An Alternative Metabolic Pathway of Glucose Oxidation Induced by Mitochondrial Complex I Inhibition: Serinogenesis and Folate Cycling
Abstract
1. Introduction
2. Results
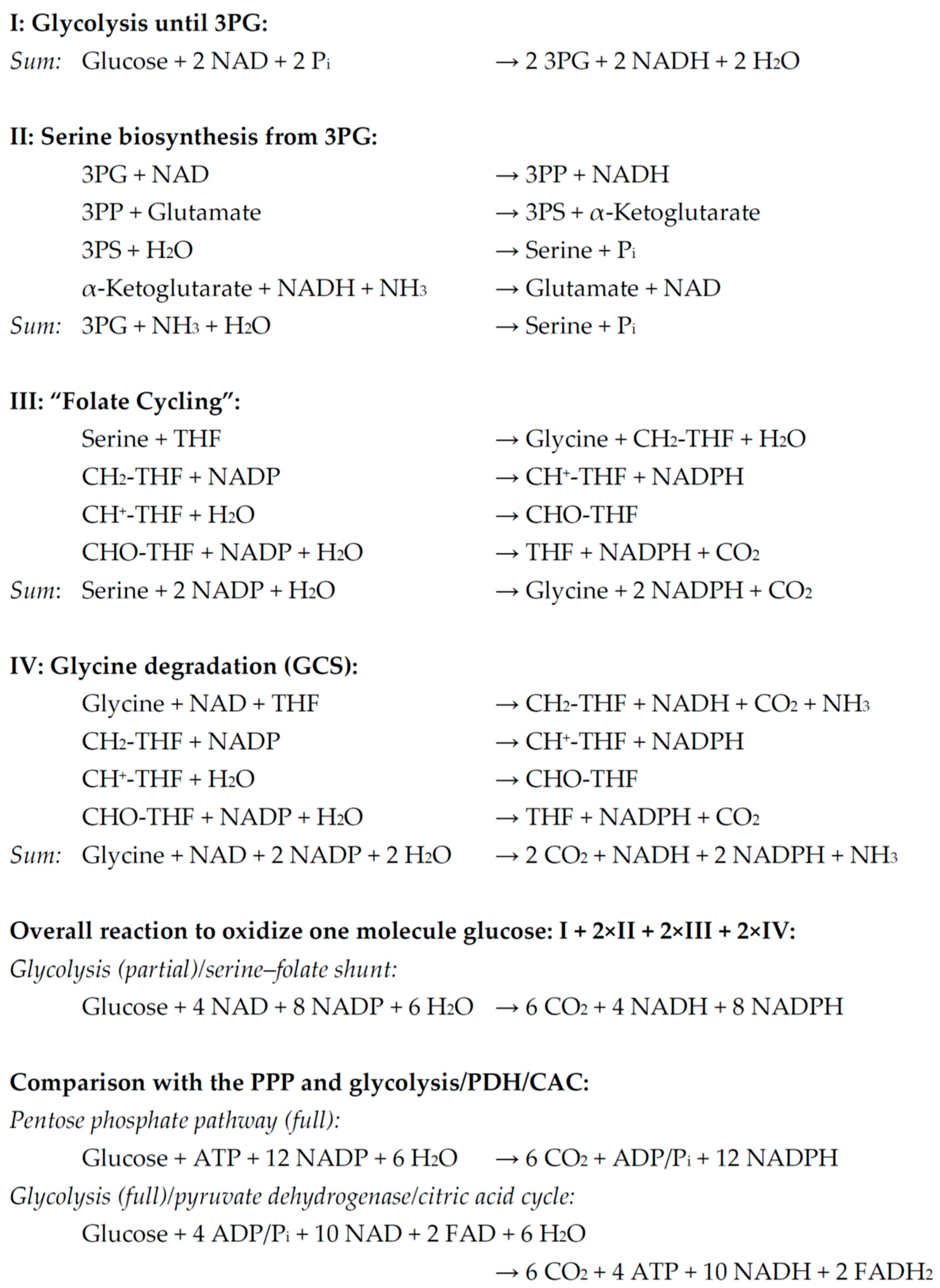
2.1. The Serine–Folate Shunt
2.2. Induction of the Serine–Folate Shunt in Three Models of Complex I Deficiency
2.3. Quantitative Metabolic Outcome of the Serine–Folate Shunt
2.4. Alternative Explanations to Account for Enhanced Serinogenesis and Folate Metabolism After Complex I Inhibition
3. Discussion
4. Materials and Methods
4.1. Model 1: MPP
4.2. Model 2: MetR
4.3. Model 3: NDUFS2
4.4. Technical and Statistical Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delp, J.; Cediel-Ulloa, A.; Suciu, I.; Kranaster, P.; van Vugt-Lussenburg, B.M.; Munic Kos, V.; van der Stel, W.; Carta, G.; Bennekou, S.H.; Jennings, P.; et al. Neurotoxicity and underlying cellular changes of 21 mitochondrial respiratory chain inhibitors. Arch. Toxicol. 2021, 95, 591–615. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, M. Inhibitors of NADH-ubiquinone reductase: An overview. Biochim. Biophys. Acta 1998, 1364, 222–235. [Google Scholar] [CrossRef]
- Schiller, J.; Zickermann, V. Binding of Natural Inhibitors to Respiratory Complex I. Pharmaceuticals 2022, 15, 1088. [Google Scholar] [CrossRef]
- Arnold, P.K.; Finley, L.W.S. Regulation and function of the mammalian tricarboxylic acid cycle. J. Biol. Chem. 2023, 299, 102838. [Google Scholar] [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Baeken, M.W.; Borlepawar, A.; Kötzner, P.; Richly, H.; Behl, C.; Moosmann, B.; Hajieva, P. Epigenetic regulation of the respiratory chain by a mitochondrial distress-related redox signal. Front. Cell Dev. Biol. 2025, 13, 1608400. [Google Scholar] [CrossRef]
- Hajieva, P.; Mocko, J.B.; Moosmann, B.; Behl, C. Novel imine antioxidants at low nanomolar concentrations protect dopaminergic cells from oxidative neurotoxicity. J. Neurochem. 2009, 110, 118–132. [Google Scholar] [CrossRef]
- Martinez, T.N.; Greenamyre, J.T. Toxin models of mitochondrial dysfunction in Parkinson’s disease. Antioxid. Redox Signal 2012, 16, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Flones, I.H.; Fernandez-Vizarra, E.; Lykouri, M.; Brakedal, B.; Skeie, G.O.; Miletic, H.; Lilleng, P.K.; Alves, G.; Tysnes, O.B.; Haugarvoll, K.; et al. Neuronal complex I deficiency occurs throughout the Parkinson’s disease brain, but is not associated with neurodegeneration or mitochondrial DNA damage. Acta Neuropathol. 2018, 135, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Van Laar, A.D.; Webb, K.R.; Keeney, M.T.; Van Laar, V.S.; Zharikov, A.; Burton, E.A.; Hastings, T.G.; Glajch, K.E.; Hirst, W.D.; Greenamyre, J.T.; et al. Transient exposure to rotenone causes degeneration and progressive parkinsonian motor deficits, neuroinflammation, and synucleinopathy. NPJ Park. Dis. 2023, 9, 121. [Google Scholar] [CrossRef]
- Gatt, A.P.; Duncan, O.F.; Attems, J.; Francis, P.T.; Ballard, C.G.; Bateman, J.M. Dementia in Parkinson’s disease is associated with enhanced mitochondrial complex I deficiency. Mov. Disord. 2016, 31, 352–359. [Google Scholar] [CrossRef]
- Swerdlow, R.H. The mitochondrial hypothesis: Dysfunction, bioenergetic defects, and the metabolic link to Alzheimer’s disease. Int. Rev. Neurobiol. 2020, 154, 207–233. [Google Scholar]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Bridges, H.R.; Blaza, J.N.; Yin, Z.; Chung, I.; Pollak, M.N.; Hirst, J. Structural basis of mammalian respiratory complex I inhibition by medicinal biguanides. Science 2023, 379, 351–357. [Google Scholar] [CrossRef]
- Brunmair, B.; Lest, A.; Staniek, K.; Gras, F.; Scharf, N.; Roden, M.; Nohl, H.; Waldhäusl, W.; Fürnsinn, C. Fenofibrate impairs rat mitochondrial function by inhibition of respiratory complex I. J. Pharmacol. Exp. Ther. 2004, 311, 109–114. [Google Scholar] [CrossRef]
- Garcia-Ruiz, I.; Solís-Muñoz, P.; Fernández-Moreira, D.; Muñoz-Yagüe, T.; Solís-Herruzo, J.A. Pioglitazone leads to an inactivation and disassembly of complex I of the mitochondrial respiratory chain. BMC Biol. 2013, 11, 88. [Google Scholar] [CrossRef]
- Tobar, N.; Rocha, G.Z.; Santos, A.; Guadagnini, D.; Assalin, H.B.; Camargo, J.A.; Gonçalves, A.E.S.S.; Pallis, F.R.; Oliveira, A.G.; Rocco, S.A.; et al. Metformin acts in the gut and induces gut-liver crosstalk. Proc. Natl. Acad. Sci. USA 2023, 120, e2211933120. [Google Scholar] [CrossRef] [PubMed]
- Alston, C.L.; Rocha, M.C.; Lax, N.Z.; Turnbull, D.M.; Taylor, R.W. The genetics and pathology of mitochondrial disease. J. Pathol. 2017, 241, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Schindeldecker, M.; Stark, M.; Behl, C.; Moosmann, B. Differential cysteine depletion in respiratory chain complexes enables the distinction of longevity from aerobicity. Mech. Ageing Dev. 2011, 132, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Loeffen, J.L.; Smeitink, J.A.; Trijbels, J.M.; Janssen, A.J.; Triepels, R.H.; Sengers, R.C.; van den Heuvel, L.P. Isolated complex I deficiency in children: Clinical, biochemical and genetic aspects. Hum. Mutat. 2000, 15, 123–134. [Google Scholar] [CrossRef]
- Kühl, I.; Miranda, M.; Atanassov, I.; Kuznetsova, I.; Hinze, Y.; Mourier, A.; Filipovska, A.; Larsson, N.G. Transcriptomic and proteomic landscape of mitochondrial dysfunction reveals secondary coenzyme Q deficiency in mammals. eLife 2017, 6, e30952. [Google Scholar] [CrossRef]
- Hozumi, K.; Sugawara, K.; Ishihara, T.; Ishihara, N.; Ogawa, W. Effects of imeglimin on mitochondrial function, AMPK activity, and gene expression in hepatocytes. Sci. Rep. 2023, 13, 746. [Google Scholar] [CrossRef]
- Perrone, C.E.; Mattocks, D.A.; Plummer, J.D.; Chittur, S.V.; Mohney, R.; Vignola, K.; Orentreich, D.S.; Orentreich, N. Genomic and metabolic responses to methionine-restricted and methionine-restricted, cysteine-supplemented diets in Fischer 344 rat inguinal adipose tissue, liver and quadriceps muscle. J. Nutr. Nutr. 2012, 5, 132–157. [Google Scholar] [CrossRef]
- Abrosimov, R.; Baeken, M.W.; Hauf, S.; Wittig, I.; Hajieva, P.; Perrone, C.E.; Moosmann, B. Mitochondrial complex I inhibition triggers NAD+-independent glucose oxidation via successive NADPH formation, “futile” fatty acid cycling, and FADH2 oxidation. Geroscience 2024, 46, 3635–3658. [Google Scholar] [CrossRef]
- Yerevanian, A.; Soukas, A.A. Metformin: Mechanisms in Human Obesity and Weight Loss. Curr. Obes. Rep. 2019, 8, 156–164. [Google Scholar] [CrossRef]
- Hasek, B.E.; Stewart, L.K.; Henagan, T.M.; Boudreau, A.; Lenard, N.R.; Black, C.; Shin, J.; Huypens, P.; Malloy, V.L.; Plaisance, E.P.; et al. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R728–R739. [Google Scholar] [CrossRef]
- Perrone, C.E.; Malloy, V.L.; Orentreich, D.S.; Orentreich, N. Metabolic adaptations to methionine restriction that benefit health and lifespan in rodents. Exp. Gerontol. 2013, 48, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.; Karan, K.R.; Monzel, A.S.; Santhanam, B.; Taivassalo, T.; Bris, C.; Ware, S.A.; Cross, M.; Towheed, A.; Higgins-Chen, A.; et al. OxPhos defects cause hypermetabolism and reduce lifespan in cells and in patients with mitochondrial diseases. Commun. Biol. 2023, 6, 22. [Google Scholar] [CrossRef]
- Krug, A.K.; Gutbier, S.; Zhao, L.; Pöltl, D.; Kullmann, C.; Ivanova, V.; Förster, S.; Jagtap, S.; Meiser, J.; Leparc, G.; et al. Transcriptional and metabolic adaptation of human neurons to the mitochondrial toxicant MPP(+). Cell Death Dis. 2014, 5, e1222. [Google Scholar] [CrossRef] [PubMed]
- Terburgh, K.; Lindeque, Z.; Mason, S.; van der Westhuizen, F.; Louw, R. Metabolomics of Ndufs4−/− skeletal muscle: Adaptive mechanisms converge at the ubiquinone-cycle. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Marmyleva, A.; Monteuuis, G.; Awadhpersad, R.; Mito, T.; Zamboni, N.; Tatsuta, T.; Vincent, A.E.; Wang, L.; Khan, N.A.; et al. De novo serine biosynthesis is protective in mitochondrial disease. Cell Rep. 2025, 44, 115710. [Google Scholar] [CrossRef]
- Nikkanen, J.; Forsström, S.; Euro, L.; Paetau, I.; Kohnz, R.A.; Wang, L.; Chilov, D.; Viinamäki, J.; Roivainen, A.; Marjamäki, P.; et al. Mitochondrial DNA Replication Defects Disturb Cellular dNTP Pools and Remodel One-Carbon Metabolism. Cell Metab. 2016, 23, 635–648. [Google Scholar] [CrossRef]
- Bao, X.R.; Ong, S.E.; Goldberger, O.; Peng, J.; Sharma, R.; Thompson, D.A.; Vafai, S.B.; Cox, A.G.; Marutani, E.; Ichinose, F.; et al. Mitochondrial dysfunction remodels one-carbon metabolism in human cells. eLife 2016, 5, e10575. [Google Scholar] [CrossRef] [PubMed]
- Lionaki, E.; Gkikas, I.; Daskalaki, I.; Ioannidi, M.K.; Klapa, M.I.; Tavernarakis, N. Mitochondrial protein import determines lifespan through metabolic reprogramming and de novo serine biosynthesis. Nat. Commun. 2022, 13, 651. [Google Scholar] [CrossRef] [PubMed]
- Nichenametla, S.N.; Mattocks, D.A.L.; Cooke, D.; Midya, V.; Malloy, V.L.; Mansilla, W.; Øvrebø, B.; Turner, C.; Bastani, N.E.; Sokolová, J.; et al. Cysteine restriction-specific effects of sulfur amino acid restriction on lipid metabolism. Aging Cell 2022, 21, e13739. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Fan, J.; Venneti, S.; Wan, Y.W.; Pawel, B.R.; Zhang, J.; Finley, L.W.; Lu, C.; Lindsten, T.; Cross, J.R.; et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 2014, 4, 1406–1417. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Caro, P.; Gómez, J.; López-Torres, M.; Sánchez, I.; Naudí, A.; Jove, M.; Pamplona, R.; Barja, G. Forty percent and eighty percent methionine restriction decrease mitochondrial ROS generation and oxidative stress in rat liver. Biogerontology 2008, 9, 183–196. [Google Scholar] [CrossRef]
- Sanchez-Roman, I.; Barja, G. Regulation of longevity and oxidative stress by nutritional interventions: Role of methionine restriction. Exp. Gerontol. 2013, 48, 1030–1042. [Google Scholar] [CrossRef]
- Perrone, C.E.; Mattocks, D.A.; Jarvis-Morar, M.; Plummer, J.D.; Orentreich, N. Methionine restriction effects on mitochondrial biogenesis and aerobic capacity in white adipose tissue, liver, and skeletal muscle of F344 rats. Metabolism 2010, 59, 1000–1011, Erratum in Metabolism 2010, 59, e20. [Google Scholar] [CrossRef]
- Verkaart, S.; Koopman, W.J.; Cheek, J.; van Emst-de Vries, S.E.; van den Heuvel, L.W.; Smeitink, J.A.; Willems, P.H. Mitochondrial and cytosolic thiol redox state are not detectably altered in isolated human NADH: Ubiquinone oxidoreductase deficiency. Biochim. Biophys. Acta 2007, 1772, 1041–1051, Correction in Biochim. Biophys. Acta 2021, 1867, 166105. [Google Scholar] [CrossRef]
- Van der Lee, R.; Szklarczyk, R.; Smeitink, J.; Smeets, H.J.; Huynen, M.A.; Vogel, R. Transcriptome analysis of complex I-deficient patients reveals distinct expression programs for subunits and assembly factors of the oxidative phosphorylation system. BMC Genom. 2015, 16, 691. [Google Scholar] [CrossRef]
- Tapias, V.; McCoy, J.L.; Greenamyre, J.T. Phenothiazine normalizes the NADH/NAD+ ratio, maintains mitochondrial integrity and protects the nigrostriatal dopamine system in a chronic rotenone model of Parkinson’s disease. Redox Biol. 2019, 24, 101164. [Google Scholar] [CrossRef]
- Anderson, K.A.; Madsen, A.S.; Olsen, C.A.; Hirschey, M.D. Metabolic control by sirtuins and other enzymes that sense NAD+, NADH, or their ratio. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Mullarky, E.; Cantley, L.C. Diverting Glycolysis to Combat Oxidative Stress. In Innovative Medicine; Nakao, K., Minato, N., Uemoto, S., Eds.; Springer: Tokyo, Japan, 2015. [Google Scholar]
- Al-Mass, A.; Poursharifi, P.; Peyot, M.L.; Lussier, R.; Chenier, I.; Leung, Y.H.; Ghosh, A.; Oppong, A.; Possik, E.; Mugabo, Y.; et al. Hepatic glycerol shunt and glycerol-3-phosphate phosphatase control liver metabolism and glucodetoxification under hyperglycemia. Mol. Metab. 2022, 66, 101609. [Google Scholar] [CrossRef]
- Ralser, M.; Wamelink, M.M.; Kowald, A.; Gerisch, B.; Heeren, G.; Struys, E.A.; Klipp, E.; Jakobs, C.; Breitenbach, M.; Lehrach, H.; et al. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J. Biol. 2007, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.E.; Mirza, I.A.; Berghuis, A.M.; Mackenzie, R.E. Magnesium and phosphate ions enable NAD binding to methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase. J. Biol. Chem. 2005, 280, 34316–34323. [Google Scholar] [CrossRef]
- Lemons, J.M.; Feng, X.J.; Bennett, B.D.; Legesse-Miller, A.; Johnson, E.L.; Raitman, I.; Pollina, E.A.; Rabitz, H.A.; Rabinowitz, J.D.; Coller, H.A. Quiescent fibroblasts exhibit high metabolic activity. PLoS Biol. 2010, 8, e1000514. [Google Scholar] [CrossRef]
- Wise, D.R.; Ward, P.S.; Shay, J.E.; Cross, J.R.; Gruber, J.J.; Sachdeva, U.M.; Platt, J.M.; DeMatteo, R.G.; Simon, M.C.; Thompson, C.B. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. USA 2011, 108, 19611–19616. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. The efficiency and plasticity of mitochondrial energy transduction. Biochem. Soc. Trans. 2005, 33 Pt 5, 897–904. [Google Scholar] [CrossRef]
- Martinov, M.V.; Vitvitsky, V.M.; Banerjee, R.; Ataullakhanov, F.I. The logic of the hepatic methionine metabolic cycle. Biochim. Biophys. Acta 2010, 1804, 89–96. [Google Scholar] [CrossRef]
- Baeken, M.W.; Moosmann, B.; Hajieva, P. Retrotransposon activation by distressed mitochondria in neurons. Biochem. Biophys. Res. Commun. 2020, 525, 570–575. [Google Scholar] [CrossRef]
- Moosmann, B.; Behl, C. Antioxidants as treatment for neurodegenerative disorders. Expert Opin. Investig. Drugs 2002, 11, 1407–1435. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Forney, L.A.; Wanders, D.; Stone, K.P.; Gettys, T.W. An integrative analysis of tissue-specific transcriptomic and metabolomic responses to short-term dietary methionine restriction in mice. PLoS ONE 2017, 12, e0177513. [Google Scholar] [CrossRef]
- Ying, Y.; Yun, J.; Guoyao, W.; Kaiji, S.; Zhaolai, D.; Zhenlong, W. Dietary L-methionine restriction decreases oxidative stress in porcine liver mitochondria. Exp. Gerontol. 2015, 65, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Li, A.M.; Ye, J. Reprogramming of serine, glycine and one-carbon metabolism in cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165841. [Google Scholar] [CrossRef]
- Yang, L.; Garcia Canaveras, J.C.; Chen, Z.; Wang, L.; Liang, L.; Jang, C.; Mayr, J.A.; Zhang, Z.; Ghergurovich, J.M.; Zhan, L.; et al. Serine Catabolism Feeds NADH when Respiration Is Impaired. Cell Metab. 2020, 31, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; TeSlaa, T.; Xu, X.; Zeng, X.; Yang, L.; Xing, G.; Tesz, G.J.; Clasquin, M.F.; Rabinowitz, J.D. Serine catabolism generates liver NADPH and supports hepatic lipogenesis. Nat. Metab. 2021, 3, 1608–1620. [Google Scholar] [CrossRef]
- Mookerjee, S.A.; Divakaruni, A.S.; Jastroch, M.; Brand, M.D. Mitochondrial uncoupling and lifespan. Mech. Ageing Dev. 2010, 131, 463–472. [Google Scholar] [CrossRef]
- Sun, Q.; Cui, X.; Yin, D.; Li, J.; Li, J.; Du, L. Molecular mechanisms of UCP1-independent thermogenesis: The role of futile cycles in energy dissipation. J. Physiol. Biochem. 2025, 81, 521–537. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Chareyron, I.; Christen, S.; Moco, S.; Valsesia, A.; Lassueur, S.; Dayon, L.; Wollheim, C.B.; Santo Domingo, J.; Wiederkehr, A. Augmented mitochondrial energy metabolism is an early response to chronic glucose stress in human pancreatic beta cells. Diabetologia 2020, 63, 2628–2640. [Google Scholar] [CrossRef] [PubMed]
- Mugabo, Y.; Zhao, S.; Seifried, A.; Gezzar, S.; Al-Mass, A.; Zhang, D.; Lamontagne, J.; Attane, C.; Poursharifi, P.; Iglesias, J.; et al. Identification of a mammalian glycerol-3-phosphate phosphatase: Role in metabolism and signaling in pancreatic β-cells and hepatocytes. Proc. Natl. Acad. Sci. USA 2016, 113, E430–E439. [Google Scholar] [CrossRef] [PubMed]
- Schindeldecker, M.; Moosmann, B. Cysteine Is the Only Universally Affected and Disfavored Proteomic Amino Acid under Oxidative Conditions in Animals. Antioxidants 2024, 13, 267. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Kowal, A.T.; Johnson, M.K.; Salach, J.I.; Singer, T.P. The inhibition site of MPP+, the neurotoxic bioactivation product of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine is near the Q-binding site of NADH dehydrogenase. Arch. Biochem. Biophys. 1987, 259, 645–649. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]

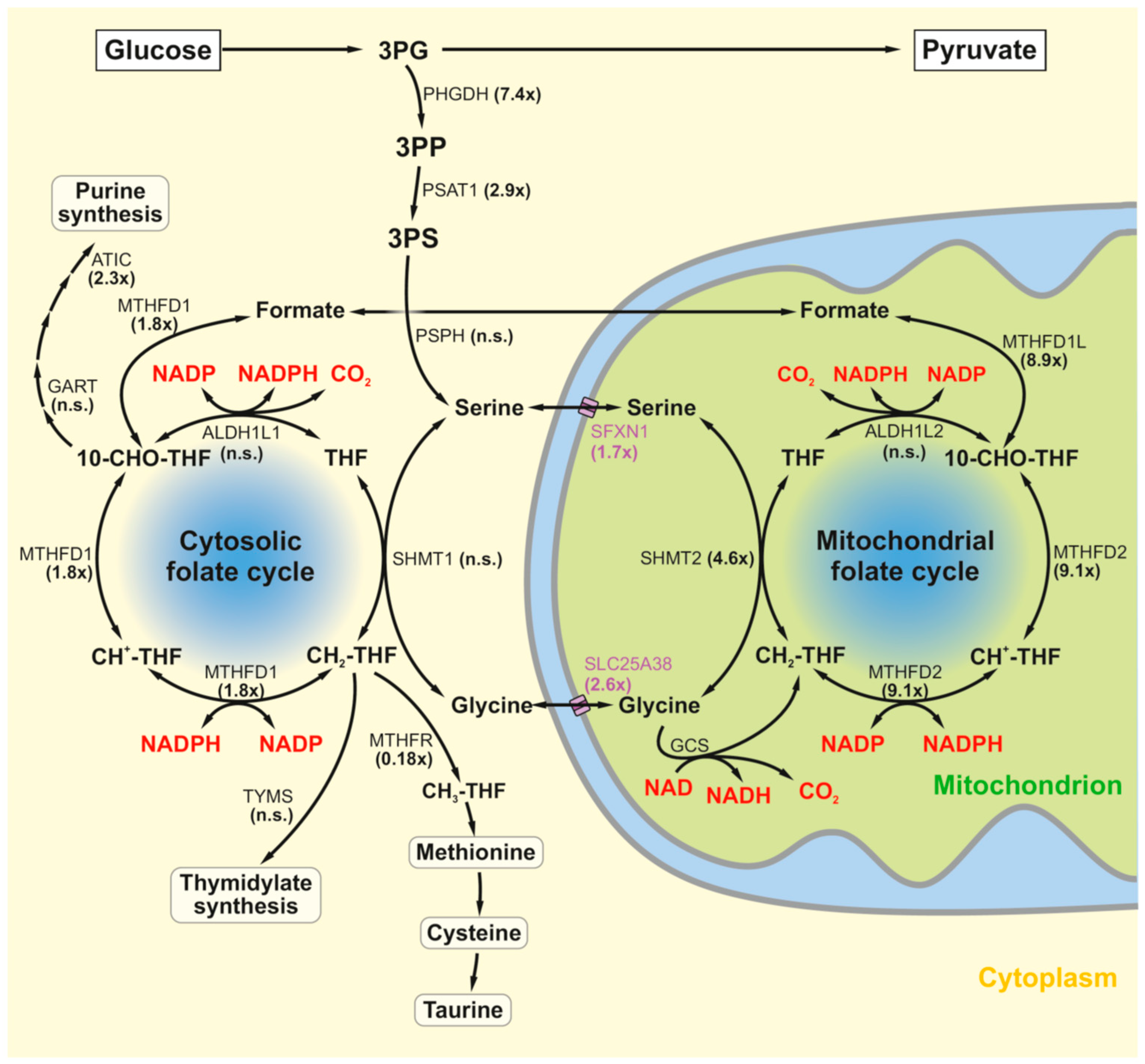
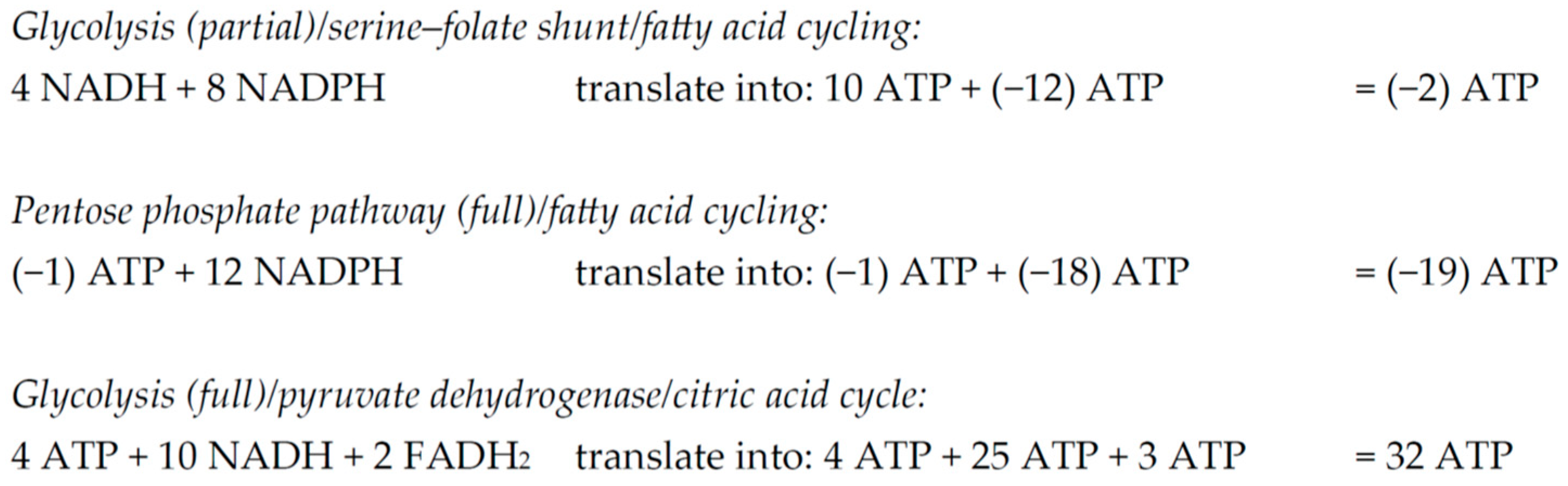
| MPP Treatment | Dietary Methionine Restriction (MetR) | Hereditary Complex I Deficiency (NDUFS2) | |||||
|---|---|---|---|---|---|---|---|
| Gene | FC | p Value | FC FC (PCR) | p Value p Value | FC | p Value | |
| Serine metabolism | PHGDH | 7.42 | 1 × 10−112 | 1.76 9.50 | 1 × 10−5 <0.001 | 9.25 | 1 × 10−104 |
| PSAT1 | 2.94 | 3 × 10−39 | 2.64 | 1 × 10−5 | 12.64 | 1 × 10−132 | |
| PSPH | 0.69 | 1 × 10−5 | 1.39 | 3 × 10−3 | 1.75 | 1 × 10−3 | |
| SFXN1 | 1.67 | 3 × 10−15 | 0.97 | 4 × 10−1 | 1.23 | 9 × 10−2 | |
| SLC25A38 | 2.56 | 1 × 10−24 | — | — | 1.11 | 5 × 10−1 | |
| Folate cycling | SHMT1 | 0.52 | 6 × 10−6 | 1.05 | 2 × 10−1 | 1.15 | 5 × 10−1 |
| SHMT2 | 4.56 | 6 × 10−93 | 1.07 | 6 × 10−2 | 1.94 | 2 × 10−13 | |
| MTHFD1 | 1.76 | 1 × 10−8 | 1.38 1.89 | 4 × 10−2 <0.001 | 0.77 | 6 × 10−3 | |
| MTHFD1L | 8.85 | 1 × 10−98 | 1.08 | 3 × 10−2 | 2.01 | 3 × 10−18 | |
| MTHFD2 | 9.06 | 4 × 10−124 | 4.54 11.68 | 1 × 10−6 <0.001 | 3.48 | 2 × 10−30 | |
| MTHFD2L | [1.19] | 2 × 10−1 | — | — | 0.46 | 1 × 10−1 | |
| ALDH1L1 | [4.13] | 1 × 10−4 | — | — | — | — | |
| ALDH1L2 | 1.40 | 5 × 10−5 | — | — | 1.80 | 1 × 10−14 | |
| Glycine cleavage system | GLDC | 1.14 | 1 × 10−1 | 1.63 2.29 | 3 × 10−3 <0.001 | — | — |
| AMT | [41.64] | 2 × 10−4 | — | — | 1.20 | 4 × 10−1 | |
| GCSH | [1.05] | 9 × 10−1 | 1.01 | 9 × 10−1 | 1.24 | 3 × 10−1 | |
| DLD | 0.52 | 5 × 10−14 | 1.08 | 3 × 10−3 | 1.04 | 7 × 10−1 | |
| NADPH export | IDH1 | 1.40 | 6 × 10−7 | 1.00 | 9 × 10−1 | 0.99 | 9 × 10−1 |
| IDH2 | 1.93 | 9 × 10−17 | 1.33 | 3 × 10−2 | 0.89 | 3 × 10−1 | |
| MPP Treatment | Dietary Methionine Restriction (MetR) | Hereditary Complex I Deficiency (NDUFS2) | |||||
|---|---|---|---|---|---|---|---|
| Gene | FC | p Value | FC FC (PCR) | p Value p Value | FC | p Value | |
| Serine usage * | SRR * | 0.75 | 2 × 10−3 | 1.00 | 1 × 10−0 | 0.87 | 3 × 10−1 |
| SPTLC1 * | 1.03 | 8 × 10−1 | 0.99 | 8 × 10−1 | 1.14 | 2 × 10−1 | |
| SPTLC2 * | 1.08 | 3 × 10−1 | — | — | 1.34 | 2 × 10−3 | |
| CBS * | [1.86] | 3 × 10−5 | 0.87 1.22 | 7 × 10−2 0.03 | 1.23 | 9 × 10−2 | |
| Folate usage * and related genes | MTFMT * | 0.36 | 4 × 10−17 | 1.07 | 5 × 10−1 | 1.01 | 9 × 10−1 |
| TYMS * | 0.79 | 1 × 10−1 | 1.16 | 8 × 10−2 | 0.55 | 1 × 10−7 | |
| DHFR* | 0.23 | 2 × 10−27 | 0.41 | 5 × 10−2 | 0.68 | 1 × 10−3 | |
| CAD | 1.00 | 1 × 10−1 | 1.19 | 2 × 10−6 | 1.16 | 1 × 10−1 | |
| GART * | 1.04 | 5 × 10−1 | 1.25 | 1 × 10−2 | 1.43 | 2 × 10−4 | |
| ATIC * | 2.25 | 2 × 10−24 | 1.44 | 2 × 10−3 | 1.15 | 1 × 10−1 | |
| PPAT | 1.24 | 3 × 10−2 | 1.57 2.64 | 8 × 10−4 <0.001 | 0.85 | 5 × 10−1 | |
| APRT | 1.03 | 2 × 10−2 | 1.08 | 1 × 10−1 | 0.95 | 7 × 10−1 | |
| HPRT1 | 2.15 | 9 × 10−12 | 1.33 | 3 × 10−3 | 1.09 | 6 × 10−1 | |
| RRM1 | 1.52 | 2 × 10−7 | 1.07 | 6 × 10−2 | 0.77 | 6 × 10−3 | |
| RRM2 | 0.90 | 6 × 10−1 | — | — | 0.53 | 8 × 10−9 | |
| MTHFR * | 0.18 | 1 × 10−89 | 1.02 1.17 | 6 × 10−1 0.49 | 0.95 | 7 × 10−1 | |
| MTR* | 0.62 | 4 × 10−6 | 1.16 1.75 | 1 × 10−1 <0.001 | 0.99 | 1 × 10−0 | |
| MAT2A | 0.12 | 4 × 10−149 | 0.92 | 5 × 10−1 | 0.90 | 2 × 10−1 | |
| NADPH usage * and related genes | GSR * | 3.05 | 1 × 10−43 | 1.69 | 4 × 10−4 | 1.40 | 2 × 10−3 |
| GPX1 | 2.80 | 7 × 10−30 | 0.76 | 7 × 10−4 | 0.48 | 1 × 10−18 | |
| GPX4 | 2.77 | 3 × 10−40 | 1.04 | 6 × 10−1 | 1.23 | 1 × 10−2 | |
| SOD1 | 2.07 | 5 × 10−16 | 0.95 | 8 × 10−2 | 0.99 | 9 × 10−1 | |
| SOD2 | 1.34 | 3 × 10−4 | 1.03 | 8 × 10−1 | 1.17 | 5 × 10−2 | |
| CAT | 3.09 | 3 × 10−39 | 0.89 | 3 × 10−3 | 0.69 | 2 × 10−5 | |
| TXNRD1 * | 1.35 | 6 × 10−6 | 1.43 | 7 × 10−5 | 1.95 | 5 × 10−24 | |
| TXNRD2 * | [0.66] | 7 × 10−3 | — | — | 0.76 | 1 × 10−1 | |
| TXN | 1.62 | 2 × 10−9 | 0.92 | 2 × 10−1 | 0.63 | 5 × 10−9 | |
| TXN2 | 2.20 | 5 × 10−21 | 1.02 | 9 × 10−1 | 1.09 | 4 × 10−1 | |
| MSRA * | 1.84 | 9 × 10−9 | — | — | 0.95 | 8 × 10−1 | |
| MSRB1 * | 0.26 | 2 × 10−53 | — | — | 1.22 | 2 × 10−1 | |
| MSRB2 * | 1.31 | 2 × 10−3 | 0.87 | 2 × 10−2 | 1.43 | 5 × 10−3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrosimov, R.; Borlepawar, A.; Hajieva, P.; Moosmann, B. An Alternative Metabolic Pathway of Glucose Oxidation Induced by Mitochondrial Complex I Inhibition: Serinogenesis and Folate Cycling. Int. J. Mol. Sci. 2025, 26, 11349. https://doi.org/10.3390/ijms262311349
Abrosimov R, Borlepawar A, Hajieva P, Moosmann B. An Alternative Metabolic Pathway of Glucose Oxidation Induced by Mitochondrial Complex I Inhibition: Serinogenesis and Folate Cycling. International Journal of Molecular Sciences. 2025; 26(23):11349. https://doi.org/10.3390/ijms262311349
Chicago/Turabian StyleAbrosimov, Roman, Ankush Borlepawar, Parvana Hajieva, and Bernd Moosmann. 2025. "An Alternative Metabolic Pathway of Glucose Oxidation Induced by Mitochondrial Complex I Inhibition: Serinogenesis and Folate Cycling" International Journal of Molecular Sciences 26, no. 23: 11349. https://doi.org/10.3390/ijms262311349
APA StyleAbrosimov, R., Borlepawar, A., Hajieva, P., & Moosmann, B. (2025). An Alternative Metabolic Pathway of Glucose Oxidation Induced by Mitochondrial Complex I Inhibition: Serinogenesis and Folate Cycling. International Journal of Molecular Sciences, 26(23), 11349. https://doi.org/10.3390/ijms262311349







