Toxicological Effects of Titanium Dioxide Nanoparticles on Human Menstrual Blood Mesenchymal Stem Cells
Abstract
1. Introduction
2. Results
2.1. Characterization of TiO2 NPs
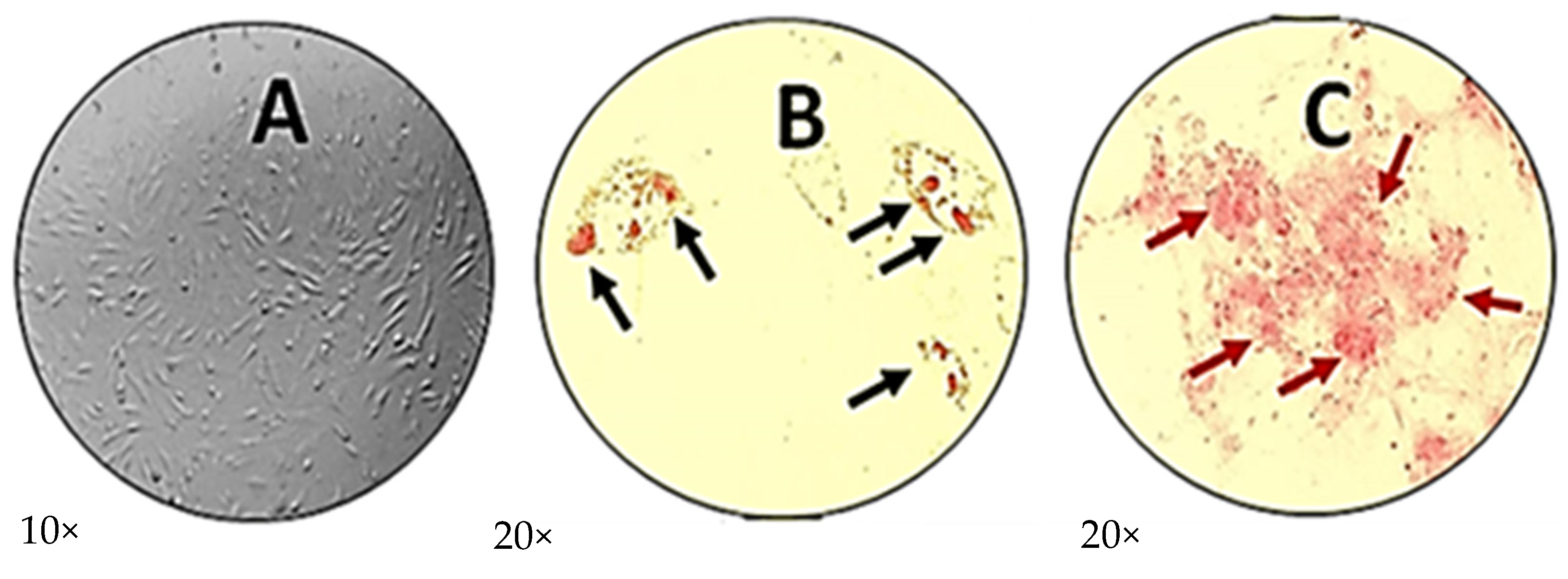
2.2. Characterization of Human Menstrual Blood Mesenchymal Stem Cells
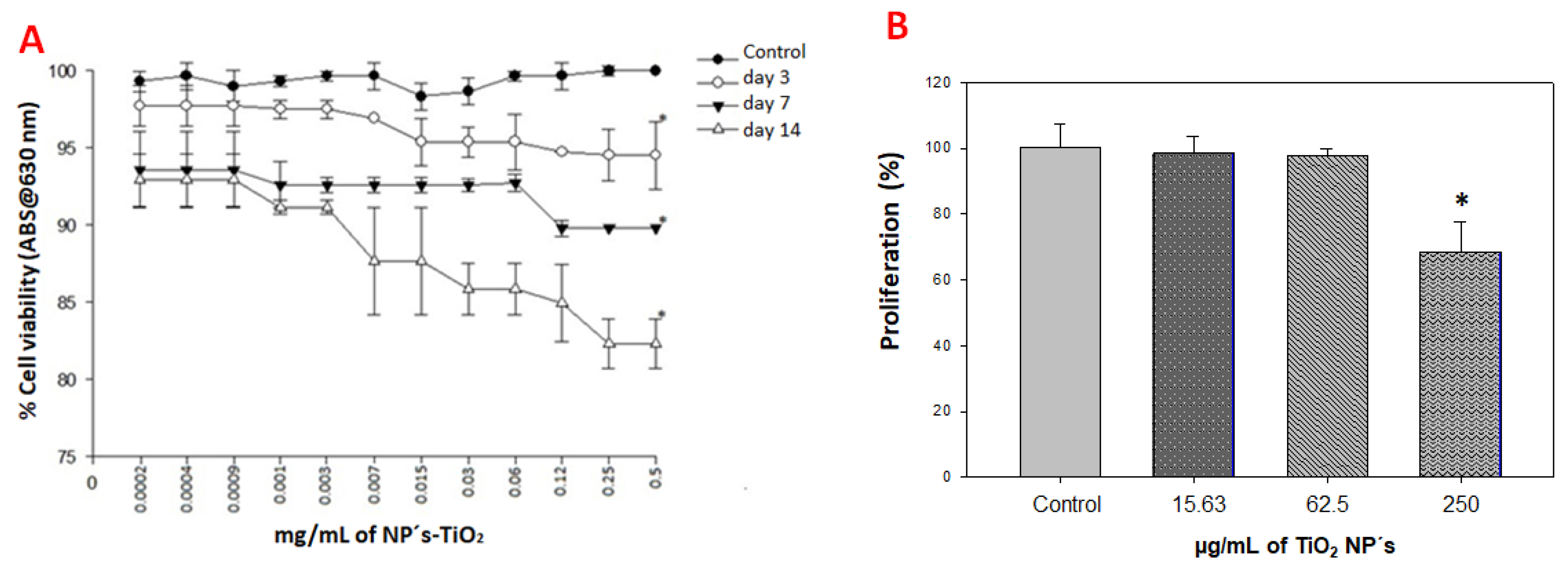
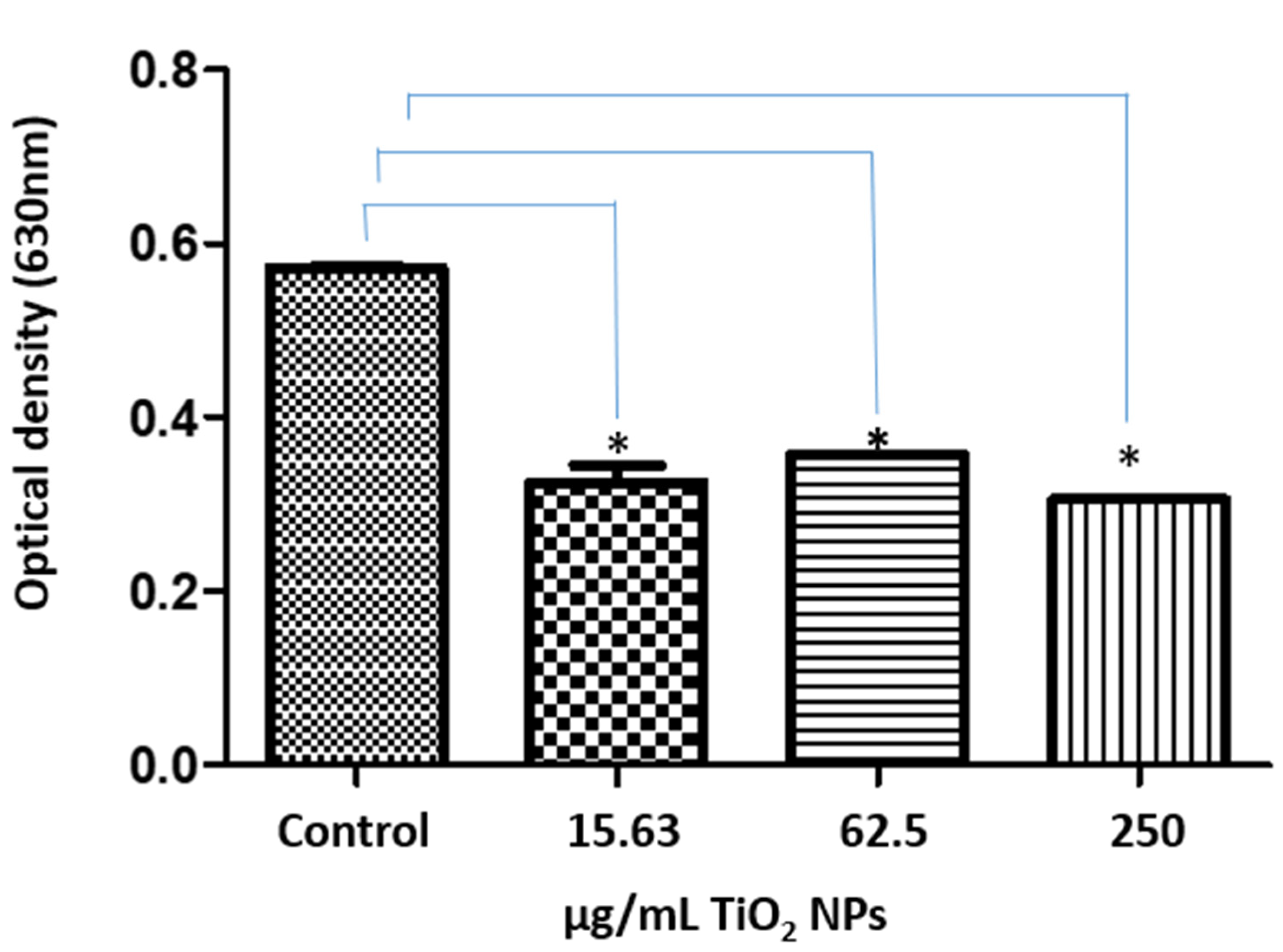
2.3. Viability and Cell Proliferation Assays
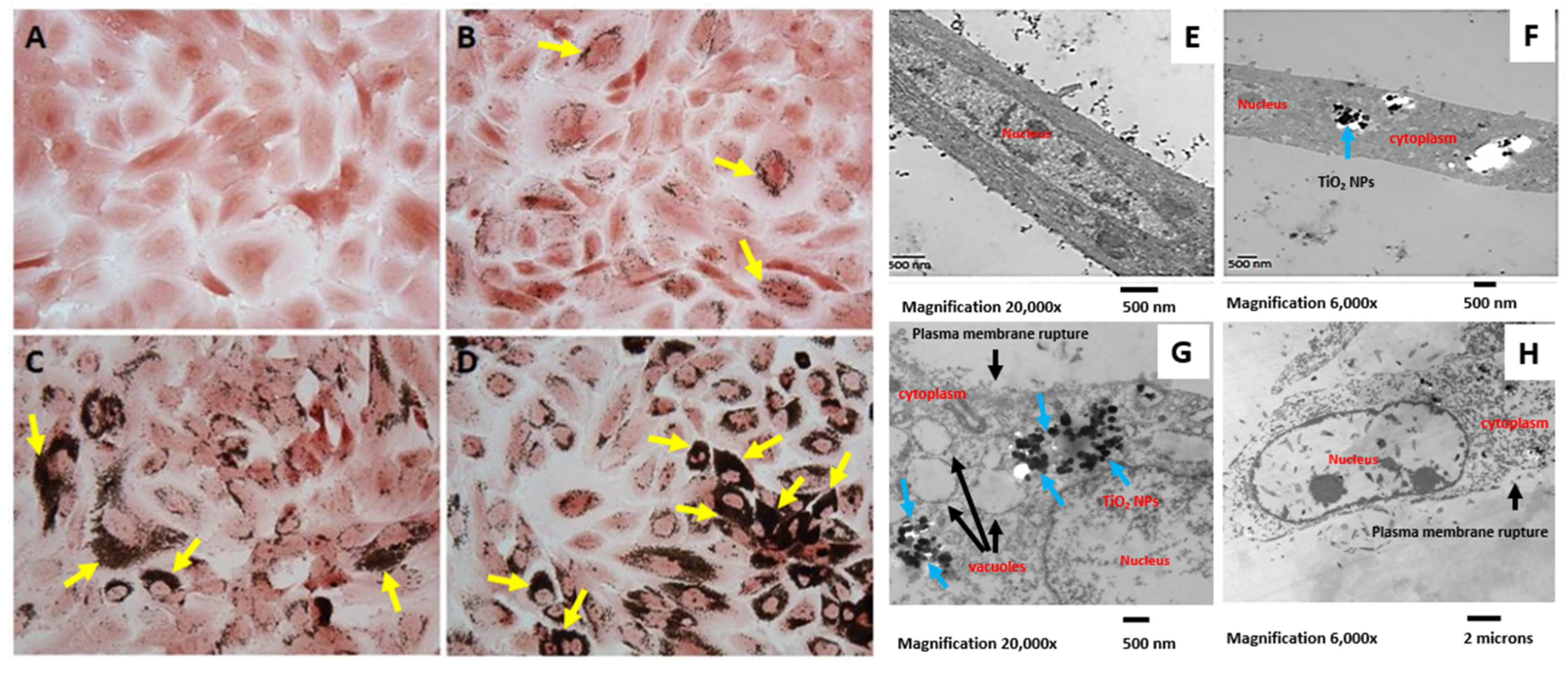
2.4. Internalization of TiO2 NPs by hMB-MSCs
2.5. Expression of Membrane Markers on hMB-MSCs Treated with TiO2 NPs
2.6. TiO2 NPs Reduced ROS Production in hMB-MSCs
3. Discussion
4. Materials and Methods
4.1. Titanium Dioxide NPs
4.2. Characterization of TiO2 NPs
4.3. Human Menstrual Blood Mesenchymal Stem Cells
4.4. Differentiation Assays (Osteogenic and Adipogenic)
4.5. Staining for Osteogenic and Adipogenic Differentiation
4.6. Cell Viability Assay
4.7. Cell Proliferation: Crystal Violet Assay
4.8. Expression of Membrane Markers
4.9. Semi-Quantitative Assay Using NBT Assay for Superoxide (ROS Proxy)
4.10. Statistics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, S.; Rajwade, M.; Paknikar, K. Nanotoxicology and in vitro studies: The need of the hour. Toxicol. Appl. Pharmacol. 2012, 258, 151–165. [Google Scholar] [CrossRef]
- Bieback, K.; Kern, S.; Klüter, H.; Eichler, H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004, 22, 625–634. [Google Scholar] [CrossRef]
- Bo, M.; Yang, X. Titanium particles enhanced osteoclast differentiation and osteoclast bone resorption activity in vitro. J. Dent. Oral Hyg. 2013, 5, 7–12. [Google Scholar]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise views: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Migita, S.; Kanehira, K.; Taniguchi, A. Role of toll-like receptors 3, 4 and 7 in cellular uptake and response to titanium dioxide nanoparticles. Sci. Technol. Adv. Mater. 2013, 14, 015008. [Google Scholar] [CrossRef]
- Dahl, M.; Syberg, S.; Jørgensen, N.R.; Pinholt, E.M. Adipose derived mesenchymal stem cells: Their osteogenicity and osteoblast in vitro mineralization on titanium granule carriers. J. Cranio-Maxillofac. Surg. 2013, 41, e213–e220. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Luyten, F.P. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001, 44, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.R.; Loitto, V.; Audinot, J.N.; Bayat, N.; Gutleb, A.C.; Cristobal, S. Dose-dependent autophagic effect of titanium dioxide nanoparticles in human HaCaT cells at non-cytotoxic levels. J. Nanobiotechnol. 2016, 14, 22. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic transplants of bone marrow. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Kuku, G.; Culha, M. Investigating the origin of toxic response in TiO2 nanoparticle-treated cells. Nanomaterials 2017, 7, 83. [Google Scholar] [CrossRef]
- Gonçalves, D.M.; Chiasson, S.; Girard, D. Activation of human neutrophils by titanium dioxide (TiO2) nanoparticles. Toxicol. In Vitr. 2010, 24, 1002–1008. [Google Scholar] [CrossRef]
- Hashemzadeh, M.R.; Eslaminejad, M.B.; Salman Yazdi, R.; Aflatoonian, R. Evaluation of toll-like receptor 4 expression in human bone marrow mesenchymal stem cells by lipopolysaccharides from Shigella. Biologicals 2018, 55, 53–58. [Google Scholar] [CrossRef]
- Lv, F.-J.; Tuan, R.S.; Cheung, K.M.C.; Leung, V.Y.L. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef]
- Fu, Y.-X.; Ji, J.; Shan, F.; Li, J.; Hu, R. Human mesenchymal stem cell treatment of premature ovarian failure: New challenges and opportunities. Stem Cell Res. Ther. 2021, 12, 161. [Google Scholar] [CrossRef]
- Najmeh, K.F.; Ali, M.; Elahe, M. How similar are human mesenchymal stem cells derived from different origins? A review of comparative studies. Curr. Stem Cell Res. Ther. 2021, 16, 980–993. [Google Scholar]
- Savkovic, V.; Li, H.; Seon, J.K.; Hacker, M.; Franz, S.; Simon, J.C. Mesenchymal stem cells in cartilage regeneration. Curr. Stem Cell Res. Ther. 2014, 9, 469–488. [Google Scholar] [CrossRef]
- Montiel-Dávalos, A.; Ventura-Gallegos, J.L.; Alfaro-Moreno, E.; Soria-Castro, E.; García-Latorre, E.; Cabañas-Moreno, J.G.; Ramos-Godinez, M.d.P.; López-Marure, R. TiO2 nanoparticles induce dysfunction and activation of human endothelial cells. Chem. Res. Toxicol. 2012, 25, 920–930. [Google Scholar] [CrossRef] [PubMed]
- In‘t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; Noort, W.A.; Claas, F.H.; Willemze, R.; Fibbe, W.E.; Kanhai, H.H. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003, 102, 1548–1549. [Google Scholar] [CrossRef] [PubMed]
- El-Said, K.S.; Ali, E.M.; Kanehira, K.; Taniguchi, A. Molecular mechanism of DNA damage induced by titanium dioxide nanoparticles in toll-like receptor 3 or 4 expressing human hepatocarcinoma cell lines. J. Nanobiotechnol. 2014, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Umer, A.; Khan, N.; Greene, D.L.; Habiba, U.E.; Shamim, S.; Khayam, A.U. The therapeutic potential of human umbilical cord derived mesenchymal stem cells for the treatment of premature ovarian failure. Stem Cell Rev. Rep. 2023, 19, 651–666. [Google Scholar] [CrossRef]
- Cong, B.; Sun, T.; Zhao, Y.; Chen, M. Current and novel therapeutics for articular cartilage repair and regeneration. Ther. Clin. Risk Manag. 2023, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Bonetta, S.; Macrì, M.; Acito, M.; Villarini, M.; Moretti, M.; Bonetta Si Bosio, D.; Mariella, G.; Bellisario, V.; Bergamaschi, E.; Carraro, E. DNA damage in workers exposed to pigment grade titanium dioxide (TiO2) and association with biomarkers of oxidative stress and inflammation. Environ. Toxicol. Pharmacol. 2024, 105, 104328. [Google Scholar] [CrossRef]
- Riedle, S.; Pele, L.C.; Otter, D.E.; Hewitt, R.E.; Singh, H.; Roy, N.C.; Powell, J.J. Pro-inflammatory adjuvant properties of pigment-grade titanium dioxide particles are augmented by a genotype that potentiates interleukin-1β processing. Part. Fibre Toxicol. 2017, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Cai, K.; Li, J.; Chen, X.; Lai, M.; Hu, Y.; Luo, Z.; Ding, X.; Xu, D. Effects of titanium nanoparticles on adhesion, migration, proliferation, and differentiation of mesenchymal stem cells. Int. J. Nanomed. 2013, 8, 3619–3630. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Jin, X.; George, S.; Xia, T.; Meng, H.; Wang, X.; Suarez, E.; Zhang, H.; Hoek, E.M.; Godwin, H.; et al. Dispersion and stability optimization of TiO2 nanoparticles in cell culture media. Environ. Sci. Technol. 2010, 44, 7309–7314. [Google Scholar] [CrossRef]
- Kongseng, S.; Yoovathaworn, K.; Wongprasert, K.; Chunhabundit, R.; Sukwong, P.; Pissuwan, D. Cytotoxic and inflammatory responses of TiO2 nanoparticles on human peripheral blood mononuclear cells. J. Appl. Toxicol. 2016, 36, 1364–1373. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, J.; Han, S.; Zheng, P.; Chen, Z.; Jia, G. Titanium dioxide nanoparticles induced reactive oxygen species (ROS) related changes of metabolomics signatures in human normal bronchial epithelial (BEAS-2B) cells. Toxicol. Appl. Pharmacol. 2022, 444, 116020. [Google Scholar] [CrossRef]
- Kheder, W.; Soumya, S.; Samsudin, A. Impact of titanium dioxide particle size on macrophage production of intracellular reactive oxygen species. Arch. Oral Biol. 2021, 127, 105133. [Google Scholar] [CrossRef]
- Bovari-Biri, J.; A Miskei, J.; Kover, Z.; Steinerbrunner-Nagy, A.; Kardos, K.; Maroti, P.E.; Pongracz, J. Titanium dioxide nanoparticle-induced autophagy and its role in stem cell differentiation. Cells 2025, 14, 145. [Google Scholar] [CrossRef]
- Afonso, P.; Castro, I.; Carvalho, M. Salt-Resilient Cowpeas: Early Identification Through Growth Parameters and Gene Expression at Germination Stage. Int. J. Mol. Sci. 2025, 26, 1892. [Google Scholar] [CrossRef]
- Im, J.H.; Lee, K.-Y.; Seo, Y.; Rhim, J.; Dho, Y.-S.; Yoo, B.C.; Park, J.B.; Shin, S.H.; Yoo, H.; Kim, J.H.; et al. Titanium dioxide nanoparticles trigger mitochondrial-mediated apoptosis and oxidative stress in human mesenchymal stem cells. Int. J. Mol. Sci. 2024, 25, 3124. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, T.; Liang, J.; Yin, J.; Xiao, M.; Han, D.; Huang, S.; Wang, S.; Meng, Y. Biodegradable and Ultra-High Expansion Ratio PPC-P Foams Achieved by Microcellular Foaming Using CO2 as Blowing Agent. Nanomaterials 2024, 14, 1120. [Google Scholar] [CrossRef]
- Nemmar, A.; Holme, J.A.; Rosas, I.; Schwarze, P.E.; Alfaro-Moreno, E. Recent advances in particulate matter and nanoparticle toxicology: A review of the in vivo and in vitro studies. BioMed Res. Int. 2013, 2013, 279371. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, N.; Zhu, M.; Lu, J.; Zhong, H.; Xue, X.; Guo, S.; Li, M.; Wei, X.; Tao, Y.; et al. TiO2 nanoparticles cause mitochondrial dysfunction, activate inflammatory responses, and attenuate phagocytosis in macrophages: A proteomic and metabolomic insight. Redox Biol. 2018, 15, 266–276. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple Applications of Alamar Blue as an Indicator of Metabolic Function and Cellular Health in Cell Viability Bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Lavenus, S.; Berreur, M.; Trichet, V.; Pilet, P.; Louarn, G.; Layrolle, P. Adhesion and osteogenic differentiation of human mesenchymal stem cells on titanium nanopores. Eur. Cell. Mater. 2011, 22, 84–96. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Alamar Blue Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-rot095489. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087379. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Barrera, A.; López-Marure, R.; Romero-López, E.; Calzada-Mendoza, C.C.; Arellano-Galindo, J.; Rangel-Martínez, R.; Gutiérrez-Iglesias, G. Toxicological Effects of Titanium Dioxide Nanoparticles on Human Menstrual Blood Mesenchymal Stem Cells. Int. J. Mol. Sci. 2025, 26, 11168. https://doi.org/10.3390/ijms262211168
Parra-Barrera A, López-Marure R, Romero-López E, Calzada-Mendoza CC, Arellano-Galindo J, Rangel-Martínez R, Gutiérrez-Iglesias G. Toxicological Effects of Titanium Dioxide Nanoparticles on Human Menstrual Blood Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2025; 26(22):11168. https://doi.org/10.3390/ijms262211168
Chicago/Turabian StyleParra-Barrera, Alberto, Rebeca López-Marure, Ernesto Romero-López, Claudia Camelia Calzada-Mendoza, José Arellano-Galindo, Ricardo Rangel-Martínez, and Gisela Gutiérrez-Iglesias. 2025. "Toxicological Effects of Titanium Dioxide Nanoparticles on Human Menstrual Blood Mesenchymal Stem Cells" International Journal of Molecular Sciences 26, no. 22: 11168. https://doi.org/10.3390/ijms262211168
APA StyleParra-Barrera, A., López-Marure, R., Romero-López, E., Calzada-Mendoza, C. C., Arellano-Galindo, J., Rangel-Martínez, R., & Gutiérrez-Iglesias, G. (2025). Toxicological Effects of Titanium Dioxide Nanoparticles on Human Menstrual Blood Mesenchymal Stem Cells. International Journal of Molecular Sciences, 26(22), 11168. https://doi.org/10.3390/ijms262211168





