Unraveling the Matrix: Proteomic Profiling Reveals Stromal ECM Dysregulation in Severe Early-Onset Fetal Growth Restriction
Abstract
1. Introduction
2. Results
2.1. Subject Demographics
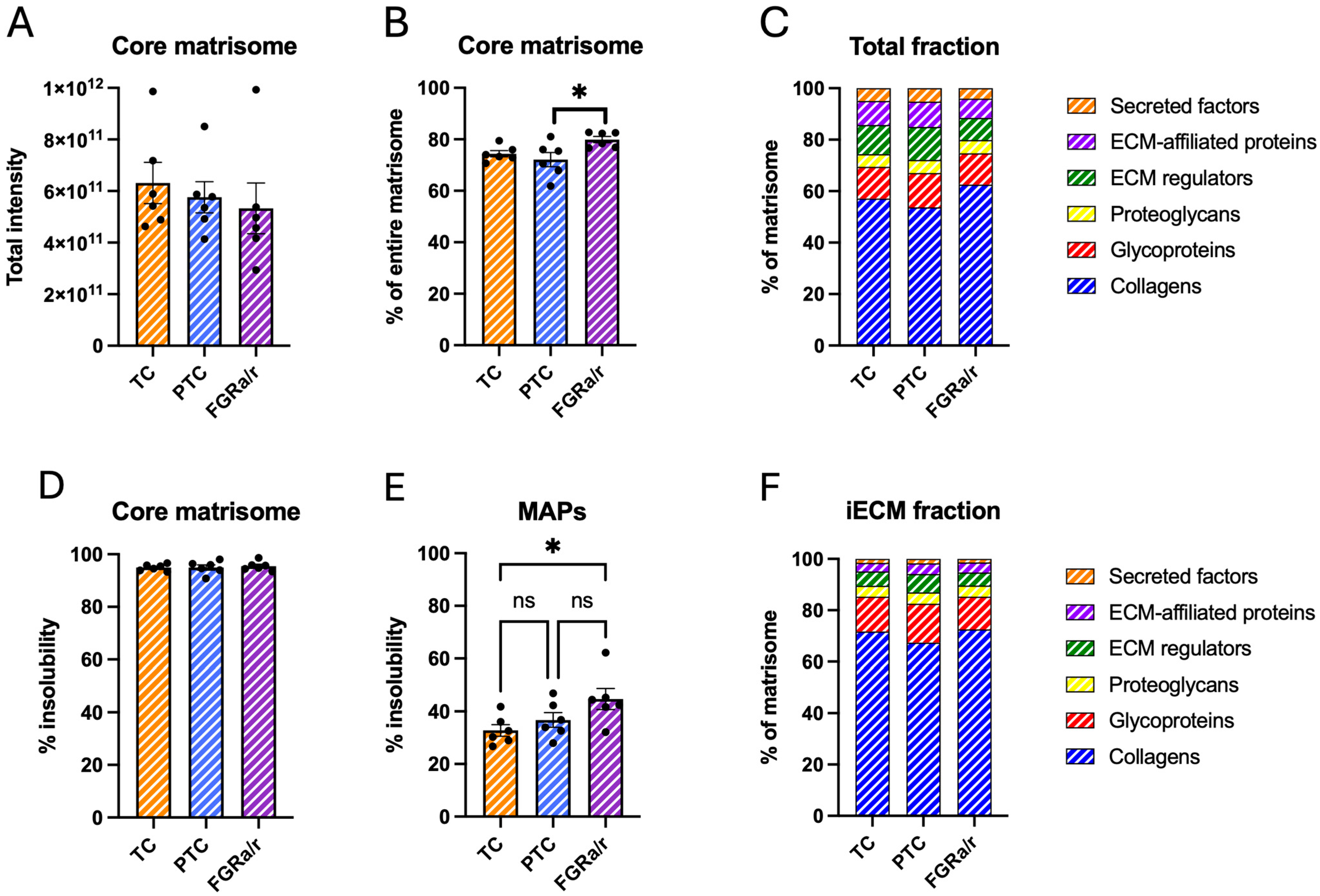
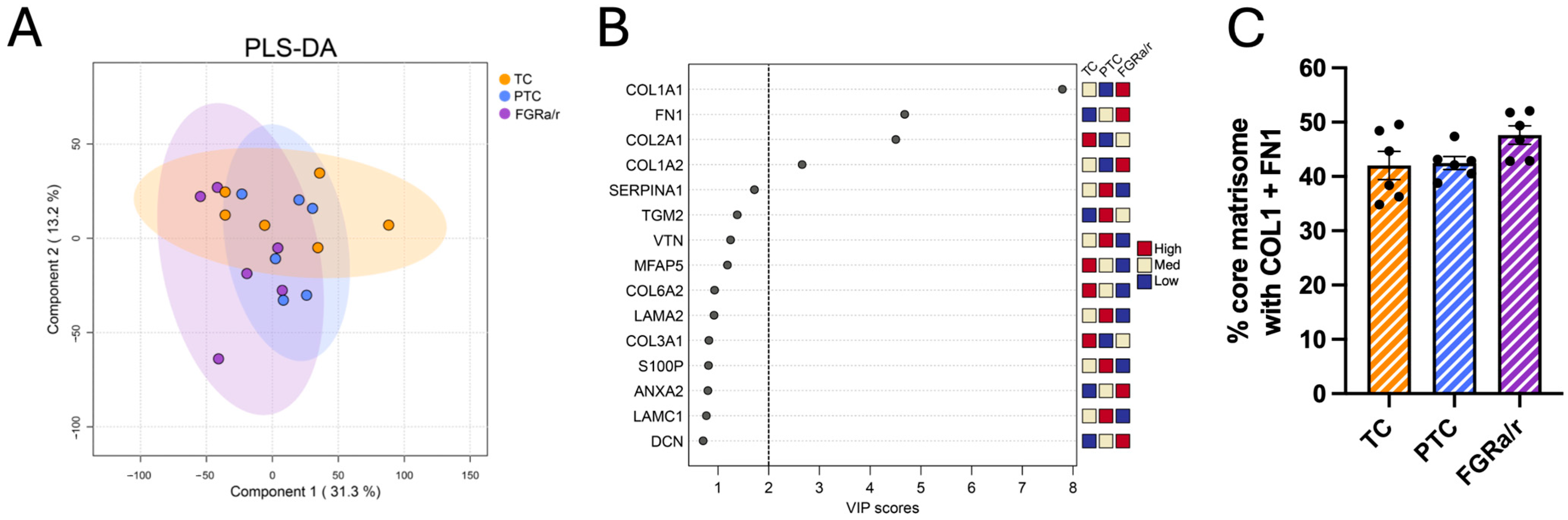
2.2. The Human Placental Matrisome
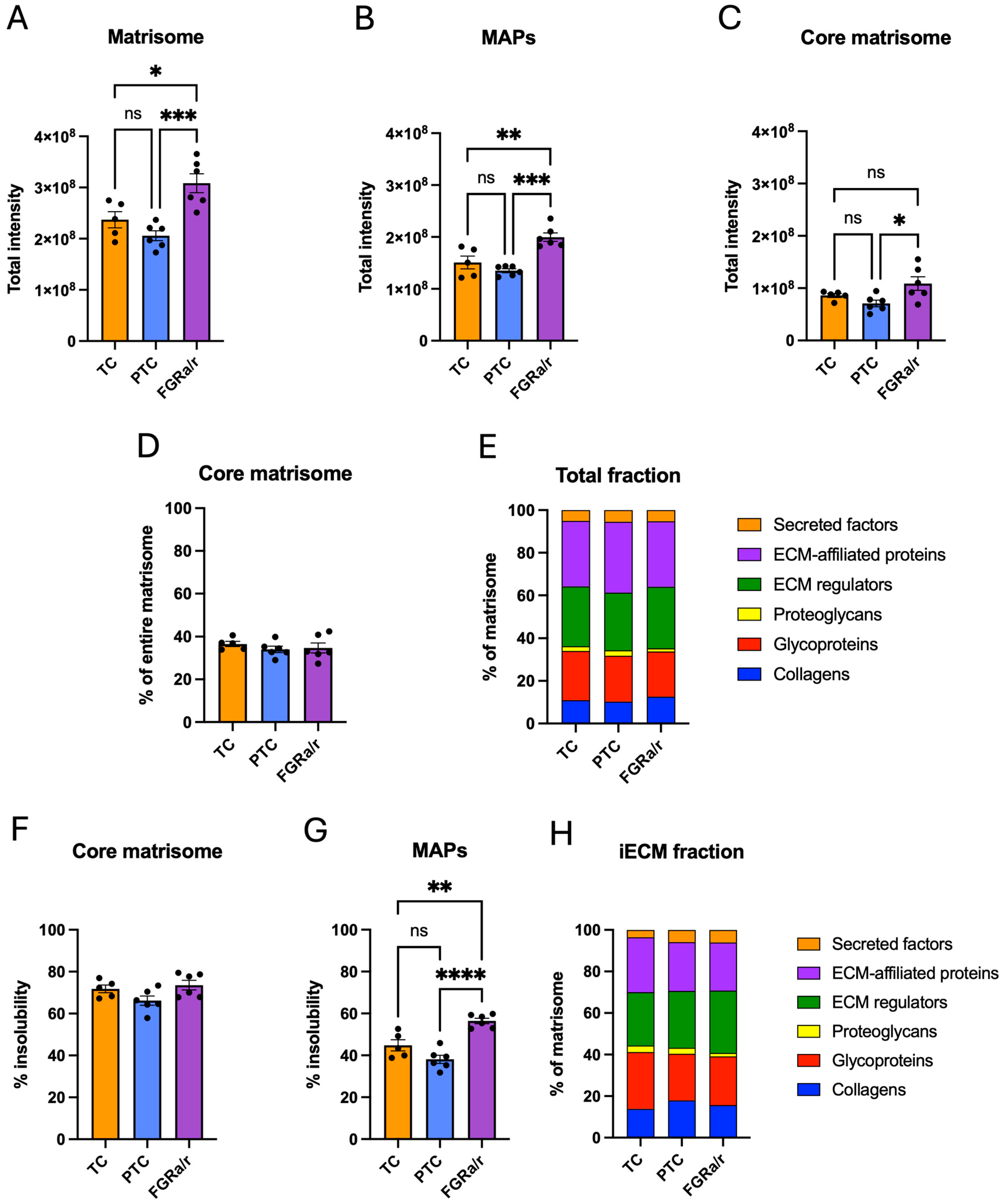
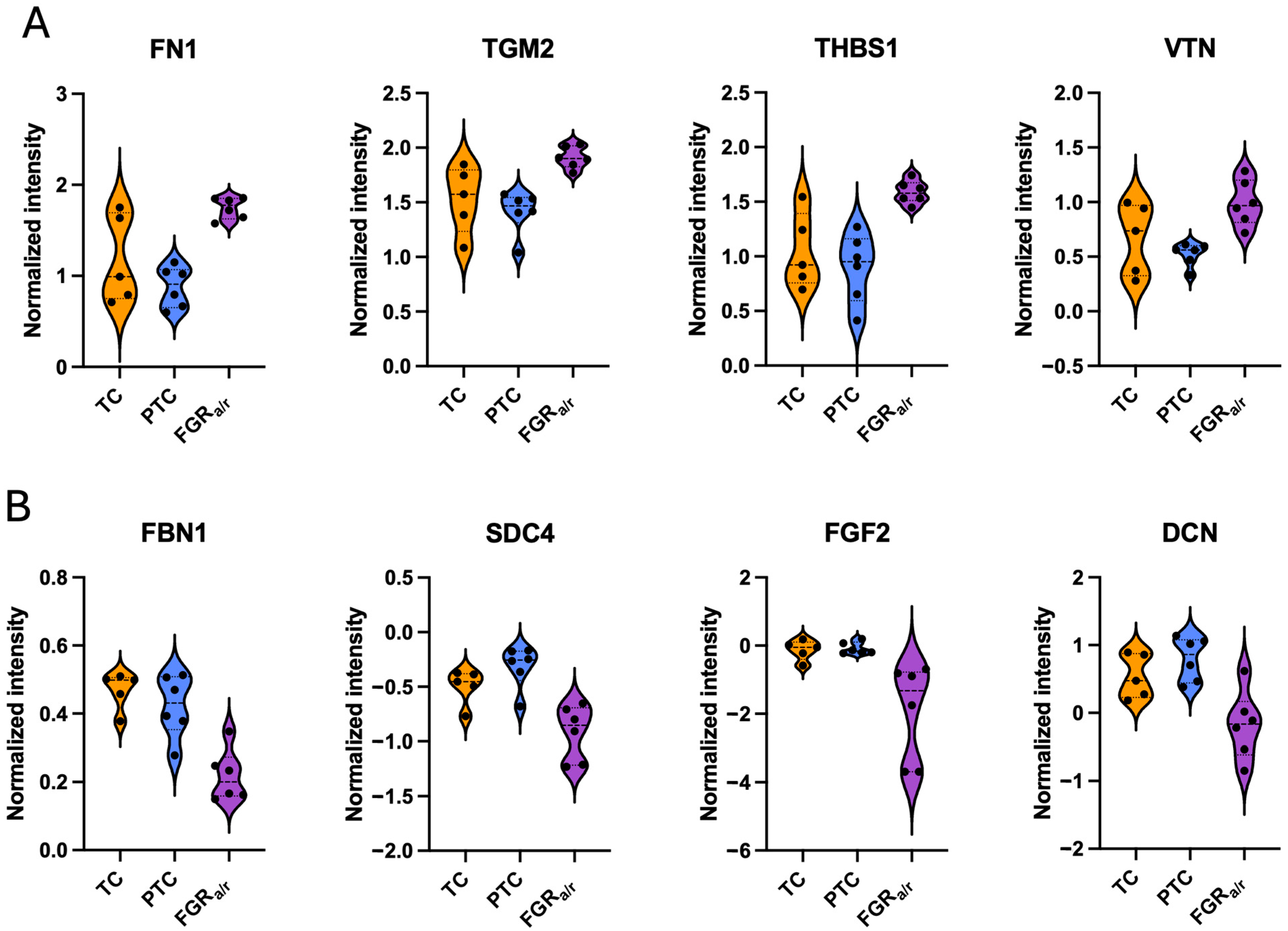
2.3. Matrisome of Placental FB-Derived CDM
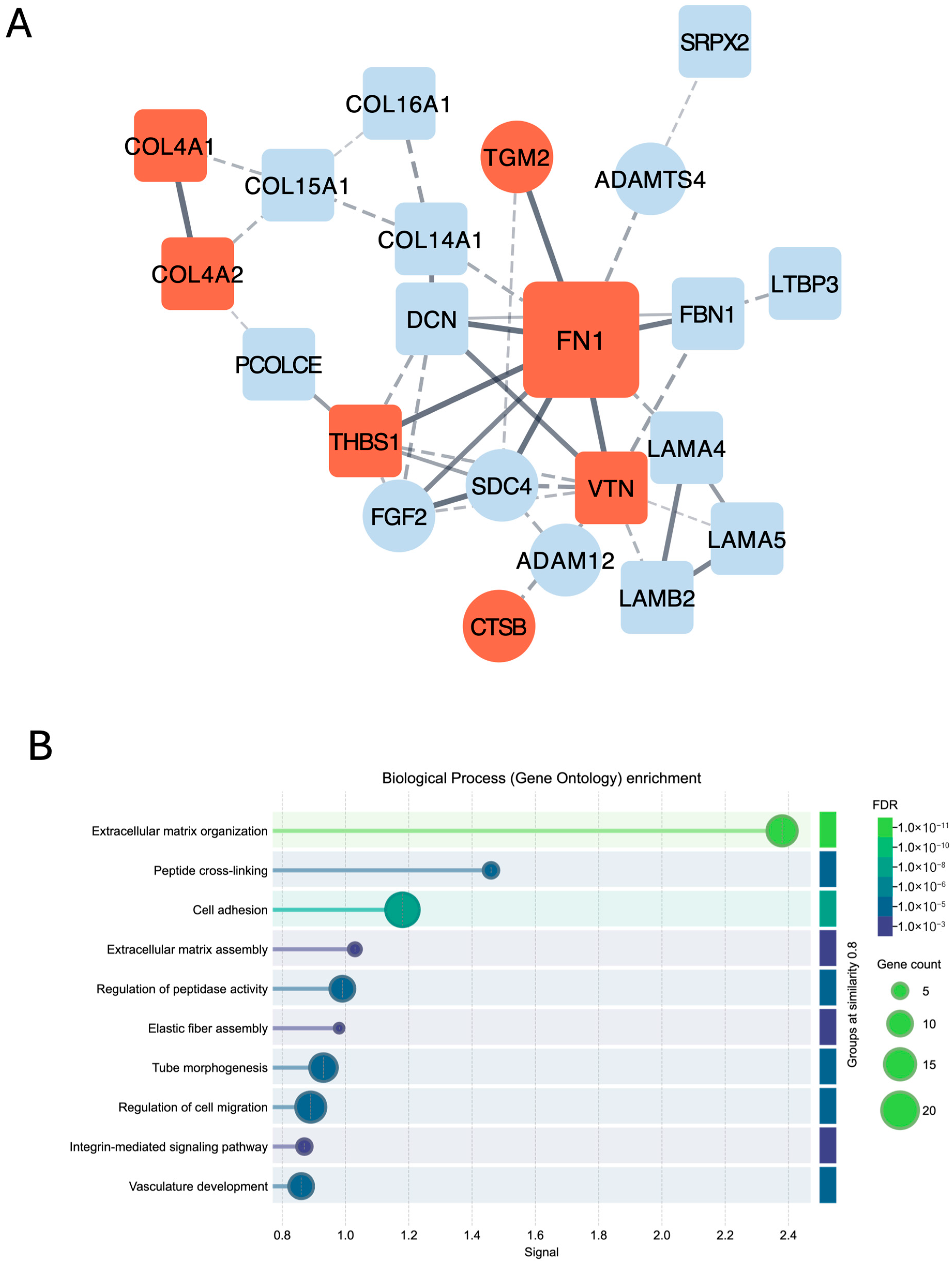
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Villous Tissue Sampling
4.3. Primary Placental Villous Stromal FB Isolation
4.4. Generation of Placental Villous Stromal FB CDMs
4.5. Sample Preparation for Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
4.5.1. Villous Tissue
4.5.2. CDM
4.6. LC-MS/MS Analysis
4.6.1. Villous Tissue
4.6.2. CDM
4.7. MS Data Processing
4.8. Statistical Analysis
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDM | Cell-derived matrices |
| EAP | ECM-affiliated proteins |
| ECM | Extracellular matrix |
| FB | Fibroblasts |
| FGR | Fetal growth restriction |
| FGRa/r | Fetal growth restriction with absent or reversed umbilical artery Doppler end-diastolic velocities |
| iECM | Insoluble ECM |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| LFQ | Label-free quantification |
| MAP | Matrix-associated proteins |
| PTC | Preterm controls |
| TC | Term controls |
References
- Hynes, R.O.; Naba, A. Overview of the matrisome—An inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Hoersch, S.; Liu, H.; Carr, S.A.; Hynes, R.O. The matrisome: In silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell Proteom. 2012, 11, M111.014647. [Google Scholar] [CrossRef]
- Naba, A. Ten Years of Extracellular Matrix Proteomics: Accomplishments, Challenges, and Future Perspectives. Mol. Cell Proteom. 2023, 22, 100528. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Gomez, C.D.; Kapoor, N.; Considine, J.M.; Grams, C.; Gao, Y.T.; Naba, A. MatrisomeDB 2.0: 2023 updates to the ECM-protein knowledge database. Nucleic Acids Res. 2023, 51, D1519–D1530. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Naba, A.; Hoersch, S.; Hynes, R.O. Towards definition of an ECM parts list: An advance on GO categories. Matrix Biol. 2012, 31, 371–372. [Google Scholar] [CrossRef]
- Marchand, M.; Monnot, C.; Muller, L.; Germain, S. Extracellular matrix scaffolding in angiogenesis and capillary homeostasis. Semin. Cell Dev. Biol. 2019, 89, 147–156. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergun, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef]
- McCabe, M.C.; Saviola, A.J.; Hansen, K.C. Mass Spectrometry-Based Atlas of Extracellular Matrix Proteins across 25 Mouse Organs. J. Proteome Res. 2023, 22, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.; Maslanka, M.D.; Darula, Z.; McCabe, M.C.; Saviola, A.J.; Schmitt, L.R.; Weaver, V.; Pitts, T.M.; Messersmith, W.A.; Hansen, K.C. Pancreatic Cancer Progression Involves Increased Lysyl Oxidase-Mediated Collagen Cross-Links as Part of the Desmoplastic Reaction. Biochemistry 2025, 64, 3515–3525. [Google Scholar] [CrossRef]
- Barrett, A.S.; Maller, O.; Pickup, M.W.; Weaver, V.M.; Hansen, K.C. Compartment resolved proteomics reveals a dynamic matrisome in a biomechanically driven model of pancreatic ductal adenocarcinoma. J. Immunol. Regen. Med. 2018, 1, 67–75. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Castellucci, M.; Classen-Linke, I.; Muhlhauser, J.; Kaufmann, P.; Zardi, L.; Chiquet-Ehrismann, R. The human placenta: A model for tenascin expression. Histochemistry 1991, 95, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Gauster, M.; Berghold, V.M.; Moser, G.; Orendi, K.; Siwetz, M.; Huppertz, B. Fibulin-5 expression in the human placenta. Histochem. Cell Biol. 2011, 135, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.L.; Kimberly, D.; Thornburg, K.; Maslen, C. Localization of fibrillin-1 in the human term placenta. J. Soc. Gynecol. Investig. 1995, 2, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Aplin, J.D. Placental extracellular matrix: Gene expression, deposition by placental fibroblasts and the effect of oxygen. Placenta 2003, 24, 316–325. [Google Scholar] [CrossRef]
- Arioz Habibi, H.; Alici Davutoglu, E.; Kandemirli, S.G.; Aslan, M.; Ozel, A.; Kalyoncu Ucar, A.; Zeytun, P.; Madazli, R.; Adaletli, I. In vivo assessment of placental elasticity in intrauterine growth restriction by shear-wave elastography. Eur. J. Radiol. 2017, 97, 16–20. [Google Scholar] [CrossRef]
- Cimsit, C.; Yoldemir, T.; Akpinar, I.N. Shear wave elastography in placental dysfunction: Comparison of elasticity values in normal and preeclamptic pregnancies in the second trimester. J. Ultrasound Med. 2015, 34, 151–159. [Google Scholar] [CrossRef]
- Kilic, F.; Kayadibi, Y.; Yuksel, M.A.; Adaletli, I.; Ustabasioglu, F.E.; Oncul, M.; Madazli, R.; Yilmaz, M.H.; Mihmanli, I.; Kantarci, F. Shear wave elastography of placenta: In vivo quantitation of placental elasticity in preeclampsia. Diagn. Interv. Radiol. 2015, 21, 202–207. [Google Scholar] [CrossRef]
- Ma, Z.; Sagrillo-Fagundes, L.; Mok, S.; Vaillancourt, C.; Moraes, C. Mechanobiological regulation of placental trophoblast fusion and function through extracellular matrix rigidity. Sci. Rep. 2020, 10, 5837. [Google Scholar] [CrossRef]
- Gumina, D.L.; Ji, S.; Flockton, A.; McPeak, K.; Stich, D.; Moldovan, R.; Su, E.J. Dysregulation of integrin alphavbeta3 and alpha5beta1 impedes migration of placental endothelial cells in fetal growth restriction. Development 2022, 149, dev200717. [Google Scholar] [CrossRef]
- Sayres, L.; Flockton, A.R.; Ji, S.; Rey Diaz, C.; Gumina, D.L.; Su, E.J. Angiogenic Function of Human Placental Endothelial Cells in Severe Fetal Growth Restriction Is Not Rescued by Individual Extracellular Matrix Proteins. Cells 2023, 12, 2339. [Google Scholar] [CrossRef]
- Su, E.J.; Xin, H.; Yin, P.; Dyson, M.; Coon, J.; Farrow, K.N.; Mestan, K.K.; Ernst, L.M. Impaired fetoplacental angiogenesis in growth-restricted fetuses with abnormal umbilical artery doppler velocimetry is mediated by aryl hydrocarbon receptor nuclear translocator (ARNT). J. Clin. Endocrinol. Metab. 2015, 100, E30–E40. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Gumina, D.; McPeak, K.; Moldovan, R.; Post, M.D.; Su, E.J. Human placental villous stromal extracellular matrix regulates fetoplacental angiogenesis in severe fetal growth restriction. Clin. Sci. 2021, 135, 1127–1143. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.C.; Schmitt, L.R.; Hill, R.C.; Dzieciatkowska, M.; Maslanka, M.; Daamen, W.F.; van Kuppevelt, T.H.; Hof, D.J.; Hansen, K.C. Evaluation and Refinement of Sample Preparation Methods for Extracellular Matrix Proteome Coverage. Mol. Cell Proteom. 2021, 20, 100079. [Google Scholar] [CrossRef] [PubMed]
- Byron, A.; Humphries, J.D.; Humphries, M.J. Defining the extracellular matrix using proteomics. Int. J. Exp. Pathol. 2013, 94, 75–92. [Google Scholar] [CrossRef]
- Lyons, T.R.; O’Brien, J.; Borges, V.F.; Conklin, M.W.; Keely, P.J.; Eliceiri, K.W.; Marusyk, A.; Tan, A.C.; Schedin, P. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat. Med. 2011, 17, 1109–1115. [Google Scholar] [CrossRef]
- Shao, X.; Taha, I.N.; Clauser, K.R.; Gao, Y.T.; Naba, A. MatrisomeDB: The ECM-protein knowledge database. Nucleic Acids Res. 2020, 48, D1136–D1144. [Google Scholar] [CrossRef]
- Franchi, M.; Piperigkou, Z.; Mastronikolis, N.S.; Karamanos, N. Extracellular matrix biomechanical roles and adaptation in health and disease. FEBS J. 2024, 291, 430–440. [Google Scholar] [CrossRef]
- McCabe, M.C.; Hill, R.C.; Calderone, K.; Cui, Y.; Yan, Y.; Quan, T.; Fisher, G.J.; Hansen, K.C. Alterations in extracellular matrix composition during aging and photoaging of the skin. Matrix Biol. Plus 2020, 8, 100041. [Google Scholar] [CrossRef]
- Yamauchi, M.; Woodley, D.T.; Mechanic, G.L. Aging and cross-linking of skin collagen. Biochem. Biophys. Res. Commun. 1988, 152, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, N.; Wu, W.; Li, H.; You, H.; Chen, W. Atlas of mildly and highly insoluble matrisome driving liver fibrosis. Front. Pharmacol. 2024, 15, 1435359. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.; Liong, C.Y.; Jemain, A.A. Partial least squares-discriminant analysis (PLS-DA) for classification of high-dimensional (HD) data: A review of contemporary practice strategies and knowledge gaps. Analyst 2018, 143, 3526–3539. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xia, Y. Pretreating and normalizing metabolomics data for statistical analysis. Genes. Dis. 2024, 11, 100979. [Google Scholar] [CrossRef]
- Kang, Y.P.; Lee, S.B.; Lee, J.M.; Kim, H.M.; Hong, J.Y.; Lee, W.J.; Choi, C.W.; Shin, H.K.; Kim, D.J.; Koh, E.S.; et al. Metabolic Profiling Regarding Pathogenesis of Idiopathic Pulmonary Fibrosis. J. Proteome Res. 2016, 15, 1717–1724. [Google Scholar] [CrossRef]
- Mendez, K.M.; Broadhurst, D.I.; Reinke, S.N. Migrating from partial least squares discriminant analysis to artificial neural networks: A comparison of functionally equivalent visualisation and feature contribution tools using jupyter notebooks. Metabolomics 2020, 16, 17. [Google Scholar] [CrossRef]
- Saraswat, M.; Joenvaara, S.; Tohmola, T.; Sutinen, E.; Vartiainen, V.; Koli, K.; Myllarniemi, M.; Renkonen, R. Label-free plasma proteomics identifies haptoglobin-related protein as candidate marker of idiopathic pulmonary fibrosis and dysregulation of complement and oxidative pathways. Sci. Rep. 2020, 10, 7787. [Google Scholar] [CrossRef]
- Stoessel, D.; Stellmann, J.P.; Willing, A.; Behrens, B.; Rosenkranz, S.C.; Hodecker, S.C.; Sturner, K.H.; Reinhardt, S.; Fleischer, S.; Deuschle, C.; et al. Metabolomic Profiles for Primary Progressive Multiple Sclerosis Stratification and Disease Course Monitoring. Front. Hum. Neurosci. 2018, 12, 226. [Google Scholar] [CrossRef]
- Akarachantachote, N.; Chadcham, S.; Saithanu, K. Cutoff threshold of variable importance in projection for variable selection. Int. J. Pure Appl. Math. 2014, 94, 307–322. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Baffet, G.; Theret, N. Molecular and tissue alterations of collagens in fibrosis. Matrix Biol. 2018, 68–69, 122–149. [Google Scholar] [CrossRef] [PubMed]
- Kubow, K.E.; Vukmirovic, R.; Zhe, L.; Klotzsch, E.; Smith, M.L.; Gourdon, D.; Luna, S.; Vogel, V. Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat. Commun. 2015, 6, 8026. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; King, B.; Hamlin, A.J.; Saniepay, M.; Gorshkov, K.; Barker, G.; Ziegler, M.; Mukundan, S.; Cvijic, M.E.; Schwarzbauer, J.E. Identification of a fibronectin-binding protein signature associated with idiopathic pulmonary fibrosis. Cells Dev. 2024, 179, 203941. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.; Raghunath, M.; Vogel, V. Fibrillar fibronectin plays a key role as nucleator of collagen I polymerization during macromolecular crowding-enhanced matrix assembly. Biomater. Sci. 2019, 7, 4519–4535. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Holze, H.; Kirsch, R.; Nastou, K.C.; Cuesta-Astroz, Y.; Rattei, T.; Szklarczyk, D.; von Mering, C.; Jensen, L.J. Cytoscape stringApp 2.0: Analysis and Visualization of Heterogeneous Biological Networks. J. Proteome Res. 2023, 22, 637–646. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Nastou, K.; Koutrouli, M.; Kirsch, R.; Mehryary, F.; Hachilif, R.; Hu, D.; Peluso, M.E.; Huang, Q.; Fang, T.; et al. The STRING database in 2025: Protein networks with directionality of regulation. Nucleic Acids Res. 2025, 53, D730–D737. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.E.; Suto, M.J. Thrombospondin-1 regulation of latent TGF-beta activation: A therapeutic target for fibrotic disease. Matrix Biol. 2018, 68–69, 28–43. [Google Scholar] [CrossRef]
- Rosini, S.; Pugh, N.; Bonna, A.M.; Hulmes, D.J.S.; Farndale, R.W.; Adams, J.C. Thrombospondin-1 promotes matrix homeostasis by interacting with collagen and lysyl oxidase precursors and collagen cross-linking sites. Sci. Signal 2018, 11, eaar2566. [Google Scholar] [CrossRef]
- Qu, Y.; Olonisakin, T.; Bain, W.; Zupetic, J.; Brown, R.; Hulver, M.; Xiong, Z.; Tejero, J.; Shanks, R.M.; Bomberger, J.M.; et al. Thrombospondin-1 protects against pathogen-induced lung injury by limiting extracellular matrix proteolysis. JCI Insight 2018, 3, e96914. [Google Scholar] [CrossRef]
- Bagavandoss, P.; Wilks, J.W. Specific inhibition of endothelial cell proliferation by thrombospondin. Biochem. Biophys. Res. Commun. 1990, 170, 867–872. [Google Scholar] [CrossRef]
- Kaur, S.; Bronson, S.M.; Pal-Nath, D.; Miller, T.W.; Soto-Pantoja, D.R.; Roberts, D.D. Functions of Thrombospondin-1 in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 4570. [Google Scholar] [CrossRef]
- Underwood, P.A.; Bean, P.A.; Cubeddu, L. Human endothelial cells grow poorly on vitronectin: Role of PAI-1. J. Cell Biochem. 2001, 82, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Weinstat-Saslow, D.L.; Zabrenetzky, V.S.; VanHoutte, K.; Frazier, W.A.; Roberts, D.D.; Steeg, P.S. Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res. 1994, 54, 6504–6511. [Google Scholar]
- Olsen, K.C.; Sapinoro, R.E.; Kottmann, R.M.; Kulkarni, A.A.; Iismaa, S.E.; Johnson, G.V.; Thatcher, T.H.; Phipps, R.P.; Sime, P.J. Transglutaminase 2 and its role in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011, 184, 699–707. [Google Scholar] [CrossRef]
- Ferdous, Z.; Wei, V.M.; Iozzo, R.; Hook, M.; Grande-Allen, K.J. Decorin-transforming growth factor- interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J. Biol. Chem. 2007, 282, 35887–35898. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, J.; Liu, Y. The extracellular matrix glycoprotein fibrillin-1 in health and disease. Front. Cell Dev. Biol. 2023, 11, 1302285. [Google Scholar] [CrossRef] [PubMed]
- Baghy, K.; Szakadati, H.; Kovalszky, I. Decorin the antifibrotic proteoglycan and its progression in therapy. Am. J. Physiol. Cell Physiol. 2025, 328, C1853–C1865. [Google Scholar] [CrossRef]
- Dolivo, D.M.; Larson, S.A.; Dominko, T. Fibroblast Growth Factor 2 as an Antifibrotic: Antagonism of Myofibroblast Differentiation and Suppression of Pro-Fibrotic Gene Expression. Cytokine Growth Factor. Rev. 2017, 38, 49–58. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef] [PubMed]
- Onyeisi, J.O.S.; Nader, H.B.; Lopes, C.C. Effects of syndecan-4 silencing on the extracellular matrix remodeling in anoikis-resistant endothelial cells. Cell Biol. Int. 2024, 48, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.S.; Wither, M.J.; Hill, R.C.; Dzieciatkowska, M.; D’Alessandro, A.; Reisz, J.A.; Hansen, K.C. Hydroxylamine Chemical Digestion for Insoluble Extracellular Matrix Characterization. J. Proteome Res. 2017, 16, 4177–4184. [Google Scholar] [CrossRef]
- Harper, J.W.; Bennett, E.J. Proteome complexity and the forces that drive proteome imbalance. Nature 2016, 537, 328–338. [Google Scholar] [CrossRef]
- Schwartz, M.A. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb. Perspect. Biol. 2010, 2, a005066. [Google Scholar] [CrossRef] [PubMed]
- Pezzoli, D.; Di Paolo, J.; Kumra, H.; Fois, G.; Candiani, G.; Reinhardt, D.P.; Mantovani, D. Fibronectin promotes elastin deposition, elasticity and mechanical strength in cellularised collagen-based scaffolds. Biomaterials 2018, 180, 130–142. [Google Scholar] [CrossRef]
- Sottile, J.; Hocking, D.C. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol. Biol. Cell 2002, 13, 3546–3559. [Google Scholar] [CrossRef]
- Sottile, J.; Shi, F.; Rublyevska, I.; Chiang, H.Y.; Lust, J.; Chandler, J. Fibronectin-dependent collagen I deposition modulates the cell response to fibronectin. Am. J. Physiol. Cell Physiol. 2007, 293, C1934–C1946. [Google Scholar] [CrossRef]
- Subramanian Balachandar, V.A.; Steward, R.L., Jr. Extracellular matrix composition alters endothelial force transmission. Am. J. Physiol. Cell Physiol. 2023, 325, C314–C323. [Google Scholar] [CrossRef]
- Mao, J.R.; Taylor, G.; Dean, W.B.; Wagner, D.R.; Afzal, V.; Lotz, J.C.; Rubin, E.M.; Bristow, J. Tenascin-X deficiency mimics Ehlers-Danlos syndrome in mice through alteration of collagen deposition. Nat. Genet. 2002, 30, 421–425. [Google Scholar] [CrossRef]
- van Dijk, F.S.; Ghali, N.; Demirdas, S.; Baker, D. TNXB-Related Classical-Like Ehlers-Danlos Syndrome. In GeneReviews((R)); Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Matsumoto, K.I.; Aoki, H. The Roles of Tenascins in Cardiovascular, Inflammatory, and Heritable Connective Tissue Diseases. Front. Immunol. 2020, 11, 609752. [Google Scholar] [CrossRef]
- Sakai, H.; Yokota, S.; Kajitani, N.; Yoneyama, T.; Kawakami, K.; Yasui, Y.; Matsumoto, K.I. A potential contribution of tenascin-X to blood vessel formation in peripheral nerves. Neurosci. Res. 2017, 124, 1–7. [Google Scholar] [CrossRef]
- Nizamoglu, M.; Koloko Ngassie, M.L.; Meuleman, R.A.; Banchero, M.; Borghuis, T.; Timens, W.; Nawijn, M.C.; Melgert, B.N.; Heijink, I.H.; Brandsma, C.A.; et al. Collagen type XIV is proportionally lower in the lung tissue of patients with IPF. Sci. Rep. 2023, 13, 19393. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, J.; Smaldone, S.; Ramirez, F. Fibrillin assemblies: Extracellular determinants of tissue formation and fibrosis. Fibrogenesis Tissue Repair. 2010, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Macara, L.; Kingdom, J.C.; Kohnen, G.; Bowman, A.W.; Greer, I.A.; Kaufmann, P. Elaboration of stem villous vessels in growth restricted pregnancies with abnormal umbilical artery Doppler waveforms. Br. J. Obs. Gynaecol. 1995, 102, 807–812. [Google Scholar] [CrossRef]
- Macara, L.; Kingdom, J.C.; Kaufmann, P.; Kohnen, G.; Hair, J.; More, I.A.; Lyall, F.; Greer, I.A. Structural analysis of placental terminal villi from growth-restricted pregnancies with abnormal umbilical artery Doppler waveforms. Placenta 1996, 17, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ohmaru-Nakanishi, T.; Asanoma, K.; Fujikawa, M.; Fujita, Y.; Yagi, H.; Onoyama, I.; Hidaka, N.; Sonoda, K.; Kato, K. Fibrosis in Preeclamptic Placentas Is Associated with Stromal Fibroblasts Activated by the Transforming Growth Factor-beta1 Signaling Pathway. Am. J. Pathol. 2018, 188, 683–695. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef]
- Harn, H.I.; Wang, Y.K.; Hsu, C.K.; Ho, Y.T.; Huang, Y.W.; Chiu, W.T.; Lin, H.H.; Cheng, C.M.; Tang, M.J. Mechanical coupling of cytoskeletal elasticity and force generation is crucial for understanding the migrating nature of keloid fibroblasts. Exp. Dermatol. 2015, 24, 579–584. [Google Scholar] [CrossRef]
- Hsu, C.K.; Lin, H.H.; Harn, H.I.; Hughes, M.W.; Tang, M.J.; Yang, C.C. Mechanical forces in skin disorders. J. Dermatol. Sci. 2018, 90, 232–240. [Google Scholar] [CrossRef]
- Perie, L.; Houel, C.; Zambon, A.; Guere, C.; Vie, K.; Leroy-Dudal, J.; Vendrely, C.; Agniel, R.; Carreiras, F.; Picot, C.R. Impaired incorporation of fibronectin into the extracellular matrix during aging exacerbates the senescent state of dermal cells. Exp. Cell Res. 2024, 442, 114251. [Google Scholar] [CrossRef]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The role of cellular senescence in skin aging and age-related skin pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, Y.; Zeng, Y.; Yang, D.; Mo, J.; Zheng, Z.; Wang, J.; Zhang, Y.; Zhou, Z.; Zhong, X.; et al. Impaired angiogenesis in ageing: The central role of the extracellular matrix. J. Transl. Med. 2023, 21, 457. [Google Scholar] [CrossRef]
- Mongiat, M.; Andreuzzi, E.; Tarticchio, G.; Paulitti, A. Extracellular Matrix, a Hard Player in Angiogenesis. Int. J. Mol. Sci. 2016, 17, 1822. [Google Scholar] [CrossRef]
- Schunk, C.T.; Wang, W.; Sabo, L.N.; Taufalele, P.V.; Reinhart-King, C.A. Matrix stiffness increases energy efficiency of endothelial cells. Matrix Biol. 2024, 133, 77–85. [Google Scholar] [CrossRef]
- Jaiman, S.; Romero, R.; Bhatti, G.; Jung, E.; Gotsch, F.; Suksai, M.; Gallo, D.M.; Chaiworapongsa, T.; Kadar, N. The role of the placenta in spontaneous preterm labor and delivery with intact membranes. J. Perinat. Med. 2022, 50, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Lien, Y.C.; Zhang, Z.; Cheng, Y.; Polyak, E.; Sillers, L.; Falk, M.J.; Ischiropoulos, H.; Parry, S.; Simmons, R.A. Human Placental Transcriptome Reveals Critical Alterations in Inflammation and Energy Metabolism with Fetal Sex Differences in Spontaneous Preterm Birth. Int. J. Mol. Sci. 2021, 22, 7899. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.K.; Tolosa, J.E.; Mele, L.; Wapner, R.J.; Spong, C.Y.; Sorokin, Y.; Dudley, D.J.; Peaceman, A.M.; Mercer, B.M.; Thorp, J.M.; et al. Placental villous hypermaturation is associated with idiopathic preterm birth. J. Matern. Fetal Neonatal Med. 2013, 26, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Visser, L.; van Buggenum, H.; van der Voorn, J.P.; Heestermans, L.; Hollander, K.W.P.; Wouters, M.; de Groot, C.J.M.; de Boer, M.A. Maternal vascular malperfusion in spontaneous preterm birth placentas related to clinical outcome of subsequent pregnancy. J. Matern. Fetal Neonatal Med. 2021, 34, 2759–2764. [Google Scholar] [CrossRef]
- Committee on Obstetric Practice American Institute of Ultrasound in Medicine Society for Maternal–Fetal Medicine. Committee Opinion No 700: Methods for Estimating the Due Date. Obs. Gynecol. 2017, 129, e150–e154. [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.; Boyd, T.K.; Brundler, M.A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Kaukonen, R.; Jacquemet, G.; Hamidi, H.; Ivaska, J. Cell-derived matrices for studying cell proliferation and directional migration in a complex 3D microenvironment. Nat. Protoc. 2017, 12, 2376–2390. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal Sample Prepara-tion Method for Proteome Analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.T.; Leprevost, F.V.; Avtonomov, D.M.; Mellacheruvu, D.; Nesvizhskii, A.I. MSFragger: Ultrafast and Comprehensive Peptide Identification in Mass Spectrometry-Based Proteomics. Nat. Methods 2017, 14, 513–520. [Google Scholar] [CrossRef]
- Mellacheruvu, D.; Wright, Z.; Couzens, A.L.; Lambert, J.P.; St-Denis, N.A.; Li, T.; Miteva, Y.V.; Hauri, S.; Sardiu, M.E.; Low, T.Y.; et al. The CRAPome: A Contaminant Repository forAffin-ity Purification-Mass Spectrometry Data. Nat. Methods 2013, 10, 730–736. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]

| TC (n = 6) | PTC (n = 6) | FGRa/r (n = 6) | |
|---|---|---|---|
| Maternal age (years) | 34 ± 1.4 | 29 ± 2.7 | 33 ± 3.2 |
| Gestational age at delivery (weeks) * | 39.0 ± 0.07 | 32.4 ± 0.7 | 28.0 ± 1.5 |
| Doppler velocimetry (absent/reversed) # | 0/6 | 0/6 | 4/2 |
| Neonatal birthweight (g) ^ | 3359 ± 157 | 1931 ± 129 | 602 ± 60 |
| Neonatal birthweight percentile @ | 53.2 ± 11.4 | 63.7 ± 6.9 | 4.3 ± 1.2 |
| Placental weight (g) & | 520 ± 30 | 288 ± 23 | 152 ± 33 |
| Neonatal sex (M/F) | 4/2 | 3/3 | 5/6 |
| Route of delivery (vaginal/C-section) # | 0/6 | 6/6 | 0/6 |
| iECM Proteins Significantly Higher in FGRa/r as Compared to Both TC and PTC | |||
| Gene Symbol | Protein | Category | FDR p-Value |
| CTSB | Cathepsin B | ECM regulator | 0.0081124 |
| FN1 | Fibronectin | Glycoprotein | 0.0091698 |
| COL4A2 | Collagen IV a2 | Network-forming collagen | 0.0097667 |
| THBS1 | Thrombospondin-1 | Glycoprotein | 0.01437 |
| TGM2 | Transglutaminase-2 | ECM regulator | 0.01972 |
| S100A11 | Protein S100-A11 | Secreted factor | 0.01972 |
| COL4A1 | Collagen IV a1 | Network-forming collagen | 0.023061 |
| VTN | Vitronectin | Glycoprotein | 0.039511 |
| iECM Proteins Significantly Lower in FGRa/r as Compared to Both TC and PTC | |||
| Gene Symbol | Protein | Category | FDR p-Value |
| TNXB | Tenascin-X | Glycoprotein | 0.00080041 |
| SVEP1 | Sushi, von Willebrand factor type A, EGF, and pentraxin domain-containing protein 1 | Glycoprotein | 0.0015087 0.0015087 |
| COL14A1 | Collagen XIV a1 | FACIT collagen | 0.0059602 |
| FBN1 | Fibrillin-1 | Glycoprotein | 0.0065528 |
| COL16A1 | Collagen XVI a1 | FACIT collagen | 0.0065528 |
| CRIM1 | Cysteine-rich motor neuron 1 protein | Glycoprotein | 0.0070293 |
| GPC6 | Glypican-6 | EAP | 0.0081931 |
| PZP | Pregnancy zone protein | ECM regulator | 0.0081931 |
| ADAM10 | Disintegrin and metalloproteinase domain-containing protein 10 | ECM regulator | 0.0091698 |
| LAMB2 | Laminin b2 | Glycoprotein | 0.0091698 |
| FGF2 | Fibroblast growth factor 2 | Secreted factor | 0.0091698 |
| LOXL4 | Lysyl oxidase homolog 4 | ECM regulator | 0.0091698 |
| ADAM12 | Disintegrin and metalloproteinase domain-containing protein 12 | ECM regulator | 0.0091698 |
| SERPINB1 | Leukocyte elastase inhibitor | ECM regulator | 0.009169 |
| AEBP1 | Adipocyte enhancer-binding protein 1 | Glycoprotein | 0.0097667 |
| SRPX2 | Sushi repeat-containing protein 2 | Glycoprotein | 0.0097667 |
| LTBP3 | Latent transforming growth factor beta-binding protein 3 | Glycoprotein | 0.01527 |
| PCOLCE | Procollagen C-endopeptidase enhancer 1 | Glycoprotein | 0.01527 |
| LAMA5 | Laminin a5 | Glycoprotein | 0.015623 |
| ANXA3 | Annexin A3 | EAP | 0.015711 |
| F13A1 | Coagulation factor XIII A chain | ECM regulator | 0.017934 |
| DCN | Decorin | Proteoglycan | 0.017934 |
| VWA1 | Von Willebrand Factor 1 | Glycoprotein | 0.019643 |
| CTSC | Dipeptidyl peptidase 1 | ECM regulator | 0.01972 |
| LAMA4 | Laminin a4 | Glycoprotein | 0.01972 |
| ADAMTS4 | A disintegrin and metalloproteinase with thrombospondin motifs 4 | ECM regulator | 0.021216 |
| SDC4 | Syndecan-4 | EAP | 0.023202 |
| LGALS8 | Galectin-8 | EAP | 0.023202 |
| PLXNB2 | Plexin B2 | EAP | 0.032235 |
| ANXA8 | Annexin A8 | EAP | 0.033602 |
| PAMR1 | Inactive serine protease PAMR1 | ECM regulator | 0.033804 |
| CTHRC1 | Collagen triple helix repeat-containing protein 1 | Glycoprotein | 0.037121 |
| THSD4 | Thrombospondin type 1-domain-containing protein 4 | Glycoprotein | 0.043657 |
| SEMA3B | Semaphorin-3B | EAP | 0.043985 |
| COL15A1 | Collagen XVa1 | Multiplexin collagen | 0.045474 |
| CRELD1 | Protein disulfide isomerase CRELD1 | Glycoprotein | 0.047579 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginocchio, S.; McCabe, M.C.; Flockton, A.R.; Gumina, D.L.; Hansen, K.C.; Ji, S.; Su, E.J. Unraveling the Matrix: Proteomic Profiling Reveals Stromal ECM Dysregulation in Severe Early-Onset Fetal Growth Restriction. Int. J. Mol. Sci. 2025, 26, 11179. https://doi.org/10.3390/ijms262211179
Ginocchio S, McCabe MC, Flockton AR, Gumina DL, Hansen KC, Ji S, Su EJ. Unraveling the Matrix: Proteomic Profiling Reveals Stromal ECM Dysregulation in Severe Early-Onset Fetal Growth Restriction. International Journal of Molecular Sciences. 2025; 26(22):11179. https://doi.org/10.3390/ijms262211179
Chicago/Turabian StyleGinocchio, Stefano, Maxwell C. McCabe, Amanda R. Flockton, Diane L. Gumina, Kirk C. Hansen, Shuhan Ji, and Emily J. Su. 2025. "Unraveling the Matrix: Proteomic Profiling Reveals Stromal ECM Dysregulation in Severe Early-Onset Fetal Growth Restriction" International Journal of Molecular Sciences 26, no. 22: 11179. https://doi.org/10.3390/ijms262211179
APA StyleGinocchio, S., McCabe, M. C., Flockton, A. R., Gumina, D. L., Hansen, K. C., Ji, S., & Su, E. J. (2025). Unraveling the Matrix: Proteomic Profiling Reveals Stromal ECM Dysregulation in Severe Early-Onset Fetal Growth Restriction. International Journal of Molecular Sciences, 26(22), 11179. https://doi.org/10.3390/ijms262211179





