Exosomal Interventions in Bone and Osteochondral Repair: Mechanisms and Outcomes
Abstract
1. Introduction
2. The Role of Different Cell-Derived Exosomes for Bone Regeneration
2.1. Stem Cell-Derived Exos
2.2. Macrophage-Derived Exos (M-Exos)
2.3. OB-Exos and Osteoclast-Derived Exos (OC-Exos)
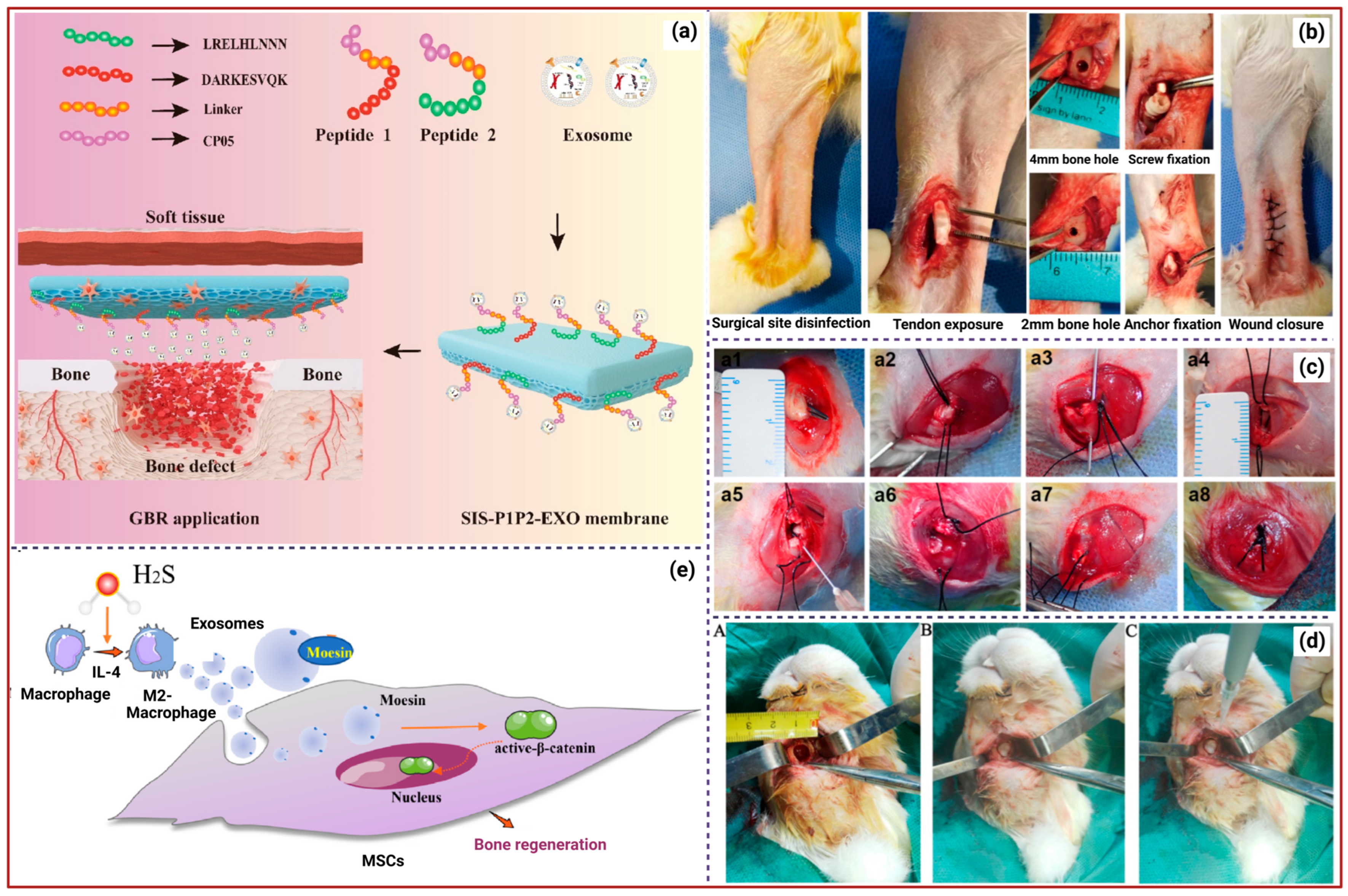
3. Exosomes for Osteoarthritis Treatment
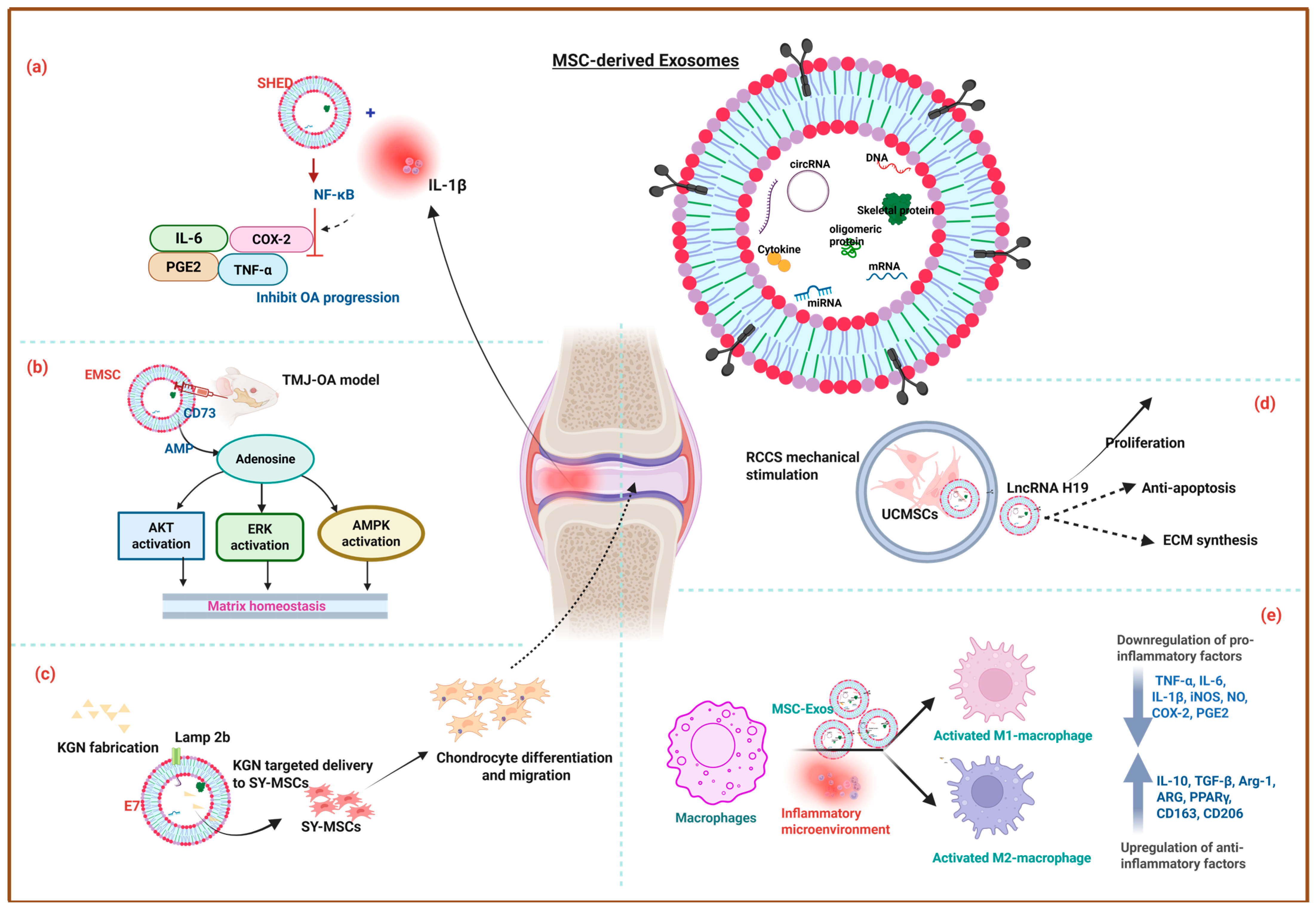
4. The Role of Exos in Osteochondral Regeneration
5. The Current Progress in EV-Based Clinical Trials
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, D.; Jian, C.; Qi, B.; Li, Z.; Yu, A. Pathological bone regeneration in soft tissues: A narrative review of traumatic heterotopic ossification. Regen. Med. Rep. 2025, 2, 130–136. [Google Scholar] [CrossRef]
- Jiang, S.; Naito, K.; Liverneaux, P. Advantages in precision, safety, and aesthetic outcomes of robot-assisted minimally invasive techniques in peripheral nerve microsurgery: A narrative review. Adv. Technol. Neurosci. 2025, 2, 122–127. [Google Scholar] [CrossRef]
- Bao, C.; He, C. The role and therapeutic potential of MSC-derived exosomes in osteoarthritis. Arch. Biochem. Biophys. 2021, 710, 109002. [Google Scholar] [CrossRef]
- Zhou, Y.; Yamamoto, Y.; Xiao, Z.; Ochiya, T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J. Clin. Med. 2019, 8, 1025. [Google Scholar] [CrossRef]
- Huang, L.; Wang, J.; Xu, J.; Bian, M.; Wang, J.; Lu, S. Ferroptosis in osteogenic differentiation: A narrative review of bone regeneration metabolism. Regen. Med. Rep. 2025, 2, 100–107. [Google Scholar] [CrossRef]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; La Shu, S.; Rio-Espinola, A.d.; Ferreira, J.R.; Bando, K.; Lemmens, M.; Pande, P.; de Wolf, C.; Chen, C.L.; Elke, E.; et al. Evaluating teratoma formation risk of pluripotent stem cell-derived cell therapy products: A consensus recommendation from the Health and Environmental Sciences Institute’s International Cell Therapy Committee. Cytotherapy 2025, 27, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Assessing Tumorigenicity in Stem Cell-Derived Therapeutic Products: A Critical Step in Safeguarding Regenerative Medicine. Bioengineering 2023, 10, 857. [Google Scholar] [CrossRef]
- Ma, Z.J.; Yang, J.J.; Lu, Y.B.; Liu, Z.Y.; Wang, X.X. Mesenchymal stem cell-derived exosomes: Toward cell-free therapeutic strategies in regenerative medicine. World J. Stem Cells 2020, 12, 814–840. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Zhu, Y.; Yang, M.; Mao, C. Human mesenchymal stem cell derived exosomes enhance cell-free bone regeneration by altering their miRNAs profiles. Adv. Sci. 2020, 7, 2001334. [Google Scholar] [CrossRef]
- Goo, J.; Lee, Y.; Lee, J.; Kim, I.S.; Jeong, C. Extracellular Vesicles in Therapeutics: A Comprehensive Review on Applications, Challenges, and Clinical Progress. Pharmaceutics 2024, 16, 311. [Google Scholar] [CrossRef]
- Ni, Z.; Zhou, S.; Li, S.; Kuang, L.; Chen, H.; Luo, X.; Ouyang, J.; He, M.; Du, X.; Chen, L. Exosomes: Roles and therapeutic potential in osteoarthritis. Bone Res. 2020, 8, 25. [Google Scholar] [CrossRef]
- Khazaei, F.; Rezakhani, L.; Alizadeh, M.; Mahdavian, E.; Khazaei, M. Exosomes and exosome-loaded scaffolds: Characterization and application in modern regenerative medicine. Tissue Cell 2023, 80, 102007. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Willing, A.E.; Ehrhart, J.; Wang, L.; Sanberg, P.R.; Borlongan, C.V. Cell-Free Extracellular Vesicles Derived from Human Bone Marrow Endothelial Progenitor Cells as Potential Therapeutics for Microvascular Endothelium Restoration in ALS. NeuroMol. Med. 2020, 22, 503–516. [Google Scholar] [CrossRef]
- Wang, W.; Qiao, S.-C.; Wu, X.-B.; Sun, B.; Yang, J.-G.; Li, X.; Zhang, X.; Qian, S.-J.; Gu, Y.-X.; Lai, H.-C. Circ_0008542 in osteoblast exosomes promotes osteoclast-induced bone resorption through m6A methylation. Cell Death Dis. 2021, 12, 628. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Jiang, H.; Yu, M. Emerging roles of extracellular vesicles in oral and maxillofacial areas. Int. J. Oral. Sci. 2025, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Chen, Z. Emerging role of exosomes in cancer therapy: Progress and challenges. Mol. Cancer 2025, 24, 13. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Lee, C.-S. Recent Progress in Hyaluronic-Acid-Based Hydrogels for Bone Tissue Engineering. Gels 2023, 9, 588. [Google Scholar] [CrossRef]
- Wan, L.; Liu, F.; Wang, A.; He, Y.; Pan, J.; Liu, Y.; Xu, J.; Xu, C.; Wu, F.; Ye, Q. PI3K/Akt pathway-mediated enhancement of bone and vascular regeneration by gelatin/hyaluronic acid/exosome composite scaffold in bone tissue engineering. Biomater. Adv. 2025, 166, 214064. [Google Scholar] [CrossRef]
- Yang, J.-X.; Xie, P.; Li, Y.-S.; Wen, T.; Yang, X.-C. Osteoclast-derived miR-23a-5p-containing exosomes inhibit osteogenic differentiation by regulating Runx2. Cell. Signal. 2020, 70, 109504. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, M.; Zhu, J.; Li, T.; Li, N.; Su, B.; Sun, G.-D.; Li, L.; Zhou, C. Exosome-loaded hyaluronic acid hydrogel composite with oxygen-producing 3D printed polylactic acid scaffolds for bone tissue repair and regeneration. Int. J. Biol. Macromol. 2024, 274, 132970. [Google Scholar] [CrossRef]
- Yu, W.; Li, S.; Guan, X.; Zhang, N.; Xie, X.; Zhang, K.; Bai, Y. Higher yield and enhanced therapeutic effects of exosomes derived from MSCs in hydrogel-assisted 3D culture system for bone regeneration. Biomater. Adv. 2022, 133, 112646. [Google Scholar] [CrossRef]
- Xiang, K.; Hao, M.; Zhang, Z.; Zhang, K.; Sun, H.; Zhang, L. Engineering 3D-BMSC exosome-based hydrogels that collaboratively regulate bone microenvironment and promote osteogenesis for enhanced cell-free bone regeneration. Mater. Today Bio 2025, 32, 101881. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Feng, T.; Cheng, S.; Chen, M.; Li, Y.; Yu, Z.; Xu, Z.; Yin, P.; Zhang, L.; Tang, P. Evaluating the defect targeting effects and osteogenesis promoting capacity of exosomes from 2D-and 3D-cultured human adipose-derived stem cells. Nano Today 2023, 49, 101789. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, Y.; Yang, Y.; Mu, Y.; Zhang, L.; Wu, J.; Li, R.; Bian, X.; Wei, P.; Jing, W. Asymmetric SIS membranes specifically loaded with exosomes through the modification of engineered recombinant peptides for guide bone regeneration. Compos. Part. B Eng. 2022, 232, 109571. [Google Scholar] [CrossRef]
- Wan, L.; He, Y.; Wang, A.; Pan, J.; Xu, C.; Fu, D.; Ye, Q.; Wu, F. Development of an integrated device utilizing exosome-hyaluronic acid-based hydrogel and investigation of its osteogenic and angiogenic characteristics. Mater. Des. 2024, 237, 112565. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Rong, Y.; Qian, D.; Chen, J.; Zhou, Z.; Luo, Y.; Jiang, D.; Cheng, L.; Zhao, S. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020, 103, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhao, P.; Xing, J.; Wang, Z.; Xu, Y.; Yan, Y.; Zhang, H.; Qu, J. GelMA encapsulating BMSCs-exosomes combined with interference screw or suture anchor promotes tendon-bone healing in a rabbit model. Sci. Rep. 2024, 14, 28212. [Google Scholar] [CrossRef]
- Fan, L.; Guan, P.; Xiao, C.; Wen, H.; Wang, Q.; Liu, C.; Luo, Y.; Ma, L.; Tan, G.; Yu, P.; et al. Exosome-functionalized polyetheretherketone-based implant with immunomodulatory property for enhancing osseointegration. Bioact. Mater. 2021, 6, 2754–2766. [Google Scholar] [CrossRef]
- Li, M.; Shi, L.; Chen, X.; Yi, D.; Ding, Y.; Chen, J.; Xing, G.; Chen, S.; Wang, L.; Zhang, Y.; et al. In-situ gelation of fibrin gel encapsulating platelet-rich plasma-derived exosomes promotes rotator cuff healing. Commun. Biol. 2024, 7, 205. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, X.; Lin, H.; Li, S.; Zhang, Z.; Wei, F.; Chen, Y. GelMA/HA-NB hydrogel encapsulating adipose-derived chondrogenic exosomes enhances enthesis regeneration in chronic rotator cuff tears. Int. J. Biol. Macromol. 2025, 309 Pt 2, 142800. [Google Scholar] [CrossRef]
- Han, L.; Hu, N.; Wang, C.; Ye, Z.; Wang, T.; Lan, F. Platelet-rich plasma-derived exosomes promote rotator cuff tendon-bone healing. Injury 2024, 55, 111212. [Google Scholar] [CrossRef]
- Han, J.; Li, G.-C.; Fang, S.-Y.; Cui, Y.-M.; Yang, H.-H. Dermal fibroblast-derived exosomes promotes bone-to-tendon interface healing of chronic rotator cuff tear in rabbit model. Arthrosc. J. Arthrosc. Relat. Surg. 2025, 41, 2761.e1–2771.e1. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Ye, Y.; Chen, D.; Song, J. SHED-derived exosomes improve the repair capacity and osteogenesis potential of hPDLCs. Oral Dis. 2023, 29, 1692–1705. [Google Scholar] [CrossRef]
- Wu, Z.; Pu, P.; Su, Z.; Zhang, X.; Nie, L.; Chang, Y. Schwann Cell-derived exosomes promote bone regeneration and repair by enhancing the biological activity of porous Ti6Al4V scaffolds. Biochem. Biophys. Res. Commun. 2020, 531, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, G.; Wu, S.; Zou, Y.; Weng, W.; Gai, T.; Chen, X.; Zhang, K.; Zhou, F.; Wang, X. Engineering preparation and sustained delivery of bone functional exosomes-laden biodegradable hydrogel for in situ bone regeneration. Compos. Part. B Eng. 2023, 261, 110803. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, L.; Zhang, J.; Chiang, C.-L.; Pan, J.; Wang, X.; Kwak, K.J.; Li, H.; Zhao, R.; Rima, X.Y.; et al. Exosomal mRNAs for Angiogenic-Osteogenic Coupled Bone Repair. Adv. Sci. 2023, 10, e2302622. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, H.; Bu, Z.; Liu, Z.; Lv, F.; Pan, M.; Huang, X.; Cheng, L. Small Extracellular Vesicles Released from Bioglass/Hydrogel Scaffold Promote Vascularized Bone Regeneration by Transferring miR-23a-3p. Int. J. Nanomed. 2022, 17, 6201–6220. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Qin, H.; Wang, Z.; Yu, M.; Liu, Z.; Peng, H.; Liang, L.; Zhang, C.; Wei, X. Bone Mesenchymal Stem Cell-Derived sEV-Encapsulated Thermosensitive Hydrogels Accelerate Osteogenesis and Angiogenesis by Release of Exosomal miR-21. Front. Bioeng. Biotechnol. 2021, 9, 829136. [Google Scholar] [CrossRef]
- Liu, R.; Wu, S.; Liu, W.; Wang, L.; Dong, M.; Niu, W. microRNAs delivered by small extracellular vesicles in MSCs as an emerging tool for bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1249860. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, H.; Lin, X.; Ma, L.; Zheng, X.; Liu, Y.; Huang, P.; Yu, S.; Zhang, W.; Lin, M.; et al. 3D Interpenetrated Graphene Foam/58S Bioactive Glass Scaffolds for Electrical-Stimulation-Assisted Differentiation of Rabbit Mesenchymal Stem Cells to Enhance Bone Regeneration. J. Biomed. Nanotechnol. 2019, 15, 602–611. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Chen, J.; Lu, R.; Liu, Z.; Zhang, Y.; Zhang, C. Pretreated exosomes by electrical stimulation accelerate bone regeneration. Bioact. Mater. 2025, 51, 383–398. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, T.; Sun, H.; Li, M.; Yang, D.; Wu, W.; Gu, Q.; Xu, A.; Li, Y.; Jiang, H. Enhanced mesenchymal stem cells-derived exosomes secretion by electrical stimulation of triboelectric nanogenerator. Nano Energy 2025, 139, 110933. [Google Scholar] [CrossRef]
- Wa, Q.; Luo, Y.; Tang, Y.; Song, J.; Zhang, P.; Linghu, X.; Lin, S.; Li, G.; Wang, Y.; Wen, Z. Mesoporous bioactive glass-enhanced MSC-derived exosomes promote bone regeneration and immunomodulation in vitro and in vivo. J. Orthop. Transl. 2024, 49, 264–282. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Si, Y.; Liang, J.; Li, M.; Wang, Z.; Qin, Y.; Sun, L. Enhancing bone regeneration and immunomodulation via gelatin methacryloyl hydrogel-encapsulated exosomes from osteogenic pre-differentiated mesenchymal stem cells. J. Colloid. Interface Sci. 2024, 672, 179–199. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Si, Y.; Dong, S.; Li, M.; Gu, J.; Luo, M.; Wang, X.; Wang, Z.; Li, X.; Zhang, C. Curcumin-encapsulated exosomes in bisphosphonate-modified hydrogel microspheres promote bone repair through macrophage polarization and DNA damage mitigation. Mater. Today Bio 2025, 32, 101874. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Omar, O.; Vazirisani, F.; Thomsen, P.; Ekström, K. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS ONE 2018, 13, e0193059. [Google Scholar] [CrossRef]
- Xu, C.; Li, Z.; Kang, M.; Chen, Y.; Sheng, R.; Aghaloo, T.; Lee, M. Hydrogel-integrated exosome mimetics derived from osteogenically induced mesenchymal stem cells in spheroid culture enhance bone regeneration. Biomaterials 2025, 317, 123088. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Tian, L.; Guo, B.; Dai, T.; Lv, Q.; Xie, J.; Liu, F.; Bao, H.; Cao, F. Exosome-capturing scaffold promotes endogenous bone regeneration through neutrophil-derived exosomes by enhancing fast vascularization. Biomaterials 2025, 319, 123215. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Y.; Wu, J.; Yang, R.; Sun, Q.; Xu, Y.; Wang, B.; Cai, M.; Xu, Y.; Zhuang, C. Adipose stem cells-derived exosomes modified gelatin sponge promotes bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1096390. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, S.; Zhang, Y. Effect of nHA/CS/PLGA delivering adipose stem cell-derived exosomes and bone marrow stem cells on bone healing—In vitro and in vivo studies. Sci. Rep. 2024, 14, 27502. [Google Scholar] [CrossRef]
- Jin, S.; Luo, Z.; Cai, Y.; Wen, J.; Lu, P.; Fu, X.; Mou, P.; Chen, A.; Meng, W.; Li, J. Exosome-functionalized heterogeneous nanofibrous scaffolds repair bone defects accompanied by muscle injury. Chem. Eng. J. 2024, 485, 149681. [Google Scholar] [CrossRef]
- Liu, A.; Lin, D.; Zhao, H.; Chen, L.; Cai, B.; Lin, K.; Shen, S.G. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials 2021, 272, 120718. [Google Scholar] [CrossRef]
- Chu, X.-Y.; Yang, K.; He, X.; Yu, K.-T.; Luan, Y.-Y.; He, Q.-B.; Li, Z.-L.; Xiang, Y.-L.; Chen, H.; Zeng, Y. Cross-linking N-succinyl chitosan-oxidated hyaluronic acid-based hydrogel loaded with bone marrow mesenchymal stem cell-derived exosomes induce bone regeneration in cranial defects. Mater. Des. 2024, 241, 112969. [Google Scholar] [CrossRef]
- Guan, X.; Li, C.; Wang, X.; Yang, L.; Lu, Y.; Guo, Z. Engineered M2 macrophage-derived exosomes: Mechanisms and therapeutic potential in inflammation regulation and regenerative medicine. Acta Biomater. 2025, 203, 38–58. [Google Scholar] [CrossRef]
- Daneshvar, A.; Nemati, P.; Azadi, A.; Amid, R.; Kadkhodazadeh, M. M2 macrophage-derived exosomes for bone regeneration: A systematic review. Arch. Oral Biol. 2024, 166, 106034. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, J.; Wang, Y.; Jiang, N.; Shi, X. Multifunctional Exosomes Derived from M2 Macrophages with Enhanced Odontogenesis, Neurogenesis and Angiogenesis for Regenerative Endodontic Therapy: An In Vitro and In Vivo Investigation. Biomedicines 2024, 12, 441. [Google Scholar] [CrossRef]
- Zhang, B.; Zha, Z.; Li, C.; Zhang, L.; Wu, T.; Lu, C.; Liu, C.; Li, D.; Feng, C.; Wei, S.; et al. M2 macrophagy-derived exosomal miRNA-26a-5p induces osteogenic differentiation of bone mesenchymal stem cells. J. Orthop. Surg. Res. 2022, 17, 137. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, Y.; Lv, Z.; Luan, Y.; Li, J.; Meng, C.; Liu, K.; Luo, X.; Chen, L.; Liu, F. Macrophage exosomes modified by miR-365-2-5p promoted osteoblast osteogenic differentiation by targeting OLFML1. Regen. Biomater. 2024, 11, rbae018. [Google Scholar] [CrossRef]
- Murakami, K.; Kikugawa, S.; Kobayashi, Y.; Uehara, S.; Suzuki, T.; Kato, H.; Udagawa, N.; Nakamura, Y. Olfactomedin-like protein OLFML1 inhibits Hippo signaling and mineralization in osteoblasts. Biochem. Biophys. Res. Commun. 2018, 505, 419–425. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Zhang, X.; Yang, Y.; Bai, S. Shear stress-mediated downregulation of miR-423-5p in M2 macrophage exosomes promotes osteogenic differentiation of bone marrow mesenchymal stem cells. Int. Immunopharmacol. 2025, 164, 115298. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Li, S.; Liu, Y.; Liu, X.; Qiu, H.; Che, Y.; Bian, L.; Zhou, M. An engineered M2 macrophage-derived exosomes-loaded electrospun biomimetic periosteum promotes cell recruitment, immunoregulation, and angiogenesis in bone regeneration. Bioact. Mater. 2025, 50, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Li, M.; Crawford, R.; Zhou, Y.; Xiao, Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater. 2019, 86, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, B.; Zhai, D.; Wu, C. Three-dimensional printing of bioceramic-induced macrophage exosomes: Immunomodulation and osteogenesis/angiogenesis. NPG Asia Mater. 2021, 13, 72. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Deng, Y.; Wu, C.; Ruan, Z.; Li, C.; Chen, Y.; Bei, C.; Zhu, L.; Yu, H. A novel adhesive dual-sensitive hydrogel for sustained release of exosomes derived from M2 macrophages promotes repair of bone defects. Mater. Today Bio 2023, 23, 100840. [Google Scholar] [CrossRef]
- Zhou, Y.-k.; Han, C.-S.; Zhu, Z.-L.; Chen, P.; Wang, Y.-M.; Lin, S.; Chen, L.-J.; Zhuang, Z.-M.; Zhou, Y.-H.; Yang, R.-L. M2 exosomes modified by hydrogen sulfide promoted bone regeneration by moesin mediated endocytosis. Bioact. Mater. 2024, 31, 192–205. [Google Scholar] [CrossRef]
- Manickam, G.; Moffatt, P.; Murshed, M. Role of SMPD3 during Bone Fracture Healing and Regulation of Its Expression. Mol. Cell Biol. 2019, 39, e00370-18. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, C.; Liang, Y.; Zou, S.; Liu, X. Osteoclasts in bone regeneration under type 2 diabetes mellitus. Acta Biomater. 2019, 84, 402–413. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Zhang, C.; Zhang, Y.; He, Y.; Liu, Y.; Ma, P.; Li, J.; Fan, Z. A blood glucose fluctuation-responsive delivery system promotes bone regeneration and the repair function of Smpd3-reprogrammed BMSC-derived exosomes. Int. J. Oral Sci. 2024, 16, 65. [Google Scholar] [CrossRef]
- Du, J.H.; Lin, S.X.; Wu, X.L.; Yang, S.M.; Cao, L.Y.; Zheng, A.; Wu, J.N.; Jiang, X.Q. The Function of Wnt Ligands on Osteocyte and Bone Remodeling. J. Dent. Res. 2019, 98, 930–938. [Google Scholar] [CrossRef]
- Guerrero, J.; Maevskaia, E.; Pfister, P.; Dominguez, A.P.; Ghayor, C.; Bhattacharya, I.; Scherberich, A.; Weber, F.E. Mineralized osteoblast-derived exosomes and 3D-printed ceramic-based scaffolds for enhanced bone healing: A preclinical exploration. Acta Biomater. 2025, 200, 686–702. [Google Scholar] [CrossRef]
- Tang, H.; He, Y.; Li, L.; Mao, W.; Chen, X.; Ni, H.; Dong, Y.; Lyu, F. Exosomal MMP2 derived from mature osteoblasts promotes angiogenesis of endothelial cells via VEGF/Erk1/2 signaling pathway. Exp. Cell Res. 2019, 383, 111541. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, X.; Chen, Y.; Chen, J.; Li, Y. Osteoblasts-derived exosomes regulate osteoclast differentiation through miR-503-3p/Hpse axis. Acta Histochem. 2021, 123, 151790. [Google Scholar] [CrossRef]
- Chen, L.; Lu, L.; Fan, C.; Zhu, X.; Pan, L.; Tang, S.; Wang, Y.; Peng, Y.; You, L. Autophagy-induced osteoblast-derived exosomes maintain bone formation and prevent osteoporosis by remodeling gut microbiota-metabolism. Biomed. J. 2025; in press. [Google Scholar] [CrossRef]
- Leng, Y.; Li, J.; Long, Z.; Li, C.; Zhang, L.; Huang, Z.; Xi, J.; Liu, Y. Osteoblast-derived exosomes promote osteogenic differentiation of osteosarcoma cells via URG4/Wnt signaling pathway. Bone 2024, 178, 116933. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Teramachi, J.; Hiasa, M.; Amachi, R.; Bat-Erdene, A.; Oda, A.; Tenshin, H.; Tanaka, M.; Nakagawa, M.; Seki, A.; et al. Myeloma cell growth suppression by osteoblast-derived extracellular vesicles: The creation of a non-permissive niche for myeloma cells by bone-forming osteoblasts. Haematologica 2025, 110, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhao, J.; Wang, J.; Jiang, Y.; Jing, Y.; Xu, K.; Su, J. Osteoblast-derived extracellular vesicles exert bone formation effects by WIF1-mediated regulation of mitophagy. Med. Plus 2024, 1, 100033. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Chen, H.; Yu, X.; Cao, X.; Zhang, H. Osteoclast-derived exosomes influence osteoblast differentiation in osteoporosis progression via the lncRNA AW011738/miR-24-2-5p/TREM1 axis. Biomed. Pharmacother. 2024, 178, 117231. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Li, W. Exosomes derived from osteoclasts under compression stress inhibit osteoblast differentiation. Biocell 2021, 45, 427–444. [Google Scholar] [CrossRef]
- Rahimi, F.; Nejati, V.; Nassadj, G.; Ziaei, B.; Mohammadi, H.K. The effect of transcranial direct stimulation as an add-on treatment to conventional physical therapy on pain intensity and functional ability in individuals with knee osteoarthritis: A randomized controlled trial. Neurophysiol. Clin. 2021, 51, 507–516. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Cao, G.; Liu, Y.; Huang, Y.; Jiang, X. Ozone therapy for knee osteoarthritis: A literature visualization analysis of research hotspots and prospects. Med. Gas Res. 2025, 15, 356–365. [Google Scholar] [CrossRef]
- Zhou, X.; Liang, H.; Hu, X.; An, J.; Ding, S.; Yu, S.; Liu, C.; Li, F.; Xu, Y. BMSC-derived exosomes from congenital polydactyly tissue alleviate osteoarthritis by promoting chondrocyte proliferation. Cell Death Discov. 2020, 6, 142. [Google Scholar] [CrossRef]
- Klyucherev, T.O.; Peshkova, M.A.; Revokatova, D.P.; Serejnikova, N.B.; Fayzullina, N.M.; Fayzullin, A.L.; Ershov, B.P.; Khristidis, Y.I.; Vlasova, I.I.; Kosheleva, N.V.; et al. The Therapeutic Potential of Exosomes vs. Matrix-Bound Nanovesicles from Human Umbilical Cord Mesenchymal Stromal Cells in Osteoarthritis Treatment. Int. J. Mol. Sci. 2024, 25, 11564. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Wu, K.-C.; Ding, D.-C. Chondrogenic Potential of Human Umbilical Cord Mesenchymal Stem Cells Cultured with Exosome-Depleted Fetal Bovine Serum in an Osteoarthritis Mouse Model. Biomedicines 2022, 10, 2773. [Google Scholar] [CrossRef] [PubMed]
- Diez-Guardia, V.; Tian, Y.; Guo, Y.; Li, J.; Cui, S.; Dreiss, C.A.; Gentleman, E.; Wang, X. Controlled Release of Human Dental Pulp Stem Cell-Derived Exosomes from Hydrogels Attenuates Temporomandibular Joint Osteoarthritis. Adv. Healthc. Mater. 2024, 2402923. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Naruphontjirakul, P.; Huang, T.-Y.; Wu, Y.-C.; Cheng, W.-H.; Su, W.-T. The Exosomes of Stem Cells from Human Exfoliated Deciduous Teeth Suppress Inflammation in Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 8560. [Google Scholar] [CrossRef]
- Fu, Y.; Cui, S.; Zhou, Y.; Qiu, L. Dental Pulp Stem Cell-Derived Exosomes Alleviate Mice Knee Osteoarthritis by Inhibiting TRPV4-Mediated Osteoclast Activation. Int. J. Mol. Sci. 2023, 24, 4926. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Crawford, R.; Xiao, Y.; Mao, X.; Prasadam, I. Osteoarthritic Subchondral Bone Release Exosomes That Promote Cartilage Degeneration. Cells 2021, 10, 251. [Google Scholar] [CrossRef]
- Song, J.E.; Kim, J.S.; Shin, J.H.; Moon, K.W.; Park, J.K.; Park, K.S.; Lee, E.Y. Role of Synovial Exosomes in Osteoclast Differentiation in Inflammatory Arthritis. Cells 2021, 10, 120. [Google Scholar] [CrossRef]
- Xu, X.; Liang, Y.; Li, X.; Ouyang, K.; Wang, M.; Cao, T.; Li, W.; Liu, J.; Xiong, J.; Li, B. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials 2021, 269, 120539. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liu, G.; Wu, X. Exosomes derived from umbilical cord mesenchymal stem cells in mechanical environment show improved osteochondral activity via upregulation of LncRNA H19. J. Orthop. Transl. 2021, 26, 111–120. [Google Scholar] [CrossRef]
- Yin, H.; Yan, Z.; Wu, J.; Li, M.; Ge, Q.; Zhang, T.; Ma, Y.; Sui, X.; Liu, S.; Guo, Q. M2 macrophages-derived exosomes combined with acellular cartilage matrix scaffolds promote osteochondral regeneration via modulatory microenvironment. Mater. Des. 2023, 226, 111672. [Google Scholar] [CrossRef]
- Tu, C.; Gao, X.; Zheng, H.; Huang, R.; Yang, F.; Dong, Y.; Jing, K.; Groth, T.; Zhao, M. Innovative injectable, self-healing, exosome cross-linked biomimetic hydrogel for cartilage regeneration. J. Control. Release 2025, 381, 113608. [Google Scholar] [CrossRef]
- Chang, L.H.; Wu, S.C.; Chen, C.H.; Chen, J.W.; Huang, W.C.; Wu, C.W.; Lin, Y.S.; Chen, Y.J.; Chang, J.K.; Ho, M.L. Exosomes Derived from Hypoxia-Cultured Human Adipose Stem Cells Alleviate Articular Chondrocyte Inflammaging and Post-Traumatic Osteoarthritis Progression. Int. J. Mol. Sci. 2023, 24, 13414. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, J.; Kim, H.K.; Kang, J.Y.; Kuppa, S.S.; Yang, H.Y.; Seon, J.K. Comparative Efficacy of Exosomes Derived from Different Mesenchymal Stem Cell Sources in Osteoarthritis Models: An In Vitro and Ex Vivo Analysis. Int. J. Mol. Sci. 2025, 26, 5447. [Google Scholar] [CrossRef] [PubMed]
- Fazaeli, H.; Kalhor, N.; Naserpour, L.; Davoodi, F.; Sheykhhasan, M.; Hosseini, S.K.E.; Rabiei, M.; Sheikholeslami, A. A comparative study on the effect of exosomes secreted by mesenchymal stem cells derived from adipose and bone marrow tissues in the treatment of osteoarthritis-induced mouse model. BioMed Res. Int. 2021, 2021, 9688138. [Google Scholar] [CrossRef]
- Meng, S.; Tang, C.; Deng, M.; Yuan, J.; Fan, Y.; Gao, S.; Feng, Y.; Yang, J.; Chen, C. Tropoelastin-Pretreated Exosomes from Adipose-Derived Stem Cells Improve the Synthesis of Cartilage Matrix and Alleviate Osteoarthritis. J. Funct. Biomater. 2023, 14, 203. [Google Scholar] [CrossRef]
- Fu, M.; Fang, L.; Xiang, X.; Fan, X.; Wu, J.; Wang, J. Microarray analysis of circRNAs sequencing profile in exosomes derived from bone marrow mesenchymal stem cells in postmenopausal osteoporosis patients. J. Clin. Lab. Anal. 2022, 36, e23916. [Google Scholar] [CrossRef]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Tian, G.; Yang, Z.; Gao, X.; Wang, F.; Li, J.; Tian, Z.; Huang, B.; Wei, F.; Sang, X. Enhancement of acellular cartilage matrix scaffold by Wharton’s jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact. Mater. 2021, 6, 2711–2728. [Google Scholar] [CrossRef]
- Yan, Z.; Yin, H.; Wu, J.; Tian, G.; Li, M.; Liao, Z.; He, S.; Deng, H.; Ning, C.; Ding, Z. Engineering exosomes by three-dimensional porous scaffold culture of human umbilical cord mesenchymal stem cells promote osteochondral repair. Mater. Today Bio 2023, 19, 100549. [Google Scholar] [CrossRef]
- Yan, L.; Wu, X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol. Toxicol. 2020, 36, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liu, G.; Wu, X. The umbilical cord mesenchymal stem cell-derived exosomal lncRNA H19 improves osteochondral activity through miR-29b-3p/FoxO3 axis. Clin. Transl. Med. 2021, 11, e255. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, Z.; Wu, M.; Xu, J.; Li, J.; Liu, J.; He, T.; Zhang, T.; Liu, B. Application of miR-29a-Exosome and multifunctional scaffold for full-thickness cartilage defects. Extracell. Vesicle 2024, 4, 100055. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.; Zhao, F.; Cao, C.; Wu, T.; Fan, Y.; Ao, Y.; Hu, X. 3D printing of microenvironment-specific bioinspired and exosome-reinforced hydrogel scaffolds for efficient cartilage and subchondral bone regeneration. Adv. Sci. 2023, 10, 2303650. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Pei, L.; Wang, Y.; Ye, M.; Sun, W.; Zhang, J.; Gao, P.; Tao, Z.; Liu, D.; Jing, J.; Guan, J. Exosome-Functionalized Hydrogels Improve Cartilage Repair by Modulating BMSCs Migration and Differentiation. ACS Appl. Mater. Interfaces 2025, 17, 41729–41746. [Google Scholar] [CrossRef]
- Zeng, J.; Sun, P.; Zhao, Y.; Fang, X.; Wu, Z.; Qi, X. Bone mesenchymal stem cell-derived exosomes involved co-delivery and synergism effect with icariin via mussel-inspired multifunctional hydrogel for cartilage protection. Asian J. Pharm. Sci. 2023, 18, 100799. [Google Scholar] [CrossRef]
- Thomas, B.L.; Eldridge, S.E.; Nosrati, B.; Alvarez, M.; Thorup, A.S.; Nalesso, G.; Caxaria, S.; Barawi, A.; Nicholson, J.G.; Perretti, M.; et al. WNT3A-loaded exosomes enable cartilage repair. J. Extracell. Vesicles 2021, 10, e12088. [Google Scholar] [CrossRef]
- Li, X.; Han, Y.; Li, G.; Zhang, Y.; Wang, J.; Feng, C. Role of Wnt signaling pathway in joint development and cartilage degeneration. Front. Cell Dev. Biol. 2023, 11, 1181619. [Google Scholar] [CrossRef]
- Lou, W.; Qiu, X.; Qin, Y.; Lu, Y.; Cao, Y.; Lu, H. 3D-printed advanced scaffold armed with exosomes derived from human skeletal stem cell identified by single-cell RNA sequencing enhances osteochondral regeneration. Bioact. Mater. 2025, 51, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Meriç, G.; Eren, O.; Yaba, A.; Aksu, B.Ç.; Başdelioğlu, K.; Ateş, U. Comparative analysis of the therapeutic effects of mesenchymal stem cells and exosomes on cartilage regeneration: Exploring their synergistic potential with hyaluronic acid for treating articular cartilage defects. Eur. J. Orthop. Surg. Traumatol. 2025, 35, 154. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.; Lai, R.; Lim, S.; Hui, J.; Toh, W. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, R.; Katagiri, W.; Endo, S.; Kobayashi, T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS ONE 2019, 14, e0225472. [Google Scholar] [CrossRef] [PubMed]
- Swanson, W.B.; Zhang, Z.; Xiu, K.; Gong, T.; Eberle, M.; Wang, Z.; Ma, P.X. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater. 2020, 118, 215–232. [Google Scholar] [CrossRef]
- Chew, J.R.J.; Chuah, S.J.; Teo, K.Y.W.; Zhang, S.; Lai, R.C.; Fu, J.H.; Lim, L.P.; Lim, S.K.; Toh, W.S. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019, 89, 252–264. [Google Scholar] [CrossRef]
- Shen, Z.; Kuang, S.; Zhang, Y.; Yang, M.; Qin, W.; Shi, X.; Lin, Z. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact. Mater. 2020, 5, 1113–1126. [Google Scholar] [CrossRef]
- Lu, J.; Yu, N.; Zhang, X.; Wu, Y.; Fan, D.; Zhen, L. Injectable thermosensitive hydrogel-loaded exosomes promote diabetic periodontal bone regeneration through mitochondrial function regulation. Chem. Eng. J. 2025, 519, 164950. [Google Scholar] [CrossRef]
- Tang, S.; Tang, T.; Gao, G.; Wei, Q.; Sun, K.; Huang, W. Bone marrow mesenchymal stem cell-derived exosomes inhibit chondrocyte apoptosis and the expression of MMPs by regulating Drp1-mediated mitophagy. Acta Histochem. 2021, 123, 151796. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, K.; Zhang, X.; Zheng, Z.; Liu, K. Exosomes derived from mature chondrocytes facilitate subcutaneous stable ectopic chondrogenesis of cartilage progenitor cells. Stem Cell Res. Ther. 2018, 9, 318. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, G.; Chen, G.; Chen, G.; Chen, K.; Fan, L.; Tu, Y.; Chen, J.; Shi, Z.; Chen, C. Novel injectable adhesive hydrogel loaded with exosomes for holistic repair of hemophilic articular cartilage defect. Bioact. Mater. 2024, 42, 85–111. [Google Scholar] [CrossRef]
- Chen, J.; Tan, Y.; Chen, Z.; Yang, H.; Li, X.; Long, X.; Han, Y.; Yang, J. Exosomes derived from primary cartilage stem/progenitor cells promote the repair of osteoarthritic chondrocytes by modulating immune responses. Int. Immunopharmacol. 2024, 143, 113397. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-W.; Huang, Y.-H.; Shao, P.-L.; Chang, L.-H.; Lu, C.-C.; Chen, C.-H.; Fu, Y.-C.; Ho, M.-L.; Chang, J.-K.; Wu, S.-C. Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Enhance Chondrocyte Function by Reducing Oxidative Stress in Chondrocytes. Int. J. Mol. Sci. 2025, 26, 7683. [Google Scholar] [CrossRef]
- Niada, S.; Giannasi, C.; Gomarasca, M.; Stanco, D.; Casati, S.; Brini, A.T. Adipose-derived stromal cell secretome reduces TNFα-induced hypertrophy and catabolic markers in primary human articular chondrocytes. Stem Cell Res. 2019, 38, 101463. [Google Scholar] [CrossRef] [PubMed]
- Bolandnazar, N.S.; Raeissadat, S.A.; Haghighatkhah, H.; Rayegani, S.M.; Oshnari, R.S.; Keshel, S.H.; Zahraei, M.; Aalipour, K.; Babaee, M.; Zamani, A. Safety and efficacy of placental mesenchymal stromal cells-derived extracellular vesicles in knee osteoarthritis: A randomized, triple-blind, placebo-controlled clinical trial. BMC Musculoskelet. Disord. 2024, 25, 856. [Google Scholar] [CrossRef]
- Abdalla Awidi Abbadi, J. Knee Osteoarthrosis (KOA) in Jordanian Patients: A Phase I Dose-finding Study Using Extracellular Vesicles (EV) for Advanced Stage III and IV KOA. 2024. Available online: https://clinicaltrials.gov/study/NCT06937528 (accessed on 5 November 2025).
- Cheng, K.; Kalluri, R. Guidelines for clinical translation and commercialization of extracellular vesicles and exosomes based therapeutics. Extracell. Vesicle 2023, 2, 100029. [Google Scholar] [CrossRef]
- Claridge, B.; Lozano, J.; Poh, Q.H.; Greening, D.W. Development of extracellular vesicle therapeutics: Challenges, considerations, and opportunities. Front. Cell Dev. Biol. 2021, 9, 734720. [Google Scholar] [CrossRef]
- Wiest, E.F.; Zubair, A.C. Generation of Current Good Manufacturing Practices-Grade Mesenchymal Stromal Cell-Derived Extracellular Vesicles Using Automated Bioreactors. Biology 2025, 14, 313. [Google Scholar] [CrossRef] [PubMed]
- Tajafrooz, F.; Ghofrani, S.; Sadeghghomi, F.; El Hadi Chamas, A.; Rahimi, N.; Mirakhor, A.; Hooshiar, M.H.; Raee, A. Exosome-based therapeutics in bone regeneration: From fundamental biology to clinical translation. Stem Cell Res. Ther. 2025, 16, 555. [Google Scholar] [CrossRef] [PubMed]
- A Phase I Study Aiming to Assess Safety and Efficacy of a Single Intra-Articular Injection of MSC-Derived Exosomes (CelliStem®OA-sEV) in Patients With Moderate Knee Osteoarthritis (ExoOA-1). 2021. Available online: https://clinicaltrials.gov/study/NCT05060107 (accessed on 5 November 2025).
- Administration of sEV Derived from UC-MSC in Patients with Osteoarthritis of the Knee: Safety Determination in a Pilot Dose-Escalation Study. 2024. Available online: https://clinicaltrials.gov/study/NCT06431152 (accessed on 5 November 2025).
- The Efficacy of Allogenic Mesenchymal Stem Cells Derived Exosomes in Osteoarthritis Patients. 2024. Available online: https://clinicaltrials.gov/study/NCT06466850 (accessed on 5 November 2025).
- Huang, H.; Xiao, L.; Fang, L.; Lei, M.; Liu, Z.; Gao, S.; Lei, Q.; Lei, J.; Wei, R.; Lei, Y. Static topographical cue combined with dynamic fluid stimulation enhances the macrophage extracellular vesicle yield and therapeutic potential for bone defects. ACS Nano 2025, 19, 8667–8691. [Google Scholar] [CrossRef] [PubMed]
| Exos Source | Delivery Vehicle | Tissue Targeting | Exos Release | Mechanical Matching | References | |
|---|---|---|---|---|---|---|
| In Vitro | In Vivo | |||||
| 3D-BMSC-Exos. | DSS6-peptide-modified GelMA (3D-SExos/DGel). | A mouse model of cranial bone defects. | At pH 7.4, 85% were released over 15 days. | 3D-Exos and 3D-SExo were released rapidly after 7 days, while for 3D-SExo/DGel, the sustained release exceeded 21 days. | The Young’s modulus of HG is comparatively low, indicating that 3D-SExos encapsulation did not affect the HG. | [24] |
| MSC-Exos | ALG-HA/MSC-Exos HG. | Model of rat cranial bone defects. | After 14 days, the HGs of ALG:HA (5%:2.5%) released 79.20 ± 2.64% of Exos, ALG:HA (5%:5%) released 59.4 ± 3.24% of Exos, and ALG:HA (2.5%:5%) HG released 41.81 ± 4.91% of Exos. | The ALG-HA/EXOs (50%) group showed the highest osteogenic and angiogenic effects, leading to improved regenerative outcomes. | The maximum Young’s modulus was observed for the ALG:HA (5%:2.5%) and further decreased with (5%:2.5%) concentration. Moreover, the increasing elastic modulus after gelation provided physical support for bone regeneration. | [27] |
| BMSC-Exos | Different densities of (GelMA-30 and GelMA-60) HG. GelMA/BMSCs-Exos. | Rabbit TBJ model. | By the seventh day, approximately 80% of the Exos had been released. | NA | After optimization over (GelMA-30 and GelMA-60) HG for tendon–bone healing, GelMA-60-HG with higher porosity was selected for better encapsulation and release of Exos. | [29] |
| SC-Exos | Ti6Al4V/SC-Exos scaffold | New Zealand white rabbits with cylindrical bone defects created in the lateral femoral epicondyle. | NA | NA | 3D porous Ti6Al4V scaffolds effectively reduce the elastic modulus of the Ti6Al4V implants, allowing them to match the natural bone tissue’s elastic modulus and reduce stress shielding. | [36] |
| BMP2-Exos. | BMP2-Exos-GelMA-HG. | Mice cranial defect model. | NA | Osteogenic differentiation and new bone formation indicate the sustained delivery of BMP2-Exos over 4–8 weeks. | The storage modulus (G’) and loss modulus (G’’) remained relatively stable with increasing angular frequency, indicating that Exos encapsulation did not alter the HG mechanical behaviour. | [37] |
| ADSC-Exos. | nHA/CS/PLGA scaffolds. | Maxillofacial bone defects. | Burst release was observed at 3 days (82.53 ± 17.91 µg), and then slowed to 105.16 ± 10.00 µg and 110.39 ± 11.75 µg on days 6 and 9, respectively. | Progressive scaffold degradation and Exos release were observed over 12 weeks. | The slow degradation rate of nHA and the inadequate mechanical strength of CS were improved when combined with PLGA, resulting in enhanced biocompatibility and osteogenic bioactivity. | [53] |
| BMSC-Exos. | 3D-printed cartilage ECM/GelMA scaffold. | Chondrocytes and macrophages. | The scaffold retained Exos for 14 days, with a retention rate of over 56%. | ECM/GelMA-HG significantly retained Exos for at least 7 days, attributed to its COL fibril D-spacing, which helped retain Exos within the HG. | The photo-crosslinked ECM/GelMA-HG exhibited proper mechanical properties, with a Young’s modulus of 33.24 ± 8.8 kPa, which is suitable for load-bearing applications and effective for OCD repair. | [102] |
| hWJMSC-Exos | ACECM scaffolds. | OCD rabbit model. | NA | NA | The Young’s modulus of repaired tissue was higher in the hWJMSC-Exos/ACECM scaffold-treated group at 3 months than in other groups, but still lower than that of normal cartilage. By 6 months, the Young’s modulus was comparable to that of normal cartilage. | [103] |
| Sources of Exos | Cell Target | Mechanism Impact | Biomedical Application | References |
|---|---|---|---|---|
| Serum-free conditioned medium-cultured BMSC-Exos | MSCs and vascular endothelial cells | Promote vascularisation and osteogenesis by upregulating angiogenic genes such as VEGF, ANG1, and ANG2, and by stimulating OB differentiation in MSCs. | Promote effective bone regeneration. | [117] |
| hDPSCs-Exos | Upregulate osteogenic markers like RUNX2, osteocalcin (OCN), bone sialoprotein (BSP), ERK phosphorylation, and ECM secretion. | Critical-sized bone defect repair. | [118] | |
| BMSC-Exos | PDLCs | Enhanced PDL cell migration and proliferation through adenosine receptor-mediated activation of AKT and ERK signalling pathways. | Cell-free treatment option for periodontal regeneration. | [119] |
| hDPSCs-Exos | Bone marrow-derived macrophages | Decrease levels of inflammatory cytokines, such as IL-1β, TNF-α, and IL-6, and downregulate the NF-κB and p38 MAPK signalling pathways. | Therapeutic periodontitis. | [120] |
| rBMSC-Exos | HUVECs | Enhanced proliferation, migration, and tube formation, upregulated VEGF and CD31, and restored mitochondrial function in HUVECs exposed to high glucose. | Effective bone regeneration in diabetic environments. | [121] |
| BMSC-Exos | Chondrocytes | Suppress apoptosis, downregulate the expression of MMP-3 and MMP-13, increase the expression of LC3-II/LC3-I and Beclin-1, and decrease (Drp1) expression. | Protect chondrocytes and promote cartilage regeneration. | [122] |
| CC-Exos | Cartilage progenitor cells | Enhanced the expression of COL-II and SOX-9, chondrocyte differentiation, and matrix maturation, and prevented hypertrophy. | Improve cartilage regeneration. | [123] |
| BMSC-Exos | RAW264.7 macrophages and chondrocytes | Promote chondrocyte migration, modulate macrophage M2 polarization, reduce inflammation, and protect chondrocytes. | Improved articular cartilage repair. | [124] |
| Cartilage stem/progenitor cell-derived Exos (CSPCs-Exos) | Rat primary chondrocytes | Regulate pathways involved in cell migration and spindle organization, and reduce TNF and IL-17 production to mitigate inflammation. | OA model cartilage tissue repair. | [125] |
| UCMSC-EVs | Chondrocytes | Upregulated SOX-9, COL-II, and AGN, and downregulated Col-I, which is associated with fibrocartilage formation and regulates oxidative stress. | Cartilage regeneration. | [126] |
| Phase | Indication | EV Source | Dosing and Route | Primary Endpoints | Current Status | References |
|---|---|---|---|---|---|---|
| RCT | Knee OA | Placental MSC-EVs | Single IA dose (≈5 mL @ 7 × 109 particles/mL) | WOMAC, MRI, safety | Completed—no superiority vs. placebo (IRCT20210423051054N1) | [128] |
| I (listed) | Knee OA/Osteoarthrosis | EV product (unspecified) | IA injection | Safety, function | Listed (NCT06937528) | [129] |
| I | Knee OA | Allogenic BM-MSC sEVs | Single IA injection (109–1010 particles/mL) | Safety, WOMAC, pain/function | Active—not recruiting (NCT05060107) | [134] |
| I | Knee OA | UCMSC-EVs (EXO-OA01) | Single IA injection | Safety, cartilage thickness (MRI) | Recruiting (NCT06431152) | [135] |
| I | Knee OA | Allogenic MSC-EVs | Single/few IA doses | Safety, pain/function | Recruiting (NCT06466850) | [136] |
| Preclinical | Cartilage Repair | MSC-EVs + PRP/fibrin scaffold | Local delivery to the defect site | Histologic repair score, integration | Translational phase (no patients) | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakthi Mohan, P.; Abdul Majid, N.B.; Susilo, R.J.K.; Kunasekaran, W.; Jin, T.L.; Ee, L.S.; Seng, C.K.; Venkatraman, G. Exosomal Interventions in Bone and Osteochondral Repair: Mechanisms and Outcomes. Int. J. Mol. Sci. 2025, 26, 11172. https://doi.org/10.3390/ijms262211172
Sakthi Mohan P, Abdul Majid NB, Susilo RJK, Kunasekaran W, Jin TL, Ee LS, Seng CK, Venkatraman G. Exosomal Interventions in Bone and Osteochondral Repair: Mechanisms and Outcomes. International Journal of Molecular Sciences. 2025; 26(22):11172. https://doi.org/10.3390/ijms262211172
Chicago/Turabian StyleSakthi Mohan, Priyadarshini, Nazia Binti Abdul Majid, Raden Joko Kuncoroningrat Susilo, Wijenthiran Kunasekaran, Tan Li Jin, Lee Siew Ee, Chua Kok Seng, and Gopinath Venkatraman. 2025. "Exosomal Interventions in Bone and Osteochondral Repair: Mechanisms and Outcomes" International Journal of Molecular Sciences 26, no. 22: 11172. https://doi.org/10.3390/ijms262211172
APA StyleSakthi Mohan, P., Abdul Majid, N. B., Susilo, R. J. K., Kunasekaran, W., Jin, T. L., Ee, L. S., Seng, C. K., & Venkatraman, G. (2025). Exosomal Interventions in Bone and Osteochondral Repair: Mechanisms and Outcomes. International Journal of Molecular Sciences, 26(22), 11172. https://doi.org/10.3390/ijms262211172





