The Effects of Several Natural Protoberberine Alkaloids and Cinnamic Acid Derivatives Used for Traditional Medicine on the Membrane Boundary Potential and Lipid Packing Stress
Abstract
1. Introduction
2. Results and Discussion
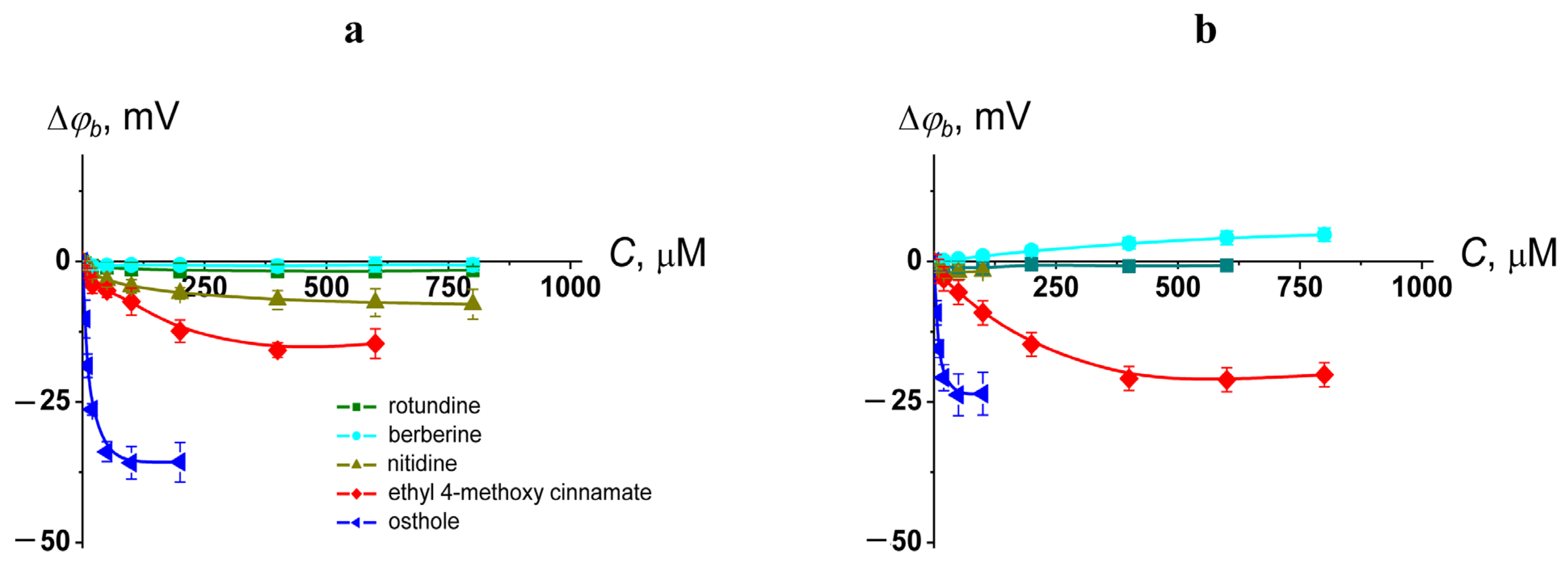
2.1. Membrane Boundary Potential
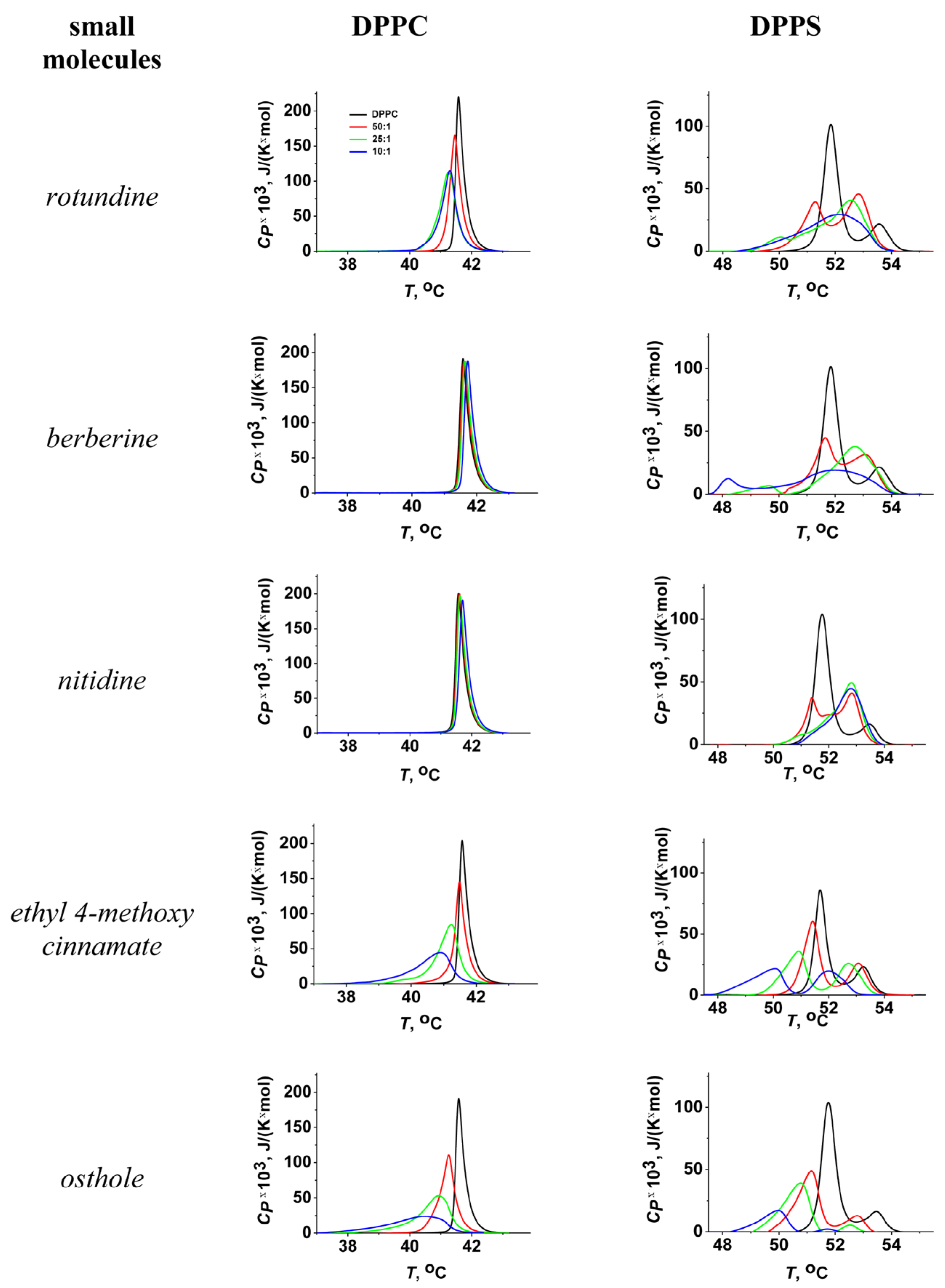
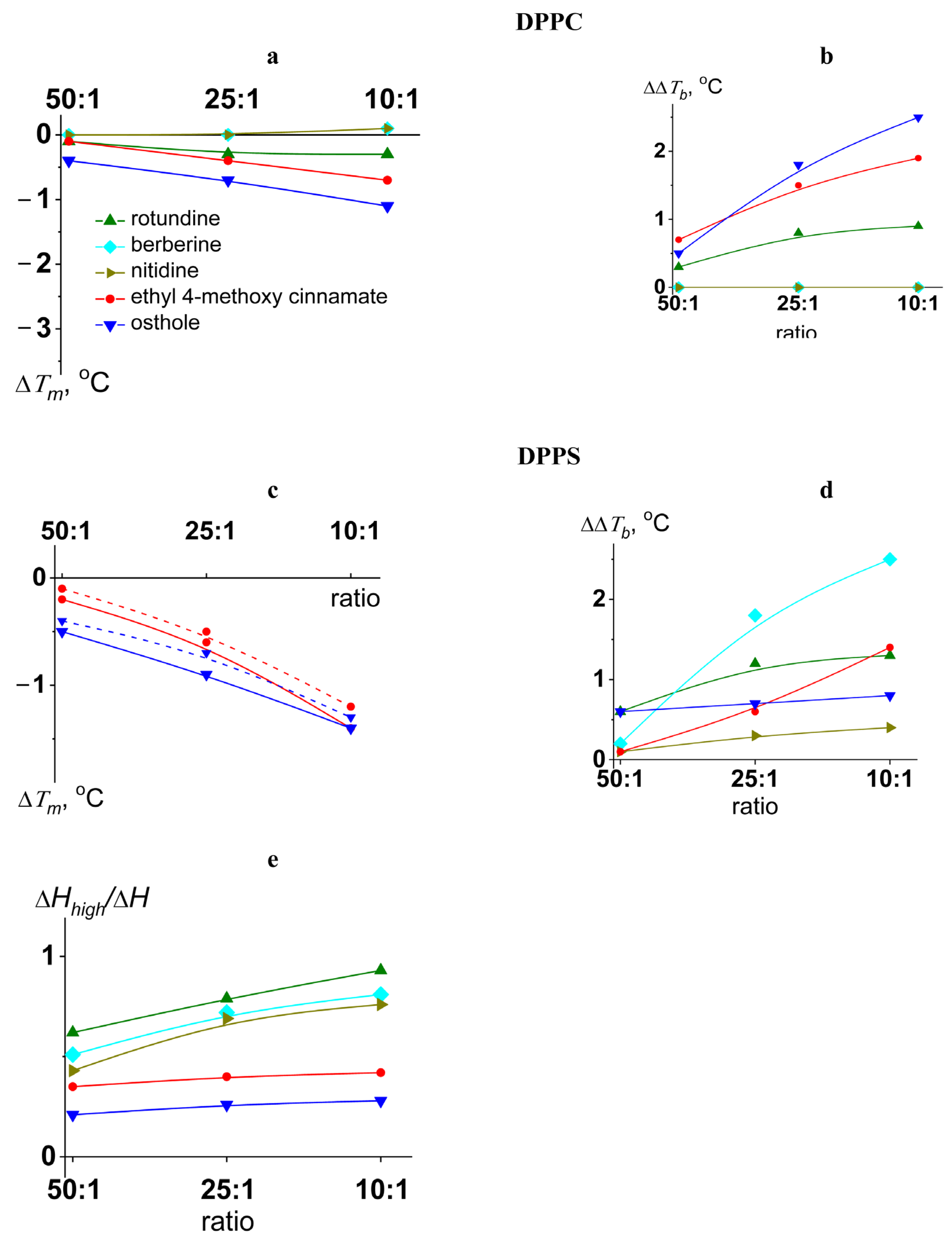
2.2. Lipid Melting
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Measurement of the Membrane Boundary Potential
3.2.2. Differential Scanning Microcalorimetry
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Safety and efficacy. Nutrition 2009, 25, 1206–1211. [Google Scholar] [CrossRef]
- Desgrouas, C.; Taudon, N.; Bun, S.S.; Baghdikian, B.; Bory, S.; Parzy, D.; Ollivier, E. Ethnobotany, phytochemistry and pharmacology of Stephania rotunda Lour. J. Ethnopharmacol. 2014, 154, 537–563. [Google Scholar] [CrossRef]
- Du, Q.; Meng, X.; Wang, S. A comprehensive review on the chemical properties, plant sources, pharmacological activities, pharmacokinetic and toxicological characteristics of tetrahydropalmatine. Front. Pharmac. 2022, 13, 890078. [Google Scholar] [CrossRef]
- Ha, L.M.; Phuong, N.T.; Du, N.V.; Tien, T.V.; Sy, N.C.; Thang, V.D.; Phuong, N.T.; Thach, B.D.; Khoi, N.V.M.; Thien, D.T. Development of a quantitative method for rotundine in fresh Stephania tubers by thin-layer chromatography combined with densitometry (TLC-scanning). J. Med. Mater. 2014, 19, 375–380. (In Vietnamese) [Google Scholar]
- He, M.; Wang, Y.; Hong, M.; Li, T. Berberine as a promising natural compound to control Penicillium italicum causing blue mold of citrus fruit. Sci. Hortic. 2022, 305, 111370. [Google Scholar] [CrossRef]
- Tuzimski, T.; Petruczynik, A.; Kaproń, B.; Makuch-Kocka, A.; Szultka-Młyńska, M.; Misiurek, J.; Szymczak, G.; Buszewski, B. Determination of cytotoxic activity of selected isoquinoline alkaloids and plant extracts obtained from various parts of Mahonia aquifolium collected in various vegetation seasons. Molecules 2021, 26, 816. [Google Scholar] [CrossRef]
- Singburaudom, N. The alkaloid berberine isolated from Coscinium fenestratum is an inhibitor of phytopathogenic fungi. J. Biopestic. 2015, 8, 28. [Google Scholar] [CrossRef]
- Taher, M.; Amri, M.S.; Susanti, D.; Abdul Kudos, M.B.; Shafawi, A.N.; Yazid, S.N. Coscinium fenestratum: A review on phytochemicals and pharmacological properties. Nat. Bio-Act. Compd. 2019, 2, 107–125. [Google Scholar]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Fan, X.; Yuan, B.; Takagi, N.; Liu, S.; Han, X.; Ren, J.; Liu, J. Berberine inhibits NLRP3 inflammasome pathway in human triple-negative breast cancer MDA-MB-231 cell. BMC Complement Altern. Med. 2019, 19, 216. [Google Scholar] [CrossRef]
- Gong, H.; Wang, L.; Zhao, J.; Wang, L.; Yu, Q.; Wan, Y. Nitidine chloride inhibits the appearance of cancer stem-like properties and regulates potential the mitochondrial membrane alterations of colon cancer cells. Ann. Transl. Med. 2020, 8, 591. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Y.; Guo, Y.; Yu, P.; Sun, Y.; Song, Y.; Zhao, L. Nitidine chloride suppresses epithelial-mesenchymal transition and stem cell-like properties in glioblastoma by regulating JAK2/STAT3 signaling. Cancer Med. 2021, 10, 3113–3128. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Liu, H. Overview of cancer stem cells and stemness for community oncologists. Target Oncol. 2017, 12, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, N.; Wang, X.; Li, Y.; Qi, W.; Zhang, H.; Li, Z.; Yang, Q. Hedgehog pathway is involved in nitidine chloride induced inhibition of epithelial-mesenchymal transition and cancer stem cells-like properties in breast cancer cells. Cell Biosci. 2016, 6, 44. [Google Scholar] [CrossRef]
- Phuong, T.H.; Quan, P.M.; Bach, P.C.; Tuyen, T.T.; Nga, N.P.; Cuc, N.T.; Thao, D.T.; Van, N.T.H. Nitidine isolated from the bark of Zanthoxylum myriacanthum and its effects on NTERA-2 cancer stem cells. Planta Med. 2025, 91, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.I.; Asmawi, M.Z.; Sadikun, A.; Atangwho, I.J.; Yam, M.F.; Altaf, R.; Ahmed, A. Bioactivity-guided isolation of ethyl-p-methoxycinnamate, an anti-inflammatory constituent, from Kaempferia galanga L. extracts. Molecules 2012, 17, 8720–8734. [Google Scholar] [CrossRef]
- Kumar, A. Phytochemistry, pharmacological activities and uses of traditional medicinal plant Kaempferia galanga L.—An overview. J. Ethnopharmacol. 2020, 253, 112667. [Google Scholar] [CrossRef]
- Dinh, D.T.; Phuong, N.T.; Thao, T.T.; Nhung, P.T.; An, N.T.; Ha, L.M. Optimisation of ultrasound-assisted extraction of ethyl p-methoxycinnamate from Kaemperia galanga L. rhizomes. Vietnam J. Sci. Technol. Eng. 2025, 67, 28–34. [Google Scholar] [CrossRef]
- Sun, M.; Sun, M.; Zhang, J. Osthole: An overview of its sources, biological activities, and modification development. Med. Chem. Res. 2021, 30, 1767–1794. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Leung, W.N.; Cheung, H.Y.; Chan, C.W. Osthole: A review on its bioactivities, pharmacological properties, and potential as alternative medicine. Evid. Based Complement Alternat Med. 2015, 2015, 919616. [Google Scholar] [CrossRef]
- Ha, L.M.; Cuong, N.M.; Van, N.T.H.; Phuong, N.T.; Ty, P.D.; Dung, N.T.; Huong, L.M.; Huong, H.T. Coumarins from the Fruits of Cnidium monnieri (L.) Cusson. J. Med. Mater. 2012, 17, 34–38. (In Vietnamese) [Google Scholar]
- Park, W.; Park, S.; Song, G.; Lim, W. Inhibitory effects of osthole on human breast cancer cell progression via induction of cell cycle arrest, mitochondrial dysfunction, and ER stress. Nutrients 2019, 11, 2777. [Google Scholar] [CrossRef]
- Yang, S.; Dai, W.; Wang, J.; Zhang, X.; Zheng, Y.; Bi, S.; Pang, L.; Ren, T.; Yang, Y.; Sun, Y.; et al. Osthole: An up-to-date review of its anticancer potential and mechanisms of action. Front. Pharmacol. 2022, 13, 945627. [Google Scholar] [CrossRef]
- Li, K.; Pi, M.S.; Li, X.T. The inhibitory effects of levo-tetrahydropalmatine on rat Kv1.5 channels expressed in HEK293 cells. Eur. J. Pharmacol. 2017, 809, 105–110. [Google Scholar] [CrossRef]
- Liu, T.T.; Qu, Z.W.; Qiu, C.Y.; Qiu, F.; Ren, C.; Gan, X.; Peng, F.; Hu, W.P. Inhibition of acid-sensing ion channels by levo-tetrahydropalmatine in rat dorsal root ganglion neurons. J. Neurosci. Res. 2015, 93, 333–339. [Google Scholar] [CrossRef]
- Meng, H.X.; Wang, B.; Liu, J.X. Effect of salvianolic acid B and tetrahydropalmatine on the L-type calcium channel of rat ventricular myocytes. Zhongguo Zhong Xi Yi Jie He Za Zhi 2011, 31, 1514–1517. (In Chinese) [Google Scholar]
- Wu, C.; Yang, K.; Liu, Q.; Wakui, M.; Jin, G.Z.; Zhen, X.; Wu, J. Tetrahydroberberine blocks ATP-sensitive potassium channels in dopamine neurons acutely-dissociated from rat substantia nigra pars compacta. Neuropharmacology 2010, 59, 567–572. [Google Scholar] [CrossRef]
- Wang, Y.J.; Dai, G.L.; Chen, P.Y.; Hang, H.X.; Bian, X.F.; Chen, Y.J.; Ju, W.Z. Tetrahydropalmatine acts on α7nAChR to regulate inflammation and polarization of BV2 microglia. Zhongguo Zhong Yao Za Zhi 2025, 50, 3117–3126. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.B.; Ma, Z.G.; Zheng, C.; Ma, X.K.; Taylor, D.H.; Gao, M.; Lukas, R.J.; Wu, J. Levo-tetrahydropalmatine inhibits α4β2 nicotinic receptor response to nicotine in cultured SH-EP1 cells. Acta Pharmacol. Sin. 2022, 43, 889–896. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhao, Z.; Hu, X.; Li, Y. NMDA Receptor Modulation in COVID-19-Associated acute respiratory syndrome in both in silico and in vitro approach. Appl. Biochem. Biotechnol. 2024, 196, 5354–5372. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Z.; Zhang, Y.; Ren, J.Y.; Zhou, Z.N. Effects of berberine of L- and T-type calcium channels in guinea pig ventricular myocytes. Zhongguo Yao Li Xue Bao 1997, 18, 515–518. [Google Scholar]
- Zhao, M.M.; Lu, J.; Li, S.; Wang, H.; Cao, X.; Li, Q.; Shi, T.T.; Matsunaga, K.; Chen, C.; Huang, H.; et al. Berberine is an insulin secretagogue targeting the KCNH6 potassium channel. Nat. Commun. 2021, 12, 5616. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, K.; Shi, Y.; Feng, L.; Lv, L.; Li, B. Mechanism and pharmacological rescue of berberine-induced hERG channel deficiency. Drug Des. Devel. Ther. 2015, 9, 5737–5747. [Google Scholar] [CrossRef]
- Alzamora, R.; O’Mahony, F.; Ko, W.H.; Yip, T.W.; Carter, D.; Irnaten, M.; Harvey, B.J. Berberine reduces cAMP-induced chloride secretion in T84 human colonic carcinoma cells through inhibition of basolateral KCNQ1 channels. Front. Physiol. 2011, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Yu, C.H.; Fu, Y.; Li, Q.; Sun, Y.Q. Berberine elicits anti-arrhythmic effects via IK1/Kir2.1 in the rat type 2 diabetic myocardial infarction model. Phytother. Res. 2011, 25, 33–37. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, H.Y.; Zhao, G.; Fu, L.Y.; Cheng, L.; Chen, J.G.; Yao, W.X. Inhibitory effects of berberine on ion channels of rat hepatocytes. World J. Gastroenterol. 2004, 10, 2842–2845. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zheng, Y.M.; Zhou, X.B. Inhibitory effects of berberine on ATP-sensitive K+ channels in cardiac myocytes. Eur. J. Pharmacol. 1996, 316, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bansal, Y.; Sodhi, R.K.; Singh, D.P.; Bishnoi, M.; Kondepudi, K.K.; Medhi, B.; Kuhad, A. Berberine attenuated olanzapine-induced metabolic alterations in mice: Targeting transient receptor potential vanilloid type 1 and 3 channels. Life Sci. 2020, 247, 117442. [Google Scholar] [CrossRef]
- Wang, J.; Guo, T.; Peng, Q.S.; Yue, S.W.; Wang, S.X. Berberine via suppression of transient receptor potential vanilloid 4 channel improves vascular stiffness in mice. J. Cell Mol. Med. 2015, 19, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Li, Y.; Li, H.R.; Ma, W.B.; Pan, T.C.; Zhu, L.Y.; Ye, W.C.; Wang, L.W.; Chen, L.X. Berberine activates volume-sensitive chloride channel in human colorectal carcinoma cells. Sheng Li Xue Bao 2011, 63, 517–524. [Google Scholar]
- Xia, Y.; Wang, X.; Lin, S.; Dong, T.T.X.; Tsim, K.W.K. Berberine and palmatine, acting as allosteric potential ligands of α7 nAChR, synergistically regulate inflammation and phagocytosis of microglial cells. FASEB J. 2024, 38, e70094. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Liu, L.; Shi, Y.; Hong, Y.; Xu, W.; Xu, H.; Feng, J.; Xie, M.; Li, Y.; et al. The Therapeutic Potential of Four Main Compounds of Zanthoxylum nitidum (Roxb.) DC: A comprehensive study on biological processes, anti-inflammatory effects, and myocardial toxicity. Pharmaceuticals 2024, 17, 524. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, C.; Wang, F.; Li, L.; Guo, Y.; Zhou, Q.; Xiong, L. Coumarins with Different Substituents from Leonurus japonicus Have Opposite Effects on Uterine Smooth Muscle. Int. J. Mol. Sci. 2024, 25, 10162. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, G.; Sun, X.; Wang, K. Inhibition of the warm temperature-activated Ca2+-permeable transient receptor potential vanilloid TRPV3 channel attenuates atopic dermatitis. Mol. Pharmacol. 2019, 96, 393–400. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, X.; Wei, N.; Wang, K. Inhibition of cutaneous heat-sensitive Ca2+ -permeable transient receptor potential vanilloid 3 channels alleviates UVB-induced skin lesions in mice. FASEB J. 2023, 37, e23309. [Google Scholar] [CrossRef]
- Leung, Y.M.; Kuo, Y.H.; Chao, C.C.; Tsou, Y.H.; Chou, C.H.; Lin, C.H.; Wong, K.L. Osthol is a use-dependent blocker of voltage-gated Na+ channels in mouse neuroblastoma N2A cells. Planta Med. 2010, 76, 34–40. [Google Scholar] [CrossRef]
- Torres, K.V.; Pantke, S.; Rudolf, D.; Eberhardt, M.M.; Leffler, A. The coumarin osthole is a non-electrophilic agonist of TRPA1. Neurosci Lett. 2022, 789, 136878. [Google Scholar] [CrossRef]
- Singhuber, J.; Baburin, I.; Ecker, G.F.; Kopp, B.; Hering, S. Insights into structure-activity relationship of GABAA receptor modulating coumarins and furanocoumarins. Eur. J. Pharmacol. 2011, 668, 57–64. [Google Scholar] [CrossRef]
- Wang, S.J.; Lin, T.Y.; Lu, C.W.; Huang, W.J. Osthole and imperatorin, the active constituents of Cnidium monnieri (L.) Cusson, facilitate glutamate release from rat hippocampal nerve terminals. Neurochem. Int. 2008, 53, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Fusi, F.; Sgaragli, G.; Hale, M.; Cuong, N.M.; Saponara, S. Mechanism of osthole inhibition of vascular Ca(v)1.2 current. Eur. J. Pharmacol. 2012, 680, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.N.; Na, W.L.; Liu, X.; Hou, S.G.; Lin, S.; Yang, H.; Ma, T.H. Identification of natural coumarin compounds that rescue defective DeltaF508-CFTR chloride channel gating. Clin. Exp. Pharmacol. Physiol. 2008, 35, 878–883. [Google Scholar] [CrossRef]
- Wu, S.N.; Lo, Y.K.; Chen, C.C.; Li, H.F.; Chiang, H.T. Inhibitory effect of the plant-extract osthole on L-type calcium current in NG108-15 neuronal cells. Biochem. Pharmacol. 2002, 63, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Errico, S.; Fani, G.; Gennari, M.; Mastricci, A.; Neri, L.; Odino, D.; Canale, C.; Relini, A.; Vendruscolo, M.; Mannini, B.; et al. Berberine mitigates neurotoxicity of misfolded protein oligomers by interacting with the cell membrane and subsequent internalization, without altering their structure. Int. J. Biol. Macromol. 2025, 322 Pt 2, 146398. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, N.; Cao, Y.; Costa, F.; Galeazzi, R.; Giacomello, A.; Hackbarth, S.; Wacker, M.G. Mapping berberine distribution in liposomes: The role of drug-phospholipid interactions in localization and release. Int. J. Pharm. 2025, 684, 126112. [Google Scholar] [CrossRef] [PubMed]
- Gawrisch, K.; Ruston, D.; Zimmerberg, J.; Parsegian, V.A.; Rand, R.P.; Fuller, N. Membrane dipole potentials, hydration forces, and the ordering of water at membrane surfaces. Biophys. J. 1992, 61, 1213–1223. [Google Scholar] [CrossRef]
- Ermakov, Y.A.; Nesterenko, A.M. Boundary potential of lipid bilayers: Methods and interpretations. J. Phys. Confer. Series 2017, 780, 012002. [Google Scholar] [CrossRef]
- Sommer, A.; Paltauf, F.; Hermetter, A. Dipolar solvent relaxation on a nanosecond time scale in ether phospholipid membranes as determined by multifrequency phase and modulation fluorometry. Biochemistry 1990, 29, 11134–11140. [Google Scholar] [CrossRef]
- Kruczek, J.; Saunders, M.; Khosla, M.; Tu, Y.; Pandit, S.A. Molecular dynamics simulations of ether- and ester-linked phospholipids. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2297–2307. [Google Scholar] [CrossRef]
- Estronca, L.M.; Moreno, M.J.; Abreu, M.S.; Melo, E.; Vaz, W.L. Solubility of amphiphiles in membranes: Influence of phase properties and amphiphile head group. Biochem. Biophys. Res. Commun. 2002, 296, 596–603. [Google Scholar] [CrossRef]
- Hauser, H.; Paltauf, F.; Shipley, G.G. Structure and thermotropic behavior of phosphatidylserine bilayer membranes. Biochemistry 1982, 21, 1061–1067. [Google Scholar] [CrossRef]
- Higashino, Y.; Matsui, A.; Ohki, K. Membrane fusion between liposomes composed of acidic phospholipids and neutral phospholipids induced by melittin: A differential scanning calorimetric study. J. Biochem. 2001, 130, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Ojogun, V.; Vyas, S.M.; Lehmler, H.J.; Knutson, B.L. Partitioning of homologous nicotinic acid ester prodrugs (nicotinates) into dipalmitoylphosphatidylcholine (DPPC) membrane bilayers. Colloids Surf. B Biointerfaces 2010, 78, 75–84. [Google Scholar] [CrossRef]
- Gobas, F.A.; Lahittete, J.M.; Garofalo, G.; Shiu, W.Y.; Mackay, D. A novel method for measuring membrane-water partition coefficients of hydrophobic organic chemicals: Comparison with 1-octanol-water partitioning. J. Pharm. Sci. 1988, 77, 265–272. [Google Scholar] [CrossRef]
- Montal, M.; Mueller, P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.S.; Finkelstein, A.; Katz, I.; Cass, A. Effect of phloretin on the permeability of thin lipid membranes. J. Gen. Physiol. 1976, 67, 749–771. [Google Scholar] [CrossRef] [PubMed]
| No. | Compound | Chemical Structure | Original Resource | Biological Activity, [References] |
|---|---|---|---|---|
| 1 | rotundine | 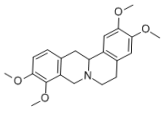 | Stephania glabra (Roxb.) Miers | sedative, analgesic [2,3,4] |
| 2 | berberine | 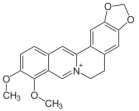 | Coscinium fenestratum | antibacterial, anti-inflammatory [7,8,9,10] |
| 3 | nitidine | 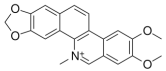 | Zanthoxylum myriacanthum | cytotoxic activity [11,12,13,14,15] |
| 4 | ethyl 4-methoxy cinnamate | 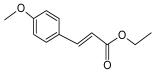 | Kaempferia galanga L. | anti-inflammatory, analgesic [16,17,18] |
| 5 | osthole | 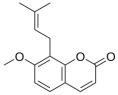 | Cnidium monnieri | anti-inflammatory [19,20,21] |
| Agent | µ $, D | LogP & | ∆φb (max), mV | |
|---|---|---|---|---|
| DOPC | DOPS | |||
| rotundine | 2.98 | 3.32 | −2 ± 2 | −1 ± 2 |
| berberine | 3.35 | −0.99 | −2 ± 2 | 5 ± 3 |
| nitidine | 2.09 | −0.88 | −7 ± 3 | −2 ± 2 |
| ethyl 4-methoxy cinnamate | 2.39 | 2.65 | −16 ± 3 | −21 ± 3 |
| osthole | 5.81 | 3.87 | −36 ± 2 | −24 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efimova, S.S.; Zlodeeva, P.D.; Pham, Q.M.; Trinh, H.T.T.; Le, H.M.; Nguyen, V.T.H.; Pham, L.Q.; Ostroumova, O.S. The Effects of Several Natural Protoberberine Alkaloids and Cinnamic Acid Derivatives Used for Traditional Medicine on the Membrane Boundary Potential and Lipid Packing Stress. Int. J. Mol. Sci. 2025, 26, 11237. https://doi.org/10.3390/ijms262211237
Efimova SS, Zlodeeva PD, Pham QM, Trinh HTT, Le HM, Nguyen VTH, Pham LQ, Ostroumova OS. The Effects of Several Natural Protoberberine Alkaloids and Cinnamic Acid Derivatives Used for Traditional Medicine on the Membrane Boundary Potential and Lipid Packing Stress. International Journal of Molecular Sciences. 2025; 26(22):11237. https://doi.org/10.3390/ijms262211237
Chicago/Turabian StyleEfimova, Svetlana S., Polina D. Zlodeeva, Quan Minh Pham, Huong Thi Thu Trinh, Ha Minh Le, Van Thị Hong Nguyen, Long Quoc Pham, and Olga S. Ostroumova. 2025. "The Effects of Several Natural Protoberberine Alkaloids and Cinnamic Acid Derivatives Used for Traditional Medicine on the Membrane Boundary Potential and Lipid Packing Stress" International Journal of Molecular Sciences 26, no. 22: 11237. https://doi.org/10.3390/ijms262211237
APA StyleEfimova, S. S., Zlodeeva, P. D., Pham, Q. M., Trinh, H. T. T., Le, H. M., Nguyen, V. T. H., Pham, L. Q., & Ostroumova, O. S. (2025). The Effects of Several Natural Protoberberine Alkaloids and Cinnamic Acid Derivatives Used for Traditional Medicine on the Membrane Boundary Potential and Lipid Packing Stress. International Journal of Molecular Sciences, 26(22), 11237. https://doi.org/10.3390/ijms262211237








