Electrodeposition of Samarium Metal, Alloys, and Oxides: Advances in Aqueous and Non-Aqueous Electrolyte Systems
Abstract
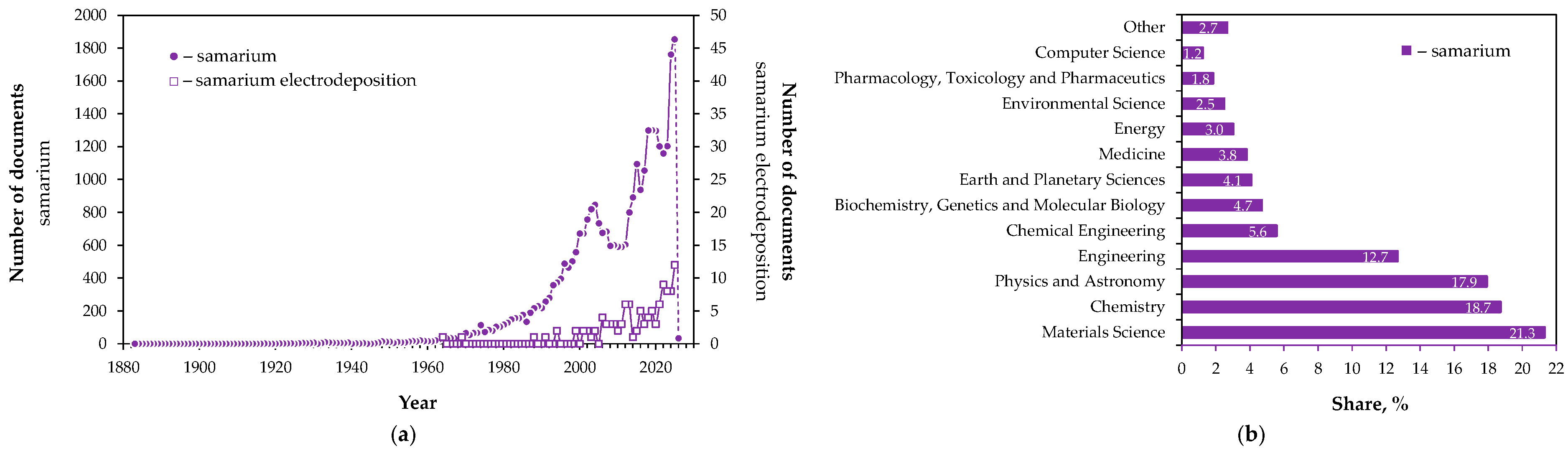
1. Introduction
2. Electrodeposition of Samarium Metal
2.1. Aqueous Solutions
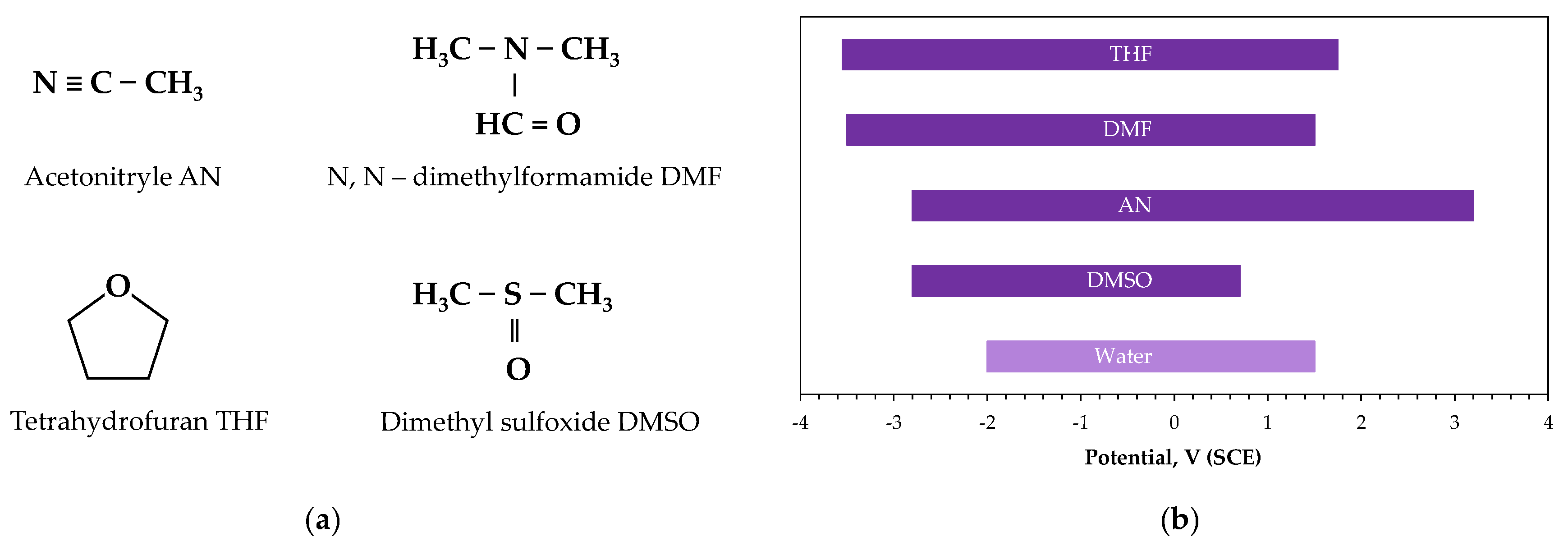
2.2. Molar Liquid Electrolytes
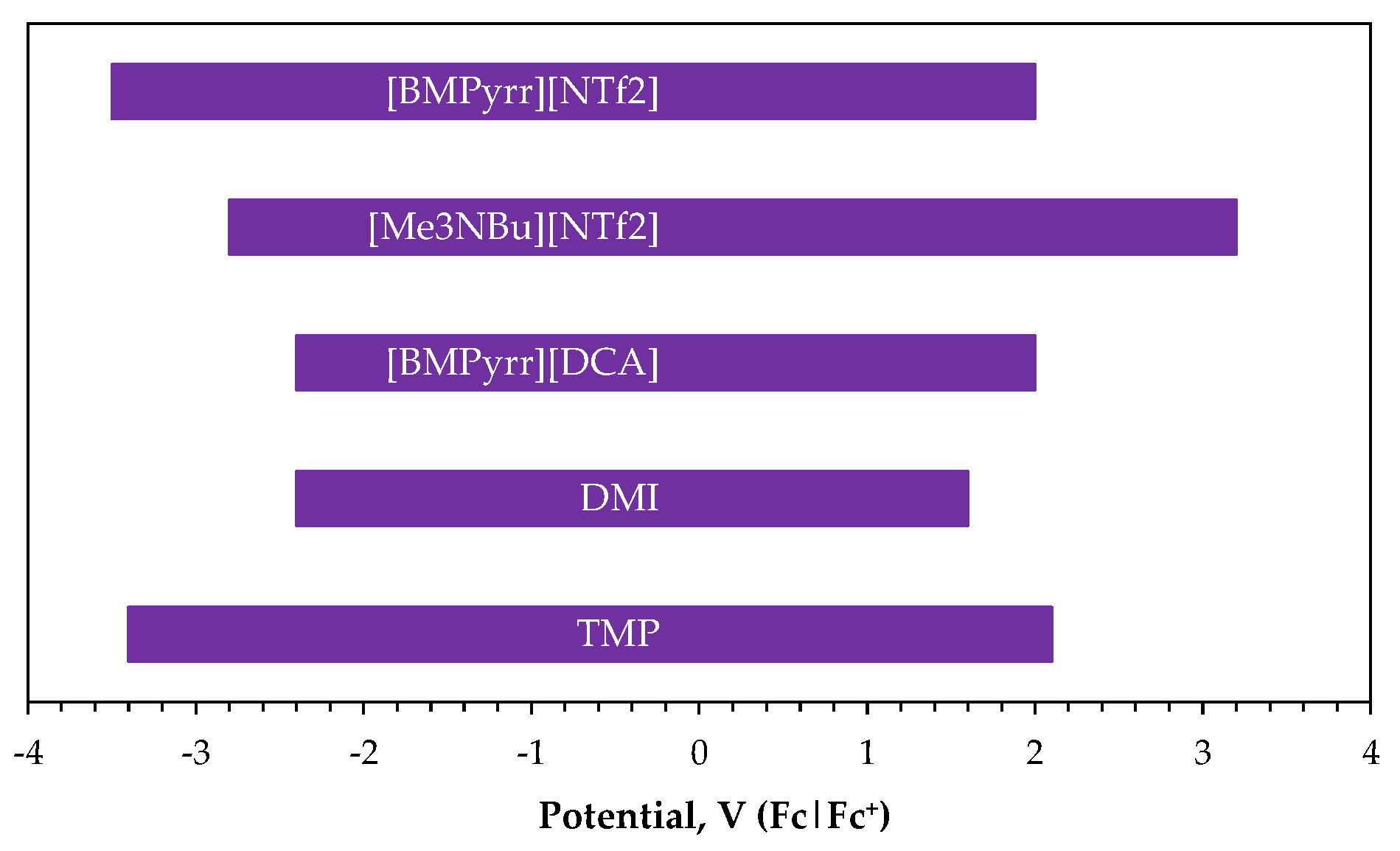
2.3. Ionic Liquid Electrolytes
2.4. Deep Eutectic Solvent Electrolytes
2.5. Molten Salt Electrolytes
3. Electrodeposition of Samarium Alloys
3.1. Sm–Co Alloys
3.1.1. Aqueous Solutions
3.1.2. Molecular Liquid Solvents
3.1.3. Ionic Liquid Solvents
3.1.4. Deep Eutectic Solvents
3.1.5. Molten Salt Electrolytes
3.2. Sm–Ni–(Fe) Alloys
3.3. Other Alloys
4. Electrodeposition of Samarium Oxides
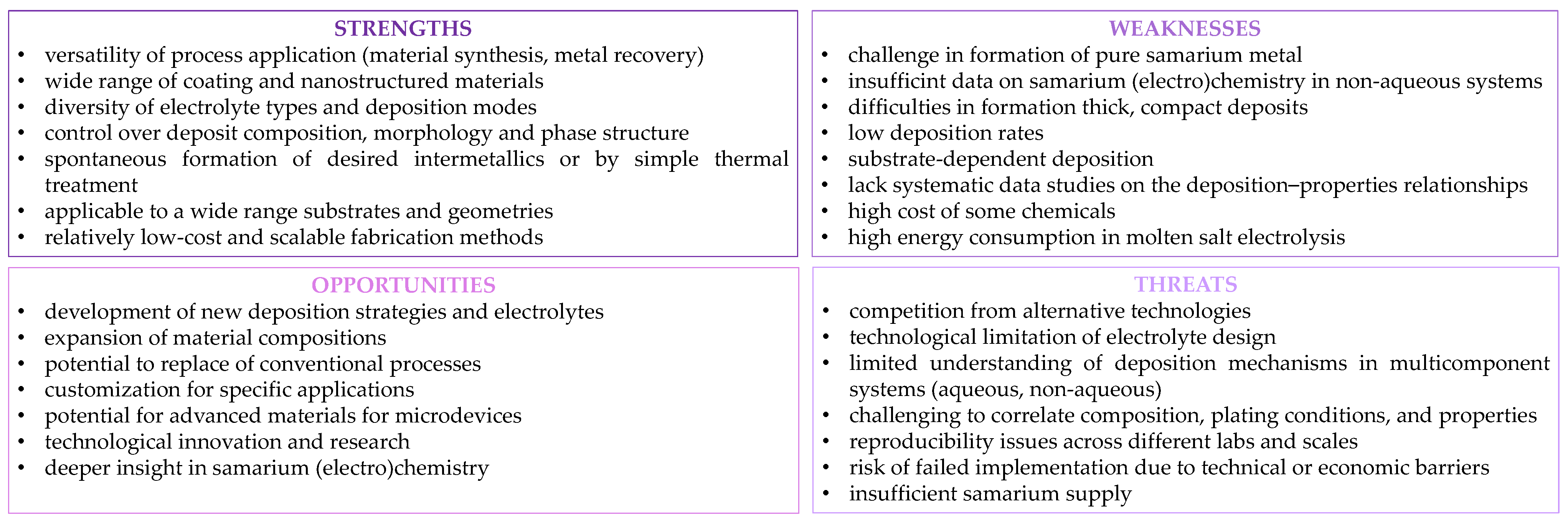
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SHE | Standard hydrogen electrode |
| SCE | Saturated calomel electrode |
| Fc/Fc+ | Ferrocene/ferrocenium redox couple |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffractometry |
| GD | Galvanostatic electrodeposition |
| PD | Potentiostatic electrodeposition |
| PCD | Pulsed current electrodeposition |
| DCV | Direct current deposition at constant voltage |
| OCP | Open circuit potential |
| GC | Glassy carbon |
References
- Critical Minerals Policy Tracker. Available online: https://www.iea.org/data-and-statistics/data-tools/critical-minerals-policy-tracker (accessed on 13 October 2025).
- Strnat, K.J. The Recent Development of Permanent Magnet Materials Containing Rare Earth Metals; Technical Report AFML-TR-69-299; Air Force Materials Laboratory, Air Force Systems Command: Wright-Patterson Air Force Base, Dayton, OH, USA, 1970. [Google Scholar]
- Moosa, I.S. History and development of permanent magnets. Int. J. Res. Dev. Technol. 2014, 2, 18–26. [Google Scholar]
- Yi, J.H. Development of samarium-cobalt rare earth permanent magnetic materials. Rare Met. 2014, 33, 633–640. [Google Scholar] [CrossRef]
- Robinson, J.J. Introducing the nominees for the greatest materials moments in history. JOM 2006, 58, 51–55. [Google Scholar] [CrossRef]
- Liu, S. Sm–Co high-temperature permanent magnets materials. Chin. Phys. B 2019, 28, 017501. [Google Scholar] [CrossRef]
- Ray, A.; Strnat, K. Easy directions of magnetization in ternary R2(Co, Fe)17 phases. IEEE Trans. Magn. 1972, 8, 516–518. [Google Scholar] [CrossRef]
- Iriyama, T.; Imaoka, N. The discovery of Sm2Fe17N3 permanent magnet material. J. Jpn. Soc. Powd. Powd. Metall. 1996, 43, 59–65. [Google Scholar] [CrossRef]
- Saito, T. Progress and prospect of Sm-Fe-N magnets. Inorganics 2025, 13, 322. [Google Scholar] [CrossRef]
- Cui, J.; Ormerod, J.; Parker, D.; Ott, R.; Palasyuk, A.; McCall, S.; Paranthaman, M.P.; Kesler, M.S.; McGuire, M.A.; Nlebedim, I.C.; et al. Manufacturing processes for permanent magnets: Part I—Sintering and casting. JOM 2022, 74, 1279–1295. [Google Scholar] [CrossRef]
- Samarium Cobalt Rare Earth Magnets Market Consumption Trends: Growth Analysis 2025–2033. Available online: https://www.marketreportanalytics.com/reports/samarium-cobalt-rare-earth-magnets-63538# (accessed on 13 October 2025).
- Rizos, V.; Righetti, E.; Kassab, A. Understanding the barriers to recycling critical raw materials for the energy transition: The case of rare earth permanents magnets. Energy Rep. 2024, 12, 1673–1682. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Ilankoon, I.M.S.K.; Dushyantha, N.; Nwaila, G.T. Rare earth permanent magnets for the green energy transition: Bottlenecks, current developments and cleaner production. Res. Conserv. Rec. 2025, 212, 107966. [Google Scholar] [CrossRef]
- Strnat, K.J.; Strnat, R.M. Rare earth–cobalt permanent magnets. J. Magn. Magn. Mater. 1991, 100, 38–56. [Google Scholar] [CrossRef]
- Factsheets—CRMS 2023. Rare Earth Elements. Available online: https://scrreen.eu/wp-content/uploads/2023/08/SCRREEN2_factsheets_REE-EUROSTAT.pdf (accessed on 13 October 2025).
- Finlay, I.G.; Mason, M.D.; Shelley, M. Radioisotopes for the palliation of metastatic bone cancer: A systematic review. Lancet Oncol. 2005, 6, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.H.; Huang, X. Review in progress of rare earth science and technology in 2024. J. Rare Earths 2025, 43, 2029–2052. [Google Scholar] [CrossRef]
- Hossain, M.K.; Raihan, G.A.; Akbar, M.A.; Rubel, M.H.K.; Ahmed, M.H.; Khan, M.I.; Hossain, S.; Sen, S.K.; Jalal, M.I.E.; El-Denglawey, A. Current application and future potential of rare earth oxides in sustainable nuclear, radiation, and energy devices: A review. ACS Appl. Electron. Mater. 2022, 4, 3327–3353. [Google Scholar] [CrossRef]
- Moreira, O. Analysis of 149Sm time evolution and the reactivity contribution in nuclear reactors. Ann. Nucl. Energy 2015, 83, 87–93. [Google Scholar] [CrossRef]
- Banik, B.K. Samarium metal in organic synthesis. Eur. J. Org. Chem. 2002, 15, 2431–2444. [Google Scholar] [CrossRef]
- Röckl, J.L.; Lundberg, H. Samarium and ytterbium in organic electrosynthesis. Synthesis 2023, 55, 1375–1384. [Google Scholar]
- Chen, R.; Bai, Y.; Wei, B. Samarium redox catalysis. Chem. Synth. 2025, 5, 62. [Google Scholar] [CrossRef]
- Carlson, R.W. Sm–Nd Dating. In Encyclopedia of Scientific Dating Methods; Rink, W., Thompson, J., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 1–20. [Google Scholar]
- Strekopytov, S. Salute to samarium. Nat. Chem. 2016, 8, 816. [Google Scholar] [CrossRef]
- Szabadváry, F. The history of discovery and separation of the rare earths. In Handbook on the Physics and Chemistry of Rare Earths. Two-Hundred-Year Impact of Rare Earths on Science; Gschneider, K.A., Erying, L.R., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 11, pp. 33–80. [Google Scholar]
- Scopus: A Comprehensive Abstract and Citation Database for Impact Markers. Available online: https://www.elsevier.com/products/scopus (accessed on 15 October 2025).
- Popov, K.I.; Djokić, S.S.; Grgur, B.N. Fundamental Aspects of Electrometallurgy; Kluwer Academic Publishers: New York, NY, USA, 2002. [Google Scholar]
- Nasirpouri, F. Electrodeposition of Nanostructured Materials; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Krishnamurthy, N.; Gupta, C.K. Rare earth metals and alloys by electrolytic methods. Min. Proc. Extract. Metall. Rev. 2001, 22, 477–507. [Google Scholar] [CrossRef]
- Akca-Guler, T.; Yuksekdag, A.; Kose-Mutlu, B.; Koyuncu, I. Advances in electrochemical methods for rare earth elements recovery: A comprehensive review. Proc. Saf. Environ. Protect. 2025, 196, 106897. [Google Scholar] [CrossRef]
- Gosh, B.; Vapink, H.; Kim, H.E.; Kim, Y.; Birawat, R.; Lu, Y.; Su, X.; Yang, H. Electrochemical separation and clean technology applications of rare earth elements. Chem. Rev. 2025, 125, 7965–8023. [Google Scholar] [CrossRef]
- de Zoubow, N.; van Muylder, J. Lanthanides. In Atlas of Electrochemical Equilibria in Aqueous Solutions; Pourbaix, M., Ed.; National Association of Corrosion Engineers: Houston, TX, USA, 1974; pp. 183–197. [Google Scholar]
- Ilkhani, H.; Ganjali, M.R.; Arvand, M.; Norouzi, P. Electrochemical and spectroscopic study of samarium ion interaction with DNA in different pHs. Int. J. Electrochem. Sci. 2010, 5, 168–176. [Google Scholar] [CrossRef]
- López, J.R.; Méndez, P.F.; Pérez Bueno, J.J.; Trejo, G.; Antaño, R.; Torres-González, J.; Stremsdoerfer, G.; Meas, Y. Samarium additive effect onto the nickel electrodeposition process. J. Electrochem. Soc. 2017, 164, D524–D531. [Google Scholar] [CrossRef]
- Atanasyants, A.G.; Seryogin, A.N. Electrochemical extraction of samarium from mixture of rare earth metals. Hydrometallurgy 1997, 44, 255–259. [Google Scholar] [CrossRef]
- Topp, N.E. The Chemistry of Rare-Earth Elements; Elsevier: Amsterdam, The Netherlands, 1965. [Google Scholar]
- Morrison, J.; Sacci, R.; Myhre, K.; Braatz, J.M. Lanthanide electrodeposition in aqueous ammonium acetate: Asurrogate approach for actinide film fabrication. J. Nucl. Mater 2025, 607, 155698. [Google Scholar] [CrossRef]
- Bratsch, S.G. Standard electrode potentials and temperature coefficients in water at 298.15 K. J. Phys. Chem. Ref. Data 1989, 18, 1–21. [Google Scholar] [CrossRef]
- Lokhande, C.D.; Madhale, R.D.; Pawar, S.H. Electrodeposition of samarium. Met. Finish. 1988, 86, 23–25. [Google Scholar]
- Jundhale, S.B.; Lokhande, C.D. Electrodeposition of samarium from tartrate bath. Mater. Chem. Phys. 1991, 27, 265–278. [Google Scholar] [CrossRef]
- Creager, S. Solvents and supporting electrolytes. In Handbook of Electrochemistry; Zoski, S.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 57–72. [Google Scholar]
- Simka, W.; Puszczyk, D.; Nawrat, G. Electrodeposition of metals from non-aqueous solutions. Electrochim. Acta 2009, 54, 5307–5319. [Google Scholar] [CrossRef]
- Polcari, D.; Dauphin–Durchame, P.; Mauzeroll, J. Scanning electrochemical microscopy: A comprehensive review of experimental parameters from 1989 to 2015. Chem. Rev. 2016, 116, 13234–13278. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, B.; Guo, F.; Liu, Y.; Zhao, J.; Shi, Z. LiNO3-assisted electrochemical extraction of metallic Sm from a molecular liquid-based electrolyte. J. Mol. Liq. 2024, 394, 123771. [Google Scholar] [CrossRef]
- da Costa, J.G.d.R.; Costa, J.M.; de Ameida Neto, A.F. Progress on electrodeposition of metals and alloys using ionic liquids as electrolytes. Metals 2022, 12, 2095. [Google Scholar] [CrossRef]
- Yeo, D.; Tini, M.I.; Joly, N.; Souvenir-Zafindrajaona, M.S.; Mbakidi, J.P.; Lequart, V.; Chaveriat, L.; Bouquillon, S.; Martin, P. Ionic liquids: History, conception, applications, and perspectives. Green Chem. Lett. Rev. 2025, 18, 2543910. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martisn, L.M.D.R.S. Application of ionic liquids in electrochemistry—Recent advances. Molecules 2020, 25, 5812. [Google Scholar] [CrossRef]
- Alreshdi, M.A.; Yadav, K.K.; Gunasekaran, S.; Gacem, A.; Sambadan, P.; Subbiah, G.; Bhutto, J.K.; Palanivel, S.; Fallatah, A.M.; El-Khair, M.A.A.; et al. A review on the evolution of ionic liquids: Sustainable synthesis, applications, and future prospects. Mater. Today Sustain. 2025, 31, 101160. [Google Scholar] [CrossRef]
- Deetlefs, M.; Seddon, K.R. Assessing the greenness of some typical laboratory ionic liquid preparations. Green Chem. 2010, 12, 17–30. [Google Scholar] [CrossRef]
- Zhang, Q.; Hua, Y.; Xu, C.; Li, Y.; Li, J.; Dong, P. Non-haloaluminate ionic liquids for low-temperature electrodeposition of rare-earth metals—A review. J. Rare Earths 2015, 33, 1017–1025. [Google Scholar] [CrossRef]
- Nahian, M.K.; Reddy, R.G. Green electrodeposition of common lanthanide rare earth metals using ionic liquids: Challenges and opportunities. J. Electrochem. Soc. 2025, 172, 022503. [Google Scholar] [CrossRef]
- Bourbos, E.; Giannopoulou, I.; Karatonis, A.; Paspaliarris, I.; Panias, D. Reduction of light rare earths and a proposed process for Nd electrorecovery based on ionic liquids. J. Sustain. Metall. 2018, 4, 395–406. [Google Scholar] [CrossRef]
- Razo-Negrete, M.; Ortega-Borges, R.; Zinovyeva, V.; Cannes, C.; Le Naour, C.; Trejo-Córdova, G.; Meas, Y. Comparison of the electrochemical behavior of some rare earth elements in butyl methyl pyrrolidinium dicyanamide ionic liquid. Int. J. Electrochem. Sci. 2019, 14, 10431–10447. [Google Scholar] [CrossRef]
- Bhatt, A.I.; May, I.; Volkovich, V.A.; Collison, D.; Helliwell, M.; Polovov, I.B.; Lewin, R.G. Structural characterization of a lanthanum bistriflimide complex, La(N(SO2CF3)2)3(H2O)3, and an investigation of La, Sm, and Eu electrochemistry in a room-temperature ionic liquid, [Me3NnBu][N(SO2CF3)2]. Inorg. Chem. 2005, 44, 4934–4940. [Google Scholar] [CrossRef]
- Yamagata, M.; Katayama, Y.; Miura, T. Electrochemical behaviour of samarium, europium, and ytterbium in hydrophobic room-temperature molten salt systems. J. Electrochem. Soc. 2006, 153, E5–E9. [Google Scholar] [CrossRef]
- Manjum, M.; Tachikawa, N.; Serizawa, N.; Katayama, Y. Electrochemical behavior of samarium species in an amide-type ionic liquid at different temperatures. J. Electrochem. Soc. 2019, 166, D483–D486. [Google Scholar] [CrossRef]
- Andrew, C.; Dhivya, M.; Jayakumar, M. Electrochemical and spectroscopic investigation of samarium in a neutral ligand based-ionic liquid. J. Electroanal. Chem. 2021, 895, 115398. [Google Scholar] [CrossRef]
- Andrew, C.; Murugesan, C.; Jayakumar, M. Electrochemical behavior of Sm(III) and electrodeposition of samarium from 1-butyl-1-methylpyrrolidinium dicyanamide ionic liquid. J. Electrochem. Soc. 2022, 169, 022503. [Google Scholar] [CrossRef]
- Andrew, C.; Jayakumar, M. Voltammeric behavior and electrodeposition of samarium in 1-butyl-1-methylpyrrolidinium bis(trifluoromethylulfonyl)imide ionic liquid. J. Electrochem. Soc. 2022, 169, 092517. [Google Scholar]
- Andrew, C.; Jayakumar, M. Electrodeposition behaviour of samarium in 1,3-dimethyl-2-imidazolidone solvent. Pure Appl. Chem. 2024, 96, 875–887. [Google Scholar] [CrossRef]
- Jagadeeswara Rao, C.; Venkatesan, K.A.; Nagarajan, K.; Srinivasan, T.G.; Vasudeva Rao, P.R. Electrochemical and thermodynamic properties of europium(III), samarium(III) and cerium(III) in 1-butyl-3-methylimidazolulium chloride ionic liquid. J. Nucl. Mater. 2010, 399, 81–86. [Google Scholar] [CrossRef]
- Pan, Y.; Hussey, C.L. Electrochemical and spectroscopic investigation of Ln3+ (Ln = Sm, Eu, and Yb) solvation in bis(trifluoromethylsulfonul)imide-based ionic liquids and coordination by N,N,N′,N′-tetraoctyl-3-oxapentane diamide (TOGA) and chloride. Inorg. Chem. 2013, 52, 3241–3252. [Google Scholar] [CrossRef] [PubMed]
- Matsumiya, M.; Suda, S.; Tsunashima, K.; Sugiya, M.; Kishioka, S.; Matsuura, H. Electrochemical behaviors of multivalent complexes in room temperature ionic liquids based on quaternary phosphonium cations. J. Electroanal. Chem. 2008, 622, 129–135. [Google Scholar] [CrossRef]
- Schoebrechts, J.P.; Gilbert, B.P.; Duyckaerts, G. Electrochemical and spectroscopic studies of the lanthanides in the AlCl3 + 1-n-butylpyridinium chloride melt at 40 °C. Part I. The Yb(III–II), Sm(III–II) systems. J. Electroanal. Chem. 1983, 145, 127–138. [Google Scholar] [CrossRef]
- Ispas, A.; Buschbeck, M.; Pitula, S.; Mudring, A.; Uhlemann, M.; Bund, A.; Endres, F. Electrodeposition of Co, Sm, and Co-Sm thin layers. ECS Trans. 2009, 16, 119–127. [Google Scholar] [CrossRef]
- Molodkina, E.B.; Ehrenburg, M.R.; Rudnev, A.V. Electrochemical codeposition of Sm and Co in a dicyanamide ionic liquid. Russ. J. Electrochem. 2022, 58, 1083–1093. [Google Scholar] [CrossRef]
- Bagri, P.; Luo, H.; Popovs, I.; Thapaliya, B.P.; Dehaudt, J.; Dai, S. Trimethyl phosphate based neutral ligand room temperature ionic liquids for electrodepositiin if rare earth elements. Electrochem. Comm. 2018, 96, 88–92. [Google Scholar] [CrossRef]
- Rout, A. Electroanalytical chemistry of lanthanides/actinides and the feasibility of direct electrodeposition in ligand containing ionic liquids: A comprehensive review. J. Electrochem. Soc. 2022, 169, 126502. [Google Scholar] [CrossRef]
- Lin, M.; Jiao, H.; Yuan, R.; Li, L.; Wang, L.; Sun, R.; Tian, D.; Jiao, S. Recent progress on electrodeposition of metal/alloy films or coatings in deep eutectic solvents. Trans. Nonferr. Met. Soc. China 2025, 35, 2803–2821. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Database of deep eutectic solvents and their physical properties: A review. J. Mol. Liq. 2023, 384, 121899. [Google Scholar] [CrossRef]
- Doneux, T.; Sorgho, A.; Soma, F.; Rayée, Q.; Bouguoma, M. Electrodeposition in deep eutectic solvents: The “obvious”, the “unexpected” and the “wonders”. Molecules 2024, 29, 3439. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs. ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Green, T.A.; Roy, S. Challenges to the adoption of deep eutectic solvents in the electrodeposition industries. ECS Adv. 2025, 4, 023001. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, J.; Li, G.; Zhao, W.; Zhao, X.; Mu, T. The electrochemical stability of ionic liquids and deep eutectic solvents. Sci. China Chem. 2016, 59, 571–577. [Google Scholar] [CrossRef]
- Hayler, H.J.; Perkin, S. The eutectic point in choline chloride and ethylene glycol mixtures. Chem. Commun. 2022, 58, 12728–12731, Erratum in Chem. Commun. 2024, 60, 1192. [Google Scholar] [CrossRef] [PubMed]
- Rudnev, A.V. Electrodeposition of lanthanides from ionic liquids and seep eutectic solvents. Russ. Chem. Rev. 2020, 89, 1463–1482. [Google Scholar] [CrossRef]
- Gómez, E.; Cojocaru, P.; Magagnin, L.; Valles, E. Electrodeposition of Co, Sm, and SmCo from a deep eutectic solvent. J. Electroanal. Chem. 2011, 658, 18–24. [Google Scholar] [CrossRef]
- Panzeri, G.; Tresoldi, M.; Rinaldi, C.; Magagnin, L. Electrodeposition of magnetic SmCo films from deep eutectic solvents and choline chloride–ethylene glycol mixtures. J. Electrochem. Soc. 2017, 164, D930–D933. [Google Scholar] [CrossRef]
- Li, J.X.; Lai, H.; Zhang, Z.C.; Zhuang, B.; Huang, Z.G. Electrodeposited Sm–Co alloy films and their magnetic properties in low temperature molten salts. Acta Phys. Chim. Sin. 2007, 23, 1301–1305. [Google Scholar]
- Liu, P.; Du, Y.; Yang, Q.; Tong, Y.; Hope, G.A. Induced codeposition of Sm–Co amorphous films in urea melt and their magnetism. J. Electrochem. Soc. 2006, 153, C57. [Google Scholar] [CrossRef]
- Habashi, F. Extractive metallurgy of rare earths. Can. Metall. Quart. 2013, 52, 224–233. [Google Scholar] [CrossRef]
- Massot, L.; Chamelot, P.; Taxil, P. Cathodic behaviour of samarium(III) in LiF−CaF2 media on molybdenum and nickel electrodes. Electrochim. Acta 2005, 50, 5510–5517. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Q.; Yan, Y.D.; Chen, L.; Zhang, M.L.; Zhang, Z.J. Cathodic behaviour of samarium(III) in LiCl–KCl melts on molybdenum and aluminum electrodes. Energy Proc. 2013, 39, 474–479. [Google Scholar] [CrossRef]
- Ge, J.; Yang, Q.; Wang, Y.; Zhuo, W.; Du, M.; Zhang, J. Selective electrodeposition of europium and samarium in molten LiCl–KCl with copper and aluminum electrodes. J. Electrochem. Soc. 2020, 167, 022501. [Google Scholar] [CrossRef]
- Li, Z.; Tang, D.; Meng, S.; Gu, L.; Dai, Y.; Liu, Z. Electrolytic separation of Dy from Sm in molten LiCl–KCl using Pb–Bi eutectic alloy cathode. Sep. Purif. Technol. 2021, 276, 119045. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, K.; Li, W.; Liu, Q.; Yu, J.; Zhu, J.; Yan, Y.; Zhang, M.; Wang, J. Electrochemical extraction of fission element samarium from molten NaCl–2CsCl eutectic salt using the liquid gallium cathode. ACS Sustain. Chem. Eng. 2023, 11, 8685–8698. [Google Scholar] [CrossRef]
- Yamamura, T.; Mehmood, M.; Maekawa, H.; Sato, Y. Electrochemical processing of rare–earth and rare metals using molten salts. Chem. Sustain. Dev. 2004, 12, 105–111. [Google Scholar]
- Zuo, Y.; Li, X.J.; Jiang, F.; She, C.F.; Huang, W.; Gong, Y. Electrochemical behavior of Sm(III)/Sm(II) and extraction of Sm on reactive electrode from molten LiF–BeF2. Sep. Purif. Technol. 2023, 315, 123737. [Google Scholar] [CrossRef]
- Castrillejo, Y.; Fernández, P.; Medina, J.; Hernández, P.; Barrado, E. Electrochemical extraction of samarium from molten chlorides in pyrochemical processes. Electrochim. Acta 2011, 56, 8638–8644. [Google Scholar] [CrossRef]
- Straka, M.; Korenko, M.; Lisy, F.; Szatmáry, L. Electrochemistry of samarium in lithium-beryllium fluoride salt mixture. J. Rare Earths 2011, 29, 798–803. [Google Scholar] [CrossRef]
- Straka, M.; Szatmáry, L. Electrochemistry of selected lanthanides in FLiBe and possibilities of their recovery on reactive cathode. Proc. Chem. 2012, 7, 804–813. [Google Scholar] [CrossRef]
- Castrillejo, Y.; de la Fuente, C.; Vega, M.; de la Rosa, F.; Pardo, R.; Barrado, E. Cathodic behaviour and oxoacidity reactions of samarium(III) in two molten chlorides with different acidity properties the eutectic LiCl–KCl and the equimolar CaCl2–NaCl melt. Electrochim. Acta 2013, 97, 120–131. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, H.; Tang, D.; Ji, N.; Dai, Y.; Gao, Z.; He, F.; Guo, K.; Zhou, J.; Liu, Z. Selective recovery of rare earth fps from radioactive molten salts using liquid In-Sn alloy cathodes and real-time monitoring techniques. J. Nucl. Mater. 2026, 618, 156179. [Google Scholar] [CrossRef]
- Cordoba, G.; Caravaca, C. An electrochemical study of samarium ions in the molten eutectic LiCl–KCl. J. Electroanal. Chem. 2004, 572, 145–151. [Google Scholar] [CrossRef]
- Yin, T.; Chen, L.; Xue, Y.; Zheng, Y.; Wang, X.; Yan, Y.; Zhang, M.; Wang, G.; Gao, F.; Qiu, M. Electrochemical behavior and underpotential deposition of Sm on reactive electrodes (Al, Ni, Cu and Zn) in a LiCl–KCl melt. Int. J. Min. Metall. Mater. 2020, 27, 1657–1665. [Google Scholar] [CrossRef]
- Xu, C.; Hu, X.; Yu, J.; Li, P.; Liu, A.; Luo, S.; Shi, Z.; Wang, Z. Raman spectroscopy and quantum chemical calculation on SmCl3–KCl–Li molten salt system. J. Mol. Liq. 2024, 394, 123693. [Google Scholar] [CrossRef]
- Bae, S.E.; Jung, T.S.; Cho, Y.H.; Kim, J.Y.; Kwak, K.; Park, T.H. Electrochemical formation of divalent samarium cation and its characteristics in LiCl–KCl melt. Inorg. Chem. 2018, 57, 8299–8306. [Google Scholar] [CrossRef]
- Jiang, S.; Ye, C.; Liu, Y.; Yang, D.; Wang, L.; Liu, Y.; Zhong, Y.; Wu, Y.; Chai, Z.; Shi, W. Insights into the effects of fluoride anions on the electrochemical behavior and solution structure of trivalent samarium in LiCl–KCl molten salt. Electrochim. Acta 2023, 439, 141733. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, Y.; Liu, X.; Huang, C.; Li, B. Cathodic behavior of samarium(III) and Sm–Al alloys preparation in fluorides melts. Electrochim. Acta 2016, 190, 208–214. [Google Scholar] [CrossRef]
- Serp, J.; Allibert, M.; Beneš, O.; Delpech, S.; Feynberg, O.; Ghetta, V.; Heuer, D.; Holcomb, D.; Ignatiev, V.; Kloosterman, J.L.; et al. The molten salt reactor (MSR) in generation IV: Overview and perspectives. Prog. Nucl. Energy 2014, 77, 308–319. [Google Scholar] [CrossRef]
- Iida, T.; Nohira, T.; Ito, Y. Electrochemical formation of Sm–Ni alloy films in a molten LiCl–KCl–SmCl3 system. Electrochim. Acta 2001, 46, 2537–2544. [Google Scholar] [CrossRef]
- Iida, T.; Nohira, T.; Ito, Y. RBS analysis of Sm–Ni alloy films in a molten salt electrochemical process. J. Alloys Compd. 2005, 386, 207–210. [Google Scholar] [CrossRef]
- Iida, T.; Nohira, T.; Ito, Y. Electrochemical formation of Sm–Co alloy films by Li codeposition method in a molten LiCl−KCl−SmCl3 system. Electrochim. Acta 2003, 48, 901–906. [Google Scholar] [CrossRef]
- Tokushige, M.; Hongo, H.; Nishikiori, T.; Ito, Y. Formation of Sm–Co intermetallic compound nanoparticles based on plasma-induced cathodic discharge electrolysis in chloride melt. J. Electrochem. Soc. 2012, 159, E5–E10. [Google Scholar] [CrossRef]
- Yan, Q.C.; Guo, X.M. Electrochemical behavior for preparation of Sm2Fe17 in CaCl2−CaF2−SmCl3 system. J. Alloys Compd. 2019, 789, 976–982. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys; Academic Press: New York, NY, USA, 1963; Volume I, pp. 75–121. [Google Scholar]
- Kröger, F.A. Cathodic deposition and characterization of metallic or semiconducting binary alloys or compounds. J. Electrochem. Soc. 1978, 125, 2028–2034. [Google Scholar] [CrossRef]
- Schwartz, M.; Myung, N.V.; Nobe, K. Electrodeposition of iron group−rare earth alloys from aqueous media. J. Electrochem. Soc. 2004, 151, C468–C477. [Google Scholar] [CrossRef]
- Wei, J.C.; Schwartz, M.; Nobe, K. Parametric aqueous electrodeposition studies of Co−Sm alloys. ECS Trans. 2006, 1, 273–278. [Google Scholar] [CrossRef]
- Wei, J.C.; Schwartz, M.; Nobe, K. Aqueous electrodeposition of SmCo alloys. I. Hull cell studies. J. Electrochem. Soc. 2008, 155, D660–C665. [Google Scholar] [CrossRef]
- Wei, J.C.; Schwartz, M.; Nobe, K. Electrodeposition of Co−Sm permanent magnets from aqueous media. ECS Trans. 2008, 11, 53–64. [Google Scholar] [CrossRef]
- Wei, J.C.; Schwartz, M.; Nobe, K. DC aqueous electrodeposition of Sm−Co permanent magnets. ECS Trans. 2009, 16, 129–140. [Google Scholar] [CrossRef]
- Wei, J.C.; Schwartz, M.; Nobe, K. Pulsed aqueous electrodeposition of Sm−Co permanent magnets. ECS Trans. 2009, 19, 75–84. [Google Scholar] [CrossRef]
- Wei, J.C.; Schwartz, M.; Nobe, K.; Myung, N.V. Aqueous electrodeposition of SmCo alloys: II. Direct current studies. Front. Chem. 2021, 9, 694726. [Google Scholar] [CrossRef] [PubMed]
- Chourabi, K.; Woytasik, M.; Dufour-Gergam, E.; Lefeuvre, E.; Moulin, J. DC electrodepositon of thick SmCo films for MEMS applications. J. Electrochem. Soc. 2012, 159, D592–D596. [Google Scholar] [CrossRef]
- Chourabi, K.; Woytasik, M.; Lefeuvre, E.; Moulin, J. SmCo micromolding in an aqueous electrolyte. Microsyst. Technol. 2013, 19, 887–893. [Google Scholar] [CrossRef]
- Moulin, J.; Woytasik, M.; Belghiti, D.; Chourabi, K. Sm–Co thick films micromolding. J. Electrochem. Soc. 2014, 161, B3034–B3037. [Google Scholar] [CrossRef]
- Xie, M.; Zhu, L.; Li, W.; Liu, H.; Zhang, T. Electrodeposition of Sm–Co alloy films with nanocrystalline/amorphous structures from a sulphamate aqueous solution. Int. J. Electrochem. Sci. 2017, 12, 11330–C11342. [Google Scholar] [CrossRef]
- Park, J.H.; Kwon, H.; Park, J.H.; Ro, J.C.; Suh, S.J. Synthesis and characterization of Sm2Co17 using electrodeposition and reduction-diffusion process. J. Alloys Compd. 2022, 901, 163669. [Google Scholar] [CrossRef]
- Long, X.; Guo, G.; Li, X.; Xia, Q.; Zhang, J. Electrodeposition of Sm–Co film with high Sm content from aqueous solution. Thin Solid Film. 2013, 548, 259–262. [Google Scholar] [CrossRef]
- Zhang, J.; Evans, P.; Zahgari, G. Electrodeposition of Sm–Co nanoparticles from aqueous solutions. J. Magn. Magn. Mater. 2004, 283, 89–94. [Google Scholar] [CrossRef]
- Yang, W.; Cui, C.; Liu, Q.; Cao, B.; Liu, L.; Zhang, Y. Fabrication and magnetic properties of Sm2Co17 and Sm2Co17/Fe7Co3 magnetic nanowires via AAO templates. J. Cryst. Growth 2014, 399, 1–6. [Google Scholar] [CrossRef]
- Herrera, E.; Riva, J.; Pozo-López, G.; Condó, A.; Urreta, S.E.; Fabietti, L.M. Very low potential electrodeposition of Sm−Co nanostructures in aqueous medium using hard templates. J. Electrochem. Soc. 2019, 166, D460–D466. [Google Scholar] [CrossRef]
- Herrera, E.; Riva, J.; Urreta, S.E.; Aquirre, M.D.C. Nucleation and growth mechanisms of bimetallic of Sm−Co nanowires and nanotubes. J. Electrochem. Soc. 2023, 170, 082504. [Google Scholar] [CrossRef]
- Sato, Y.; Ishida, H.; Kobayakawa, K.; Abe, Y. Electrodeposition of Sm−Co from formamide solution. Chem. Lett. 1990, 19, 1471–1474. [Google Scholar] [CrossRef]
- Sato, Y.; Takazawa, T.; Takashi, M.; Ishida, H.; Kobayakawa, K. Electrolytic preparation of Sm-Co thin films and their magnetic properties. Plat. Surf. Finish. 1993, 80, 72–74. [Google Scholar]
- Ali, S.S.; Cheng, C.; Parajuli, S.; Zhang, X.; Feng, J.; Han, X.F. Non-aqueous electro-deposition of novel one-dimensional Sm2Co17 nanostructures and magnetic field annealing effect on structural and magnetic properties. J. Magn. Magn. Mater. 2022, 547, 168836. [Google Scholar] [CrossRef]
- Manjum, M.; Serizawa, N.; Ispas, A.; Bund, A.; Katayama, Y. Electrochemical preparation of cobalt−samarium nanoparticles in aprotic ionic liquid. J. Electrochem. Soc. 2020, 167, 042505. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, L.; Yang, N.; Tian, L.; Xie, G.; Li, B. Ternary PtSmCo NPs electrocatalysts with enhanced oxygen reduction reaction. J. Rare Earth 2020, 38, 1305–1311. [Google Scholar]
- Chen, Y.; Wang, H.; Li, B. Electrodeposition of SmCo alloy nanowires with a large length-diameter ratio from SmCl3−CoCl2−1-ethyl-3-methylimidazolium chloride ionic liquid. RSC Adv. 2015, 5, 39620–39624. [Google Scholar] [CrossRef]
- Panzeri, G.; Magagnin, L. Electrodeposition of magnetic SmCo films from deep eutectic solvents. ECS Trans. 2016, 75, 31–35. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yan, Y.D.; Zhang, M.L.; Liang, M.L.; Liang, Y.; Qu, J.M.; Li, P.; Ji, D.B.; Xue, Y.; Jing, X.Y.; et al. Electrochemical synthesis of Sm−Co metal magnetic materials by co-reduction of Sm(III) and Co(II) in LiCl−KCl−SmCl3−CoCl2 melt. Electrochim. Acta 2017, 249, 278–289. [Google Scholar] [CrossRef]
- Takeda, O.; Ideno, T.; Nakamura, E.; Sato, Y. Electrochemical production of Sm−Co thin film in molten LiCl−KCl system. ECS Trans. 2009, 16, 431–439. [Google Scholar] [CrossRef]
- Iida, T.; Nohira, T.; Ito, Y. Electrochemical formation of Sm-Co alloy films by codeposition of Sm and Co in a molten LiCl−KCl−SmCl3−CoCl2 system. Electrochim. Acta 2003, 48, 2517–2521. [Google Scholar] [CrossRef]
- Murali Krishna, G.; Jeyabharathi, C.; Jayakumar, M. Electrochemical deposition of nickel−samarium coatings from deep eutectic solvent for hydrogen evolution reaction. Ionics 2025, 31, 5951–5963. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yan, Y.D.; Zhang, M.L.; Zheng, J.N.; Zhao, Y.; Wang, P.; Yin, T.Q.; Xue, Y.; Jing, X.Y.; Han, W. Electrochemical synthesis of Sm−Ni alloy magnetic materials by co-reduction of Sm(III) and Ni(II) in LiCl−KCl−SmCl3−NiCl2 melt. J. Electrochem. Soc. 2016, 163, D672–D681. [Google Scholar] [CrossRef]
- Kou, Y.; Zhang, W.; Lou, C.; Lv, X. Influence of current density on microstructure and magnetic property of electrodeposited Sm−Fe alloy film. J. Funct. Mater. 2013, 44, 98–101. [Google Scholar]
- Lou, C.; Kou, Y.; Lyu, X.; Zhang, W. Electrodeposition of Sm−Fe thin film in aqueous solution under magnetic field. Int. J. Electrochem. Sci. 2015, 10, 9687–9694. [Google Scholar] [CrossRef]
- Li, J.; Lai, H.; Fan, B.; Zhuang, B.; Guan, L.; Huang, Z. Electrodeposition of RE−TM (RE = La, Sm, Gd; TM = Fe, Co, Ni) films and magnetic properties in urea melt. J. Alloys Compd. 2009, 477, 547–551. [Google Scholar] [CrossRef]
- Gandhi, A.A.; Rahman, I.Z.; Mousavi, M.V.K.; Rahman, M.A. Sm-doped NiFe thin films: Structural and magnetic characterization. Adv. Mater. Res. 2011, 264–265, 518–523. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.L.; Yuan, L.Y.; He, H.; Yang, Z.Y.; Zhao, X.L.; Chai, Z.F.; Shi, W.Q. Electroextraction of samarium from Sm2O3 in chloride melts. Electrochim. Acta 2014, 129, 401–409. [Google Scholar] [CrossRef]
- Xue, Y.; Zhou, Z.P.; Yan, Y.D.; Zhang, M.L.; Li, X.; Ji, D.B.; Tang, H.; Zhang, Z.J. Electrochemistry of Zn and co-reduction of Zn and Sm from LiCl−KCl melt. RSC Adv. 2015, 5, 23114–23121. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yuan, L.Y.; Liu, K.; Ye, G.A.; Zhang, M.L.; He, H.; Tang, H.B.; Lin, R.S.; Chai, Z.F.; Shi, W.Q. Electrochemical extraction of samarium from LiCl−KCl melt by forming Sm−Zn alloys. Electrochim. Acta 2014, 120, 369–378. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yan, Y.D.; Zhang, M.L.; Ji, D.B.; Li, P.; Yin, T.Q.; Wang, P.; Xue, Y.; Jing, X.Y.; Han, W.; et al. Electrochemical synthesis of Sm−Cu dendritic catalysts by co-reduction of Sm(III) and Cu(II) in LiCl−KCl−SmCl3−CuCl2 melt. J. Alloys Compd. 2019, 772, 978–987. [Google Scholar] [CrossRef]
- Wei, H.; Tian, Y.; Zhang, M.; Ye, K.; Zhao, Q.; Wei, S. Preparing different phases of Mg-Li-Sm alloys by molten salt electrolysis in LiCl−KCl−MgCl2−SmCl3 melts. J. Rare Earths 2010, 28, 227–231. [Google Scholar]
- Huang, J.; Ma, X.; Cao, L.; Wu, J.; He, H. Electrodeposition of SmS optical thin films on ITO glass substrate. Mater. Lett. 2007, 61, 3920–3922. [Google Scholar] [CrossRef]
- Jundale, S.B.; Lokhande, C.D. Electrosynthesis of SmTe films. Mater. Chem. Phys. 1994, 37, 333–337. [Google Scholar] [CrossRef]
- Jundale, S.B.; Lokhande, C.D. Studies on electrosynthesis of Sm−Se films. Mater. Chem. Phys. 1994, 38, 325–331. [Google Scholar] [CrossRef]
- Devadas, B.; Cheemalapati, S.; Chen, S.M.; Rajkumar, M. Investigation of morphologies and characterization of rare earth metal samarium hexacyanoferrate and its composite with surfactant intercalated graphene oxide for sensor applications. RSC Adv. 2014, 4, 45895–45902. [Google Scholar] [CrossRef]
- Burns, J.D.; Myhre, K.C.; Sims, N.J.; Stracener, D.W.; Boll, R.A. Effects of annealing temperature on morphology and thickness of samarium electrodeposited thin films. Nucl. Instrum. Methods Phys. Res. A 2016, 830, 92–101. [Google Scholar] [CrossRef]
- Ruiz, E.J.; Ortega-Borges, R.; Godínez, L.A.; Chapman, T.W.; Meas-Vong, Y. Mechanism of the electrochemical deposition of samarium-based coatings. Electrochim. Acta 2006, 52, 914–920. [Google Scholar] [CrossRef]
- Lair, V.; Živković, L.S.; Lupan, O.; Ringuedé, A. Synthesis and characterization of electrodeposited samaria and samaria-doped ceria thin films. Electrochim. Acta 2011, 56, 4638–4644. [Google Scholar] [CrossRef]
- Yousefi, T.; Mostaedi, M.T.; Ghasemi, M.; Ghadirifar, A. A simple way to synthesize of samarium oxide nanoparticles: Characterization and effect of pH on morphology. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2016, 46, 137–142. [Google Scholar] [CrossRef]
- Ursaki, V.V.; Lair, V.; Živković, L.S.; Cassir, M.; Ringuedé, A.; Lupan, O. Optical properties of Sm-doped ceria nanostructured film grown by electrodeposition at low temperature. Opt. Mater. 2012, 34, 1897–1901. [Google Scholar] [CrossRef]
- Matei, E.; Enculescu, M.; Enculescu, I. Single bath electrodeposition of samarium oxide/zinc oxide nanostructured films with intense, broad luminescence. Electrochim. Acta 2013, 95, 170–178. [Google Scholar] [CrossRef]
- Ravichandiran, C.; Sakthivelu, A.; Kumar, K.D.A.; Davidprabu, R.; Valanarasu, S.; Kathalingam, A.; Ganesh, V.; Shkir, M.; Algarni, H.; AlFaify, S. Influence of rare earth element (Sm3+) doping on the properties of electrodeposited Cu2O films for optoelectronics. J. Mater. Sci. Mat. Electron. 2019, 30, 2530–2537. [Google Scholar] [CrossRef]
- Buizon, L.P.; Marquez, M.C. Supercapacitive performance of electrochemically synthesized samarium cobalt oxide nanosheets and nanoflowers. Mater. Sci. Forum 2022, 1053, 125–130. [Google Scholar] [CrossRef]
- Bučko, M.; Stupar, S.; Bajat, J.B. The influence of Sm content on the surface morphology and corrosion behavior of Zn-Co-Sm composite coatings. Metals 2023, 13, 481. [Google Scholar] [CrossRef]
- Parker, W.; Bildstein, H.; Getoff, N.; Fischer−Colbrie, H.; Regal, H. Molecular plating II. A rapid and quantitative method for the electrodeposition of the rare-earth elements. Nucl. Instrum. Methods 1964, 26, 61–65. [Google Scholar] [CrossRef]
- Ingelbrecht, C.; Ambeck-Madsen, J.; Teipel, K.; Robouch, P.; Arana, G.; Pomme, S. The electrodeposition of 149Sm targets for (n,α) studies. Nucl. Instrum. Methods Phys. Res. A 1999, 438, 36–39. [Google Scholar] [CrossRef]


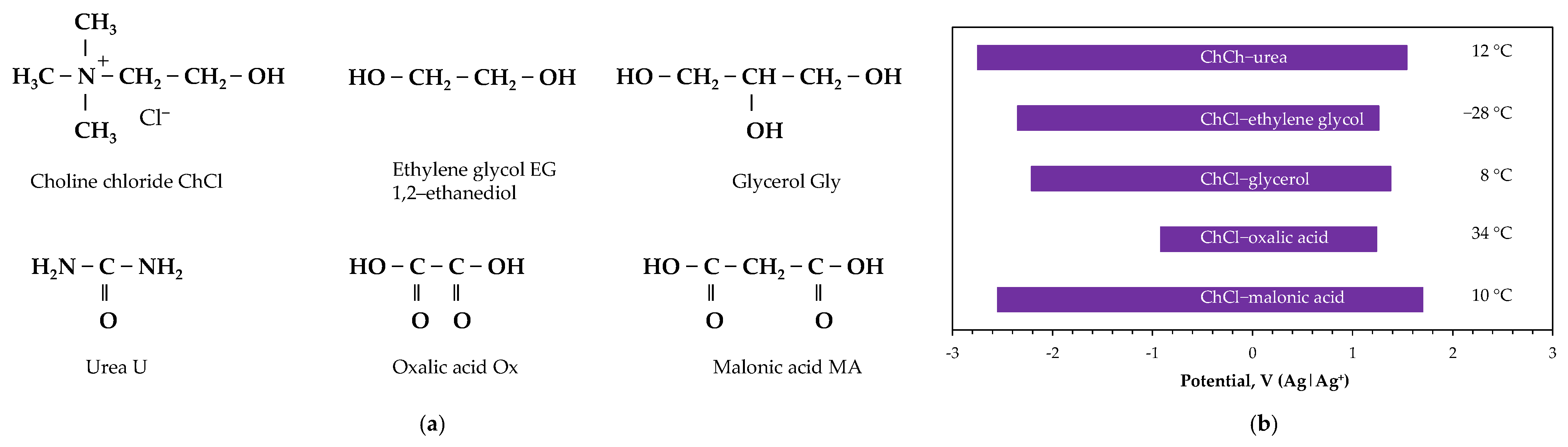
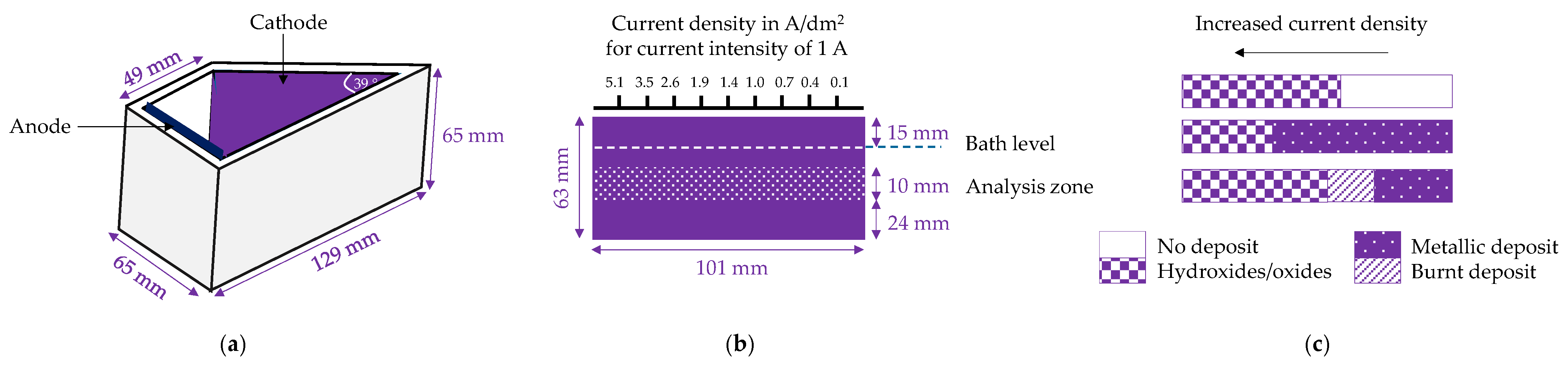
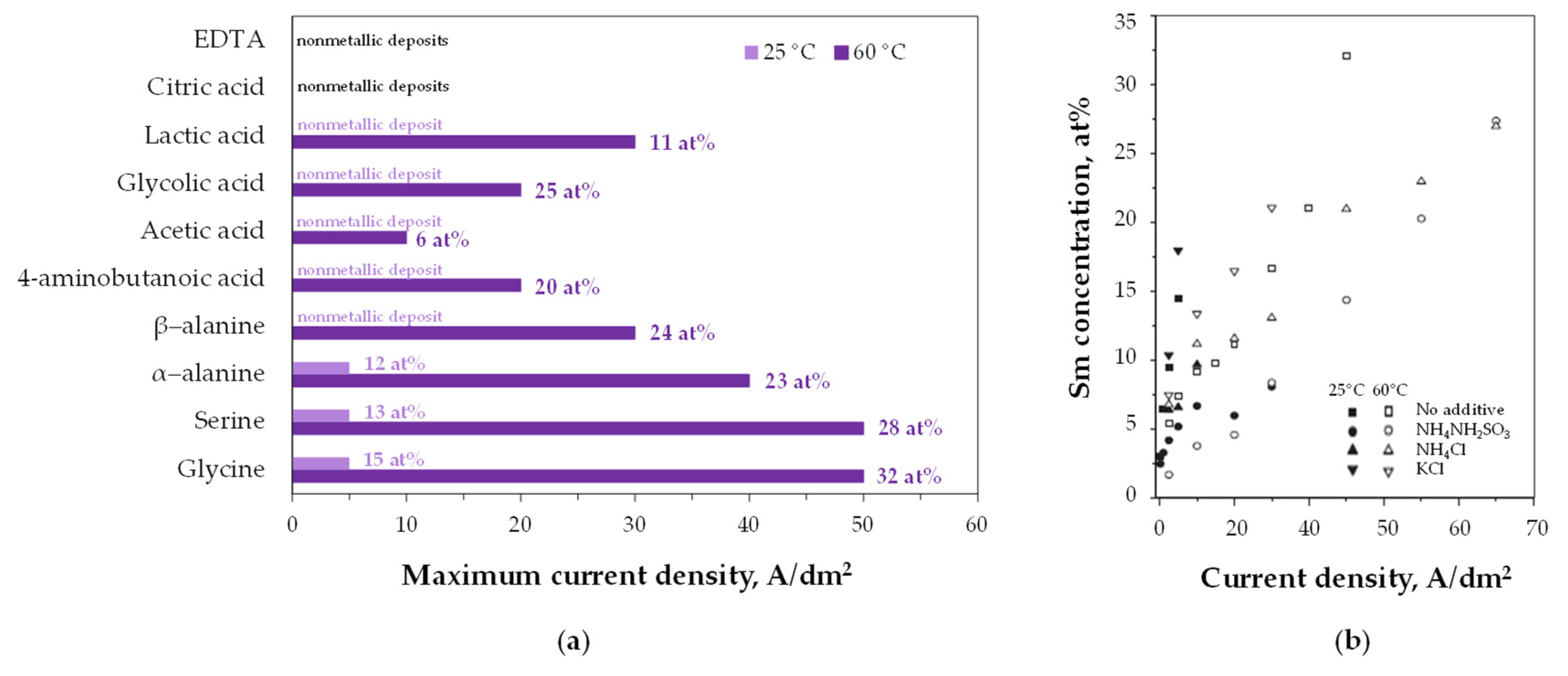


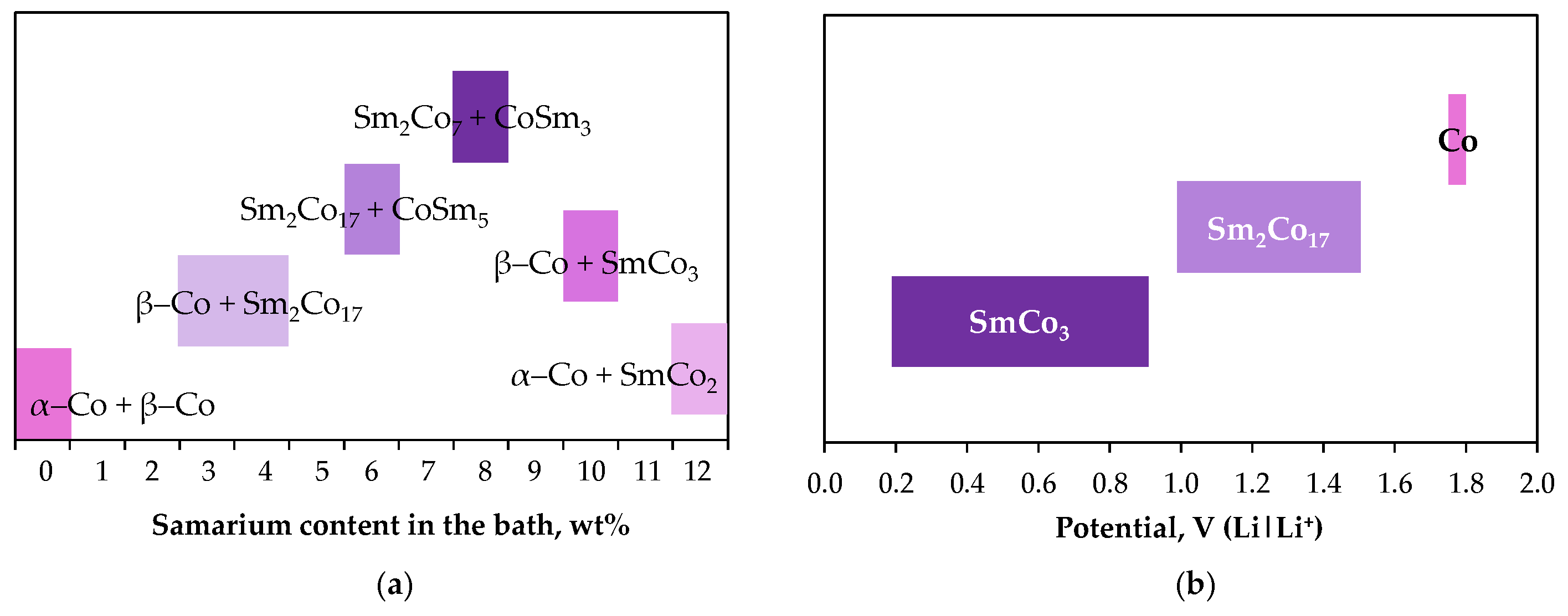
| Electrode Equilibrium * | Standard Potential E°, V |
|---|---|
| Acid solution (pH = 0) | |
| Sm3+|Sm2+ | −1.33 |
| SmOH2+, H+|Sm(c) | −2.15 |
| Sm3+|Sm(c) | −2.30 |
| Sm2+|Sm(c) | −2.68 |
| Alkaline solution (pH = 13.996) | |
| Sm(OH)3(pt) |Sm(c), OH− | −2.75 |
| Sm(OH)2∙H2O(c)|Sm(c), OH− | −2.77 |
| Sm(OH)3(c)|Sm(c), OH− | −2.78 |
| Sm(OH)3(c)|Sm(OH)2∙H2O(c), OH− | −2.80 |
| Electrolyte Composition | Electrolysis Conditions | Metal Formation (Evidence Method) | Application Context | Ref. |
|---|---|---|---|---|
| Electrodeposition | ||||
| 0.06M Sm(OTf)3 in [BMP][NTf2] | PD: −3.1 V vs. Ag/Ag+, 25 °C, 5 h, Cu substrate | yes (EDS) | metal electrowinning | [52] |
| 0.06M Sm(OTf)3 in [Me3NBu][NTf2] | ||||
| 0.01M Sm(NTf2)3 in [BMP][NTf2] | PD: −1.6 or −2.5 V vs. Ag/Ag+, 100 °C, 3C, GC substrate | yes (EDS, TEM) | metal recovery | [56] |
| 0.4M Sm(NTf2)3 in TMP | PD: −3.2/−2.5 V vs. Pt, 30 °C, 3 h, GC/Cu substrate | yes (EDS, XPS) | magnetic materials | [57] |
| 0.1M Sm(OTf)3 or Sm(NO3)3∙H2O or SmCl3 in [BMP][DCA] | PD: −2.0 to −2.8 V vs. Ag/Ag+, 25–60 °C, 4 h, GC/Ni substrate | yes (XPS) | metal recovery | [58] |
| 0.5M Sm(NTf2)3 in [BMP][NTf2] | PD: −2.5 V vs. Ag/Ag+, 100 °C, 5 h, Cu substrate | yes (XPS) | metal recovery | [59] |
| 0.1M Sm(OTf)3 or Sm(NO3)3∙H2O in DMI | PD: −3.0 V vs. Ag/Ag+, 70 °C, 5 h, Cu substrate | yes (XPS) | metal electrowinning | [60] |
| 0.1M Sm(NTf2)3 in [BMP][NTf2] | PD: −2.6 V vs. Ag/Ag+ or GD: 0.01 A/dm2 120 °C, 1.1C, Au substrate | yes (XRD) | magnetic materials | [65] |
| 0.01M Sm(OTf)3 in [BMP][DCA] | PD: −1.8 V vs. Ag/AgCl, 24 °C, 1 h, Pt substrate | yes (XPS) | magnetic materials | [66] |
| Cyclic Voltammetry | ||||
| 0.03M Sm(NTf2)3 in [BMP][DCA] | −0.35 to −2.3 V vs. Fc/Fc+, 100 mV/s, 25 °C, Pt substrate | possible (CV) | selective separation | [53] |
| 0.22M Sm(NTf2)3(H2O)3 in [Me3NBu][NTf2] | 1 to −3.2 V vs. Ag, 100 mV/s, 25 °C, Pt substrate | possible (CV) | selective separation in nuclear industry | [54] |
| 0.1M Sm(NTf2)3 in [BMP][NTf2] | 0 to −2.5 V vs. Ag, 10 mV/s, 25 °C, GC substrate | no (CV) | redox batteries | [55] |
| 0.03M Sm3+ in [Me3NBu][NTf2] | 1.5 to −2.5 V vs. Ag/Ag+, 25 °C, Pt substrate | possible (visual) | selective separation in nuclear industry | [62] |
| Electrolyte Composition | Electrolysis Conditions | Metal Formation (Evidence Method) | Ref. |
|---|---|---|---|
| 0.045M Sm(NO3)3 in ChCl–U (1:2) | PD: −1.9 V vs. Ag/AgCl, 70 °C, 0.5 h, GC substrate | yes (SEM) | [77] |
| 0.04M or 0.08M SmCl3∙6H2O in ChCl–EG (1:2 or 1:4.5) | CV: 0.5 to −1.5 V vs. Ag, 70 °C, 10 mV/s, Pt substrate | no (CV) | [78] |
| 0.1M SmCl3 in U–AT(34%)–NaBr(14.5%)–KBr(1.5%) * | CV: 0.85 to −1.0 V vs. Ag/AgCl, 80 °C, Pt substrate | no (CV) | [79] |
| PD: −0.6 to −1.0 V vs. Ag/AgCl, 80 °C, Pt substrate | no (visual) | ||
| 0.1M SmCl3 in U–AT(50%)–NaBr(15%) * | CV: 0.6 to −1.4 V vs. Ag, 70 °C, 100 mV/s, Pt substrate | no (CV) | [80] |
| Electrolyte Composition | Electrolysis Conditions | Cathode Substrate | Metal Formation (Evidence Method) | Ref. |
|---|---|---|---|---|
| Chloride Systems | ||||
| 0.1M SmCl3 in LiCl–KCl eutectic | CV: 0 to −2.5 V vs. Ag/AgCl, 530 °C, 100 mV/s | Mo 1 | no (CV) | [83] |
| CV: 0 to −2.5 V vs. Ag/AgCl, 530–600 °C, 100 mV/s | Al 2 | yes: SmxAly (OCP) | ||
| 1 wt% SmCl3 in LiCl–KCl eutectic | PD: −2.4 V vs. Ag/Ag+, 500 °C, 3 h | Cu 2 | yes: SmxCuy (EDS, CV) | [84] |
| PD: −1.9 or −2.0 V vs. Ag/Ag+, 500 °C, 0.5 h | Al 2 | yes: SmxAly (EDS, CV) | ||
| 0.07M SmCl3 in LiCl–KCl eutectic | PP: −1.4 to –0.4 V vs. Ag/AgCl, 500 °C, 15 h | PbBiliq 2 | yes: SmBi (XRD, CV) | [85] |
| 0.1M SmCl3 in NaCl–2CsCl eutectic | CV: 0.7 to −2.5 V vs. Ag/AgCl, 550–650 °C, 50–300 mV/s | Mo 1 | no (CV) | [86] |
| PD: −1.75 V vs. Ag/Ag+, 550 °C, 8 h | Galiq 2 | yes: SmGa6 (XRD, EDS) | ||
| min. 0.03M SmCl3 in KCl | CV: 0.2 to −1.7 V vs. Ag/AgCl, 815 °C, 100 mV/s | Au | possible (CV) | [87] |
| min. 0.03M SmCl3 in LiCl–KCl–(KF) | CV: 0.2 to −2.8 V vs. Ag/AgCl, 550 °C, 25 mV/s | W 1 | no (CV) | |
| 0.1M SmCl3 in LiCl–KCl eutectic | PD: −2.2 V vs. Ag/AgCl, 450 °C, 3 h | Al 2 | yes: SmAl3, SmAl2 (EDS, XRD) | [89] |
| 0.001M SmCl3 in NaCl–CaCl2 eqmol melt | CV: 1.2 to −2.6 V vs. Ag/Ag+, 550 °C, 20 mV/s | W 1 | no (CV) | [92] |
| 0.1M SmCl3 in LiCl–KCl eutectic | PD: −1.6 or −2.0 V vs. Ag/AgCl, 500 °C, 20 h | SnInliq 2 | yes: SmSn2, Sm(In1.5 Sn1.5) (XRD, EDS, CV) | [93] |
| 1wt% SmCl3 in LiCl–KCl eutectic | PD: −1.6 V vs. Ag/AgCl, 500 °C, 10 h | Znliq 2 | yes: Sm2Zn17 (XRD, SEM) | [95] |
| GD: 15 A/dm2, 500 °C, 8 h | ||||
| 0.5 mol SmCl3 in LiCl–KCl eutectic | PD: 0.1 V vs. Li/Li+, 500 °C, 72 h | Ni 2 | yes: SmNi2 (XRD) | [101] |
| GD: 5 A/dm2, 500 °C, 1 h | ||||
| 0.5mol% SmCl3 in LiCl–KCl eutectic | GD: 5 A/dm2, 450 °C, 24 h | Co 2 | yes: SmCo2 (XRD) | [103,104] |
| PD: 0.2 V vs. Li/Li+, 450 °C, 1 h; | ||||
| Fluoride Systems | ||||
| 0.5M SmF3 in LiF–CaF2 eutectic | CV: 0.4 to −2.0 V vs. Pt, 850 °C, 100 mV/s | Mo 1 | no (CV) | [82] |
| GD: 200 A/dm2, 850 °C, 1 h | Ni 2 | yes: SmxNiy (EDS, SEM) | ||
| 0.094M SmF3 in 2LiF–BeF2 eutectic | CV: 0.8 to −0.15 V vs. Be/Be2+, 600 °C | W 1 | no (CV) | [88] |
| PD: −0.14 V vs. Be/Be2+, 600 °C, 1 h | Cu 2 | yes: SmCu6 (XRD) | ||
| PD: −0.14 V vs. Be/Be2+, 600 °C, 1 h | Ni 2 | yes: Sm2Ni17 (XRD) | ||
| PD: −0.07 V vs. Be/Be2+, 600 °C, 1 h | Al 2 | yes: SmAl4 (XRD) | ||
| 0.001M SmF3 in 2LiF–BeF2 eutectic | CV: 0.2 to −1.7 V vs. Pt, 540 °C, 50 mV/s | Mo 1 | no (CV) | [90,91] |
| Chloride–Fluoride Systems | ||||
| 0.1M SmCl in LiF–CaF2 eutectic | GD: 200 A/dm2, 1100 °C, 1 h | Fe 2 | yes: Sm2Fe17 (XRD) | [105] |
| Electrolyte Composition | Electrolysis Conditions | Alloy Deposit | Ref. |
|---|---|---|---|
| Aqueous Solutions | |||
| 0.9M Sm(NH2SO3)3, 0.12M Co(NH2SO3)2, 0.36M C2H5NO2, 1M NH4NH2SO3; pH 7 | GD: 20–45 A/dm2, 23 °C | 3–8 at% Sm; film | [108] |
| PCD: 40 A/dm2, 10 Hz, ton/(ton + toff) 0.1–1, 23 °C | 3–7 at% Sm; film | ||
| 1M Sm(NH2SO3)3, 0.05M CoSO4, 0.15M C2H5NO2, 1M NH4NH2SO3; pH 5.2 | GD: 35–70 A/dm2, 50 C, 25 or 60 °C | 3–8 at% Sm; film: amorphous or Sm2Co17 | [109] |
| 0.05M Sm2O3, 0.3M HNH2SO3, 0.07M CoSO4, 0.21M C2H5NO2, 1M NH4NH2SO3; pH 6 | GD: 2.8–6 A/dm2, 60 °C, 0.3 h | 2–9 at% Sm; amorphous film, Sm2Co17 after reduction-diffusion | [119] |
| Sm(NH2SO3)3, 0.1M CoSO4, 0.3M C2H5NO2, 0.5M H3BO3; pH 2.5 | PD: −1.6 to −2.1 V vs. Ag/AgCl, 5 or 35 °C | 5–40 at% Sm; film, amorphous: Sm2Co17 after annealing | [120] |
| 0.2M SmCl3, 0.1M CoCl2, 0.7M H3BO3, 0.2M C2H5NO2, 0.05M C6H8O6, 1M HCl; pH 2 | DCV: 2.5 V | 3 at% Sm; amorphous nanowires, Sm2Co17 after annealing | [122] |
| 0.06M SmCl3, 0.06M CoSO4, 0.5M H3BO3; pH 3 | PD: −0.8 to −3.0 V vs. Ag/AgCl, 27 °C, 0.3 h | 10–50 at% Sm; amorphous nanowires or nanotubes | [123,124] |
| Molecular Liquid Solvents | |||
| 0.05M SmCl3, 0.075M CoCl2, 0.015M LiNO3 in DMF | PD: −2.8 vs. Ag, 50 °C, 0.5 h | 16 at% Sm; film | [44] |
| SmCl3, CoCl2, ethylenediamine in CH3NO | GD: 1 A/dm2, 25 °C, 1 h | 21% Sm, amorphous | [125,126] |
| SmCl3, CoCl2 in CH3NO | GD: 25 °C | nanowires, Sm2Co17 | [127] |
| Ionic Liquid Solvents | |||
| 0.05M Sm(NTf2)3, 0.05M Co(NTf2)2 in [BMP][NTf2] | PPD: −0.8 V 1s/ −2 V 1s vs. Pt, 120 °C, 2 h | 21/41 at% Sm; film | [65] |
| 0.01M Sm(OTf)3, 0.01M CoCl2, in [BMP][DCA] | PD: −1.8 V vs. Ag/AgCl, 24 °C, 1 h | film (XPS) | [66] |
| 0.005M Sm(NTf2)3, 0.03M Co(NTf2)2 in [BMP][NTf2] | PD: −1.5 to −2.5 V vs. Ag/Ag+, 25 °C, 1.2 C | SmCo7 nanoparticles | [128] |
| 1.25–2.5mol% SmCl3, 60%mol CoCl2 in [EMI][Cl] | PD: −0.6 to −0.7 V vs. Ag/Ag+, 120 °C, 0.1–0.3 h | Co:Sm 2–35; nanowires | [130] |
| Deep Eutectic Solvents | |||
| 0.04M SmCl3∙6H2O, 0.04MCoCl2∙6H2O, (0.12M C2H5NO2) in ChCl–EG | PD: −0.7 to −0.97 V vs. Ag, 70 °C | 0.5–44 wt% Sm; film; Sm2Co17 after annealing | [78,131] |
| 0.045M Sm(NO3)3, 0.018M CoCl2 in ChCl–U | PD: −1.6 to −1.9 V vs. Ag/AgCl, 70 °C, 0.25–0.7 h | 27–75 wt% Sm; film | [77] |
| 0.1M SmCl3, 0.04M CoCl2 in U–AT(34%)–NaBr(14.5%)–KBr(1.5%) | PD: −0.7 to −0.9 V vs. Ag/AgCl, 80 °C, 0.5 h | up to 50 wt% Sm; amorphous film, after annealing: SmCo5, Sm2Co7, Sm2Co17 | [79] |
| 0.1M SmCl3, 0.2M CoCl2 in U–AT(50%)–NaBr(15%) | PD: −1.15 to −1.35 V vs. Ag, 70 °C | 8–79 wt% Sm; amorphous film, Sm2Co17 after annealing | [80] |
| Molten Salt Electrolytes | |||
| 2–12 wt% SmCl3, 0.5–3 wt% CoCl2 in LiCl–KCl eutectic | GD: 594 A/dm2, 700 °C, 2 h; Mo substrate | 22–33 wt% Sm; flaky: Sm2Co17, SmCo5, Sm2Co7, SmCo3 | [132] |
| 2–12 wt% SmCl3, 0.5–3 wt% CoCl2 in LiCl–KCl eutectic | GD: 30 A/dm2, 550–750 °C; PD: −2.7 V vs. Ag; 0.5 h; W substrate | film: SmCo2 | [133] |
| 0.5mol% SmCl3, 0.1 mol% CoCl2 in LiCl–KCl eutectic | PD: 0.2 to 1.5 V vs. Li/Li+, 450 °C, 1 h; Cu substrate | nonuniform layer: Sm2Co17, SmCo3 | [134] |
| Electrolyte Composition | Electrolysis Conditions | Deposit | Application | Ref. |
|---|---|---|---|---|
| Sm–Ni Alloys | ||||
| 0.2M SmCl3, 0.05M NiCl2, 0.12M C2H5NO2 in ChCl–EG eutectic | GD: 0.1–0.5 A/dm2, 70 °C, 2 h | 2–6 wt% Sm; metallic with oxides | hydrogen evolution catalyst | [135] |
| 2 wt% SmCl3, 2 wt% NiCl2 in LiCl–KCl eutectic | PD: −1.5 to −2.2 V vs. Ag/Ag+, 700 °C, 6 h; Mo substrate | 19–55 wt% Sm; SmNi5, SmNi2, SmNi, SmOCl | magnetic materials | [136] |
| Sm–Fe Alloys | ||||
| 0.6M SmCl3, 0.1M FeCl2, 0.5M H3BO3, 0.06M HNH2SO3, 0.21M C2H5NO2, (0.4M NaCl); pH 3 | GD: 0.2–35 A/dm2, 25 °C, 0.2 h; (magnetic field) | 0.5–8 at% Sm; SmFe12, Sm3Fe5O12 | magnetic materials | [137,138] |
| 0.1M SmCl3, 0.04M FeCl2 in U–AT(50%)–NaBr(14%)–KBr(2%) | PD: −0.9 to −1.2 V vs. Ag/Ag+, 80 °C, 0.4 h | SmxFe x = 0.05–0.4; amorphous | magnetic materials | [139] |
| Sm–Ni–Fe Alloys | ||||
| 0.007–0.03M Sm2(SO4)3, 1M NiSO4, 0.03M FeSO4, 0.8 g/L H3BO3 | GD: 1 A/dm2 | 10–25 at% Sm | magnetic materials | [140] |
| Aspect | Aqueous Solutions | Molecular Liquids | Ionic Liquids | Deep Eutectic Solvents | Molten Salts |
|---|---|---|---|---|---|
| Solvent Type | Inorganic | Organic | Organic | Organic | Inorganic |
| Pure Samarium Deposition | No | Yes/No 1 | Yes | Yes 1 | No |
| Samarium Alloy Deposition | Yes 2 | Yes 2 | Yes 2 | Yes 2 | Yes |
| Samarium Oxide Deposition | Yes | Possible 3 | Not reported | Not reported | Not reported |
| Operation Temperature | 20–60 °C | 25–60 °C | 25–120 °C | 70–80 °C | 450–750 °C |
| Deposition Potential (Ag/Ag+) | −0.8 to −2.0 V | −2.8 V | −0.5 to −3.5 V | −0.7 to −1.9 V | −1.6 to −2.4 V |
| Current Density | 0.5 to 70 A/dm2 | 1 A/dm2 | Not reported | 0.1 to 0.5 A/dm2 | 5 to 200 A/dm2 |
| Advantages | easy to handle, low cost, high conductivity | wider electrochemical windows, pure samarium deposition | high efficiency, thick deposits | ||
| Disadvantages | low efficiency, only some alloys 2 | moisture-sensitive, lower conductivity, higher costs, unknown efficiency | energy-intensive, only for alloys | ||
| volatile, toxic | expensive, toxic | low toxicity | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudnik, E. Electrodeposition of Samarium Metal, Alloys, and Oxides: Advances in Aqueous and Non-Aqueous Electrolyte Systems. Int. J. Mol. Sci. 2025, 26, 11176. https://doi.org/10.3390/ijms262211176
Rudnik E. Electrodeposition of Samarium Metal, Alloys, and Oxides: Advances in Aqueous and Non-Aqueous Electrolyte Systems. International Journal of Molecular Sciences. 2025; 26(22):11176. https://doi.org/10.3390/ijms262211176
Chicago/Turabian StyleRudnik, Ewa. 2025. "Electrodeposition of Samarium Metal, Alloys, and Oxides: Advances in Aqueous and Non-Aqueous Electrolyte Systems" International Journal of Molecular Sciences 26, no. 22: 11176. https://doi.org/10.3390/ijms262211176
APA StyleRudnik, E. (2025). Electrodeposition of Samarium Metal, Alloys, and Oxides: Advances in Aqueous and Non-Aqueous Electrolyte Systems. International Journal of Molecular Sciences, 26(22), 11176. https://doi.org/10.3390/ijms262211176






