrogen dioxide radical and carbonate radical by tea polyphenol derivatives: A pulse radiolysis study. Radiat. Phys. Chem. 2001, 60, 163–168. [Google Scholar] [CrossRef]
- Calleja-Amador, C.; Ogilvie, J.F.; Blanco, R. Kosmotropic Chromatography of Proteins. Theory and practice. In Advances in Chromatography; Grinberg, N., Carr, P.W., Eds.; CRC Press: Boca Raton, FL, USA, 2020; Volume 57, pp. 101–127. [Google Scholar]
- Zilberg, S.; Mizrahi, A.; Meyerstein, D.; Kornweitz, H. Carbonate and carbonate anion radicals in aqueous solutions exist as CO3(H2O)62− and CO3(H2O)6•− respectively: The crucial role of the inner hydration sphere of anions in explaining their properties. Phys. Chem. Chem. Phys. 2018, 20, 9429–9435. [Google Scholar] [CrossRef]
- Hebert, S.P.; Schlegel, H.B. Computational study of the pH-dependent competition between carbonate and thymine addition to the guanine radical. Chem. Res. Toxicol. 2019, 32, 195–210. [Google Scholar] [CrossRef]
- Dooley, M.R.; Vyas, S. Role of explicit solvation and level of theory in predicting the aqueous reduction potential of carbonate radical anion by DFT. Phys. Chem. Chem. Phys. 2025, 27, 6867–6874. [Google Scholar] [CrossRef]
- Walton, J.C. Microhydration and the enhanced acidity of free radicals. Molecules 2018, 23, 423. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.K.; Mukherjee, T.; Maity, D.K. Theoretical study on the spectroscopic properties of CO3•−.nH2O clusters: Extrapolation to bulk. ChemPhysChem 2008, 9, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.K.; Maity, D.K. Distinctive IR signature of CO3•− and CO32− hydrated clusters: A theoretical study. J. Phys. Chem. A 2009, 113, 13443–13447. [Google Scholar] [CrossRef]
- Ghosh, D.; Roy, A.; Seidel, R.; Winter, B.; Bradforth, S.; Krylov, A.I. A first-principle protocol for calculating ionization energies and redox potentials of solvated molecules and ions: Theory and application to aqueous phenol and phenolate. J. Phys. Chem. B 2012, 116, 7269–7280. [Google Scholar] [CrossRef]
- Mitarai, A.; Ouchi, A.; Mukai, K.; Tokunaga, A.; Mukai, K.; Abe, K. Kinetic studies of the free radical-scavenging actions of tocopherol metabolites (α-, γ-, and δ-carboxyethyl-6-hydroxychroman) and Trolox in ethanol and micellar solutions. J. Agric. Food Chem. 2008, 56, 84–91. [Google Scholar] [CrossRef]
- Wang, X.-B.; Kass, S.R. Anion A−• HX clusters with reduced electron binding energies: Proton vs hydrogen atom relocation upon electron detachment. J. Am. Chem. Soc. 2014, 136, 17332–17336. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, Z.; Kong, X.; Ling, Z.; Zhang, H.; Cheng, L.; Wang, X.-B. Photodetachment photoelectron spectroscopy shows isomer-specific proton-coupled electron transfer reactions in phenolic nitrate complexes. Commun. Chem. 2024, 7, 176. [Google Scholar] [CrossRef] [PubMed]
- Makurat, S.; Yuan, Q.; Czub, J.; Chomicz-Manka, L.; Cao, W.; Wang, X.-B.; Rak, J. Guanosine dianions hydrated by one to four water molecules. J. Phys. Chem. Lett. 2022, 13, 3230–3236. [Google Scholar] [CrossRef]
- Perez-Gonzalez, A.; Alvarez-Idaboy, J.R.; Galano, A. Dual antioxidant/pro-oxidant behavior of the tryptophan metabolite 3-hydroxyanthranilic acid: A theoretical investigation of reaction mechanisms and kinetics. New J. Chem. 2017, 41, 3829–3845. [Google Scholar] [CrossRef]
- Amić, A.; Milanović, Ž.; Mastiľák Cagardová, D. Protective roles of α-tocopherol, α-CEHC, and gallic acid against CO3•−-induced DNA damage and their effect on OGG1 and pro-oxidant enzymes. J. Mol. Liq. 2025, 438, 128847. [Google Scholar] [CrossRef]
- Amić, A.; Mastiľák Cagardová, D. A DFT study of the antioxidant potency of α-tocopherol and its derivatives: PMHC, Trolox, and α-CEHC. J. Mol. Liq. 2024, 403, 124796. [Google Scholar] [CrossRef]
- Hrovat, D.A.; Hou, G.-L.; Chen, B.; Wang, X.-B.; Borden, W.T. Negative ion photoelectron spectroscopy confirms the prediction that D3h carbon trioxide (CO3) has a singlet ground state. Chem. Sci. 2016, 7, 1142–1150. [Google Scholar] [CrossRef]
- Cappa, C.D.; Elrod, M.J. A computational investigation of the electron affinity of CO3 and the thermodynamic feasibility of CO3−(H2O)n + ROOH reactions. Phys. Chem. Chem. Phys. 2001, 3, 2986–2994. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241, Erratum in Theor. Chem. Acc. 2008, 119, 525. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Exploring the limit of accuracy of the global hybrid meta density functional for main-group thermochemistry, kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2008, 4, 1849–1868. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Alvarez-Idaboy, J.R. Computational strategies for predicting free radical scavengers’ protection against oxidative stress: Where are we and what might follow? Int. J. Quantum Chem. 2018, 119, e25665. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Dzib, E.; Cabellos, J.L.; Ortiz-Chi, F.; Pan, S.; Galano, A.; Merino, G. Eyringpy: A program for computing rate constants in the gas phase and in solution. Int. J. Quantum Chem. 2019, 119, e25686. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron transfer reactions in chemistry. Theory and experiment. Angew. Chem. Int. Ed. 1993, 32, 1111–1121. [Google Scholar] [CrossRef]
- Collins, F.C.; Kimball, G.E. Diffusion-controlled reaction rates. J. Colloid Sci. 1949, 4, 425–437. [Google Scholar] [CrossRef]
- Silverstein, T.P. Marcus theory: Thermodynamics CAN control the kinetics of electron transfer reactions. J. Chem. Ed. 2012, 89, 1159–1167. [Google Scholar] [CrossRef]
- Czapski, G.; Lymar, S.V.; Schwarz, H.A. Acidity of the carbonate radical. J. Phys. Chem. A 1999, 103, 3447–3450. [Google Scholar] [CrossRef]
- Mishra, P.M. Electron affinity calculation for selected PAHs using DFT: Effect of cyclopenta ring fusion and aromaticity. Comput. Theor. Chem. 2015, 1068, 165–171. [Google Scholar] [CrossRef]
- Klein, E.; Lukeš, V.; Ilčin, M. DFT/B3LYP study of tocopherols and chromans antioxidant action energetics. Chem. Phys. 2007, 336, 51–57. [Google Scholar] [CrossRef]
- Donald, W.A.; Demireva, M.; Leib, R.D.; Aiken, M.J.; Williams, E.R. Electron hydration and ion-electron pairs in water clusters containing trivalent metal ions. J. Am. Chem. Soc. 2010, 132, 4633–4640. [Google Scholar] [CrossRef] [PubMed]

 | + |  | ⟶ |  | + | 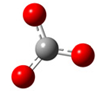 | kapp = 7.10 × 109 = 5.39 × 109 ΔrG = −2.0 ΔG≠ = 2.8 |
| Trolox(H2O)6− | CO3•− | Trolox(H2O)6• | CO32− | λ = 14.7 | |||
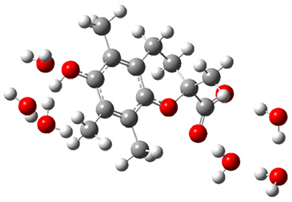
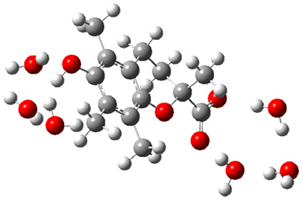 | + | 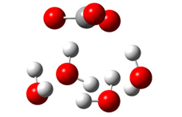 | ⟶ | 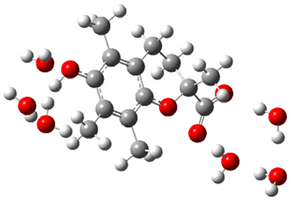 | + | 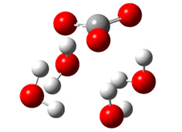 | kapp = 7.50 × 109 = 5.70 × 109 ΔrG = −10.8 ΔG≠ = 1.5 |
| Trolox(H2O)6− | CO3(H2O)4•− | Trolox(H2O)6• | CO3(H2O)42− | λ = 22.5 | |||
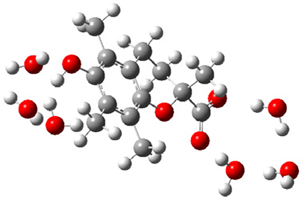 | + | 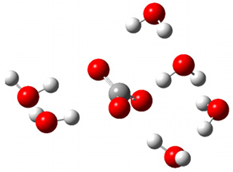 | ⟶ | 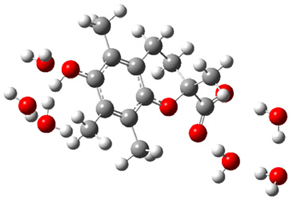 | + |  | kapp = 7.50 × 109 = 5.70 × 109 ΔrG = −16.7 ΔG≠ = 0.8 |
| Trolox(H2O)6− | CO3(H2O)6•− | Trolox(H2O)6• | CO3(H2O)62− | λ = 25.5 | |||
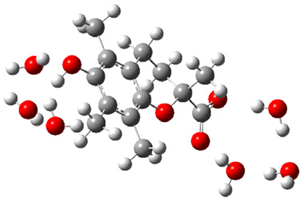 | + | 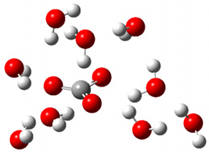 | ⟶ | 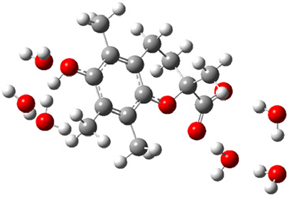 | + | 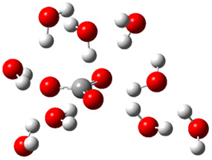 | kapp = 7.50 × 109 = 5.70 × 109 ΔrG = −19.1 ΔG≠ = 0.0 |
| Trolox(H2O)6− | CO3(H2O)9•− | Trolox(H2O)6• | CO3(H2O)92− | λ = 21.1 |
| a | 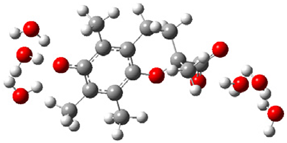 | + |  | ⟶ | 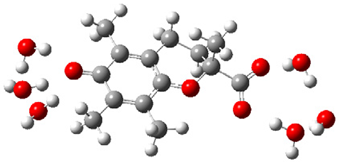 | + | 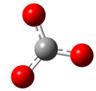 | kapp = 7.90 × 109 = 1.90 × 109 ΔrG = −18.1 ΔG≠ = 1.5 |
| Trolox(H2O)62− | CO3•− | Trolox(H2O)6•− | CO32− | λ = 10.0 | ||||
| b | 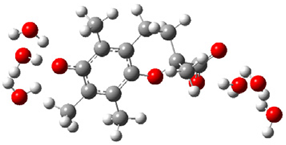 | + | 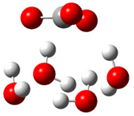 | ⟶ | 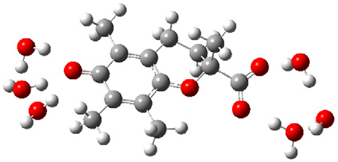 | + | 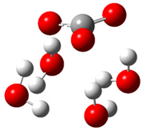 | kapp = 7.50 × 109 = 1.80 × 109 ΔrG = −26.9 ΔG≠ = 1.2 |
| Trolox(H2O)62− | CO3(H2O)4•− | Trolox(H2O)6•− | CO3(H2O)42− | λ = 17.8 | ||||
| c | 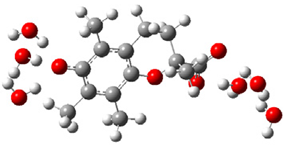 | + | 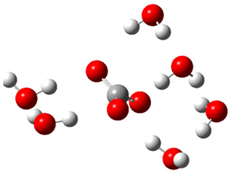 | ⟶ | 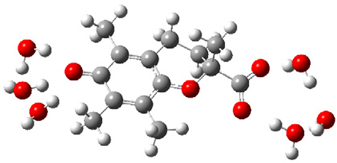 | + | 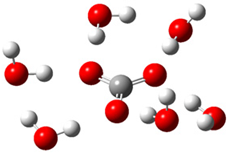 | kapp = 7.40 × 109 = 1.78 × 109 ΔrG = −32.8 ΔG≠ = 1.7 |
| Trolox(H2O)62− | CO3(H2O)6•− | Trolox(H2O)6•− | CO3(H2O)62− | λ = 20.8 | ||||
| d | 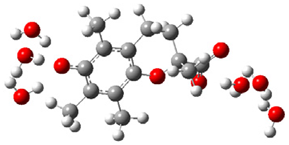 | + | 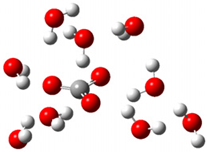 | ⟶ | 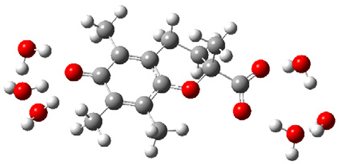 | + | 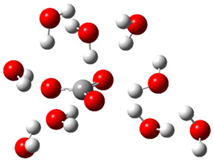 | kapp = 6.10 × 108 = 1.47 × 108 ΔrG = −35.2 ΔG≠ = 5.4 |
| Trolox(H2O)62− | CO3(H2O)9•− | Trolox(H2O)6•− | CO3(H2O)92− | λ = 16.4 |
| (a) | (b) | |||||
|---|---|---|---|---|---|---|
| CO3(H2O)n•− | Trolox(H2O)6− | Trolox(H2O)62− | Trolox− | Trolox2− | ||
| n | koverall | koverall | ||||
| 0 | 5.39 × 109 | 1.90 × 109 | 7.29 × 109 | 5.62 × 109 | 1.25 × 109 | 6.87 × 109 |
| 4 | 5.85 × 109 | 1.83 × 109 | 7.68 × 109 | 5.70 × 109 | 8.89 × 107 | 5.79 × 109 |
| 6 | 5.70 × 109 | 1.78 × 109 | 7.48 × 109 | 5.62 × 109 | 6.01 × 107 | 5.68 × 109 |
| 9 | 5.70 × 109 | 1.47 × 108 | 5.85 × 109 | 5.62 × 109 | 6.25 × 102 | 5.62 × 109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amić, A.; Mastil’ák Cagardová, D. Role of Explicit Hydration in Scavenging of CO3•− by Trolox: A DFT Study. Int. J. Mol. Sci. 2025, 26, 11342. https://doi.org/10.3390/ijms262311342
Amić A, Mastil’ák Cagardová D. Role of Explicit Hydration in Scavenging of CO3•− by Trolox: A DFT Study. International Journal of Molecular Sciences. 2025; 26(23):11342. https://doi.org/10.3390/ijms262311342
Chicago/Turabian StyleAmić, Ana, and Denisa Mastil’ák Cagardová. 2025. "Role of Explicit Hydration in Scavenging of CO3•− by Trolox: A DFT Study" International Journal of Molecular Sciences 26, no. 23: 11342. https://doi.org/10.3390/ijms262311342
APA StyleAmić, A., & Mastil’ák Cagardová, D. (2025). Role of Explicit Hydration in Scavenging of CO3•− by Trolox: A DFT Study. International Journal of Molecular Sciences, 26(23), 11342. https://doi.org/10.3390/ijms262311342







