Wine Industry Waste as a Source of Bioactive Compounds for Drug Use
Abstract
1. Introduction
2. Winery Industries
2.1. Historical Context
2.2. Winery Procedure
2.2.1. Destemming
2.2.2. Crushing
2.2.3. Maceration
2.2.4. Pressing
2.2.5. Must Preparation
2.2.6. Alcoholic Fermentation
2.2.7. Malolactic Fermentation
2.2.8. Clarification
2.2.9. Maturation
2.2.10. Filtration
2.2.11. Stabilization
2.2.12. Bottling
2.3. Actual Wine Context
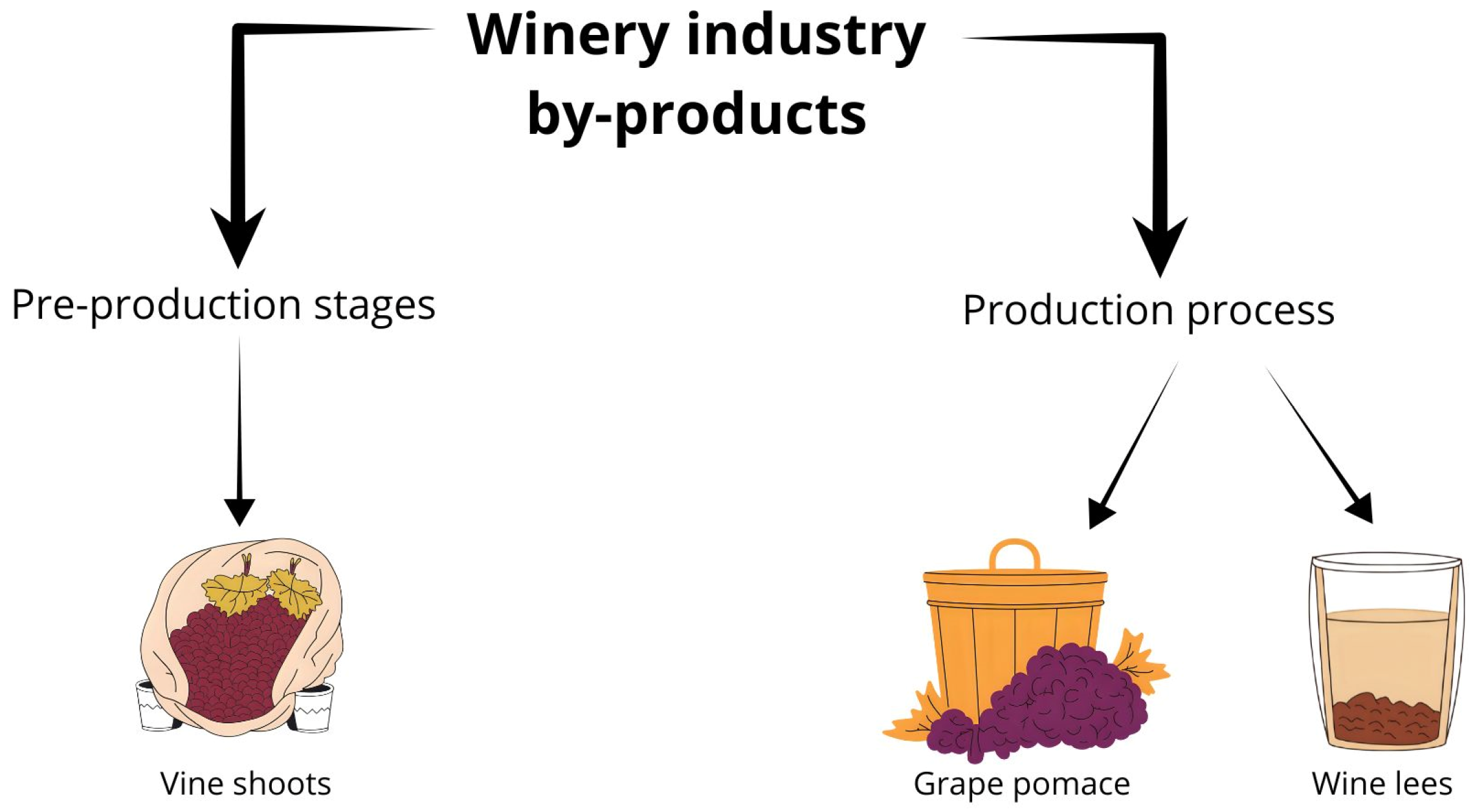
3. Winery Industries By-Products
3.1. Vine Shoots
3.2. Grape Pomace
3.3. Wine Lees
3.4. Winery By-Products and Circular Economy
4. Natural Drugs from Wine Industry Waste Through Fermentation
4.1. Polyphenols
4.1.1. Flavonoids
- Anthocyanins
- Catechins
- Tannins
4.1.2. Resveratrol
4.1.3. Phenolic Acids
4.2. Alcohols
4.3. Enzymes
5. Winery Residues in Pharmaceutical Industry Potential Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Organisation of Vine and Wine. State of the World Vine and Wine Sector 2023; International Organisation of Vine and Wine: Dijon, France, 2024. [Google Scholar]
- Food and Agriculture Organization. FAO STAT. 2025. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 8 May 2025).
- Constantin, O.E.; Stoica, F.; Rațu, R.N.; Stănciuc, N.; Bahrim, G.E.; Râpeanu, G. Bioactive Components, Applications, Extractions, and Health Benefits of Winery By-Products from a Circular Bioeconomy Perspective: A Review. Antioxidants 2024, 13, 100. [Google Scholar] [CrossRef]
- Siller-Sánchez, A.; Luna-Sánchez, K.A.; Bautista-Hernández, I.; Chávez-González, M.L. Use of Grape Pomace from the Wine Industry for the Extraction of Valuable Compounds with Potential Use in the Food Industry. Curr. Food Sci. Technol. Rep. 2024, 2, 7–16. [Google Scholar] [CrossRef]
- Melo, F.D.O.; Ferreira, V.C.; Barbero, G.F.; Carrera, C.; Ferreira, E.D.S.; Umsza-Guez, M.A. Extraction of Bioactive Compounds from Wine Lees: A Systematic and Bibliometric Review. Foods 2024, 13, 2060. [Google Scholar] [CrossRef]
- Coghlan, C.; Proulx, P.; Salazar, K. A Food-Circular Economy-Women Nexus: Lessons from Guelph-Wellington. Sustainability 2022, 14, 192. [Google Scholar] [CrossRef]
- Ioannidou, S.P.; Margellou, A.G.; Petala, M.D.; Triantafyllidis, K.S. Pretreatment/Fractionation and Characterization of Winery Waste Streams within an Integrated Biorefinery Concept. Sustain. Chem. Pharm. 2022, 27, 100670. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Iyyappan, J.; Jayamuthunagai, J.; Kumar, R.P.; Sirohi, R.; Gnansounou, E.; Pandey, A. Critical Review on Bioconversion of Winery Wastes into Value-Added Products. Ind. Crops Prod. 2020, 158, 112954. [Google Scholar] [CrossRef]
- Yepes-Betancur, D.P.; Márquez-Cardozo, C.J.; Cadena-Chamorro, E.M.; Martinez-Saldarriaga, J.; Torres-León, C.; Ascacio-Valdes, A.; Aguilar, C.N. Solid-State Fermentation—Assisted Extraction of Bioactive Compounds from Hass Avocado Seeds. Food Bioprod. Process. 2021, 126, 155–163. [Google Scholar] [CrossRef]
- Lemes, A.C.; Egea, M.B.; de Oliveira Filho, J.G.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological Approaches for Extraction of Bioactive Compounds from Agro-Industrial By-Products: A Review. Front. Bioeng. Biotechnol. 2022, 9, 802543. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Wine Science: Principles and Applications, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Jackson, R.S. (Ed.) Introduction. In Food Science and Technology; Academic Press: New York, NY, USA, 2020; pp. 1–20. [Google Scholar] [CrossRef][Green Version]
- Norrie, P.A. The History of Wine as a Medicine. In Wine; CRC Press: Boca Raton, FL, USA, 2002; pp. 21–55. [Google Scholar][Green Version]
- Harutyunyan, M.; Malfeito-Ferreira, M. Historical and Heritage Sustainability for the Revival of Ancient Wine-Making Techniques and Wine Styles. Beverages 2022, 8, 10. [Google Scholar] [CrossRef]
- Pagnoux, C.; Bouby, L.; Valamoti, S.M.; Bonhomme, V.; Ivorra, S.; Gkatzogia, E.; Karathanou, A.; Kotsachristou, D.; Kroll, H.; Terral, J.F. Local Domestication or Diffusion? Insights into Viticulture in Greece from Neolithic to Archaic Times, Using Geometric Morphometric Analyses of Archaeological Grape Seeds. J. Archaeol. Sci. 2021, 125, 105263. [Google Scholar] [CrossRef]
- Joshi, V.K.; Panesar, P.S.; Rana, V.S.; Kaur, S. Science and Technology of Fruit Wines: An Overview; Academic Press: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Gründemann, D.; Harlfinger, S.; Golz, S.; Geerts, A.; Lazar, A.; Berkels, R.; Jung, N.; Rubbert, A.; Schömig, E. Discovery of the Ergothioneine Transporter. Proc. Natl. Acad. Sci. USA 2005, 102, 5256–5261. [Google Scholar] [CrossRef]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Wine as a Biological Fluid: History, Production, and Role in Disease Prevention. J. Clin. Lab. Anal. 1997, 11, 287–313. [Google Scholar] [CrossRef]
- Suriano, S.; Alba, V.; Di Gennaro, D.; Basile, T.; Tamborra, M.; Tarricone, L. Major Phenolic and Volatile Compounds and Their Influence on Sensorial Aspects in Stem-Contact Fermentation Winemaking of Primitivo Red Wines. J. Food Sci. Technol. 2016, 53, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Wimalasiri, P.M.; Olejar, K.J.; Harrison, R.; Hider, R.; Tian, B. Whole Bunch Fermentation and the Use of Grape Stems: Effect on Phenolic and Volatile Aroma Composition of Vitis vinifera Cv. Pinot Noir Wine. Aust. J. Grape Wine Res. 2022, 28, 395–406. [Google Scholar] [CrossRef]
- Luciano, A.; Picariello, L.; Forino, M.; Moio, L.; Gambuti, A. Anthocyanins and Nucleation Seeds Are Key Factors Affecting Quercetin Precipitation in Red Wines. J. Sci. Food Agric. 2024, 104, 5163–5175. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. (Ed.) Fermentation. In Food Science and Technology, 5th ed.; Academic Press: New York, NY, USA, 2020; pp. 461–572. [Google Scholar] [CrossRef]
- Grainger, K.; Tattersall, H. Wine Production: Vine to Bottle; Blackwell Publishing Ltd.: Oxford, UK, 2005. [Google Scholar]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Rinaldi, A.; Louazil, P.; Iturmendi, N.; Moine, V.; Moio, L. Effect of Marc Pressing and Geographical Area on Sangiovese Wine Quality. LWT 2020, 118, 108728. [Google Scholar] [CrossRef]
- Shanshiashvili, G.; Baviera, M.; Fracassetti, D. Exploring Grape Pressing for Sparkling Wine Production: A Comprehensive Literature Review on Physicochemical Parameters and Technological Applications. Appl. Food Res. 2024, 4, 100454. [Google Scholar] [CrossRef]
- Kelanne, N. Effects of Wine Yeast to Chemical Composition of Black Currant Wine—Anthocyanins and Alcohols. Ph.D. Thesis, Technology University of Turku, Turku, Finland, 2016. [Google Scholar]
- Volschenk, H.; Van Vuuren, H.J.J.; Viljoen-Bloom, M. Malic Acid in Wine: Origin, Function and Metabolism During Vinification. S. Afr. J. Enol. Vitic. 2006, 27, 123–136. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Stasinou, V.; Tzamourani, A.; Kotseridis, Y.; Dimopoulou, M. Malolactic Fermentation—Theoretical Advances and Practical Considerations. Fermentation 2022, 8, 521. [Google Scholar] [CrossRef]
- Balmaseda, A.; Rozès, N.; Leal, M.Á.; Bordons, A.; Reguant, C. Impact of Changes in Wine Composition Produced by Non-Saccharomyces on Malolactic Fermentation. Int. J. Food Microbiol. 2021, 337, 108954. [Google Scholar] [CrossRef]
- Zamora, F. Barrel Aging; Types of Wood. In Red Wine Technology; Academic Press: New York, NY, USA, 2019; pp. 125–147. [Google Scholar]
- Zhang, D.; Wei, Z.; Han, Y.; Duan, Y.; Shi, B.; Ma, W. A Review on Wine Flavour Profiles Altered by Bottle Aging. Molecules 2023, 28, 6522. [Google Scholar] [CrossRef]
- Echave, J.; Barral, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Bottle Aging and Storage of Wines: A Review. Molecules 2021, 26, 713. [Google Scholar] [CrossRef]
- Khan, N.; Fahad, S.; Naushad, M.; Faisal, S. Grape Production Critical Review in the World. 2020. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3595842 (accessed on 1 June 2025).
- Ohana-Levi, N.; Netzer, Y. Long-Term Trends of Global Wine Market. Agriculture 2023, 13, 224. [Google Scholar] [CrossRef]
- Meraz-Ruiz, L.; Olague, J.T.; Flores-Villanueva, C.A.; Perez-Cruz, O.A. The Role of Innovation and Reference Groups on Emotions and Purchasing Decision on Consumers of Mexican Wine. J. Wine Res. 2023, 34, 1–19. [Google Scholar] [CrossRef]
- Reyes Martin, C. El Mercado del Vino en Mexico; ICEX España Exportación e Inversiones: Mexico City, Mexico, 2022. [Google Scholar]
- STATISTA. World Wine Consume. Available online: https://www.statista.com/outlook/cmo/alcoholic-drinks/wine/worldwide (accessed on 20 October 2024).
- Cebrián-Tarancón, C.; Fernández-Roldán, F.; Sánchez-Gómez, R.; Alonso, G.L.; Salinas, M.R. Pruned Vine-Shoots as a New Enological Additive to Differentiate the Chemical Profile of Wines. Food Res. Int. 2022, 156, 111195. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.; Mota, A.C.; Pereira, A.S.; Fernandes, A.M.; Lopes, M.; Belo, I. Rice Husk, Brewer’s Spent Grain, and Vine Shoot Trimmings as Raw Materials for Sustainable Enzyme Production. Materials 2024, 17, 935. [Google Scholar] [CrossRef] [PubMed]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive Compounds from Vine Shoots, Grape Stalks, and Wine Lees: Their Potential Use in Agro-Food Chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef]
- Duarte, H.; Aliaño-González, M.J.; Cantos-Villar, E.; Faleiro, L.; Romano, A.; Medronho, B. Sustainable Extraction of Polyphenols from Vine Shoots Using Deep Eutectic Solvents: Influence of the Solvent, Vitis Sp., and Extraction Technique. Talanta 2024, 267, 125135. [Google Scholar] [CrossRef]
- Noviello, M.; Caputi, A.F.; Squeo, G.; Paradiso, V.M.; Gambacorta, G.; Caponio, F. Vine Shoots as a Source of Trans-Resveratrol and ε-Viniferin: A Study of 23 Italian Varieties. Foods 2022, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Kokkinomagoulos, E.; Kandylis, P. Grape Pomace, an Undervalued by-Product: Industrial Reutilization Within a Circular Economy Vision. Rev. Environ. Sci. Biotechnol. 2023, 22, 739–773. [Google Scholar] [CrossRef]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable Green Processing of Grape Pomace for the Production of Value-Added Products: An Overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and Future Strategies for Wine Yeast Lees Valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef]
- de Andrade Bulos, R.B.; da Gama Paz, F.; Machado, C.G.; Tavares, P.P.L.G.; de Souza, C.O.; Umsza-Guez, M.A. Scientific and Technological Research on the Use of Wine Lees. Food Prod. Process. Nutr. 2023, 5, 25. [Google Scholar] [CrossRef]
- Gabur, G.-D.; Teodosiu, C.; Fighir, D.; Cotea, V.V.; Gabur, I. From Waste to Value in Circular Economy: Valorizing Grape Pomace Waste Through Vermicomposting. Agriculture 2024, 14, 1529. [Google Scholar] [CrossRef]
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Sisti, L.; Marchese, P.; Celli, A.; Tassoni, A. From Winery Waste to Bioactive Compounds and New Polymeric Biocomposites: A Contribution to the Circular Economy Concept. J. Adv. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Amaya-Chantaca, D.; Flores-Gallegos, A.C.; Iliná, A.; Aguilar, C.N.; Sepúlveda-Torre, L.; Ascacio-Vadlés, J.A.; Chávez-González, M.L. Comparative Extraction Study of Grape Pomace Bioactive Compounds by Submerged and Solid-state Fermentation. J. Chem. Technol. Biotechnol. 2022, 97, 1494–1505. [Google Scholar] [CrossRef]
- Saldaña-Mendoza, S.A.; Chavez-González, M.L.; Aguilar Gonzalez, C.N. Protocol for the Production of Trichoderma Spores for Use as a Biological Control Agent Through the Revalorization of Agro-Industrial Waste. In Food Waste Conversion (Methods and Protocols in Food Science); Aguilar-Gonzalez, C.N., Gómez-García, R., Kuddus, M., Eds.; Humana: New York, NY, USA, 2023; Volume 1, pp. 169–176. [Google Scholar] [CrossRef]
- Saldaña-Mendoza, S.A.; Pacios-Michelena, S.; Palacios-Ponce, A.S.; Chávez-González, M.L.; Aguilar, C.N. Trichoderma as a Biological Control Agent: Mechanisms of Action, Benefits for Crops and Development of Formulations. World J. Microbiol. Biotechnol. 2023, 39, 269. [Google Scholar] [CrossRef] [PubMed]
- Paz-Arteaga, S.L.; Cadena-Chamorro, E.; Goméz-García, R.; Serna-Cock, L.; Aguilar, C.N.; Torres-León, C. Unraveling the Valorization Potential of Pineapple Waste to Obtain Value-Added Products towards a Sustainable Circular Bioeconomy. Sustainability 2024, 16, 7236. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.; Serna-Cock, L.; dos Santos Correia, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-State Fermentation with Aspergillus Niger to Enhance the Phenolic Contents and Antioxidative Activity of Mexican Mango Seed: A Promising Source of Natural Antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- Saldaña-Mendoza, S.A.; Martínez-Hernandez, J.L.; Rodríguez-Herrera, R.; Palacios-Ponce, A.S.; Sugathan, S.; Aguilar, C.N. Use of Coffee Waste for the Production of Biofuels. Environ. Qual. Manag. 2022, 32, 463–471. [Google Scholar] [CrossRef]
- Saldaña-Mendoza, S.A.; Ascacio-Valdés, J.A.; Palacios-Ponce, A.S.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Ruiz, H.A.; Martínez-Hernandez, J.L.; Sugathan, S.; Aguilar, C.N. Use of Wastes from the Tea and Coffee Industries for the Production of Cellulases Using Fungi Isolated from the Western Ghats of India. Syst. Microbiol. Biomanuf. 2021, 1, 33–41. [Google Scholar] [CrossRef]
- Siller-Sánchez, A.; Aguilar, C.N.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Kumar Verma, D.; Aguilar-González, M. Solid-State Fermentation-Assisted Extraction of Flavonoids from Grape Pomace Using Co-Cultures. Processes 2024, 12, 2027. [Google Scholar] [CrossRef]
- Méndez-Carmona, J.Y.; Ramírez-Guzman, K.N.; Ascacio-Valdes, J.A.; Sepúlveda, L.; Sandoval-Cortés, J.; Aguilar, C.N. Evaluation of the Pretreatment of Tomato Waste as Support for the Recovery of Carotenoids Through Solid-State Fermentation Assisted Extraction (SSFAE). Waste Biomass Valorization 2024, 15, 1701–1709. [Google Scholar] [CrossRef]
- Aguilar-Zárate, P.; Gutiérrez-Sánchez, G.; Michel, M.R.; Bergmann, C.W.; Buenrostro-Figueroa, J.J.; Ascacio-Valdés, J.A.; Contreras-Esquivel, J.C.; Aguilar, C.N. Production of a Fungal Punicalagin-Degrading Enzyme by Solid-State Fermentation: Studies of Purification and Characterization. Foods 2023, 12, 903. [Google Scholar] [CrossRef]
- Cerda-Cejudo, N.D.; Buenrostro-Figueroa, J.J.; Sepúlveda, L.; Estrada-Gil, L.E.; Torres-León, C.; Chávez-González, M.L.; Aguilar, C.N.; Ascacio-Valdés, J.A. Enhancing the Release of Ellagic Acid from Mexican Rambutan Peel Using Solid-State Fermentation. Biomass 2024, 4, 1005–1016. [Google Scholar] [CrossRef]
- Saldaña-Mendoza, S.A.; Palacios-Ponce, A.S.; Ruiz, H.A.; Ascacio-Valdés, J.A.; Aguilar, C.N. Revalorization of Green Tea Waste through the Production of Cellulases by Solid-State Fermentation Using a Aspergillus Niger 28A. Biomass Convers. Biorefin. 2024, 14, 16711–16724. [Google Scholar] [CrossRef]
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Valorization of Agro-Industrial Wastes to Produce Hydrolytic Enzymes by Fungal Solid-State Fermentation. Waste Manag. Res. 2019, 37, 149–156. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Capeplucci, G.; Miraldi, E. Polyphenols: From Theory to Practice Alberto. Foods 2021, 10, 2595. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef]
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and Purification of Anthocyanins: A Review. J. Agric. Food Res. 2022, 8, 100306. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Ghasemlou, M.; Ariffin, F.; Singh, Z.; Al-Hassan, A.A. Natural Anthocyanins: Sources, Extraction, Characterization, and Suitability for Smart Packaging. Food Packag. Shelf Life 2022, 33, 100872. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Solverson, P. Anthocyanin Bioactivity in Obesity and Diabetes: The Essential Role of Glucose Transporters in the Gut and Periphery. Cells 2020, 9, 2515. [Google Scholar] [CrossRef]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Rev. Int. 2023, 39, 4581–4609. [Google Scholar] [CrossRef]
- Li, P.; Feng, D.; Yang, D.; Li, X.; Sun, J.; Wang, G.; Tian, L.; Jiang, X.; Bai, W. Protective Effects of Anthocyanins on Neurodegenerative Diseases. Trends Food Sci. Technol. 2021, 117, 205–217. [Google Scholar] [CrossRef]
- Overall, J.; Bonney, S.A.; Wilson, M.; Beermann, A.; Grace, M.H.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Metabolic Effects of Berries with Structurally Diverse Anthocyanins. Int. J. Mol. Sci. 2017, 18, 422. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Braga, A.R.C.; de Oliveira, B.R.; Gomes, F.P.; Moreira, V.L.; Pereira, V.A.C.; Egea, M.B. The Potential of Anthocyanins in Smart, Active, and Bioactive Eco-Friendly Polymer-Based Films: A Review. Food Res. Int. 2021, 142, 110202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Zambrano, C.; Kotogán, A.; Bencsik, O.; Papp, T.; Vágvölgyi, C.; Mondal, K.C.; Krisch, J.; Takó, M. Mobilization of Phenolic Antioxidants from Grape, Apple and Pitahaya Residues via Solid State Fungal Fermentation and Carbohydrase Treatment. LWT 2018, 89, 457–465. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and Grape Polyphenols—A Chemical Perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Yang, C.; Han, Y.; Tian, X.; Sajid, M.; Mehmood, S.; Wang, H.; Li, H. Phenolic Composition of Grape Pomace and Its Metabolism. Crit. Rev. Food Sci. Nutr. 2024, 64, 4865–4881. [Google Scholar] [CrossRef]
- Chedea, V.S.; Tomoiagǎ, L.L.; Macovei, Ş.O.; Mǎgureanu, D.C.; Iliescu, M.L.; Bocsan, I.C.; Buzoianu, A.D.; Voşloban, C.M.; Pop, R.M. Antioxidant/Pro-Oxidant Actions of Polyphenols from Grapevine and Wine By-Products-Base for Complementary Therapy in Ischemic Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 750508. [Google Scholar] [CrossRef]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Rezaei-Tavirani, H.A.M. Resveratrol: A Miraculous Natural Compound for Diseases Treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Gao, M.; Cheng, Y.; Yang, B.; Kuang, H.; Wang, Z.; Yi, S.; Wang, B.; Fu, Y. Surfactant-Assisted and Ionic Liquid Aqueous System Pretreatment for Biocatalysis of Resveratrol from Grape Seed Residue Using an Immobilized Microbial Consortia. J. Food Process Preserv. 2021, 45, e15279. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, D.; Zhang, J.; Shen, J.; Cao, J.; Gu, H.; Cui, M.; He, L.; Chen, G.; Liu, S.; et al. Improving Soluble Phenolic Profile and Antioxidant Activity of Grape Pomace Seeds Through Fungal Solid-State Fermentation. Foods 2024, 13, 1158. [Google Scholar] [CrossRef]
- Arias, A.; Costa, C.E.; Moreira, M.T.; Feijoo, G.; Domingues, L. Environmental and Techno-Economic Assessment on the Valorization of Vine-Side Streams to Produce Resveratrol. J. Clean. Prod. 2023, 429, 139622. [Google Scholar] [CrossRef]
- Rompkovksi, C.; Agustini, B.C.; Deffert, F.; Stadtlober, M.G.A.; Brand, D.; da Silva, G.A.; Bonfim, T.M.B. Microbial Dynamics in Industrial-Scale Wine Fermentation Employing Hanseniaspora Uvarum β-Glucosidase-Producer Strain. J. Food Sci. Technol. 2022, 59, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Karpe, A.V.; Beale, D.J.; Godhani, N.B.; Morrison, P.D.; Harding, I.H.; Palombo, E.A. Untargeted Metabolic Profiling of Winery-Derived Biomass Waste Degradation by Penicillium chrysogenum. J. Agric. Food Chem. 2015, 63, 10696–10704. [Google Scholar] [CrossRef]
- Salgado, J.M.; Rodríguez, N.; Cortés, S.; Domínguez, J.M. Improving Downstream Processes to Recover Tartaric Acid, Tartrate and Nutrients from Vinasses and Formulation of Inexpensive Fermentative Broths for Xylitol Production. J. Sci. Food Agric. 2010, 90, 2168–2177. [Google Scholar] [CrossRef]
- Gasmi Benahmed, A.; Gasmi, A.; Arshad, M.; Shanaida, M.; Lysiuk, R.; Peana, M.; Pshyk-Titko, I.; Adamiv, S.; Shanaida, Y.; Bjørklund, G.; et al. Health Benefits of Xylitol. Appl. Microbiol. Biotechnol. 2020, 104, 7225–7237. [Google Scholar] [CrossRef]
- Tomonobu, N.; Komalasari, N.L.G.Y.; Sumardika, I.W.; Jiang, F.; Chen, Y.; Yamamoto, K.I.; Kinoshita, R.; Murata, H.; Inoue, Y.; Sakaguchi, M. Xylitol Acts as an Anticancer Monosaccharide to Induce Selective Cancer Death via Regulation of the Glutathione Level. Chem. Biol. Interact. 2020, 324, 109085. [Google Scholar] [CrossRef] [PubMed]
- Baptista, S.L.; Romaní, A.; Cunha, J.T.; Domingues, L. Multi-Feedstock Biorefinery Concept: Valorization of Winery Wastes by Engineered Yeast. J. Environ. Manag. 2023, 326, 116623. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of Bioethanol—A Review of Factors Affecting Ethanol Yield. Fermentation 2021, 7, 268. [Google Scholar] [CrossRef]
- Mobin Siddique, M.B.; Khairuddin, N.; Ali, N.A.; Hassan, M.A.; Ahmed, J.; Kasem, S.; Tabassum, M.; Afrouzi, H.N. A Comprehensive Review on the Application of Bioethanol/Biodiesel in Direct Injection Engines and Consequential Environmental Impact. Clean. Eng. Technol. 2021, 3, 100092. [Google Scholar] [CrossRef]
- Garita-Cambronero, J.; Paniagua-García, A.I.; Hijosa-Valsero, M.; Díez-Antolínez, R. Biobutanol Production from Pruned Vine Shoots. Renew. Energy 2021, 177, 124–133. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chávez, D.W.H.; Oliveira, R.A.; Bon, E.P.S.; Terzi, S.C.; Souza, E.F.; Gottschalk, L.M.F.; Tonon, R.V. Use of Grape Pomace for the Production of Hydrolytic Enzymes by Solid-State Fermentation and Recovery of Its Bioactive Compounds. Food Res. Int. 2019, 120, 441–448. [Google Scholar] [CrossRef]
- Papadaki, A.; Kachrimanidou, V.; Papanikolaou, S.; Philippoussis, A.; Diamantopoulou, P. Upgrading Grape Pomace Through Pleurotus Spp. Cultivation for the Production of Enzymes and Fruiting Bodies. Microorganisms 2019, 7, 207. [Google Scholar] [CrossRef] [PubMed]
- Khatami, S.H.; Vakili, O.; Movahedpour, A.; Ghesmati, Z.; Ghasemi, H.; Taheri-Anganeh, M. Laccase: Various Types and Applications. Biotechnol. Appl. Biochem. 2022, 69, 2658–2672. [Google Scholar] [CrossRef]
- Filipe, D.; Fernandes, H.; Castro, C.; Peres, H.; Oliva-Teles, A.; Belo, I.; Salgado, J.M. Improved Lignocellulolytic Enzyme Production and Antioxidant Extraction Using Solid-State Fermentation of Olive Pomace Mixed with Winery Waste. Biofuels Bioprod. Biorefining 2020, 14, 78–91. [Google Scholar] [CrossRef]
- Hamrouni, R.; Regus, F.; Claeys-Bruno, M.; Farnet Da Silva, A.M.; Orsière, T.; Laffont-Schwob, I.; Boudenne, J.L.; Dupuy, N. Statistical Experimental Design as a New Approach to Optimize a Solid-State Fermentation Substrate for the Production of Spores and Bioactive Compounds from Trichoderma asperellum. J. Fungi 2023, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Verri, T.; Barca, A.; Gerardi, C.; Giovinazzo, G.; Carluccio, M.A. Grape Pomace Extract Attenuates Inflammatory Response in Intestinal Epithelial and Endothelial Cells: Potential Health-Promoting Properties in Bowel Inflammation. Nutrients 2022, 14, 1175. [Google Scholar] [CrossRef]
- Perdicaro, D.J.; Rodriguez Lanzi, C.; Fontana, A.R.; Antoniolli, A.; Piccoli, P.; Miatello, R.M.; Diez, E.R.; Vazquez Prieto, M.A. Grape Pomace Reduced Reperfusion Arrhythmias in Rats with a High-Fat-Fructose Diet. Food Funct. 2017, 8, 3501–3509. [Google Scholar] [CrossRef]
- Balea, Ş.S.; Pârvu, A.E.; Pop, N.; Marín, F.Z.; Andreicuț, A.; Pârvu, M. Phytochemical Profiling, Antioxidant and Cardioprotective Properties of Pinot Noir Cultivar Pomace Extracts. Farmacia 2018, 66, 432–441. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Silva, P. The Wine Industry By-Products: Applications for Food Industry and Health Benefits. Antioxidants 2022, 11, 2025. [Google Scholar] [CrossRef] [PubMed]

| Bioactive Compound | Uses | Substrate | Fermentative Process |
|---|---|---|---|
| Polyphenolic compounds: -Anthocyanins | Alleviating metabolic dysfunction induced by obesity and diabetes, anticancer activity [75] | Grape pomace [56] | Solid and liquid fermentation (Aspergillus niger GH1) [56] |
| Alcohols: -Xylitol | Osteoporosis prevention, anticancer activity [91,92] | Vine shoots, grape pomace, and wine less [93] | Solid fermentation (Saccharomyces cerevisiae) [93] |
| Enzymes: -Laccase | Bioremediation processes, wastewater detoxification, and the food industry [99] | Grape pomace [98] | Solid fermentation (Pleurotus ostruatus) [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesta-Corral, M.; Ramirez-Guzman, N.; Aguillón-Gutiérrez, D.; Torres-León, C.; Aguirre-Joya, J. Wine Industry Waste as a Source of Bioactive Compounds for Drug Use. Int. J. Mol. Sci. 2025, 26, 10820. https://doi.org/10.3390/ijms262210820
Mesta-Corral M, Ramirez-Guzman N, Aguillón-Gutiérrez D, Torres-León C, Aguirre-Joya J. Wine Industry Waste as a Source of Bioactive Compounds for Drug Use. International Journal of Molecular Sciences. 2025; 26(22):10820. https://doi.org/10.3390/ijms262210820
Chicago/Turabian StyleMesta-Corral, Mariana, Nathiely Ramirez-Guzman, David Aguillón-Gutiérrez, Cristian Torres-León, and Jorge Aguirre-Joya. 2025. "Wine Industry Waste as a Source of Bioactive Compounds for Drug Use" International Journal of Molecular Sciences 26, no. 22: 10820. https://doi.org/10.3390/ijms262210820
APA StyleMesta-Corral, M., Ramirez-Guzman, N., Aguillón-Gutiérrez, D., Torres-León, C., & Aguirre-Joya, J. (2025). Wine Industry Waste as a Source of Bioactive Compounds for Drug Use. International Journal of Molecular Sciences, 26(22), 10820. https://doi.org/10.3390/ijms262210820










