Therapeutic Potential of Irisin in Neurodegenerative Diseases
Abstract
1. Introduction
2. Irisin Is a Novel Exercise-Induced Molecule
3. Regulation of Irisin Secretion
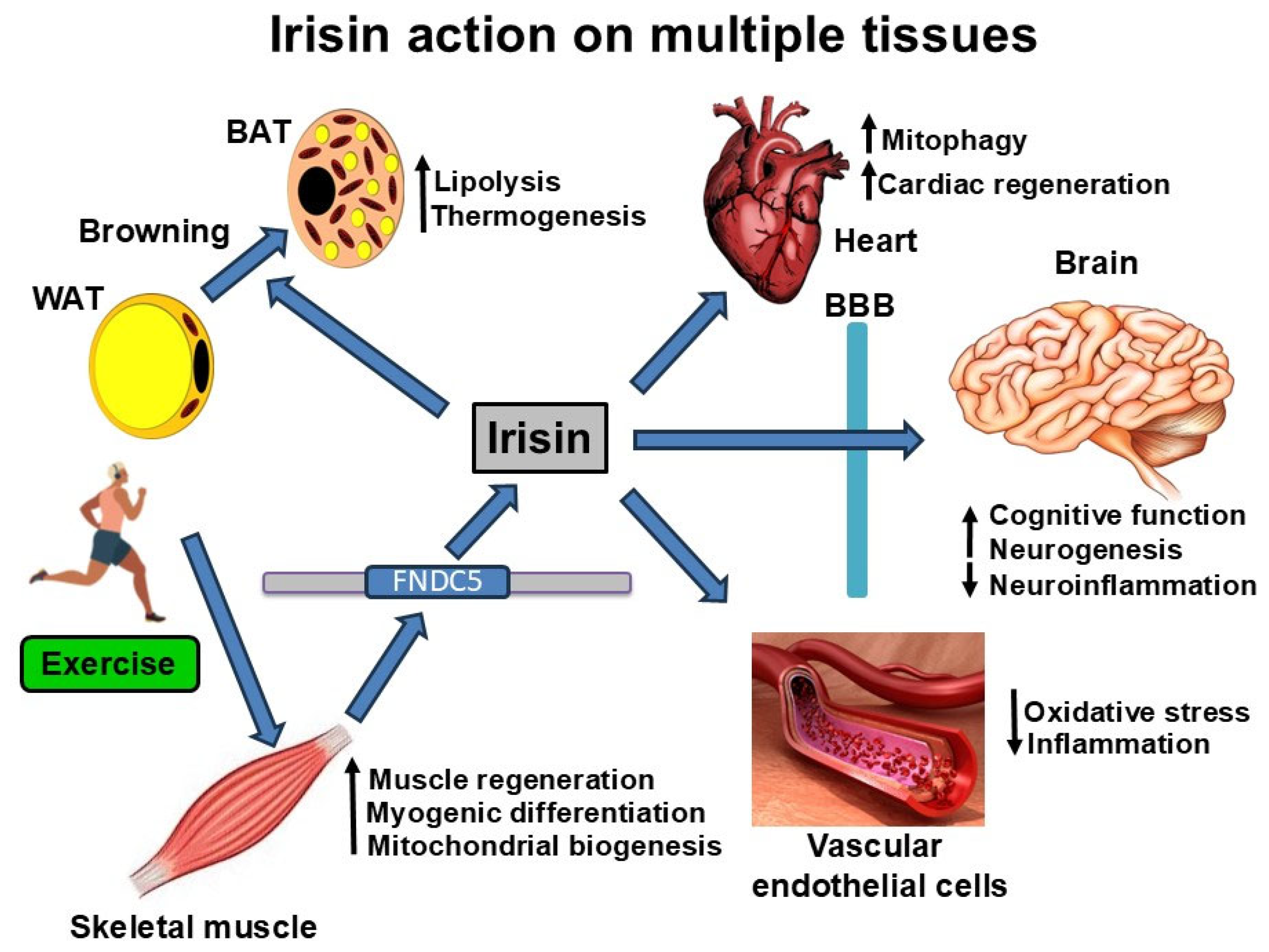
4. Biological Actions of Irisin
4.1. Irisin Actions in Skeletal Muscle
4.2. Irisin Actions in the Brain
5. Irisin in the Prevention of Aging-Associated Diseases
5.1. Exercise, Irisin, and Aging
5.2. Exercise, Irisin, and Alzheimer’s Disease
5.3. Irisin and Other Neurodegenerative Diseases
6. Irisin and Comorbidities of AD
6.1. Irisin and Diabetes
6.2. Irisin and Obesity
6.3. Irisin and Cardiovascular Diseases (CVDs)
7. Mechanism of Irisin Action
Irisin and Sirtuins
8. Therapeutic Potential of Irisin
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pratt, M.; Norris, J.; Lobelo, F.; Roux, L.; Wang, G. The cost of physical inactivity: Moving into the 21st century. Br. J. Sports Med. 2014, 48, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Kivipelto, M.; von Strauss, E. Epidemiology of Alzheimer’s disease: Occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. 2009, 11, 111–128. [Google Scholar] [CrossRef]
- Reiman, E.M. Alzheimer’s disease and other dementias: Advances in 2013. Lancet Neurol. 2014, 13, 3–5. [Google Scholar] [CrossRef]
- Blair, S.N. Physical inactivity: The biggest public health problem of the 21st century. Br. J. Sports Med. 2009, 43, 1–2. [Google Scholar]
- Voss, M.W.; Soto, C.; Yoo, S.; Sodoma, M.; Vivar, C.; van Praag, H. Exercise and Hippocampal Memory Systems. Trends Cogn. Sci. 2019, 23, 318–333. [Google Scholar] [CrossRef]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef]
- Morris, J.K.; Vidoni, E.D.; Johnson, D.K.; Van Sciver, A.; Mahnken, J.D.; Honea, R.A.; Wilkins, H.M.; Brooks, W.M.; Billinger, S.A.; Swerdlow, R.H.; et al. Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. PLoS ONE 2017, 12, e0170547. [Google Scholar] [CrossRef]
- Adlard, P.A.; Perreau, V.M.; Pop, V.; Cotman, C.W. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J. Neurosci. 2005, 25, 4217–4221. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, O.; Robinson, J.; Tang, Y.P.; Hairston, I.S.; Korade-Mirnics, Z.; Lee, V.M.; Hersh, L.B.; Sapolsky, R.M.; Mirnics, K.; Sisodia, S.S. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell 2005, 120, 701–713. [Google Scholar] [CrossRef]
- De Sousa, R.A.L.; Rodrigues, C.M.; Mendes, B.F.; Improta-Caria, A.C.; Peixoto, M.F.D.; Cassilhas, R.C. Physical exercise protocols in animal models of Alzheimer’s disease: A systematic review. Metab. Brain Dis. 2021, 36, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Campos, H.C.; Ribeiro, D.E.; Hashiguchi, D.; Glaser, T.; Milanis, M.D.S.; Gimenes, C.; Suchecki, D.; Arida, R.M.; Ulrich, H.; Monteiro Longo, B. Neuroprotective effects of resistance physical exercise on the APP/PS1 mouse model of Alzheimer’s disease. Front. Neurosci. 2023, 17, 1132825. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, J.M.T.; Yan, T.; Zhang, Y.; Chen, Y.; Chang, R.C.C.; Wong, G.T.C. Short-term resistance exercise inhibits neuroinflammation and attenuates neuropathological changes in 3xTg Alzheimer’s disease mice. J. Neuroinflamm. 2020, 17, 4. [Google Scholar] [CrossRef]
- Tsai, C.L.; Pan, C.Y.; Tseng, Y.T.; Chen, F.C.; Chang, Y.C.; Wang, T.C. Acute effects of high-intensity interval training and moderate-intensity continuous exercise on BDNF and irisin levels and neurocognitive performance in late middle-aged and older adults. Behav. Brain Res. 2021, 413, 113472. [Google Scholar] [CrossRef]
- Leem, Y.H.; Lim, H.J.; Shim, S.B.; Cho, J.Y.; Kim, B.S.; Han, P.L. Repression of tau hyperphosphorylation by chronic endurance exercise in aged transgenic mouse model of tauopathies. J. Neurosci. Res. 2009, 87, 2561–2570. [Google Scholar] [CrossRef]
- Belarbi, K.; Burnouf, S.; Fernandez-Gomez, F.J.; Laurent, C.; Lestavel, S.; Figeac, M.; Sultan, A.; Troquier, L.; Leboucher, A.; Caillierez, R.; et al. Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like Tau pathology. Neurobiol. Dis. 2011, 43, 486–494. [Google Scholar] [CrossRef]
- Leem, Y.H.; Lee, Y.I.; Son, H.J.; Lee, S.H. Chronic exercise ameliorates the neuroinflammation in mice carrying NSE/htau23. Biochem. Biophys. Res. Commun. 2011, 406, 359–365. [Google Scholar] [CrossRef]
- Nichol, K.E.; Poon, W.W.; Parachikova, A.I.; Cribbs, D.H.; Glabe, C.G.; Cotman, C.W. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J. Neuroinflamm. 2008, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yang, D.; Wen, P.; Li, Y.; Ge, Y.; Ma, P.; Yuan, J.; Zhang, P.; Zhu, Z.; Luo, X.; et al. Expression analysis of irisin during different development stages of skeletal muscle in mice. Gene Expr. Patterns 2022, 46, 119287. [Google Scholar] [CrossRef]
- Islam, M.R.; Valaris, S.; Young, M.F.; Haley, E.B.; Luo, R.; Bond, S.F.; Mazuera, S.; Kitchen, R.R.; Caldarone, B.J.; Bettio, L.E.B.; et al. Exercise hormone irisin is a critical regulator of cognitive function. Nat. Metab. 2021, 3, 1058–1070, Erratum in Nat. Metab. 2021, 3, 1432. [Google Scholar] [CrossRef]
- Lima-Filho, R.; Fortuna, J.S.; Cozachenco, D.; Isaac, A.R.; Lyra, E.S.N.; Saldanha, A.; Santos, L.E.; Ferreira, S.T.; Lourenco, M.V.; De Felice, F.G. Brain FNDC5/Irisin Expression in Patients and Mouse Models of Major Depression. eNeuro 2023, 10, 1–9. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via alphaV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cui, F.; Ning, K.; Wang, Z.; Fu, P.; Wang, D.; Xu, H. Role of irisin in physiology and pathology. Front. Endocrinol. 2022, 13, 962968. [Google Scholar] [CrossRef]
- Aydin, S. Three new players in energy regulation: Preptin, adropin and irisin. Peptides 2014, 56, 94–110. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436–456. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Anastasilakis, A.D.; Hofbauer, L.C.; Rauner, M.; Lademann, F. Irisin and Bone in Sickness and in Health: A Narrative Review of the Literature. J. Clin. Med. 2022, 11, 6863. [Google Scholar] [CrossRef]
- Loffler, D.; Muller, U.; Scheuermann, K.; Friebe, D.; Gesing, J.; Bielitz, J.; Erbs, S.; Landgraf, K.; Wagner, I.V.; Kiess, W.; et al. Serum irisin levels are regulated by acute strenuous exercise. J. Clin. Endocrinol. Metab. 2015, 100, 1289–1299. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Goncalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, P.A.; Fernandez-Real, J.M. Metabolism: Irisin, the metabolic syndrome and follistatin in humans. Nat. Rev. Endocrinol. 2014, 10, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Siopi, A.; Mougios, V.; Park, K.H.; Mantzoros, C.S. Irisin in response to exercise in humans with and without metabolic syndrome. J. Clin. Endocrinol. Metab. 2015, 100, E453–E457. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ando, D.; Takamatsu, K.; Goto, K. Resistance exercise induces a greater irisin response than endurance exercise. Metabolism 2015, 64, 1042–1050. [Google Scholar] [CrossRef]
- Zhao, J.; Su, Z.; Qu, C.; Dong, Y. Effects of 12 Weeks Resistance Training on Serum Irisin in Older Male Adults. Front. Physiol. 2017, 8, 171. [Google Scholar] [CrossRef]
- Winn, N.C.; Grunewald, Z.I.; Liu, Y.; Heden, T.D.; Nyhoff, L.M.; Kanaley, J.A. Plasma Irisin Modestly Increases during Moderate and High-Intensity Afternoon Exercise in Obese Females. PLoS ONE 2017, 12, e0170690. [Google Scholar] [CrossRef]
- Bilek, F.; Cetisli-Korkmaz, N.; Ercan, Z.; Deniz, G.; Demir, C.F. Aerobic exercise increases irisin serum levels and improves depression and fatigue in patients with relapsing remitting multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2022, 61, 103742. [Google Scholar] [CrossRef]
- Makiel, K.; Suder, A.; Targosz, A.; Maciejczyk, M.; Haim, A. Effect of Exercise Interventions on Irisin and Interleukin-6 Concentrations and Indicators of Carbohydrate Metabolism in Males with Metabolic Syndrome. J. Clin. Med. 2023, 12, 369. [Google Scholar] [CrossRef]
- Belviranli, M.; Okudan, N.; Kabak, B.; Erdogan, M.; Karanfilci, M. The relationship between brain-derived neurotrophic factor, irisin and cognitive skills of endurance athletes. Phys. Sportsmed. 2016, 44, 290–296. [Google Scholar] [CrossRef]
- Belviranli, M.; Okudan, N. Exercise training increases cardiac, hepatic and circulating levels of brain-derived neurotrophic factor and irisin in young and aged rats. Horm. Mol. Biol. Clin. Investig. 2018, 36, 20180053. [Google Scholar] [CrossRef] [PubMed]
- Brenmoehl, J.; Albrecht, E.; Komolka, K.; Schering, L.; Langhammer, M.; Hoeflich, A.; Maak, S. Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise. Int. J. Biol. Sci. 2014, 10, 338–349. [Google Scholar] [CrossRef]
- Uysal, N.; Yuksel, O.; Kizildag, S.; Yuce, Z.; Gumus, H.; Karakilic, A.; Guvendi, G.; Koc, B.; Kandis, S.; Ates, M. Regular aerobic exercise correlates with reduced anxiety and incresed levels of irisin in brain and white adipose tissue. Neurosci. Lett. 2018, 676, 92–97. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, W. Resistance training increases fibroblast growth factor-21 and irisin levels in the skeletal muscle of Zucker diabetic fatty rats. J. Exerc. Nutr. Biochem. 2017, 21, 50–54. [Google Scholar] [CrossRef]
- Shi, H.; Hao, X.; Sun, Y.; Zhao, Y.; Wang, Y.; Cao, X.; Gong, Z.; Ji, S.; Lu, J.; Yan, Y.; et al. Exercise-inducible circulating extracellular vesicle irisin promotes browning and the thermogenic program in white adipose tissue. Acta Physiol. 2024, 240, e14103. [Google Scholar] [CrossRef] [PubMed]
- Farrash, W.; Brook, M.; Crossland, H.; Phillips, B.E.; Cegielski, J.; Wilkinson, D.J.; Constantin-Teodosiu, D.; Greenhaff, P.L.; Smith, K.; Cleasby, M.; et al. Impacts of rat hindlimb Fndc5/irisin overexpression on muscle and adipose tissue metabolism. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E943–E955. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef]
- Reza, M.M.; Sim, C.M.; Subramaniyam, N.; Ge, X.; Sharma, M.; Kambadur, R.; McFarlane, C. Irisin treatment improves healing of dystrophic skeletal muscle. Oncotarget 2017, 8, 98553–98566. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Gannon, N.P.; Barberena, M.A.; Garcia-Smith, R.; Bisoffi, M.; Mermier, C.M.; Conn, C.A.; Trujillo, K.A. Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes. Metab. 2014, 16, 711–718. [Google Scholar] [CrossRef]
- Ye, X.; Shen, Y.; Ni, C.; Ye, J.; Xin, Y.; Zhang, W.; Ren, Y. Irisin reverses insulin resistance in C2C12 cells via the p38-MAPK-PGC-1alpha pathway. Peptides 2019, 119, 170120. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.O.; Kim, N.; Kim, J.K.; Kim, H.I.; Lee, Y.W.; Kim, S.J.; Choi, J.I.; Oh, Y.; Kim, J.H.; et al. Irisin, a Novel Myokine, Regulates Glucose Uptake in Skeletal Muscle Cells via AMPK. Mol. Endocrinol. 2015, 29, 873–881. [Google Scholar] [CrossRef]
- Jodeiri Farshbaf, M.; Alvina, K. Multiple Roles in Neuroprotection for the Exercise Derived Myokine Irisin. Front. Aging Neurosci. 2021, 13, 649929. [Google Scholar] [CrossRef]
- Papp, C.; Pak, K.; Erdei, T.; Juhasz, B.; Seres, I.; Szentpeteri, A.; Kardos, L.; Szilasi, M.; Gesztelyi, R.; Zsuga, J. Alteration of the irisin-brain-derived neurotrophic factor axis contributes to disturbance of mood in COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2023–2033. [Google Scholar] [CrossRef]
- Wang, J.; Gao, S.; Fu, S.; Li, Y.; Su, L.; Li, X.; Wu, G.; Jiang, J.; Zhao, Z.; Yang, C.; et al. Irisin reprograms microglia through activation of STAT6 and prevents cognitive dysfunction after surgery in mice. Brain Behav. Immun. 2024, 125, 68–91. [Google Scholar] [CrossRef]
- Yardimci, A.; Ertugrul, N.U.; Ozgen, A.; Ozbeg, G.; Ozdede, M.R.; Ercan, E.C.; Canpolat, S. Effects of chronic irisin treatment on brain monoamine levels in the hypothalamic and subcortical nuclei of adult male and female rats: An HPLC-ECD study. Neurosci. Lett. 2023, 806, 137245. [Google Scholar] [CrossRef]
- Natalicchio, A.; Marrano, N.; Biondi, G.; Dipaola, L.; Spagnuolo, R.; Cignarelli, A.; Perrini, S.; Laviola, L.; Giorgino, F. Irisin increases the expression of anorexigenic and neurotrophic genes in mouse brain. Diabetes Metab. Res. Rev. 2020, 36, e3238. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Mikami, T. Exercise hormone irisin prevents physical inactivity-induced cognitive decline in mice. Behav. Brain Res. 2022, 433, 114008. [Google Scholar] [CrossRef] [PubMed]
- Zarzissi, S.; Bouzid, M.A.; Zghal, F.; Rebai, H.; Hureau, T.J. Aging reduces the maximal level of peripheral fatigue tolerable and impairs exercise capacity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R617–R625. [Google Scholar] [CrossRef]
- Jakovljevic, D.G. Physical activity and cardiovascular aging: Physiological and molecular insights. Exp. Gerontol. 2018, 109, 67–74. [Google Scholar] [CrossRef]
- Fleg, J.L.; Morrell, C.H.; Bos, A.G.; Brant, L.J.; Talbot, L.A.; Wright, J.G.; Lakatta, E.G. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 2005, 112, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, Z.; Pantelic, S.; Trajkovic, N.; Sporis, G.; Kostic, R.; James, N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin. Interv. Aging 2013, 8, 549–556, Erratum in Clin. Interv. Aging 2014, 9, 979. [Google Scholar] [CrossRef]
- Gouni-Berthold, I.; Berthold, H.K.; Huh, J.Y.; Berman, R.; Spenrath, N.; Krone, W.; Mantzoros, C.S. Effects of lipid-lowering drugs on irisin in human subjects in vivo and in human skeletal muscle cells ex vivo. PLoS ONE 2013, 8, e72858. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, L.; Zhang, Y.; Zhang, L.; An, Y.; Liu, J.; Wang, G. Effect of Sitagliptin on Serum Irisin Levels in Patients with Newly Diagnosed Type 2 Diabetes Mellitus. Diabetes Ther. 2021, 12, 1029–1039. [Google Scholar] [CrossRef]
- Li, D.J.; Huang, F.; Lu, W.J.; Jiang, G.J.; Deng, Y.P.; Shen, F.M. Metformin promotes irisin release from murine skeletal muscle independently of AMP-activated protein kinase activation. Acta Physiol. 2015, 213, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Varela-Rodriguez, B.M.; Pena-Bello, L.; Juiz-Valina, P.; Vidal-Bretal, B.; Cordido, F.; Sangiao-Alvarellos, S. FNDC5 expression and circulating irisin levels are modified by diet and hormonal conditions in hypothalamus, adipose tissue and muscle. Sci. Rep. 2016, 6, 29898. [Google Scholar] [CrossRef] [PubMed]
- Rachid, T.L.; Penna-de-Carvalho, A.; Bringhenti, I.; Aguila, M.B.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Fenofibrate (PPARalpha agonist) induces beige cell formation in subcutaneous white adipose tissue from diet-induced male obese mice. Mol. Cell. Endocrinol. 2015, 402, 86–94, Erratum in Mol. Cell. Endocrinol. 2015, 413, 249. [Google Scholar] [CrossRef]
- Amengual, J.; Garcia-Carrizo, F.J.; Arreguin, A.; Musinovic, H.; Granados, N.; Palou, A.; Bonet, M.L.; Ribot, J. Retinoic Acid Increases Fatty Acid Oxidation and Irisin Expression in Skeletal Muscle Cells and Impacts Irisin In Vivo. Cell. Physiol. Biochem. 2018, 46, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017, 127, 3240–3249. [Google Scholar] [CrossRef]
- Wilkins, H.M.; Swerdlow, R.H. Relationships Between Mitochondria and Neuroinflammation: Implications for Alzheimer’s Disease. Curr. Top. Med. Chem. 2016, 16, 849–857. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Hu, X.; Zhang, L.; Xiong, Z.; Bai, Y.; Zeng, J.; Xu, F. Effective dosage and mode of exercise for enhancing cognitive function in Alzheimer’s disease and dementia: A systematic review and Bayesian Model-Based Network Meta-analysis of RCTs. BMC Geriatr. 2024, 24, 480. [Google Scholar] [CrossRef]
- Jensen, C.S.; Simonsen, A.H.; Siersma, V.; Beyer, N.; Frederiksen, K.S.; Gottrup, H.; Hoffman, K.; Hogh, P.; Frikke-Schmidt, R.; Sobol, N.A.; et al. Patients with Alzheimer’s disease who carry the APOE epsilon4 allele benefit more from physical exercise. Alzheimers Dement. 2019, 5, 99–106. [Google Scholar] [CrossRef]
- Wan, Y.W.; Al-Ouran, R.; Mangleburg, C.G.; Perumal, T.M.; Lee, T.V.; Allison, K.; Swarup, V.; Funk, C.C.; Gaiteri, C.; Allen, M.; et al. Meta-Analysis of the Alzheimer’s Disease Human Brain Transcriptome and Functional Dissection in Mouse Models. Cell Rep. 2020, 32, 107908. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.M.; Elizondo-Montemayor, L. The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines, and Modulation of Inflammation for Energy Substrate Redistribution and Fat Mass Loss: A Review. Nutrients 2020, 12, 1899. [Google Scholar] [CrossRef]
- Barros-Aragao, F.G.Q.; Januszkiewicz, E.; Hunter, T.; Lyra, E.S.N.M.; De Felice, F.G. Physical activity in Alzheimer’s disease prevention: Sex differences and the roles of BDNF and irisin. Front. Neuroendocrinol. 2025, 77, 101189. [Google Scholar] [CrossRef] [PubMed]
- Dicarlo, M.; Pignataro, P.; Zecca, C.; Dell’Abate, M.T.; Urso, D.; Gnoni, V.; Giugno, A.; Borlizzi, F.; Zerlotin, R.; Oranger, A.; et al. Irisin Levels in Cerebrospinal Fluid Correlate with Biomarkers and Clinical Dementia Scores in Alzheimer Disease. Ann. Neurol. 2024, 96, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Bretland, K.A.; Lin, L.; Bretland, K.M.; Smith, M.A.; Fleming, S.M.; Dengler-Crish, C.M. Irisin treatment lowers levels of phosphorylated tau in the hippocampus of pre-symptomatic female but not male htau mice. Neuropathol. Appl. Neurobiol. 2021, 47, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.I.; Park, H.; Chou, S.C.; Van Vranken, J.G.; Mittenbuhler, M.J.; Kim, H.; A, M.; Choi, Y.R.; Biswas, D.; Wang, J.; et al. Amelioration of pathologic alpha-synuclein-induced Parkinson’s disease by irisin. Proc. Natl. Acad. Sci. USA 2022, 119, e2204835119. [Google Scholar] [CrossRef]
- Li, N.; Wang, B.; Wang, Y.; Tian, X.; Lin, J.; Sun, X.; Sun, Y.; Zhang, X.; Xu, H.; Li, M.; et al. Exercise Ameliorates Dysregulated Mitochondrial Fission, Mitochondrial Respiration, and Neuronal Apoptosis in Parkinson’s Disease Mice via the Irisin/AMPK/SIRT1 Pathway. Mol. Neurobiol. 2025, 62, 8843–8856. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Liu, J.; Dalamaga, M. Could exercise hormone irisin be a therapeutic agent against Parkinson’s and other neurodegenerative diseases? Metabol. Open 2023, 17, 100233. [Google Scholar] [CrossRef]
- Wallin, M.T.; Culpepper, W.J.; Campbell, J.D.; Nelson, L.M.; Langer-Gould, A.; Marrie, R.A.; Cutter, G.R.; Kaye, W.E.; Wagner, L.; Tremlett, H.; et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 2019, 92, e1029–e1040, Erratum in Neurology 2019, 93. [Google Scholar] [CrossRef]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Zhang, L.J.; Zhao, N.; Yang, L. Irisin restrains neuroinflammation in mouse experimental autoimmune encephalomyelitis via regulating microglia activation. Front. Pharmacol. 2025, 16, 1561939. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhang, Z.; Ke, J.; Wang, Y.; Wu, H. Exercise-Linked Irisin Prevents Mortality and Enhances Cognition in a Mice Model of Cerebral Ischemia by Regulating Klotho Expression. Oxidative Med. Cell. Longev. 2021, 2021, 1697070. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, R.; Ying, J.; Li, X.; Lu, R.; Qu, Y.; Mu, D. Irisin prevents hypoxic-ischemic brain damage in rats by inhibiting oxidative stress and protecting the blood-brain barrier. Peptides 2023, 161, 170945. [Google Scholar] [CrossRef]
- Guo, P.; Jin, Z.; Wu, H.; Li, X.; Ke, J.; Zhang, Z.; Zhao, Q. Effects of irisin on the dysfunction of blood-brain barrier in rats after focal cerebral ischemia/reperfusion. Brain Behav. 2019, 9, e01425. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Q.; Li, M.; Wang, N.; Li, C.; Song, D.; Shen, X.; Luo, L.; Fan, Y.; Xie, H.; et al. Early Post-Stroke Electroacupuncture Promotes Motor Function Recovery in Post-Ischemic Rats by Increasing the Blood and Brain Irisin. Neuropsychiatr. Dis. Treat. 2021, 17, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, G.; Ding, Q.; Tao, L.; Li, J.; Sun, L.; Sun, X.; Yang, Y. Irisin Protects Brain against Ischemia/Reperfusion Injury through Suppressing TLR4/MyD88 Pathway. Cerebrovasc. Dis. 2020, 49, 346–354. [Google Scholar] [CrossRef]
- Erden, Y.; Tekin, S.; Sandal, S.; Onalan, E.E.; Tektemur, A.; Kirbag, S. Effects of central irisin administration on the uncoupling proteins in rat brain. Neurosci. Lett. 2016, 618, 6–13. [Google Scholar] [CrossRef]
- Guo, P.; Jin, Z.; Wang, J.; Sang, A.; Wu, H. Irisin Rescues Blood-Brain Barrier Permeability following Traumatic Brain Injury and Contributes to the Neuroprotection of Exercise in Traumatic Brain Injury. Oxidative Med. Cell. Longev. 2021, 2021, 1118981. [Google Scholar] [CrossRef]
- Asadi, Y.; Gorjipour, F.; Behrouzifar, S.; Vakili, A. Irisin Peptide Protects Brain Against Ischemic Injury Through Reducing Apoptosis and Enhancing BDNF in a Rodent Model of Stroke. Neurochem. Res. 2018, 43, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Green, M.F.; Hirschey, M.D. SIRT3 weighs heavily in the metabolic balance: A new role for SIRT3 in metabolic syndrome. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Dalle Grave, R.; Calugi, S.; Centis, E.; Marzocchi, R.; El Ghoch, M.; Marchesini, G. Lifestyle modification in the management of the metabolic syndrome: Achievements and challenges. Diabetes Metab. Syndr. Obes. 2010, 3, 373–385. [Google Scholar] [CrossRef]
- Tyagi, A.; Mirita, C.; Shah, I.; Reddy, P.H.; Pugazhenthi, S. Effects of Lipotoxicity in Brain Microvascular Endothelial Cells During Sirt3 Deficiency-Potential Role in Comorbid Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 716616. [Google Scholar] [CrossRef]
- Tyagi, A.; Mirita, C.; Taher, N.; Shah, I.; Moeller, E.; Tyagi, A.; Chong, T.; Pugazhenthi, S. Metabolic syndrome exacerbates amyloid pathology in a comorbid Alzheimer’s mouse model. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165849. [Google Scholar] [CrossRef]
- Tyagi, A.; Musa, M.; Labeikovsky, W.; Pugazhenthi, S. Sirt3 deficiency induced down regulation of insulin degrading enzyme in comorbid Alzheimer’s disease with metabolic syndrome. Sci. Rep. 2022, 12, 19808. [Google Scholar] [CrossRef]
- Tyagi, A.; Nguyen, C.U.; Chong, T.; Michel, C.R.; Fritz, K.S.; Reisdorph, N.; Knaub, L.; Reusch, J.E.B.; Pugazhenthi, S. SIRT3 deficiency-induced mitochondrial dysfunction and inflammasome formation in the brain. Sci. Rep. 2018, 8, 17547. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, C.; Kong, C.Y.; Song, P.; Wu, H.M.; Xu, S.C.; Yuan, Y.P.; Deng, W.; Ma, Z.G.; Tang, Q.Z. FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ. 2020, 27, 540–555. [Google Scholar] [CrossRef]
- Marrano, N.; Biondi, G.; Borrelli, A.; Cignarelli, A.; Perrini, S.; Laviola, L.; Giorgino, F.; Natalicchio, A. Irisin and Incretin Hormones: Similarities, Differences, and Implications in Type 2 Diabetes and Obesity. Biomolecules 2021, 11, 286. [Google Scholar] [CrossRef]
- Behera, J.; Ison, J.; Voor, M.J.; Tyagi, N. Exercise-Linked Skeletal Irisin Ameliorates Diabetes-Associated Osteoporosis by Inhibiting the Oxidative Damage-Dependent miR-150-FNDC5/Pyroptosis Axis. Diabetes 2022, 71, 2777–2792. [Google Scholar] [CrossRef]
- Zhou, L.; Pinho, R.; Gu, Y.; Radak, Z. The Role of SIRT3 in Exercise and Aging. Cells 2022, 11, 2596. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, C.; Wang, Y.; Tian, X.; Lin, J.; Zhu, B.; Zhou, Y.; Zhang, X.; Li, N.; Sun, Y.; et al. Exercise ameliorating myocardial injury in type 2 diabetic rats by inhibiting excessive mitochondrial fission involving increased irisin expression and AMP-activated protein kinase phosphorylation. J. Diabetes 2024, 16, e13475. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abdelmonsif, D.A.; Zeitoun, T.M.; El-Sayed, N.S.; Samy, D.M. Swimming exercise versus L-carnosine supplementation for Alzheimer’s dementia in rats: Implication of circulating and hippocampal FNDC5/irisin. J. Physiol. Biochem. 2022, 78, 109–124. [Google Scholar] [CrossRef]
- Mai, S.; Grugni, G.; Mele, C.; Vietti, R.; Vigna, L.; Sartorio, A.; Aimaretti, G.; Scacchi, M.; Marzullo, P. Irisin levels in genetic and essential obesity: Clues for a potential dual role. Sci. Rep. 2020, 10, 1020. [Google Scholar] [CrossRef] [PubMed]
- Bernal Rivas, C.; Llamunao Tropa, A.; Reyes Barria, A.; Halabi, D.; Pavicic, F.; Ehrenfeld, P.; Martinez Huenchullan, S. Effects of exercise on irisin in subjects with overweight or obesity. A systematic review of clinical studies. Nutr. Hosp. 2022, 39, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, H.; Shen, S.W.; Shen, Z.H.; Xu, M.; Yang, C.J.; Li, F.; Feng, Y.B.; Yun, J.T.; Wang, L.; et al. Swimming exercise increases serum irisin level and reduces body fat mass in high-fat-diet fed Wistar rats. Lipids Health Dis. 2016, 15, 93. [Google Scholar] [CrossRef]
- Seo, D.Y.; Bae, J.H.; Kim, T.N.; Kwak, H.B.; Kha, P.T.; Han, J. Exercise-Induced Circulating Irisin Level Is Correlated with Improved Cardiac Function in Rats. Int. J. Environ. Res. Public Health 2020, 17, 3863. [Google Scholar] [CrossRef]
- Tine Kartinah, N.; Rosalyn Sianipar, I.; Nafi’ah; Rabia. The Effects of Exercise Regimens on Irisin Levels in Obese Rats Model: Comparing High-Intensity Intermittent with Continuous Moderate-Intensity Training. BioMed Res. Int. 2018, 2018, 4708287. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Chen, D.; Sun, H.J.; Ding, L.; Wang, J.J.; Chen, Q.; Li, Y.H.; Zhou, Y.B.; Han, Y.; Zhang, F.; et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim. Biophys. Acta 2015, 1852, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, A.H.; Hajinia, M.; Askari, R.; Abbasian, S.; Goldfied, G. Effect of high-intensity interval training and high-intensity resistance training on irisin and fibroblast growth factor 21 in men with overweight and obesity. Can. J. Physiol. Pharmacol. 2022, 100, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Yuan, H.; Li, J.; Fan, J.J.; Jia, S.H.; Kou, X.J.; Chen, N. Swimming intervention mitigates HFD-induced obesity of rats through PGC-1alpha-irisin pathway. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2123–2130. [Google Scholar]
- Chen, Y.; Ding, J.; Zhao, Y.; Ju, S.; Mao, H.; Peng, X.G. Irisin induces white adipose tissue browning in mice as assessed by magnetic resonance imaging. Exp. Biol. Med. 2021, 246, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Corina-Sosa, B.; Basurto, L.; Luqueno, E.; Robledo, A.; Mendieta-Zeron, H.; Oros-Pantoja, R. The colors of adipose tissue and the relationship with irisin. Cir. Cir. 2020, 88, 664–671. [Google Scholar] [CrossRef]
- Huerta-Delgado, A.S.; Roffe-Vazquez, D.N.; Luna-Ceron, E.; Gonzalez-Gil, A.M.; Casillas-Fikentscher, A.; Villarreal-Calderon, J.R.; Enriquez, C.; de la Pena-Almaguer, E.; Castillo, E.C.; Silva-Platas, C.; et al. Association of irisin levels with cardiac magnetic resonance, inflammatory, and biochemical parameters in patients with chronic heart failure versus controls. Magn. Reson. Imaging 2022, 93, 62–72. [Google Scholar] [CrossRef]
- Alzoughool, F.; Al-Zghoul, M.B.; Ghanim, B.Y.; Gollob, M.; Idkaidek, N.; Qinna, N.A. The Role of Interventional Irisin on Heart Molecular Physiology. Pharmaceuticals 2022, 15, 863. [Google Scholar] [CrossRef]
- Li, H.; Qin, S.; Liang, Q.; Xi, Y.; Bo, W.; Cai, M.; Tian, Z. Exercise Training Enhances Myocardial Mitophagy and Improves Cardiac Function via Irisin/FNDC5-PINK1/Parkin Pathway in MI Mice. Biomedicines 2021, 9, 701. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Wang, J.; Yano, N.; Zhang, L.X.; Wang, H.; Zhang, S.; Qin, G.; Dubielecka, P.M.; Zhuang, S.; Liu, P.Y.; et al. Irisin promotes cardiac progenitor cell-induced myocardial repair and functional improvement in infarcted heart. J. Cell. Physiol. 2019, 234, 1671–1681. [Google Scholar] [CrossRef]
- Chi, C.; Fu, H.; Li, Y.H.; Zhang, G.Y.; Zeng, F.Y.; Ji, Q.X.; Shen, Q.R.; Wang, X.J.; Li, Z.C.; Zhou, C.C.; et al. Exerkine fibronectin type-III domain-containing protein 5/irisin-enriched extracellular vesicles delay vascular ageing by increasing SIRT6 stability. Eur. Heart J. 2022, 43, 4579–4595. [Google Scholar] [CrossRef]
- Bi, J.; Yang, L.; Wang, T.; Zhang, J.; Li, T.; Ren, Y.; Wang, M.; Chen, X.; Lv, Y.; Wu, R. Irisin Improves Autophagy of Aged Hepatocytes via Increasing Telomerase Activity in Liver Injury. Oxidative Med. Cell. Longev. 2020, 2020, 6946037. [Google Scholar] [CrossRef]
- A, M.; Wales, T.E.; Zhou, H.; Draga-Coleta, S.V.; Gorgulla, C.; Blackmore, K.A.; Mittenbuhler, M.J.; Kim, C.R.; Bogoslavski, D.; Zhang, Q.; et al. Irisin acts through its integrin receptor in a two-step process involving extracellular Hsp90alpha. Mol. Cell 2023, 83, 1903–1920.e12. [Google Scholar] [CrossRef] [PubMed]
- Oguri, Y.; Shinoda, K.; Kim, H.; Alba, D.L.; Bolus, W.R.; Wang, Q.; Brown, Z.; Pradhan, R.N.; Tajima, K.; Yoneshiro, T.; et al. CD81 Controls Beige Fat Progenitor Cell Growth and Energy Balance via FAK Signaling. Cell 2020, 182, 563–577.e20. [Google Scholar] [CrossRef]
- Kim, E.; Kim, H.; Jedrychowski, M.P.; Bakiasi, G.; Park, J.; Kruskop, J.; Choi, Y.; Kwak, S.S.; Quinti, L.; Kim, D.Y.; et al. Irisin reduces amyloid-beta by inducing the release of neprilysin from astrocytes following downregulation of ERK-STAT3 signaling. Neuron 2023, 111, 3619–3633.e18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, M.; Tan, J.; Pei, X.; Lu, C.; Xin, Y.; Deng, S.; Zhao, F.; Gao, Y.; Gong, Y. Irisin ameliorates neuroinflammation and neuronal apoptosis through integrin alphaVbeta5/AMPK signaling pathway after intracerebral hemorrhage in mice. J. Neuroinflamm. 2022, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Kobilo, T.; Guerrieri, D.; Zhang, Y.; Collica, S.C.; Becker, K.G.; van Praag, H. AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learn. Mem. 2014, 21, 119–126. [Google Scholar] [CrossRef]
- Kobilo, T.; Yuan, C.; van Praag, H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn. Mem. 2011, 18, 103–107. [Google Scholar] [CrossRef]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.X.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; et al. AMPK and PPARdelta agonists are exercise mimetics. Cell 2008, 134, 405–415. [Google Scholar] [CrossRef]
- Lourenco, M.V.; de Freitas, G.B.; Raony, I.; Ferreira, S.T.; De Felice, F.G. Irisin stimulates protective signaling pathways in rat hippocampal neurons. Front. Cell. Neurosci. 2022, 16, 953991. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef]
- He, W.; Newman, J.C.; Wang, M.Z.; Ho, L.; Verdin, E. Mitochondrial sirtuins: Regulators of protein acylation and metabolism. Trends Endocrinol. Metab. 2012, 23, 467–476. [Google Scholar] [CrossRef]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschop, M.H. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef] [PubMed]
- Dumke, C.L.; Mark Davis, J.; Angela Murphy, E.; Nieman, D.C.; Carmichael, M.D.; Quindry, J.C.; Travis Triplett, N.; Utter, A.C.; Gross Gowin, S.J.; Henson, D.A.; et al. Successive bouts of cycling stimulates genes associated with mitochondrial biogenesis. Eur. J. Appl. Physiol. 2009, 107, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Rinaldi, B.; Corbi, G.; Conti, V.; Stiuso, P.; Boccuti, S.; Rengo, G.; Rossi, F.; Filippelli, A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008, 11, 139–150. [Google Scholar] [CrossRef]
- Safarpour, P.; Daneshi-Maskooni, M.; Vafa, M.; Nourbakhsh, M.; Janani, L.; Maddah, M.; Amiri, F.S.; Mohammadi, F.; Sadeghi, H. Vitamin D supplementation improves SIRT1, Irisin, and glucose indices in overweight or obese type 2 diabetic patients: A double-blind randomized placebo-controlled clinical trial. BMC Fam. Pract. 2020, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.; Munoz-Pinto, M.F.; Cano, M. Irisin enhances longevity by boosting SIRT1, AMPK, autophagy and telomerase. Expert Rev. Mol. Med. 2022, 25, e4. [Google Scholar] [CrossRef]
- Lin, J.Y.; Kuo, W.W.; Baskaran, R.; Kuo, C.H.; Chen, Y.A.; Chen, W.S.; Ho, T.J.; Day, C.H.; Mahalakshmi, B.; Huang, C.Y. Swimming exercise stimulates IGF1/ PI3K/Akt and AMPK/SIRT1/PGC1alpha survival signaling to suppress apoptosis and inflammation in aging hippocampus. Aging 2020, 12, 6852–6864. [Google Scholar] [CrossRef]
- Johnson, M.L.; Irving, B.A.; Lanza, I.R.; Vendelbo, M.H.; Konopka, A.R.; Robinson, M.M.; Henderson, G.C.; Klaus, K.A.; Morse, D.M.; Heppelmann, C.; et al. Differential Effect of Endurance Training on Mitochondrial Protein Damage, Degradation, and Acetylation in the Context of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1386–1393. [Google Scholar] [CrossRef]
- Ge, Y.; Wu, X.; Cai, Y.; Hu, Q.; Wang, J.; Zhang, S.; Zhao, B.; Cui, W.; Wu, Y.; Wang, Q.; et al. FNDC5 prevents oxidative stress and neuronal apoptosis after traumatic brain injury through SIRT3-dependent regulation of mitochondrial quality control. Cell Death Dis. 2024, 15, 364, Erratum in Cell Death Dis. 2024, 15, 527. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Wang, Z.; Ma, A.; Ni, Y.; Wu, D.; Zhou, Y.; Zhang, N.; Zhang, L.; Chang, Y.; et al. Irisin Alleviates Impaired Mitochondrial Fusion via Enhancing PKA/SIRT3/mTOR Pathway in Hepatic Steatosis. J. Gastroenterol. Hepatol. 2025, 40, 1616–1630. [Google Scholar] [CrossRef]
- Cong, Y.; Guo, R.; Li, C.; Li, Q.; Qi, S. Irisin protects against cerebral ischemia reperfusion injury in a SIRT3-dependent manner. Front. Pharmacol. 2025, 16, 1558457. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzaffar, S.; Tyagi, A.; Pugazhenthi, S. Therapeutic Potential of Irisin in Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 11348. https://doi.org/10.3390/ijms262311348
Muzaffar S, Tyagi A, Pugazhenthi S. Therapeutic Potential of Irisin in Neurodegenerative Diseases. International Journal of Molecular Sciences. 2025; 26(23):11348. https://doi.org/10.3390/ijms262311348
Chicago/Turabian StyleMuzaffar, Sania, Alpna Tyagi, and Subbiah Pugazhenthi. 2025. "Therapeutic Potential of Irisin in Neurodegenerative Diseases" International Journal of Molecular Sciences 26, no. 23: 11348. https://doi.org/10.3390/ijms262311348
APA StyleMuzaffar, S., Tyagi, A., & Pugazhenthi, S. (2025). Therapeutic Potential of Irisin in Neurodegenerative Diseases. International Journal of Molecular Sciences, 26(23), 11348. https://doi.org/10.3390/ijms262311348




