Mechanistic Evaluation of Radical Scavenging Pathways in Ginger Phenolics: A DFT Study of 6-Gingerol, 6-Shogaol, and 6-Paradol
Abstract
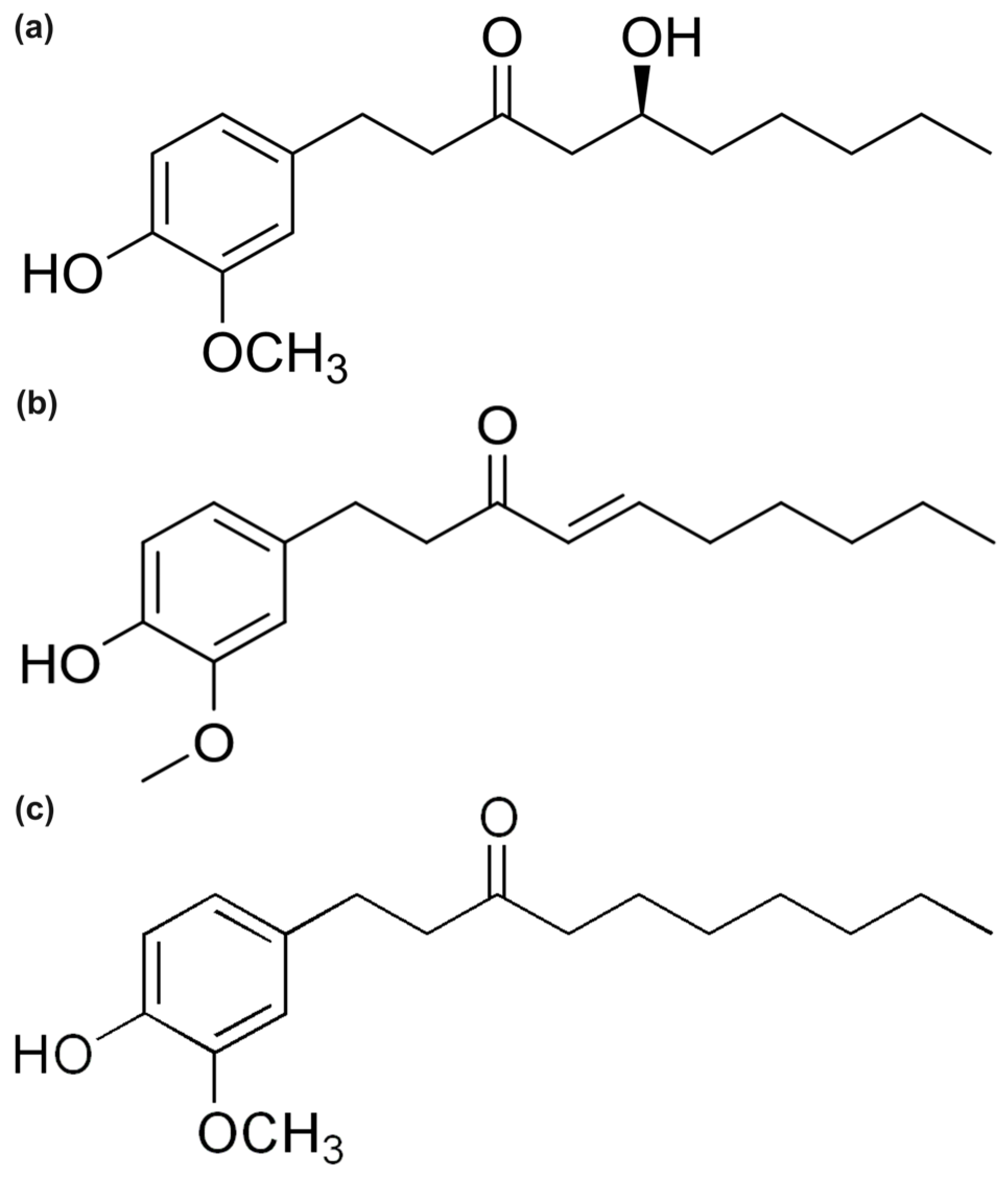
1. Introduction
2. Results and Discussion
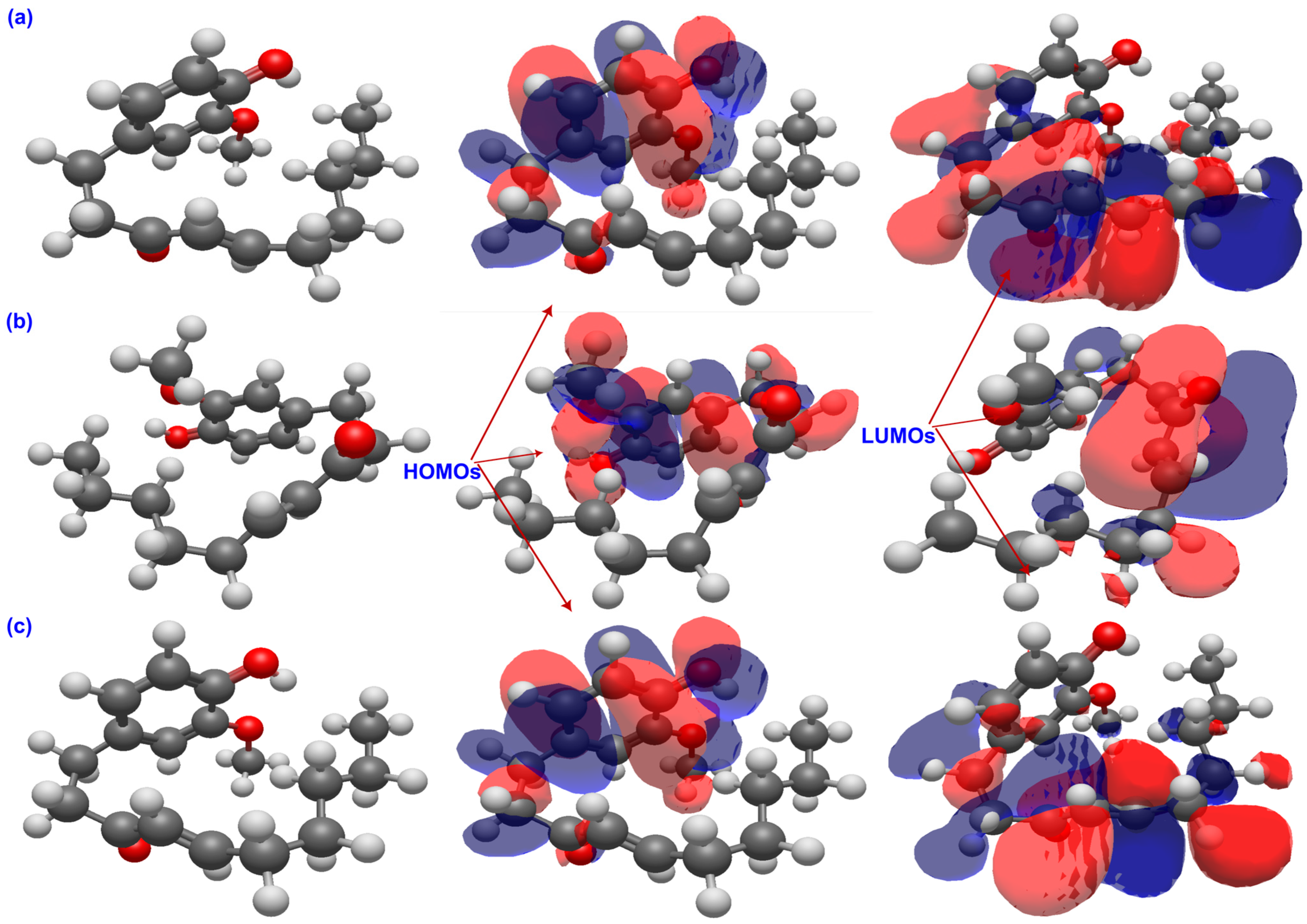
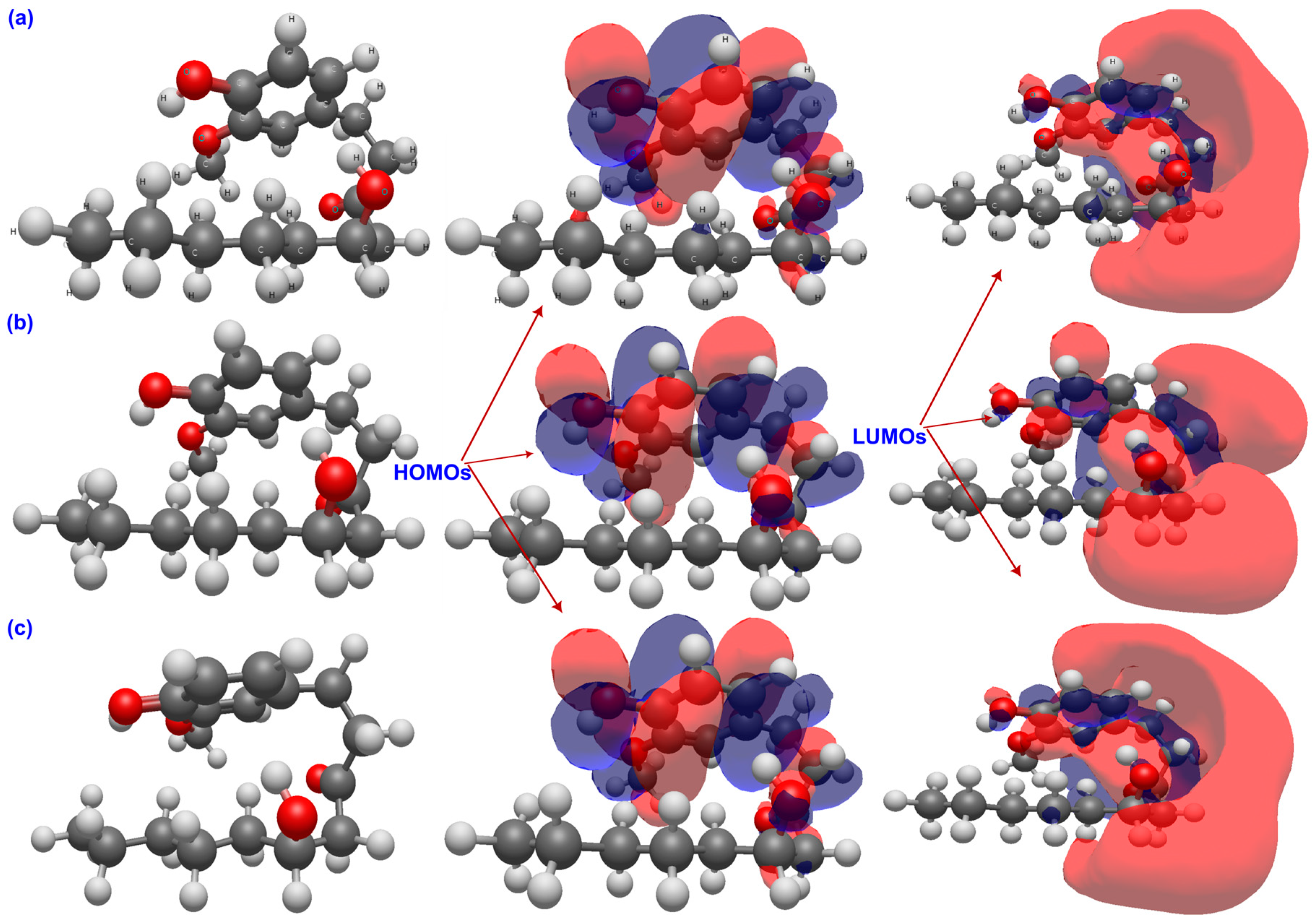
2.1. Chemical Reactivity Descriptors
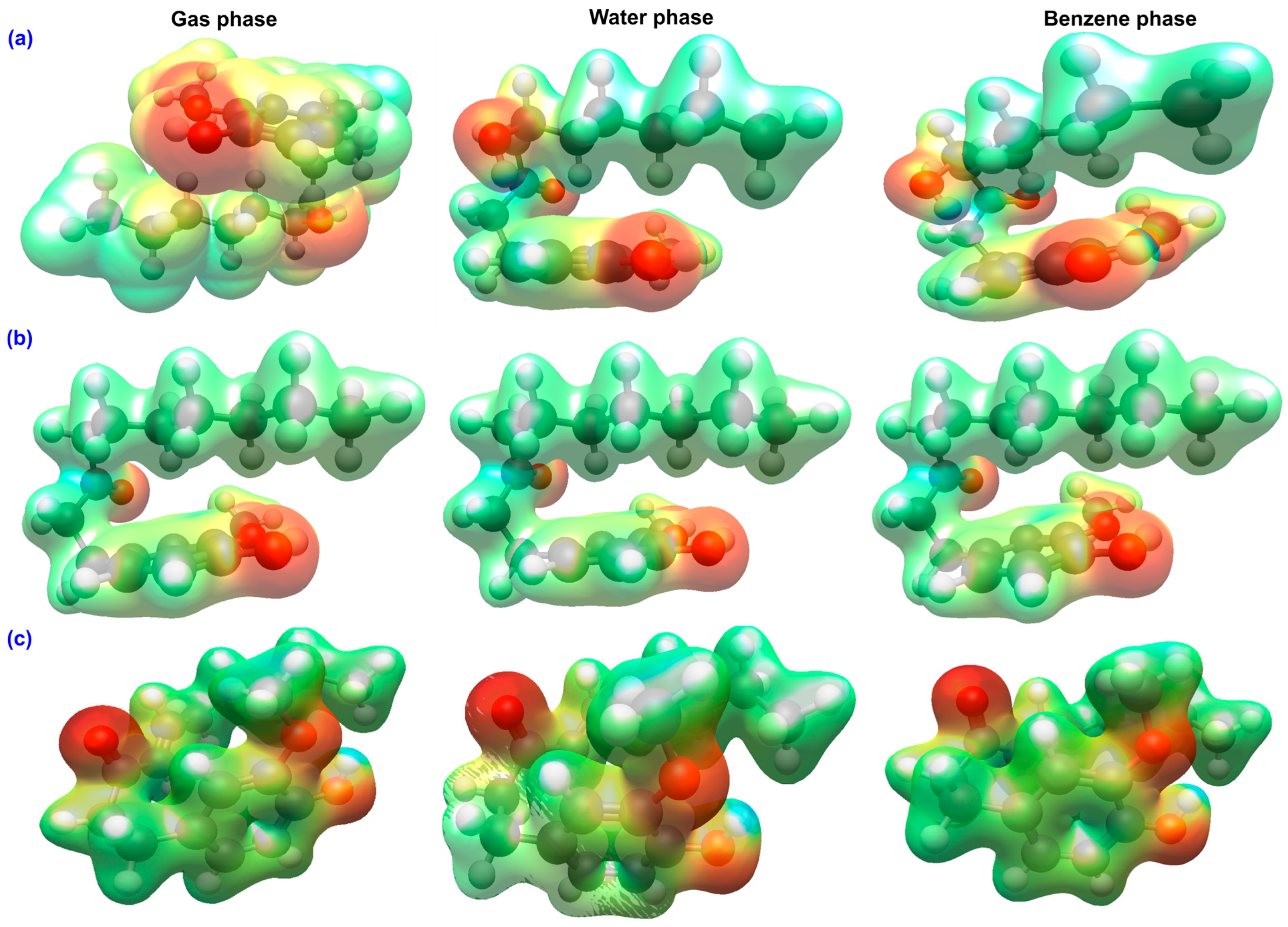
2.2. Molecular Electrostatic Potential
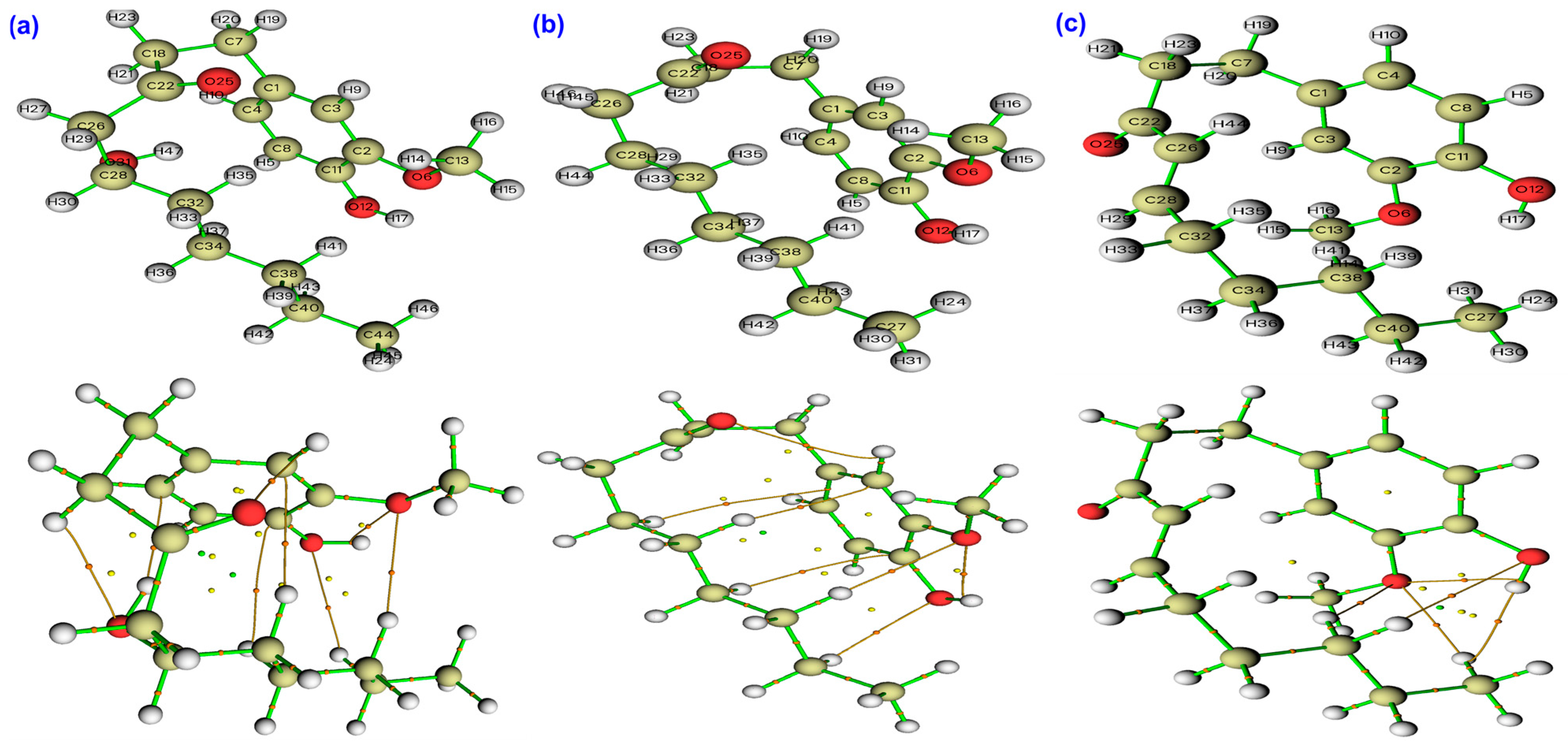
2.3. QTAIM Analysis of Bonding and Intramolecular Contacts
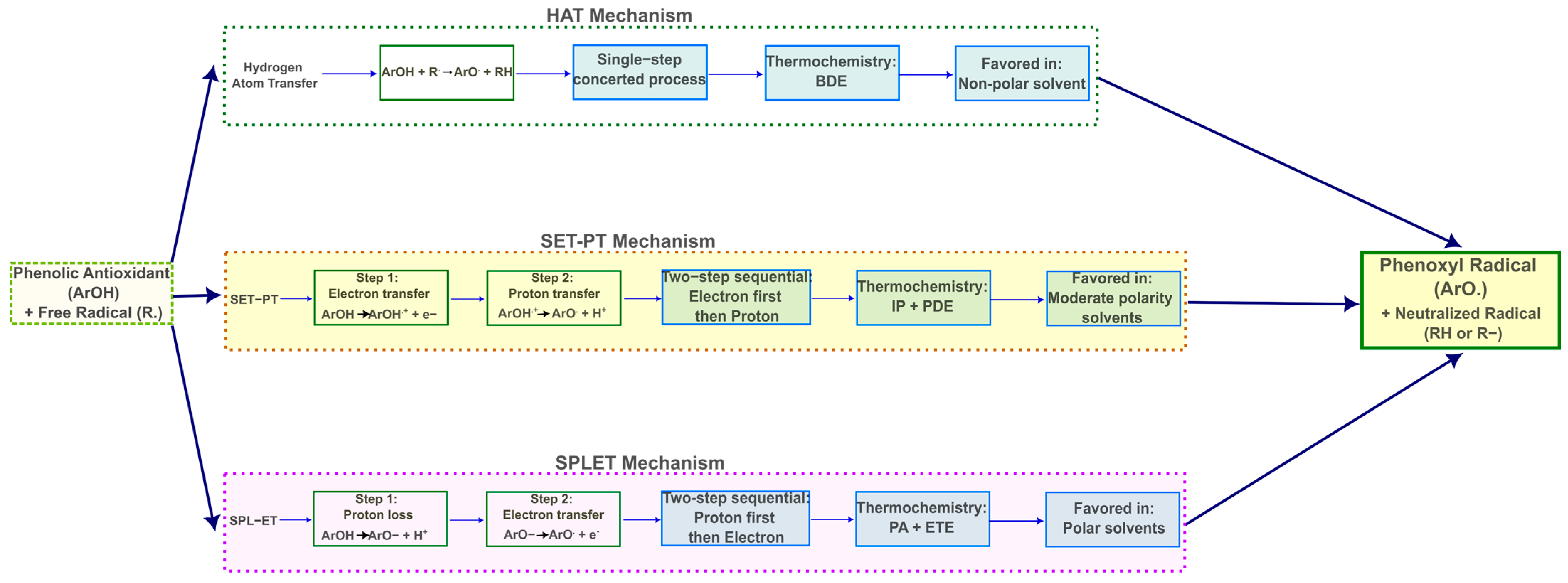
2.4. Gas-Phase Antioxidant Mechanisms
2.5. Antioxidant Mechanisms in Water
2.6. Antioxidant Mechanisms in Benzene
3. Materials and Methods
3.1. Computational Methodology
3.2. Conformer Search
3.3. Thermochemical Framework
3.4. Electronic and Topological Analyses
- Shared-shell (covalent) bonds: high ρ, negative ∇2ρ.
- Closed-shell (hydrogen-bond-like) contacts: low ρ, positive ∇2ρ, and |V|/G < 1.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for Measuring Reactive Oxygen Species and Oxidative Damage in Cells and in Vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S.; Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Oxygen Species in Living Systems: Source, Biochemistry, and Role in Human Disease. Am. J. Med. 1991, 91, S14–S22. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 552535. [Google Scholar] [CrossRef]
- Mosley, R.L.; Benner, E.J.; Kadiu, I.; Thomas, M.; Boska, M.D.; Hasan, K.; Laurie, C.; Gendelman, H.E. Neuroinflammation, Oxidative Stress, and the Pathogenesis of Parkinson’s Disease. Clin. Neurosci. Res. 2006, 6, 261–281. [Google Scholar] [CrossRef]
- Miller, J.K.; Brzezinska-Slebodzinska, E.; Madsen, F.C. Oxidative Stress, Antioxidants, and Animal Function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Sonboli, A.; Mojarrad, M.; Nejad Ebrahimi, S.; Enayat, S. Free Radical Scavenging Activity and Total Phenolic Content of Methanolic Extracts from Male Inflorescence of Salix Aegyptiaca Grown in Iran. Iran. J. Pharm. Res. 2010, 9, 293–296. [Google Scholar]
- Lien, E.J.; Ren, S.; Bui, H.-H.; Wang, R. Quantitative Structure-Activity Relationship Analysis of Phenolic Antioxidants. Free Radic. Biol. Med. 1999, 26, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I.R.N.A. Phytochemical and Antioxidant Analysis of Medicinal and Food Plants towards Bioactive Food and Pharmaceutical Resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Star, B.S.; van der Slikke, E.C.; Ransy, C.; Schmitt, A.; Henning, R.H.; Bouillaud, F.; Bouma, H.R. GYY4137-Derived Hydrogen Sulfide Donates Electrons to the Mitochondrial Electron Transport Chain via Sulfide: Quinone Oxidoreductase in Endothelial Cells. Antioxidants 2023, 12, 587. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B.; Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; et al. Bioactive Compounds and Bioactivities of Ginger (Zingiber Officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Ayustaningwarno, F.; Anjani, G.; Ayu, A.M.; Fogliano, V. A Critical Review of Ginger’s (Zingiber officinale) Antioxidant, Anti-Inflammatory, and Immunomodulatory Activities. Front. Nutr. 2024, 11, 1364836. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, M.N.; Nazir, A.; Fallico, B.; Shaukat, M.N.; Nazir, A.; Fallico, B. Ginger Bioactives: A Comprehensive Review of Health Benefits and Potential Food Applications. Antioxidants 2023, 12, 2015. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Al Juhaimi, F.; Özcan, M.M.; Uslu, N.; Babiker, E.E.; Mohamed Ahmed, I.A. Total Phenolics, Total Carotenoids, Individual Phenolics and Antioxidant Activity of Ginger (Zingiber officinale) Rhizome as Affected by Drying Methods. LWT 2020, 126, 109354. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative Antioxidant and Anti-Inflammatory Effects of [6]-Gingerol, [8]-Gingerol, [10]-Gingerol and [6]-Shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Wei, C.-K.; Tsai, Y.-H.; Korinek, M.; Hung, P.-H.; El-Shazly, M.; Cheng, Y.-B.; Wu, Y.-C.; Hsieh, T.-J.; Chang, F.-R.; Wei, C.-K.; et al. 6-Paradol and 6-Shogaol, the Pungent Compounds of Ginger, Promote Glucose Utilization in Adipocytes and Myotubes, and 6-Paradol Reduces Blood Glucose in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2017, 18, 168. [Google Scholar] [CrossRef]
- Sapkota, A.; Park, S.J.; Choi, J.W. Neuroprotective Effects of 6-Shogaol and Its Metabolite, 6-Paradol, in a Mouse Model of Multiple Sclerosis. Biomol. Ther. 2018, 27, 152. [Google Scholar] [CrossRef] [PubMed]
- Attallah, K.A.; El-Dessouki, A.M.; Abd-Elmawla, M.A.; Ghaiad, H.R.; Abo-Elghiet, F.; Mustafa, A.M.; El-Shiekh, R.A.; Elosaily, H. The Therapeutic Potential of Naturally Occurring 6-Shogaol: An Updated Comprehensive Review. Inflammopharmacology 2025. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Park, K.-H.; Park, J.; Kim, B.S.; Kim, G.-S.; Hwang, D.G.; Kim, J.E.; Park, K.-H.; Park, J.; Kim, B.S.; et al. Immunomodulatory Potential of 6-Gingerol and 6-Shogaol in Lactobacillus Plantarum-Fermented Zingiber Officinale Extract on Murine Macrophages. Int. J. Mol. Sci. 2025, 26, 2159. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. The Antioxidant and Free Radical Scavenging Activities of Processed Cowpea (Vigna unguiculata (L.) Walp.) Seed Extracts. Food Chem. 2007, 101, 10–19. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant Properties of Phenolic Compounds: H-Atom versus Electron Transfer Mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Fifen, J.J.; Nsangou, M.; Dhaouadi, Z.; Motapon, O.; Jaidane, N. Solvent Effects on the Antioxidant Activity of 3,4-Dihydroxyphenylpyruvic Acid: DFT and TD-DFT Studies. Comput. Theor. Chem. 2011, 966, 232–243. [Google Scholar] [CrossRef]
- Yoosefian, M.; Esmaeili, A.; Abass, K.S. Pharmacokinetic, Toxicological, and Molecular Interaction Assessment of Ginger-Derived Phenolics for SARS-CoV-2 Main Protease Inhibition. Sci. Rep. 2025, 15, 27390. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, Y.; Ni, Y.; Zhang, T.; Duan, C.; Huang, C.; Zhao, Y.; Gao, L.; Li, S. Protective Effects of Lactobacillus Plantarum C88 on Chronic Ethanol-Induced Liver Injury in Mice. J. Funct. Foods 2017, 35, 97–104. [Google Scholar] [CrossRef]
- Spiegel, M. Current Trends in Computational Quantum Chemistry Studies on Antioxidant Radical Scavenging Activity. J. Chem. Inf. Model. 2022, 62, 2639–2658. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Mu, J.; Wang, W.; Liu, Y.; Lu, X.; Sun, J.; Wang, J.; Ma, Q. Effects and Mechanism of Natural Phenolic Acids/Fatty Acids on Copigmentation of Purple Sweet Potato Anthocyanins. Curr. Res. Food Sci. 2022, 5, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Canneaux, S.; Hammaecher, C.; Ribaucour, M. An Extensive Methodological Theoretical Study of the Kinetics of the Benzylperoxy Radical Isomerization. Comput. Theor. Chem. 2012, 1002, 64–70. [Google Scholar] [CrossRef]
- Fassihi, A.; Abedi, D.; Saghaie, L.; Sabet, R.; Fazeli, H.; Bostaki, G.; Deilami, O.; Sadinpour, H. Synthesis, Antimicrobial Evaluation and QSAR Study of Some 3-Hydroxypyridine-4-One and 3-Hydroxypyran-4-One Derivatives. Eur. J. Med. Chem. 2009, 44, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Rohman, R.; Nath, R.; Kar, R. Revisiting the Hydrogen Atom Transfer Reactions through a Simple and Accurate Theoretical Model: Role of Hydrogen Bond Energy in Polyphenolic Antioxidants. Comput. Theor. Chem. 2023, 1223, 114097. [Google Scholar] [CrossRef]
- Vo, Q.V.; Nam, P.C.; Bay, M.V.; Thong, N.M.; Cuong, N.D.; Mechler, A. Density Functional Theory Study of the Role of Benzylic Hydrogen Atoms in the Antioxidant Properties of Lignans. Sci. Rep. 2018, 8, 12361. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the Activity of Phenolic Antioxidants: Theoretical Method, Analysis of Substituent Effects, and Application to Major Families of Antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef]
- Klein, E.; Rimarčík, J.; Senajová, E.; Vagánek, A.; Lengyel, J. Deprotonation of Flavonoids Severely Alters the Thermodynamics of the Hydrogen Atom Transfer. Comput. Theor. Chem. 2016, 1085, 7–17. [Google Scholar] [CrossRef]
- Farrokhnia, M. Density Functional Theory Studies on the Antioxidant Mechanism and Electronic Properties of Some Bioactive Marine Meroterpenoids: Sargahydroquionic Acid and Sargachromanol. ACS Omega 2020, 5, 20382–20390. [Google Scholar] [CrossRef]
- Al-Sou’od, K.A. Mechanism Engineering of Polyphenol Antioxidants: DFT/TD-DFT Evidence for HAT ↔ SPLET Switching via Targeted Functional Blocking in Curcumin, Quercetin, and Resveratrol Derivatives. Comput. Theor. Chem. 2025, 1254, 115470. [Google Scholar] [CrossRef]
- Soltani, S.; Sowlati-Hashjin, S.; Feugmo, C.G.T.; Karttunen, M.; Soltani, S.; Sowlati-Hashjin, S.; Feugmo, C.G.T.; Karttunen, M. Structural Investigation of DHICA Eumelanin Using Density Functional Theory and Classical Molecular Dynamics Simulations. Molecules 2022, 2, 8417. [Google Scholar] [CrossRef]
- Bakheit, A.H.; Wani, T.A.; Al-Majed, A.A.; Alkahtani, H.M.; Alanazi, M.M.; Alqahtani, F.R.; Zargar, S. Theoretical Study of the Antioxidant Mechanism and Structure-Activity Relationships of 1,3,4-Oxadiazol-2-Ylthieno [2,3-d]Pyrimidin-4-Amine Derivatives: A Computational Approach. Front. Chem. 2024, 12, 1443718. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, T.S.; Rajan, V.K.; Sabira, K.; Muraleedharan, K. DFT and QTAIM Based Investigation on the Structure and Antioxidant Behavior of Lichen Substances Atranorin, Evernic Acid and Diffractaic Acid. Comput. Biol. Chem. 2019, 80, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Akhtari, K.; Hassanzadeh, K.; Fakhraei, B.; Fakhraei, N.; Hassanzadeh, H.; Zarei, S.A. A Density Functional Theory Study of the Reactivity Descriptors and Antioxidant Behavior of Crocin. Comput. Theor. Chem. 2013, 1013, 123–129. [Google Scholar] [CrossRef]
- Akintemi, E.O.; Govender, K.K.; Singh, T. A DFT Study of the Chemical Reactivity Properties, Spectroscopy and Bioactivity Scores of Bioactive Flavonols. Comput. Theor. Chem. 2022, 1210, 113658. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, Y.; Qiao, M.; Yuan, W.; Li, X.; Wang, X.; Sheng, J.; Zi, C. Structure–Antioxidant Activity Relationships of Dendrocandin Analogues Determined Using Density Functional Theory. Struct. Chem. 2022, 33, 795–805. [Google Scholar] [CrossRef]
- Morin, J.; Pelletier, J.M. Density Functional Theory: Principles, Applications and Analysis; Nova Science Pub Inc.: New York, NY, USA, 2013; 322p. [Google Scholar]
- Li, M.-J.; Zhang, L.-M.; Liu, W.-X.; Lu, W.-C. DFT Study on Molecular Structures and ROS Scavenging Mechanisms of Novel Antioxidants from Lespedeza Virgata. Chin. J. Chem. Phys. 2011, 24, 173–180. [Google Scholar] [CrossRef]
- Alam, M.S.; Lee, D.-U. Quantum-Chemical Studies to Approach the Antioxidant Mechanism of Nonphenolic Hydrazone Schiff Base Analogs: Synthesis, Molecular Structure, Hirshfeld and Density Functional Theory Analyses. Bull. Korean Chem. Soc. 2015, 36, 682–691. [Google Scholar] [CrossRef]
- Lahmidi, S.; Anouar, E.H.; El Hafi, M.; Boulhaoua, M.; Ejjoummany, A.; Jemli, M.; Essassi, E.M.; Mague, J.T. Synthesis, X-Ray, Spectroscopic Characterization, DFT and Antioxidant Activity of 1,2,4-Triazolo[1,5-a]Pyrimidine Derivatives. J. Mol. Struct. 2019, 1177, 131–142. [Google Scholar] [CrossRef]
- Choudhary, V.; Bhatt, A.; Dash, D.; Sharma, N. DFT Calculations on Molecular Structures, HOMO–LUMO Study, Reactivity Descriptors and Spectral Analyses of Newly Synthesized Diorganotin(IV) 2-Chloridophenylacetohydroxamate Complexes. J. Comput. Chem. 2019, 40, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Nalewajski, R.F.; Korchowiec, J.; Zhou, Z. Molecular Hardness and Softness Parameters and Their Use in Chemistry. Int. J. Quantum Chem. 1988, 34, 349–366. [Google Scholar] [CrossRef]
- Messali, M.; Jhaa, G.; Alrashdi, A.A.; Lee, H.; Kaya, S.; Sabik, A.; Abualrejal, M.M.A.; Lgaz, H. Computational Exploration of a Chlorinated Tetrahydroisoquinoline: Geometry, Reactivity, Noncovalent Interactions, and Molecular Docking Evaluation. Results Phys. 2025, 75, 108331. [Google Scholar] [CrossRef]
- Pratihar, S. Electrophilicity and Nucleophilicity of Commonly Used Aldehydes. Org. Biomol. Chem. 2014, 12, 5781–5788. [Google Scholar] [CrossRef] [PubMed]
- Campodonico, P.; Santos, J.G.; Andres, J.; Contreras, R. Relationship between Nucleophilicity/Electrophilicity Indices and Reaction Mechanisms for the Nucleophilic Substitution Reactions of Carbonyl Compounds. J. Phys. Org. Chem. 2004, 17, 273–281. [Google Scholar] [CrossRef]
- Ram Kumar, A.; Selvaraj, S.; Azam, M.; Sheeja Mol, G.P.; Kanagathara, N.; Alam, M.; Jayaprakash, P. Spectroscopic, Biological, and Topological Insights on Lemonol as a Potential Anticancer Agent. ACS Omega 2023, 8, 31548–31566. [Google Scholar] [CrossRef]
- Umamaheswari, S.; Senthilkumar, S. Hydrazide Compounds Incorporating with Aldehyde Moiety: Synthesis, Spectroscopy, Crystal Structure Analysis, Theoretical Studies and Molecular Docking Studies of (E)-4-Amino-N’-(Substituted Benzylidene) Benzohydrazide. J. Mol. Struct. 2025, 1346, 143102. [Google Scholar] [CrossRef]
- Ragi, C.; Muraleedharan, K. Antioxidant Activity of Hibiscetin and Hibiscitrin: Insight from DFT, NCI, and QTAIM. Theor. Chem. Acc. 2023, 142, 30. [Google Scholar] [CrossRef]
- Tamafo Fouegue, A.D.; Nono, J.H.; Nkungli, N.K.; Ghogomu, J.N. A Theoretical Study of the Structural and Electronic Properties of Some Titanocenes Using DFT, TD-DFT, and QTAIM. Struct. Chem. 2021, 32, 353–366. [Google Scholar] [CrossRef]
- Baryshnikov, G.V.; Minaev, B.F.; Minaeva, V.A.; Baryshnikova, A.T.; Pittelkow, M. DFT and QTAIM Study of the Tetra-Tert-Butyltetraoxa[8]Circulene Regioisomers Structure. J. Mol. Struct. 2012, 1026, 127–132. [Google Scholar] [CrossRef]
- Moosavi, M.; Banazadeh, N.; Torkzadeh, M. Structure and Dynamics in Amino Acid Choline-Based Ionic Liquids: A Combined QTAIM, NCI, DFT, and Molecular Dynamics Study. J. Phys. Chem. B 2019, 123, 4070–4084. [Google Scholar] [CrossRef]
- Sarkar, A.; Middya, T.R.; Jana, A.D. A QSAR Study of Radical Scavenging Antioxidant Activity of a Series of Flavonoids Using DFT Based Quantum Chemical Descriptors—The Importance of Group Frontier Electron Density. J. Mol. Model. 2012, 18, 2621–2631. [Google Scholar] [CrossRef]
- Sadasivam, K.; Jayaprakasam, R.; Kumaresan, R. A DFT Study on the Role of Different Oh Groups in the Radical Scavenging Process. J. Theor. Comput. Chem. 2012, 11, 871–893. [Google Scholar] [CrossRef]
- Rong, Y.Z.; Wang, Z.W.; Zhao, B. A DFT Study on the Structural and Antioxidant Properties of Three Flavonols. Food Biophys. 2013, 8, 90–94. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Hou, B.; Zhang, P.; Wang, Q.; Zhang, B.-L.; Huang, Y.-W.; Wang, Y.; Xiang, Z.-M.; Zi, C.-T. Synthesis, Antioxidant Activity, and Density Functional Theory Study of Catechin Derivatives. RSC Adv. 2017, 7, 54136–54141. [Google Scholar] [CrossRef]
- Cândido-Júnior, J.R.; Soares Romeiro, L.A.S.; Marinho, E.S.; de Kássio Vieira Monteiro, N.K.V.; de Lima Neto, P. Antioxidant Activity of Eugenol and Its Acetyl and Nitroderivatives: The Role of Quinone Intermediates—A DFT Approach of DPPH Test. J. Mol. Model. 2022, 28, 133. [Google Scholar] [CrossRef] [PubMed]
- Amić, A.; Mastiľák Cagardová, D. A DFT Study on the Kinetics of HOO•, CH3OO•, and O2•− Scavenging by Quercetin and Flavonoid Catecholic Metabolites. Antioxidants 2023, 12, 1154. [Google Scholar] [CrossRef]
- Akhtari, K.; Hassanzadeh, K.; Fakhraei, B.; Fakhraei, N.; Hassanzadeh, H.; Akhtari, G.; Zarei, S.A.; Hassanzadeh, K. Mechanisms of the Hydroxyl and Superoxide Anion Radical Scavenging Activity and Protective Effect on Lipid Peroxidation of Thymoquinone: A DFT Study. Monatshefte Chem. 2015, 146, 601–611. [Google Scholar] [CrossRef]
- Rossi, M.; Caruso, F.; Thieke, N.; Belli, S.; Kim, A.; Damiani, E.; Morresi, C.; Bacchetti, T. Examining the Antioxidant and Superoxide Radical Scavenging Activity of Anise, (Pimpinella anisum L. Seeds), Esculetin, and 4-Methyl-Esculetin Using X-Ray Diffraction, Hydrodynamic Voltammetry and DFT Methods. Pharmaceuticals 2024, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Huong, D.Q.; Quang, D.T.; Dang, N.T.T.; Linh, N.L.M.; Thong, N.M.; Tam, N.M.; Vo, Q.V.; Nam, P.C. Theoretical Study on the Influence of OH Group Position on the Free Radical Scavenging Ability of Tryptamine Derivatives. RSC Adv. 2025, 15, 11417–11430. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Density Functional Computations of the Energetic and Spectroscopic Parameters of Quercetin and Its Radicals in the Gas Phase and in Solvent. Theor. Chem. Acc. 2004, 111, 210–216. [Google Scholar] [CrossRef]
- Leopoldini, M.; Pitarch, I.P.; Russo, N.; Toscano, M. Structure, Conformation, and Electronic Properties of Apigenin, Luteolin, and Taxifolin Antioxidants. A First Principle Theoretical Study. J. Phys. Chem. A 2004, 108, 92–96. [Google Scholar] [CrossRef]
- Boudiaf, H.; Latelli, N.; Khelifi, R.; Hamadouche, S.; Merzoud, L.; Morell, C.; Chermette, H. Investigation of Free Radical Scavenging Activities of Some Isatin Schiff Bases (OH versus NH). A DFT Study. Chem. Phys. Impact 2025, 11, 100904. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 6.0. WIREs Comput. Mol. Sci. 2025, 15, e70019. [Google Scholar] [CrossRef]
- Neese, F. An Improvement of the Resolution of the Identity Approximation for the Formation of the Coulomb Matrix. J. Comput. Chem. 2003, 24, 1740–1747. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. The SHARK Integral Generation and Digestion System. J. Comput. Chem. 2023, 44, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, Approximate and Parallel Hartree–Fock and Hybrid DFT Calculations. A ‘Chain-of-Spheres’ Algorithm for the Hartree–Fock Exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Bykov, D.; Petrenko, T.; Izsák, R.; Kossmann, S.; Becker, U.; Valeev, E.; Neese, F. Efficient Implementation of the Analytic Second Derivatives of Hartree–Fock and Hybrid DFT Energies: A Detailed Analysis of Different Approximations. Mol. Phys. 2015, 113, 1961–1977. [Google Scholar] [CrossRef]
- Garcia-Ratés, M.; Neese, F. Efficient Implementation of the Analytical Second Derivatives of Hartree–Fock and Hybrid DFT Energies within the Framework of the Conductor-like Polarizable Continuum Model. J. Comput. Chem. 2019, 40, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ratés, M.; Neese, F. Effect of the Solute Cavity on the Solvation Energy and Its Derivatives within the Framework of the Gaussian Charge Scheme. J. Comput. Chem. 2020, 41, 922–939. [Google Scholar] [CrossRef] [PubMed]
- Helmich-Paris, B.; de Souza, B.; Neese, F.; Izsák, R. An Improved Chain of Spheres for Exchange Algorithm. J. Chem. Phys. 2021, 155, 104109. [Google Scholar] [CrossRef]
- Khelifi, R.; Latelli, N.; Charifi, Z.; Morell, C.; Chermette, H. Predicting the Activity of Methoxyphenol Derivatives Antioxidants: II—Importance of the Nature of the Solvent on the Mechanism, a DFT Study. J. Comput. Chem. 2024, 45, 886–897. [Google Scholar] [CrossRef]
- Khelifi, R.; Latelli, N.; Charifi, Z.; Baaziz, H.; Chermette, H. Predicting the Activity of Methoxyphenol Derivatives Antioxidants: I. Structure and Reactivity of Methoxyphenol Derivatives, a DFT Approach. Comput. Theor. Chem. 2023, 1229, 114287. [Google Scholar] [CrossRef]
- Saïd, A.E.-H.; Mekelleche, S.M. Investigation of Reaction Mechanisms and Kinetics of the Radical Scavenging Ability of 5-Tert-Butylbenzene-1,2,3-Triol and 3,5-Di-Tert-Butylbenzene-1,2-Diol Compounds Towards OOH Radical. Prog. React. Kinet. Mech. 2018, 43, 101–111. [Google Scholar] [CrossRef]
- La Rocca, M.V.; Rutkowski, M.; Ringeissen, S.; Gomar, J.; Frantz, M.-C.; Ngom, S.; Adamo, C. Benchmarking the DFT Methodology for Assessing Antioxidant-Related Properties: Quercetin and Edaravone as Case Studies. J. Mol. Model. 2016, 22, 250. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- De Souza, B. GOAT: A Global Optimization Algorithm for Molecules and Atomic Clusters. Angew. Chem. Int. Ed. 2025, 64, e202500393. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended TIGHT-BINDING Quantum Chemistry Methods. WIREs Comput. Mol. Sci. 2021, 11, e1493. [Google Scholar] [CrossRef]
- Xu, S.-C.; Ren, Y.; Wan, L.; Li, W.-K.; Wong, N.-B.; Zhang, J.-X.; Liao, Q.; Ji, L. DFT Insight into the UV-Vis Spectra and Radical Scavenging Activity of Aurantio-Obtusin. J. Theor. Comput. Chem. 2013, 12, 1350024. [Google Scholar] [CrossRef]
- Senthil Kumar, K.; Kumaresan, R. A DFT Study on the Structural, Electronic Properties and Radical Scavenging Mechanisms of Calycosin, Glycitein, Pratensein and Prunetin. Comput. Theor. Chem. 2012, 985, 14–22. [Google Scholar] [CrossRef]
- Nenadis, N.; Sigalas, M.P. A DFT Study on the Radical Scavenging Activity of Maritimetin and Related Aurones. J. Phys. Chem. A 2008, 112, 12196–12202. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. A Comprehensive Electron Wavefunction Analysis Toolbox for Chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Astani, E.K.; Chen, N.-C.; Huang, Y.-C.; Bahrami, A.; Chen, L.-Y.; Lin, P.-R.; Guan, H.-H.; Lin, C.-C.; Chuankhayan, P.; Hadipour, N.L.; et al. DFT, QTAIM, and NBO Studies on the Trimeric Interactions in the Protrusion Domain of a Piscine Betanodavirus. J. Mol. Graph. Model. 2017, 78, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Obot, I.B.; Onyeachu, I.B.; Kaya, S. Density Functional Theory: Practical Application and Their Limitations in Corrosion Inhibition Research. In Corrosion Science; Apple Academic Press: New York, NY, USA, 2023; pp. 173–198. [Google Scholar] [CrossRef]
- Kaya, S.; von Szentpaly, L.; Serdaroglu, G.; Guo, L. Chemical Reactivity: Volume 1: Theories and Principles; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 978-0-323-90612-8. [Google Scholar]
- Kaya, S. Chapter 3—Conceptual Density Functional Theory–Based Applications in Extraction Studies. In Green Analytical Chemistry; Locatelli, M., Kaya, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 43–58. ISBN 978-0-443-16122-3. [Google Scholar] [CrossRef]

| Mol | EHOMO | ELUMO | IP | EA | Gap | η | S (eV−1) | χ | μ | ω | ΔNmax | ω− | ω+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gas phase | |||||||||||||
| GIN | −7.384 | −0.048 | 7.384 | 0.048 | 7.336 | 3.668 | 0.136 | 3.716 | −3.716 | 1.882 | 1.013 | 4.199 | 0.483 |
| SHO | −7.169 | −0.673 | 7.169 | 0.673 | 6.496 | 3.248 | 0.154 | 3.921 | −3.921 | 2.367 | 1.207 | 4.733 | 0.812 |
| PAR | −7.155 | 0.033 | 7.155 | −0.033 | 7.188 | 3.594 | 0.139 | 3.561 | −3.561 | 1.764 | 0.991 | 3.994 | 0.433 |
| Water (SMD) | |||||||||||||
| GIN | −7.274 | 0.036 | 7.274 | −0.036 | 7.310 | 3.655 | 0.137 | 3.619 | −3.619 | 1.792 | 0.990 | 4.058 | 0.439 |
| SHO | −7.237 | −0.842 | 7.237 | 0.842 | 6.395 | 3.198 | 0.156 | 4.040 | −4.040 | 2.552 | 1.263 | 4.971 | 0.932 |
| PAR | −7.198 | 0.167 | 7.198 | −0.167 | 7.365 | 3.682 | 0.136 | 3.516 | −3.516 | 1.678 | 0.955 | 3.896 | 0.381 |
| Benzene (SMD) | |||||||||||||
| GIN | −7.243 | 0.091 | 7.243 | −0.091 | 7.334 | 3.667 | 0.136 | 3.576 | −3.576 | 1.744 | 0.975 | 3.990 | 0.414 |
| SHO | −7.136 | −0.637 | 7.136 | 0.637 | 6.499 | 3.250 | 0.154 | 3.886 | −3.886 | 2.324 | 1.196 | 4.674 | 0.787 |
| PAR | −7.099 | 0.179 | 7.099 | −0.179 | 7.278 | 3.639 | 0.137 | 3.460 | −3.460 | 1.645 | 0.951 | 3.830 | 0.370 |
| Phase | Bond | Pair | ρ (a.u.) | ∇2ρ (a.u.) | |V|/G | ε | Class |
|---|---|---|---|---|---|---|---|
| Benzene | 47H–31O | O–H | 0.3560 | −2.0545 | 8.81 | 0.026 | Shared-shell |
| 12O–17H | O–H | 0.3559 | −2.1322 | 9.68 | 0.022 | Shared-shell | |
| 21H–31O | O–H | 0.0098 | 0.0337 | 0.88 | 0.149 | Closed-shell | |
| 6O–41H | O–H | 0.0095 | 0.0306 | 0.94 | 0.154 | Closed-shell | |
| 12O–43H | O–H | 0.0046 | 0.0165 | 0.78 | 0.346 | Closed-shell | |
| 25O–22C | C–O | 0.3956 | 0.4897 | 1.84 | 0.031 | Intermediate | |
| 11C–12O | C–O | 0.2879 | −0.2103 | 2.14 | 0.010 | Shared-shell | |
| 2C–6O | C–O | 0.2820 | −0.1866 | 2.12 | 0.008 | Shared-shell | |
| 31O–28C | C–O | 0.2543 | −0.4207 | 2.40 | 0.012 | Shared-shell | |
| 6O–13C | C–O | 0.2439 | −0.2000 | 2.17 | 0.002 | Shared-shell | |
| 8C–11C | C–C | 0.3164 | −0.8908 | 4.15 | 0.245 | Shared-shell | |
| 2C–11C | C–C | 0.3126 | −0.8731 | 4.34 | 0.268 | Shared-shell | |
| 3C–2C | C–C | 0.3116 | −0.8567 | 4.05 | 0.246 | Shared-shell | |
| 1C–4C | C–C | 0.3088 | −0.8274 | 4.05 | 0.211 | Shared-shell | |
| 4C–8C | C–C | 0.3057 | −0.8106 | 4.06 | 0.206 | Shared-shell | |
| 1C–3C | C–C | 0.3039 | −0.8012 | 4.03 | 0.201 | Shared-shell | |
| Gas | 12O–17H | O–H | 0.3590 | −2.1470 | 9.57 | 0.022 | Shared-shell |
| 47H–31O | O–H | 0.3573 | −2.0593 | 8.74 | 0.025 | Shared-shell | |
| 40H–31O | O–H | 0.0125 | 0.0422 | 0.86 | 0.102 | Closed-shell | |
| 42H–12O | O–H | 0.0110 | 0.0367 | 0.87 | 0.129 | Closed-shell | |
| 9H–31O | O–H | 0.0100 | 0.0317 | 0.86 | 0.122 | Closed-shell | |
| 25O–22C | C–O | 0.4035 | 0.5876 | 1.82 | 0.038 | Intermediate | |
| 2C–6O | C–O | 0.2898 | −0.1744 | 2.08 | 0.010 | Shared-shell | |
| 11C–12O | C–O | 0.2871 | −0.2011 | 2.12 | 0.010 | Shared-shell | |
| 31O–28C | C–O | 0.2597 | −0.3911 | 2.37 | 0.011 | Shared-shell | |
| 6O–13C | C–O | 0.2399 | −0.1647 | 2.11 | 0.003 | Shared-shell | |
| 8C–11C | C–C | 0.3191 | −0.9072 | 4.15 | 0.244 | Shared-shell | |
| 3C–2C | C–C | 0.3168 | −0.8873 | 4.05 | 0.247 | Shared-shell | |
| 1C–4C | C–C | 0.3143 | −0.8573 | 4.06 | 0.209 | Shared-shell | |
| 4C–8C | C–C | 0.3106 | −0.8306 | 4.07 | 0.205 | Shared-shell | |
| 1C–3C | C–C | 0.3088 | −0.8151 | 4.04 | 0.205 | Shared-shell | |
| 2C–11C | C–C | 0.3051 | −0.7897 | 4.00 | 0.200 | Shared-shell | |
| Water | 47H–31O | O–H | 0.3527 | −2.0762 | 9.24 | 0.024 | Shared-shell |
| 12O–17H | O–H | 0.3504 | −2.1547 | 10.26 | 0.021 | Shared-shell | |
| 9H–31O | O–H | 0.0128 | 0.0374 | 0.86 | 0.120 | Closed-shell | |
| 40H–31O | O–H | 0.0118 | 0.0380 | 0.86 | 0.106 | Closed-shell | |
| 42H–12O | O–H | 0.0117 | 0.0378 | 0.86 | 0.132 | Closed-shell | |
| 21H–31O | O–H | 0.0115 | 0.0358 | 0.84 | 0.137 | Closed-shell | |
| 25O–22C | C–O | 0.3873 | 0.4329 | 1.85 | 0.002 | Intermediate | |
| 2C–6O | C–O | 0.2813 | −0.2231 | 2.15 | 0.011 | Shared-shell | |
| 11C–12O | C–O | 0.2797 | −0.2483 | 2.17 | 0.010 | Shared-shell | |
| 31O–28C | C–O | 0.2488 | −0.3870 | 2.38 | 0.022 | Shared-shell | |
| 6O–13C | C–O | 0.2400 | −0.1832 | 2.15 | 0.003 | Shared-shell | |
| 8C–11C | C–C | 0.3167 | −0.8925 | 4.14 | 0.245 | Shared-shell | |
| 2C–11C | C–C | 0.3132 | −0.8759 | 4.34 | 0.273 | Shared-shell | |
| 3C–2C | C–C | 0.3108 | −0.8537 | 4.06 | 0.242 | Shared-shell | |
| 1C–4C | C–C | 0.3088 | −0.8286 | 4.06 | 0.206 | Shared-shell | |
| 4C–8C | C–C | 0.3065 | −0.8232 | 4.08 | 0.202 | Shared-shell | |
| 1C–3C | C–C | 0.3052 | −0.8101 | 4.05 | 0.202 | Shared-shell |
| Phase | Bond | Pair | ρ (a.u.) | ∇2ρ (a.u.) | |V|/G | ε | Class |
|---|---|---|---|---|---|---|---|
| Gas | 12O-17H | O-H | 0.3577 | −2.1343 | 9.56 | 0.022 | Shared-shell |
| 6O-41H | O-H | 0.0103 | 0.0325 | 0.95 | 0.116 | Closed-shell | |
| 9H-25O | O-H | 0.0080 | 0.0296 | 0.81 | 1.262 | Closed-shell | |
| 12O-43H | O-H | 0.0079 | 0.0271 | 0.87 | 0.168 | Closed-shell | |
| 22C-25O | C-O | 0.3982 | 0.5075 | 1.84 | 0.039 | Intermediate | |
| 11C-12O | C-O | 0.2871 | −0.2152 | 2.14 | 0.009 | Shared-shell | |
| 2C-6O | C-O | 0.2787 | −0.1821 | 2.12 | 0.007 | Shared-shell | |
| 6O-13C | C-O | 0.2473 | −0.2246 | 2.19 | 0.007 | Shared-shell | |
| 11C-2C | C-C | 0.3132 | −0.8762 | 4.34 | 0.270 | Shared-shell | |
| 3C-2C | C-C | 0.3126 | −0.8614 | 4.04 | 0.250 | Shared-shell | |
| 8C-11C | C-C | 0.3170 | −0.8944 | 4.15 | 0.247 | Shared-shell | |
| 4C-1C | C-C | 0.3101 | −0.8348 | 4.06 | 0.214 | Shared-shell | |
| 4C-8C | C-C | 0.3070 | −0.8268 | 4.10 | 0.204 | Shared-shell | |
| Water | 12O-17H | O-H | 0.3516 | −2.1612 | 10.24 | 0.021 | Shared-shell |
| 12O-41H | O-H | 0.0076 | 0.0281 | 0.84 | 2.380 | Closed-shell | |
| 12O-43H | O-H | 0.0054 | 0.0196 | 0.80 | 0.658 | Closed-shell | |
| 12O-24H | O-H | 0.0041 | 0.0162 | 0.74 | 1.023 | Closed-shell | |
| 22C-25O | C-O | 0.3869 | 0.4294 | 1.85 | 0.006 | Intermediate | |
| 2C-6O | C-O | 0.2803 | −0.2214 | 2.15 | 0.001 | Shared-shell | |
| 11C-12O | C-O | 0.2793 | −0.2414 | 2.17 | 0.009 | Shared-shell | |
| 6O-13C | C-O | 0.2405 | −0.1803 | 2.15 | 0.001 | Shared-shell | |
| 11C-2C | C-C | 0.3131 | −0.8752 | 4.34 | 0.274 | Shared-shell | |
| 8C-11C | C-C | 0.3169 | −0.8935 | 4.13 | 0.247 | Shared-shell | |
| 3C-2C | C-C | 0.3108 | −0.8525 | 4.05 | 0.244 | Shared-shell | |
| 4C-1C | C-C | 0.3095 | −0.8323 | 4.06 | 0.208 | Shared-shell | |
| 1C-3C | C-C | 0.3044 | −0.8062 | 4.05 | 0.201 | Shared-shell | |
| Benzene | 12O-17H | O-H | 0.3561 | −2.1328 | 9.66 | 0.022 | Shared-shell |
| 6O-41H | O-H | 0.0091 | 0.0299 | 0.93 | 0.228 | Closed-shell | |
| 9H-25O | O-H | 0.0076 | 0.0282 | 0.81 | 1.074 | Closed-shell | |
| 12O-43H | O-H | 0.0068 | 0.0236 | 0.84 | 0.191 | Closed-shell | |
| 12O-24H | O-H | 0.0032 | 0.0135 | 0.67 | 20.920 | Closed-shell | |
| 22C-25O | C-O | 0.3960 | 0.4937 | 1.84 | 0.030 | Intermediate | |
| 11C-12O | C-O | 0.2868 | −0.2081 | 2.14 | 0.010 | Shared-shell | |
| 2C-6O | C-O | 0.2808 | −0.1853 | 2.12 | 0.006 | Shared-shell | |
| 6O-13C | C-O | 0.2446 | −0.2030 | 2.17 | 0.003 | Shared-shell | |
| 11C-2C | C-C | 0.3125 | −0.8722 | 4.34 | 0.269 | Shared-shell | |
| 3C-2C | C-C | 0.3121 | −0.8587 | 4.04 | 0.248 | Shared-shell | |
| 8C-11C | C-C | 0.3167 | −0.8924 | 4.14 | 0.248 | Shared-shell | |
| 4C-1C | C-C | 0.3097 | −0.8325 | 4.06 | 0.212 | Shared-shell | |
| 4C-8C | C-C | 0.3065 | −0.8241 | 4.09 | 0.202 | Shared-shell |
| Phase | Bond | Pair | Rho (a.u.) | ∇2ρ (a.u.) | |V|/G | ε | Class |
|---|---|---|---|---|---|---|---|
| Gas | 12O-17H | O-H | 0.3569 | −2.1401 | 9.61 | 0.022 | Shared-shell |
| 6O-31H | O-H | 0.0103 | 0.0336 | 0.93 | 0.044 | Closed-shell | |
| 41H-6O | O-H | 0.0098 | 0.0322 | 0.94 | 0.067 | Closed-shell | |
| 12O-39H | O-H | 0.0035 | 0.0125 | 0.76 | 0.337 | Closed-shell | |
| 22C-25O | C-O | 0.3957 | 0.4408 | 1.86 | 0.038 | Intermediate | |
| 12O-11C | C-O | 0.2881 | −0.2173 | 2.14 | 0.009 | Shared-shell | |
| 2C-6O | C-O | 0.2787 | −0.1728 | 2.11 | 0.007 | Shared-shell | |
| 6O-13C | C-O | 0.2479 | −0.2315 | 2.19 | 0.008 | Shared-shell | |
| 26C-28C | C-C | 0.3431 | −1.0084 | 3.90 | 0.312 | Shared-shell | |
| 8C-11C | C-C | 0.3171 | −0.8946 | 4.15 | 0.247 | Shared-shell | |
| 2C-3C | C-C | 0.3140 | −0.8689 | 4.05 | 0.252 | Shared-shell | |
| 11C-2C | C-C | 0.3127 | −0.8745 | 4.35 | 0.267 | Shared-shell | |
| 4C-1C | C-C | 0.3109 | −0.8387 | 4.05 | 0.217 | Shared-shell | |
| 8C-4C | C-C | 0.3067 | −0.8254 | 4.10 | 0.202 | Shared-shell | |
| Water | 12O-17H | O-H | 0.3506 | −2.1584 | 10.22 | 0.021 | Shared-shell |
| 6O-31H | O-H | 0.0096 | 0.0315 | 0.92 | 0.031 | Closed-shell | |
| 41H-6O | O-H | 0.0084 | 0.0287 | 0.90 | 0.055 | Closed-shell | |
| 12O-39H | O-H | 0.0036 | 0.0128 | 0.76 | 0.204 | Closed-shell | |
| 22C-25O | C-O | 0.3834 | 0.3543 | 1.88 | 0.004 | Intermediate | |
| 2C-6O | C-O | 0.2815 | −0.2163 | 2.15 | 0.014 | Shared-shell | |
| 12O-11C | C-O | 0.2793 | −0.2500 | 2.17 | 0.010 | Shared-shell | |
| 6O-13C | C-O | 0.2405 | −0.1863 | 2.16 | 0.003 | Shared-shell | |
| 26C-28C | C-C | 0.3410 | −0.9988 | 3.92 | 0.298 | Shared-shell | |
| 8C-11C | C-C | 0.3166 | −0.8917 | 4.13 | 0.247 | Shared-shell | |
| 11C-2C | C-C | 0.3129 | −0.8744 | 4.34 | 0.273 | Shared-shell | |
| 2C-3C | C-C | 0.3124 | −0.8620 | 4.06 | 0.244 | Shared-shell | |
| 4C-1C | C-C | 0.3101 | −0.8356 | 4.06 | 0.210 | Shared-shell | |
| 8C-4C | C-C | 0.3065 | −0.8245 | 4.09 | 0.200 | Shared-shell | |
| Benzene | 12O-17H | O-H | 0.3554 | −2.1368 | 9.69 | 0.022 | Shared-shell |
| 6O-31H | O-H | 0.0100 | 0.0326 | 0.93 | 0.037 | Closed-shell | |
| 41H-6O | O-H | 0.0090 | 0.0300 | 0.92 | 0.056 | Closed-shell | |
| 12O-39H | O-H | 0.0029 | 0.0103 | 0.75 | 0.913 | Closed-shell | |
| 22C-25O | C-O | 0.3934 | 0.4269 | 1.86 | 0.030 | Intermediate | |
| 12O-11C | C-O | 0.2877 | −0.2086 | 2.13 | 0.010 | Shared-shell | |
| 2C-6O | C-O | 0.2809 | −0.1819 | 2.12 | 0.009 | Shared-shell | |
| 6O-13C | C-O | 0.2450 | −0.2087 | 2.17 | 0.004 | Shared-shell | |
| 26C-28C | C-C | 0.3422 | −1.0036 | 3.91 | 0.308 | Shared-shell | |
| 8C-11C | C-C | 0.3167 | −0.8923 | 4.14 | 0.247 | Shared-shell | |
| 2C-3C | C-C | 0.3132 | −0.8649 | 4.05 | 0.249 | Shared-shell | |
| 11C-2C | C-C | 0.3124 | −0.8722 | 4.35 | 0.268 | Shared-shell | |
| 4C-1C | C-C | 0.3104 | −0.8365 | 4.06 | 0.215 | Shared-shell | |
| 8C-4C | C-C | 0.3064 | −0.8238 | 4.10 | 0.201 | Shared-shell |
| Molecule/Site | ΔG′ (HAT) | ΔH′ (HAT) | ΔG′ (IP) | ΔH′ (IP) | ΔG′ (PDE) | ΔH′ (PDE) | ΔG′ (PA) | ΔH′ (PA) | ΔG′ (ETE) | ΔH′ (ETE) |
|---|---|---|---|---|---|---|---|---|---|---|
| GIN—phenolic O–H | 402.5 | 405.8 | 167.5 | 172.5 | 235.0 | 233.3 | 337.3 | 340.7 | 65.2 | 65.1 |
| GIN—aliphatic O–H | 410.2 | 414.8 | 167.5 | 172.5 | 242.7 | 242.3 | 355.1 | 359.3 | 55.1 | 55.5 |
| PAR—phenolic O–H | 396.8 | 396.9 | 173.7 | 174.1 | 223.1 | 222.8 | 345.2 | 344.7 | 51.6 | 52.2 |
| SHO—phenolic O–H | 396.6 | 396.8 | 173.7 | 174.0 | 222.9 | 222.9 | 344.9 | 344.0 | 51.8 | 52.8 |
| Molecule/Site | ΔG° (Overall) | ΔH° (Overall) |
|---|---|---|
| GIN—phenolic O–H | +7.7 | +10.6 |
| GIN—aliphatic O–H | +15.4 | +19.6 |
| PAR—phenolic O–H | +2.0 | +1.7 |
| SHO—phenolic O–H | +1.8 | +1.6 |
| Molecule/Site | ΔG′ (HAT) | ΔH′ (HAT) | ΔG′ (IP) | ΔH′ (IP) | ΔG′ (PDE) | ΔH′ (PDE) | ΔG′ (PA) | ΔH′ (PA) | ΔG′ (ETE) | ΔH′ (ETE) |
|---|---|---|---|---|---|---|---|---|---|---|
| GIN—phenolic O–H | 392.6 | 393.0 | 130.3 | 131.3 | 262.3 | 261.9 | 290.1 | 289.8 | 102.5 | 103.4 |
| GIN—aliphatic O–H | 415.4 | 415.9 | 130.3 | 131.3 | 285.1 | 284.4 | 302.6 | 302.8 | 112.8 | 113.0 |
| PAR—phenolic O–H | 392.9 | 392.7 | 130.5 | 130.5 | 262.4 | 262.0 | 291.0 | 290.4 | 101.9 | 102.3 |
| SHO—phenolic O–H | 393.0 | 393.0 | 130.7 | 131.0 | 262.3 | 262.0 | 290.7 | 290.4 | 102.4 | 102.8 |
| Molecule/Site | ΔG° (Overall) | ΔH° (Overall) |
|---|---|---|
| GIN—phenolic O–H | −4.3 | −4.3 |
| GIN—aliphatic O–H | +18.5 | +18.6 |
| PAR—phenolic O–H | −4.0 | −4.6 |
| SHO—phenolic O–H | −3.9 | −4.3 |
| Molecule/Site | ΔG′ (HAT) | ΔH′ (HAT) | ΔG′ (IP) | ΔH′ (IP) | ΔG′ (PDE) | ΔH′ (PDE) | ΔG′ (PA) | ΔH′ (PA) | ΔG′ (ETE) | ΔH′ (ETE) |
|---|---|---|---|---|---|---|---|---|---|---|
| GIN—phenolic O–H | 397.8 | 396.2 | 150.3 | 149.4 | 247.5 | 246.6 | 320.4 | 319.5 | 77.4 | 76.7 |
| GIN—aliphatic O–H | 414.7 | 414.8 | 150.3 | 149.4 | 264.5 | 265.6 | 329.6 | 328.9 | 85.1 | 85.7 |
| PAR—phenolic O–H | 395.1 | 395.4 | 149.4 | 149.8 | 245.7 | 245.6 | 322.6 | 322.4 | 72.5 | 72.9 |
| SHO—phenolic O–H | 395.3 | 395.4 | 150.0 | 150.2 | 245.3 | 245.4 | 323.9 | 322.5 | 71.4 | 72.8 |
| Molecule/Site | ΔG° (Overall) | ΔH° (Overall) |
|---|---|---|
| GIN—phenolic O–H | +2.9 | +0.9 |
| GIN—aliphatic O–H | +19.8 | +19.5 |
| PAR—phenolic O–H | +0.2 | +0.1 |
| SHO—phenolic O–H | +0.4 | +0.1 |
| Symbol | Descriptor | Formula | Units |
|---|---|---|---|
| vIP | Vertical ionization potential | −EHOMO | eV |
| vEA | Vertical electron affinity | −ELUMO | eV |
| ΔE | HOMO–LUMO energy gap | ELUMO − EHOMO | eV |
| η | Chemical hardness | (vIP − vEA)/2 | eV |
| S | Chemical softness | 1/(2η) | eV−1 |
| χ | Electronegativity | (vIP + vEA)/2 | eV |
| μ | Chemical potential | −χ | eV |
| ω | Global electrophilicity index | Χ2/(2η) | eV |
| ΔNmax | Maximum charge acceptance | −μ/η | – |
| ω− | Electro-donating power | (3vIP + vEA)2/[16(vIP − vEA)] | eV |
| ω⁺ | Electro-accepting power | (vIP + 3vEA)2/[16(vIP − vEA)] | eV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lgaz, H.; Messali, M.; Lee, H.-s. Mechanistic Evaluation of Radical Scavenging Pathways in Ginger Phenolics: A DFT Study of 6-Gingerol, 6-Shogaol, and 6-Paradol. Int. J. Mol. Sci. 2025, 26, 11217. https://doi.org/10.3390/ijms262211217
Lgaz H, Messali M, Lee H-s. Mechanistic Evaluation of Radical Scavenging Pathways in Ginger Phenolics: A DFT Study of 6-Gingerol, 6-Shogaol, and 6-Paradol. International Journal of Molecular Sciences. 2025; 26(22):11217. https://doi.org/10.3390/ijms262211217
Chicago/Turabian StyleLgaz, Hassane, Mouslim Messali, and Han-seung Lee. 2025. "Mechanistic Evaluation of Radical Scavenging Pathways in Ginger Phenolics: A DFT Study of 6-Gingerol, 6-Shogaol, and 6-Paradol" International Journal of Molecular Sciences 26, no. 22: 11217. https://doi.org/10.3390/ijms262211217
APA StyleLgaz, H., Messali, M., & Lee, H.-s. (2025). Mechanistic Evaluation of Radical Scavenging Pathways in Ginger Phenolics: A DFT Study of 6-Gingerol, 6-Shogaol, and 6-Paradol. International Journal of Molecular Sciences, 26(22), 11217. https://doi.org/10.3390/ijms262211217








